Abstract
Borrelia burgdorferi OspC is required for the spirochete to establish infection in a mammal by tick transmission or needle inoculation. After a brief essential period, the protein no longer is required and the gene can be shut off. Using a system in which spirochetes contain only an unstable wild-type copy of the ospC gene, we can obtain mice persistently infected with bacteria lacking OspC. We implanted pieces of infected mouse skin subcutaneously in naïve mice, using donors carrying wild-type or ospC mutant spirochetes, and found that both could infect mice by this method, with similar numbers of wild-type or ospC mutant spirochetes disseminated throughout the tissues of recipient mice. Recipient mouse immune responses to tissue transfer-mediated infection with wild-type or ospC mutant spirochetes were similar. These experiments demonstrate that mammalian host-adapted spirochetes can infect and disseminate in mice in the absence of OspC, thereby circumventing this hallmark of tick-derived or in vitro-grown spirochetes. We propose a model in which OspC is one of a succession of functionally equivalent, essential proteins that are synthesized at different stages of mammalian infection. In this model, another protein uniquely present on host-adapted spirochetes performs the same essential function initially fulfilled by OspC. The strict temporal control of B. burgdorferi outer surface protein gene expression may reflect immunological constraints rather than distinct functions.
Vector-borne pathogens, like Borrelia burgdorferi, must adapt to two very different environments to be maintained in their natural infectious cycles. B. burgdorferi, which cycles between ticks and small mammals, dramatically alters its protein synthesis patterns as it moves from vector to host and back again (11, 32, 37, 45). One of the B. burgdorferi proteins whose synthesis is differentially regulated throughout the infectious cycle is outer surface protein C (OspC), whose production increases when infected ticks feed (44, 45) and shuts off within the first few weeks of mammalian infection (11, 31). OspC was first identified as a B. burgdorferi surface protein that was recognized by antibodies produced early during mammalian infection (7, 53, 54). The analysis of B. burgdorferi mutants has demonstrated that the OspC protein is required for the spirochete to establish infection in a mammal (22, 50-52), following either tick bite or needle inoculation. During persistent infection, however, the OspC protein is dispensable (52), consistently with the abovementioned cessation of protein and transcript production during the course of a normal mammalian infection. In fact, the continued expression of the gene is deleterious for B. burgdorferi when the spirochete infects an immunocompetent animal (57).
When inoculated intradermally into a mouse, ospC mutant spirochetes can be reisolated within the first 24 h of infection but not subsequently (51). In addition to the early requirement implicated by the inability of ospC mutant spirochetes to survive within a mammal, OspC also has been hypothesized to play roles in transmission by ticks (21, 38), dissemination (14, 30, 42, 48, 55), and the colonization of heart tissue (3). These potential roles remain controversial, since either confounding evidence has been presented or the studies rely on correlations that have yet to be conclusively confirmed by experimental data (2, 15, 22, 52).
Since OspC production is not required for persistent infection by B. burgdorferi, it is possible to derive mice infected with ospC mutant spirochetes by inoculating them with the complemented mutant ospCK1/pBSV2G-ospC. The complementing plasmid (pBSV2G-ospC) is unstable during mammalian infection (52), perhaps because it confers the continued production of the OspC protein, so the host immune response selects for a population that has lost pBSV2G-ospC. One explanation for how these host-adapted spirochetes are able to survive in the absence of OspC is that the protein performs a discrete function required only in the early stages of infection. Alternatively, OspC may perform a function that is required throughout infection but that is performed by another protein after infection has been established. These two models are difficult to distinguish experimentally, although characterizing infection by host-adapted spirochetes should yield insight.
To study the early steps of the B. burgdorferi infection of mammals, ideally one wants a uniform population of bacteria primed for infection. Natural infection by tick feeding leads, by definition, to a primed population, although even this population is somewhat heterogeneous (37). Unfortunately, tick feeding yields a small and variable inoculum, and the timing of transmission is difficult to synchronize. Infection with bacteria that have undergone various degrees of host adaptation is an alternative, although the production of OspC and other products differs between tick-transmitted and host-adapted bacteria (1, 24, 37). One source of host-adapted bacteria is spirochetes that have been grown in dialysis membrane chambers (DMCs) implanted in rats or mice (1, 12, 13). Bacteria within DMCs are exposed to low-molecular-weight host factors capable of passing through the dialysis membrane, but they are protected from host cells and antibodies. Growth under these conditions leads to the decreased production of OspA and the increased production of OspC and some of the other proteins whose synthesis is upregulated in the mammalian host (1). A defined inoculum of host-adapted bacteria derived by growth in DMCs can be needle inoculated into naïve animals. A second way to obtain host-adapted bacteria is to use tissue from a persistently infected animal. Infection by these bacteria can be directly transferred from one animal to another by placing a disk of infected skin beneath the skin of a recipient mouse (4, 5, 13). We previously have used both of these methods to address the role of OspC in mouse infection. In the first study, spirochetes with an insertion in the ospC gene (ospC7) were grown in DMCs in rats until their protein profiles altered to reflect host adaptation. Such host-adapted bacteria were directly inoculated into outbred mice, in which case ospC mutant spirochetes were found to be noninfectious (50). In a second study, using an ospC deletion mutant (ospCK1), ear tissue from outbred mice persistently infected with the mutant was transferred to naïve outbred mice. Again, the ospC mutant spirochetes did not infect mice (52). Since host adaptation within DMCs likely is incomplete and tissue transfer between outbred mice may stimulate an immune response to the foreign tissue, in addition to a response to the spirochetes, we readdressed the role of OspC during infection with host-adapted spirochetes.
In the current study, we performed tissue transfers with wild-type and ospC mutant spirochetes within and between various inbred and outbred mouse strains. We found, in contrast to our earlier study, that the ospC mutant and wild-type spirochetes were equally capable of infection by tissue transfer. Since the outcomes of infections with wild-type and ospC mutant spirochetes using this method were indistinguishable, we demonstrate that this artificial method allows the bypass of the essential function of OspC. We conclude that OspC, whose production is essential for the infection of mice by tick bite and needle inoculation, is dispensable for infection by tissue transfer with host-adapted spirochetes. Furthermore, these results suggest that either OspC plays no role in dissemination or tissue colonization in a mammal, or another protein produced by host-adapted spirochetes can substitute for this function of OspC.
MATERIALS AND METHODS
B. burgdorferi strains and culture conditions.
B. burgdorferi strains were derived from the infectious B31 clone A3 (19). The ospCK1 mutant has a complete deletion of the ospC coding region, which was replaced with a flaBpkan fusion (8, 52). The mutant was complemented using a wild-type copy of the ospC gene cloned into the shuttle vector pBSV2G to make pBSV2G-ospC (18, 52). This complementation construct retains the native ospC promoter, including the putative ospC operator sequence (56).
To assess the serological response to OspC by infected animals, we constructed and made lysates of a B. burgdorferi strain that reproducibly produced OspC during growth in culture. This step was necessary because neither our wild-type strain (A3) nor the complemented mutant (ospCK1/pBSV2G-ospC), which we used for the in vivo studies, consistently produces OspC during growth in culture (52). The constitutive strain contains a plasmid that confers the production of ospC (pBSV2G-flaBpospC), which was constructed by the following process. We amplified the flaB promoter with primers 1 and 2 and amplified the ospC gene with primers 3 and 5 (Table 1). The resultant PCR products were separately cloned into pCR2.1-Topo (Invitrogen, Carlsbad, CA). A pCR2.1-ospC plasmid was linearized by digestion with NdeI and XhoI and ligated with an NdeI-XhoI fragment containing the flaB promoter. The resultant flaBpospC fusion was excised with XhoI and HindIII and ligated with SalI- and HindIII-digested pBSV2G (18), resulting in pBSV2G-flaBpospC. This plasmid was purified, confirmed to have the correct sequence, and transformed into ospCK1 by electroporation (19, 43) after modification by growth in another B. burgdorferi strain that contained lp25 (28), yielding strain ospCK1/pBSV2G-flaBpospC. A similar plasmid was constructed previously and used by Xu and colleagues to demonstrate that continued production of OspC during B. burgdorferi infection of an immunocompetent animal is deleterious (57). B. burgdorferi strains were grown in Barbour-Stoenner-Kelly II (BSKII) medium at 35°C with 2.5% CO2.
TABLE 1.
Oligonucleotides and TaqMan probes used in this study
| No. | Name | Sequence (5′-3′) | Reference or source |
|---|---|---|---|
| Oligonucleotide | |||
| 1 | 5′flaBp-XhoI | CCGCTCGAGCTGTCGCCTCTTGTGGCTTC | 26 |
| 2 | 3′flaBp-NdeI | GATTGATAATCATATGTCATTCCTCCATG | 8 |
| 3 | 5′ospC-NdeI | CATATGAAAAAGAATACATTAAGTGCA | This work |
| 4 | ospC33 | CAAGATATTGAAGAATTTGA | 52 |
| 5 | ospC34 | GACTTTATTTTTCCAGTTAC | 52 |
| 6 | flg5′-AvrII | CCTAGGTAATACCCGAGCTTCAAGGAG | This work |
| 7 | aacC13′-NheI | GCTAGCCGATCTCGGCTTGAACG | 18 |
| 8 | flaB-for | TCTTTTCTCTGGTGAGGGAGCT | 27 |
| 9 | flaB-rev | TCCTTCCTGTTGAACACCCTCT | 27 |
| 10 | nid-for | CACCCAGCTTCGGCTCAGTA | 27 |
| 11 | nid-rev | TCCCCAGGCCATCGGT | 27 |
| TaqMan probe | |||
| 1 | flaB | AAACTGCTCAGGCTGCACCGGTTC | 27 |
| 2 | nid | CGCCTTTCCTGGCTGACTTGGACA | 27 |
Assessing seroreactivity to B. burgdorferi proteins and OspC by immunoblotting.
To assess infection and seroreactivity to OspC, we used lysates of B. burgdorferi strains that produce no OspC (ospCK1) and that constitutively produce OspC (ospCK1/pBSV2G-flaBpospC; described above). To confirm seroreactivity with OspC, we used lysates of Escherichia coli containing a pET29b-His-ospC plasmid (from which the production of His-tagged nonlipidated OspC protein is induced after the addition of isopropyl-β-d-thiogalactopyranoside [IPTG; a gift from R. Gilmore, CDC, Fort Collins, CO]) or pET29b with no insert. These lysates were run on 12.5% acrylamide sodium dodecyl sulfate (SDS) gels, and proteins were electroblotted to nitrocellulose membranes and incubated with mouse sera (diluted 1:200) or rabbit anti-OspC (diluted 1:1,000 [46]), washed with TBS-Tween, incubated with peroxidase-conjugated goat anti-mouse immunoglobulins (Ig) or goat anti-rabbit IgG (Sigma, St. Louis, MO), and washed as previously described (22). Bound antibodies were detected using the Supersignal substrate (Thermo Scientific, Rockford, IL), followed by exposure to X-ray film.
OspC protein purification.
A 5-ml overnight culture of E. coli BL21(DE3)/pLysS containing pET29b-His-ospC (a gift from R. Gilmore) was diluted into 500 ml of L broth supplemented with 40 μg/ml kanamycin and grown to an optical density at 600 nm of ∼0.5. IPTG was added to 1 mM, and the culture was grown for an additional 2.5 h. Cells were pelleted, washed twice with phosphate-buffered saline (PBS) (pH 7.5) containing 900 mM CaCl2 and 500 mM MgCl2, and frozen. The pellet was resuspended in 20 ml of the PBS-CaCl2-MgCl2 buffer, sonicated, and clarified by centrifugation. The protein lysate was stabilized by adding 100 μM phenylmethylsulfonyl fluoride and 2 mM β-mercaptoethanol. A 1.5-ml slurry of Talon Superflow Metal Affinity Reagent (BD Biosciences Clontech, Palo Alto, CA) was equilibrated into wash buffer (PBS [pH 7.5], 2 mM β-mercaptoethanol, 10 mM imidazole, 300 mM NaCl). The slurry then was mixed with the clarified lysate, rocked for 1 h at 4°C, and applied to a chromatography column. The column was washed with approximately 50 column volumes of wash buffer and then eluted with the same buffer containing 200 mM imidazole. Fractions were assayed for the presence of OspC by Coomassie blue-stained SDS-polyacrylamide gel electrophoresis and immunoblotting. Although a significant amount of the OspC protein present in the crude lysate was found in the flowthrough and wash fractions, the eluate contained two proteins visible by Coomassie blue staining, both of which reacted with rabbit anti-OspC and pooled serum from mice infected with B. burgdorferi. Based on Coomassie blue staining, the purified protein was approximately 95% pure. Imidazole was removed by repeated dilution into PBS, followed by being spun through Amicon Ultra centrifugal filter devices (Millipore, Billerica, MA).
VlsE C6 peptide ELISA.
A commercially available kit was used for the VlsE C6 peptide enzyme-linked immunosorbent assay (ELISA) (Immunetics, Boston, MA). The tests were performed according to the instructions, with the following exceptions. First, the sera were tested at a dilution of 1:100, and second, the secondary antibody was peroxidase-conjugated goat anti-mouse IgG (diluted 1:10,000; Sigma).
ELISA with purified OspC.
The procedures for ELISAs with purified OspC and whole-cell lysate were modified from a previously described protocol (40). Purified OspC protein was incubated on an Ni-nitrilotriacetic acid (Ni-NTA) plate (Qiagen) for 2 h at room temperature and overnight at 4°C (200 μl/well; 320 μg/ml). The plate was washed three times with PBS-Tween 20 (0.05%) and blocked for at least 1 h with PBS-Tween containing 5% horse serum and 0.001% dextran sulfate (diluent). The plate was again washed three times with PBS-Tween, and duplicate wells were incubated for at least 1 h with various sera diluted in diluent. The wells were washed three times with PBS-Tween and incubated for at least 1 h with peroxidase-conjugated goat anti-mouse Ig (diluted 1:10,000; Sigma). After a final three washes with PBS-Tween, wells were incubated with substrate [50% 2,2′-azino-di-(3-ethyl-benzthiazoline sulfonate)] until color developed, at which time the absorbance at 405 nm was determined using a Labsystems Multiskan Plus microtiter plate reader.
ELISA with whole-cell antigen.
B. burgdorferi whole-cell lysate was prepared from a 500-ml culture of B31 A3. The bacteria were pelleted, washed twice in PBS, and resuspended in 30 ml PBS. After sonication for 2 min at a setting of 5, the protein concentration was quantitated by Bio-Rad protein assay. The protein was diluted to at least 10 μg/ml, and 50 μl per well was placed in Immulon 2 plates (Thermo Scientific) and allowed to dry overnight. Subsequent steps were the same as those described for the OspC ELISA.
Tissue transfer-mediated infection.
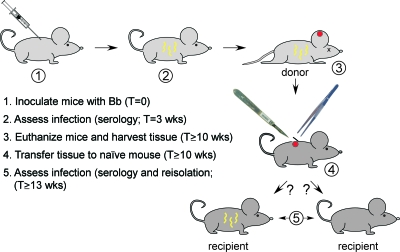
All animal experiments were performed using protocols approved by the Animal Care and Use Committee of the Rocky Mountain Laboratories and according to the guidelines of the National Institutes of Health. Rocky Mountain Laboratories is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice were purchased from Harlan Sprague-Dawley (for C3H/HeN, C3H/HeN SCID, and C57BL/6; Indianapolis, IN) or reared at the Rocky Mountain Laboratories (RML mice). See Fig. 1 for a schematic of the entire process of infection by tissue transfer. Mice were needle inoculated with 5 × 103 B. burgdorferi organisms (4 × 103 intraperitoneally and 1 × 103 subcutaneously). A previous study determined the 50% infectious doses of A3 and ospCK1/pBSV2G-ospC to be ∼1,000 to 3,000 spirochetes by needle inoculation (52). Three weeks postinoculation, mice were retroorbitally bled and their sera assessed for serological reactivity with B. burgdorferi proteins. During the process of infection, spirochetes within immunocompetent mice inoculated with ospCK1/pBSV2G-ospC lose the complementing plasmid (pBSV2G-ospC) (52). We use the name ospCK1* to describe ospC mutant spirochetes derived by this process. We previously determined that the complete loss of pBSV2G-ospC from spirochetes infecting immunocompetent mice inoculated with ospCK1/pBSV2G-ospC had occurred by 10 weeks postinoculation (52). Accordingly, mice were euthanized after at least 10 weeks of infection, and 3-mm punch biopsies were excised from one ear. Two punch biopsies, each containing approximately 500 to 1,000 spirochetes (as determined by the quantitative PCR of DNA from single biopsies or the equivalent proportion of an ear; see below) were placed beneath the dorsal lumbar skin of an individual recipient mouse. The reisolation of spirochetes was attempted from the remainders of the ears, along with bladders and ankle joints of donor mice. To confirm the loss of the complementing plasmid from spirochetes in persistently infected donor mice that had been inoculated with ospCK1/pBSV2G-ospC, reisolates from all tissues were plated in solid medium, and 20 to 24 single colonies per reisolate were screened for the presence of pBSV2G-ospC using primers 6 and 7 (Table 1). DNA also was prepared from reisolate cultures and PCR screened for the ospC locus (primers 4 and 5) and the gentamicin resistance cassette (aacC1; primers 6 and 7). Only the mutant ospC locus was detected, and the screening for aacC1 was negative, except for a few weak bands. Ambiguous aacC1 screening was shown to be spurious by passing the reisolate cultures into medium containing gentamicin. No detectable spirochetes survived, confirming the absence of the complementation shuttle vector that confers gentamicin resistance. After 3 weeks, recipient mice were euthanized and reisolation was attempted from ears, bladders, ankle joints, and tissue transfer sites. In a few cases, tissues were frozen for DNA extraction and quantitative PCR.
FIG. 1.
Tissue transfer-mediated infection. Schematic showing major steps of tissue transfer-mediated infection. The time between steps 2 and 3 allows the loss of pBSV2G-ospC from spirochetes in mice inoculated with ospCK1/pBSV2G-ospC. At step 3, in addition to tissue transfer, spirochetes were isolated from tissues and plasmid loss confirmed. Likewise, at step 5, spirochetes were reisolated from tissues to confirm infection and lack of pBSV2G-ospC.
DNA isolation and TaqMan analysis.
DNA was prepared from ear punch biopsies and other tissues by a method involving collagenase, proteinase K, and RNase digestion, combined with phenol-chloroform and chloroform extractions and ethanol precipitations (27, 47). After the final precipitation, DNA samples were resuspended in 200 μl H2O and further purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA). Samples were diluted to 50 μg/ml DNA, and B. burgdorferi and mouse genome copies were assessed using TaqMan analysis with primer-probe sets for flaB (primers 8 and 9 and probe 1) and nid (primers 10 and 11 and probe 2) (Table 1) (27).
RESULTS
Successful infection by tissue transfer between inbred immunocompetent and SCID mice for both wild-type and ospC mutant bacteria.
Since our previous study suggested that the ospC mutant was unable to infect by tissue transfer between outbred mice (52), we next tested whether inbred mice could be infected with the ospC mutant by tissue transfer. We also assessed the role of acquired immunity in the recipient mouse by attempting tissue transfer-mediated infection of recipients with severe combined immunodeficiency (SCID). To accomplish this, we used a previously described protocol to produce mice persistently infected with wild-type or ospC mutant spirochetes (52). In this scenario, mice are inoculated with the complemented ospC mutant ospCK1/pBSV2G-ospC. The presence of the complementing plasmid (pBSV2G-ospC) is detrimental to the spirochete during the persistent infection of immunocompetent mice, so the plasmid is lost over time from the spirochete population, yielding mice infected only with the ospC mutant spirochetes we call ospCK1*.
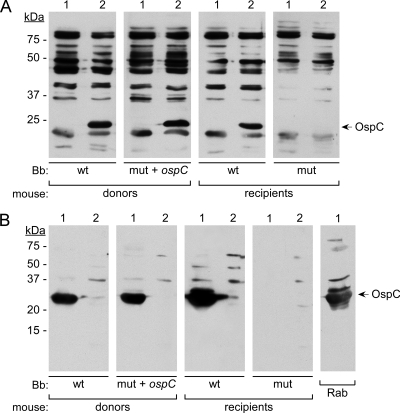
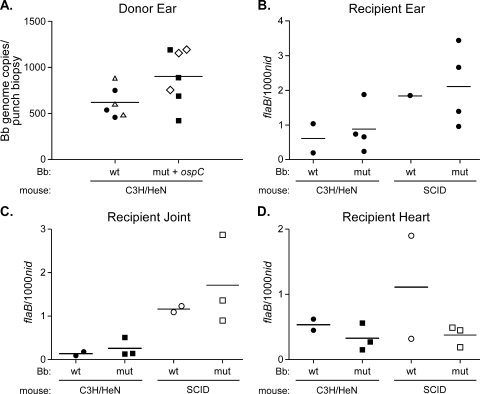
To initiate the tissue transfer study (Fig. 1), wild-type C3H/HeN mice were inoculated with either A3 (wild-type) or ospCK1/pBSV2G-ospC bacteria at a dose of 5 × 103 spirochetes per animal. After 3 weeks, the mice were bled and the infection status was determined by assessing seroreactivity to B. burgdorferi proteins (Fig. 2A). Fifteen weeks later, when pBSV2G-ospC had been lost from spirochetes persisting in mice inoculated with ospCK1-pBSV2G-ospC, seropositive donor mice were euthanized and 3-mm ear punch biopsies were removed from one ear for transfer to recipient mice. Two ear punch biopsies, each of which contains approximately 500 to 1,000 spirochetes (as determined by quantitative PCR) (Fig. 3A), were placed beneath the dorsal lumbar skin of individual wild-type C3H/HeN or isogenic SCID mice. We successfully cultured spirochetes from ankle joint, bladder, and the remaining ear of seropositive donor mice, confirming that they were infected. We also confirmed that reisolates from mice initially inoculated with ospCK1/pBSV2G-ospC had indeed lost the complementing plasmid (i.e., carried ospCK1*), as described in Materials and Methods.
FIG. 2.
Representative immunoblot showing reactivity of seropositive mouse sera to B. burgdorferi and recombinant OspC protein. (A) Immunoblot showing reactivity of seropositive mouse sera to lysates of B. burgdorferi lacking OspC (lanes 1) or producing OspC (lanes 2). Lanes were derived from a blot of a single gel that was cut into strips for subsequent incubation with various sera. Donors were infected by needle inoculation with the B. burgdorferi (Bb) strains indicated below the lanes, and recipients were infected by subcutaneous implantation of infected ear tissue from the corresponding donor mice. Reactivity to OspC was not found for any sera (0/13 tested) from recipients infected with ospCK1* bacteria. (B) Immunoblot showing the reactivity of representative mouse sera and rabbit anti-OspC antibody (46) to E. coli BL21(DE3)/pLysS containing either pET29b-ospC (lanes 1) or pET29b alone (lanes 2) after induction with IPTG. Rab, rabbit anti-OspC; wt, A3; mut+ospC, ospCK1/pBSV2G-ospC; mut, ospCK1*. kDa, migration positions of molecular mass markers with the indicated sizes (in kilodaltons). The mobility of OspC is indicated on the right.
FIG. 3.
Spirochete loads in infected mouse tissues. (A) DNA was extracted from individual ear punch biopsies from infected mice 3 weeks postinoculation (open symbols) or remaining ear tissue from donor mice 18 weeks postinoculation (closed symbols). Spirochete genome copies per ear punch biopsy (or the equivalent proportion of an ear) were determined by TaqMan analysis, using primer-probe sets flaB (for B. burgdorferi [Bb] genomes) and nid (for mouse genomes). The numbers of spirochetes per ear punch biopsy in mice inoculated with wild-type (wt) or ospCK1/pBSV2G-ospC (mut+ospC) are not significantly different, as assessed by an unpaired two-tailed t test using the program GraphPad Prism 5. Spirochete densities in the transferred mouse ear tissue, as assessed by flaB copies/1,000 nid copies, were approximately 10-fold lower than densities 3 weeks after the tissue transfer shown in panel B (an average of 0.03 copies flaB/1,000 copies of nid, as opposed to 0.8 copies flaB/1,000 copies of nid for recipient mice). (B to D) Three weeks postinfection by tissue transfer with wild-type (A3) or ospC mutant (ospCK1*) bacteria, mice were euthanized and the heart, one ear, and one ankle joint were removed from each mouse and frozen. Infection was confirmed by serological analysis and the culture of bladder and remaining ears and ankle joints. DNA was isolated from infected ears, joints, and hearts, and the numbers of spirochete genome equivalents in tissues were determined by TaqMan analysis using primer-probe sets flaB (for B. burgdorferi genomes) and nid (for mouse genomes). Numbers are expressed as flaB copies per 1,000 nid copies. Circles, A3 (wt); squares, ospCK1* (mut); filled symbols, C3H/HeN mice; open symbols, C3H SCID mice. No statistically significant differences were observed between levels of wild-type and ospC mutant spirochete genomes derived from comparable tissues of the same mouse strain, as determined by one-way analysis of variance followed by Tukey's post test applied using the program GraphPad Prism 5.
Three weeks after tissue transfer, recipient mice were bled and euthanized. Infection was assessed by immunoblot analysis of serum (Fig. 2A), and the reisolation of spirochetes from ear, bladder, joint, and tissue transfer sites was attempted (Table 2). Both B. burgdorferi strains were able to infect both wild-type and SCID mice by tissue transfer. When recipient mice were infected, spirochetes were reisolated from every tissue tested. These results suggest that OspC is not required for spirochetes to infect either immunodeficient or immunocompetent mice by tissue transfer when both donors and recipients have the same inbred background. The ability of ospC mutant spirochetes to infect by tissue transfer in this experiment differed from our previous result (52), suggesting that some aspect of the inflammatory or innate immune response of the outbred mouse recipient to the transferred tissue was relevant to the requirement for OspC.
TABLE 2.
Tissue transfer-mediated infection of inbred wild-type and immunodeficient mice
| B. burgdorferi strain transferred | Mouse strain
|
Recipient infection
|
|||||
|---|---|---|---|---|---|---|---|
| Donor | Recipient | Micea | Earb | Bladderb | Jointb | Transfer siteb | |
| A3 (wild type) | C3H/HeN | C3H/HeN | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 |
| A3 (wild type) | C3H/HeN | C3H/HeN SCID | 2/2 | 2/2 | 1/1c | 2/2 | 2/2 |
| ospCK1* (ospC mutant)d | C3H/HeN | C3H/HeN | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| ospCK1* (ospC mutant)d | C3H/HeN | C3H/HeN SCID | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
Number of mice infected/number of mice receiving tissue transfer, confirmed by serology and reisolation.
Number of tissues positive/number of tissues from which reisolation was attempted.
One bladder culture was contaminated.
Spirochetes from donor mice inoculated with ospCK1/pBSV2G-ospC were demonstrated to have lost the complementing plasmid, as described in Materials and Methods, confirming that the mice were persistently infected with ospC mutant spirochetes, called ospCK1*.
Infection by tissue transfer between and within inbred and outbred strains of mice.
Since ospC mutant spirochetes were able to successfully infect naïve mice by tissue transfer between C3H/HeN mice, we wondered if such infections would be possible within other inbred strains, between quite different inbred strains, and between inbred and outbred strains. To test these possibilities, we inoculated the following mouse strains with wild-type (A3) or complemented ospC mutant (ospCK1/pBSV2G-ospC) spirochetes: C3H/HeN (H2k), C57BL/6 (H2b) (both inbred, but with different major histocompatibility locus alleles and many other genetic differences [39]), and RML (outbred). After checking for seroconversion (Fig. 2A) and allowing time for the complementing plasmid to be lost in mice inoculated with ospCK1/pBSV2G-ospC, ear punch biopsies from these mice were used for tissue transfer to various recipients (Tables 3 and 4).
TABLE 3.
Tissue transfer-mediated infection within inbred and outbred mouse strains
| B. burgdorferi strain transferred | Donor and recipient mouse strain | Recipient infection
|
||||
|---|---|---|---|---|---|---|
| Micea | Earb | Bladderb | Jointb | Transfer siteb | ||
| A3 (wild type) | C3H/HeN | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 |
| A3 (wild type) | C57BL/6 | 3/5 | 3/5 | 3/5 | 3/5 | 2/5 |
| A3 (wild type) | RML | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| ospCK1* (ospC mutant)c | C3H/HeN | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 |
| ospCK1* (ospC mutant)c | C57BL/6 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 |
| ospCK1* (ospC mutant)c | RML | 4/6 | 4/6 | 4/6 | 4/6 | 4/6 |
Number of mice infected/number of mice receiving tissue transfer, confirmed by serology and reisolation.
Number of tissues positive/number of tissues from which reisolation was attempted.
Spirochetes from donor mice inoculated with ospCK1/pBSV2G-ospC were demonstrated to have lost the complementing plasmid, as described in Materials and Methods, confirming that the mice were persistently infected with ospC mutant spirochetes, called ospCK1*.
TABLE 4.
Tissue transfer-mediated infection between various inbred and outbred mouse strains
| B. burgdorferi strain transferred | Mouse strain
|
Recipient infection
|
|||||
|---|---|---|---|---|---|---|---|
| Donor | Recipient | Micea | Earb | Bladderb | Jointb | Transfer siteb | |
| A3 (wild type) | C3H/HeN | C57BL/6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| A3 (wild type) | C57BL/6 | C3H/HeN | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 |
| A3 (wild type) | RML | C3H/HeN | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| A3 (wild type) | RML | C57BL/6 | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 |
| ospCK1* (ospC mutant)c | C3H/HeN | C57BL/6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| ospCK1* (ospC mutant)c | C57BL/6 | C3H/HeN | 3/5 | 2/5 | 3/5 | 3/5 | 3/5 |
| ospCK1* (ospC mutant)c | RML | C3H/HeN | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| ospCK1* (ospC mutant)c | RML | C57BL/6 | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 |
Number of mice infected/number of mice receiving tissue transfer, confirmed by serology and reisolation.
Number of tissues positive/number of tissues from which reisolation was attempted.
Spirochetes from donor mice inoculated with ospCK1/pBSV2G-ospC were demonstrated to have lost the complementing plasmid, as described in Materials and Methods, confirming that the mice were persistently infected with ospC mutant spirochetes, called ospCK1*.
When tissue was transferred from donor mice to recipients of the same mouse strain, infection with both A3 and ospCK1* occurred (Table 3, Fig. 2A). Although the frequency of infection transfer ranged from 40 to 100%, infection was passed successfully for both inbred strains tested and for the outbred strain (RML), with which we previously failed to transfer infection with ospCK1* spirochetes (52). Again, spirochetes were reisolated from every tissue tested, with the exception of one transfer site. One possible explanation for the now-successful tissue transfer-mediated infection of RML mice by ospCK1* was that the spirochetes had undergone a mutation that compensated for the lack of OspC. To address the possibility of a frequently occurring suppressor mutation, we attempted to reinfect mice with in vitro-grown ospCK1* isolated from RML mice that had been infected by tissue transfer. None of the three reisolates tested was able to infect naïve mice by needle inoculation with 5 × 103 spirochetes, the standard inoculum used to infect donor mice (0 infected out of 12 mice inoculated, as assessed by seroconversion and reisolation). The recipient mice from which these reisolates came originally were infected by the transfer of a similar number of spirochetes (approximately 1,000 to 2,000, as assessed by the quantitative PCR analysis of DNA derived from ear punch biopsies) (Fig. 3A). The inability of reisolates from tissue transfer recipients to infect naïve mice supports the idea that host adaptation, rather than a compensatory mutation that happened during persistent infection, allows the ospCK1* spirochetes to infect by tissue transfer.
Transfer of infection between different inbred strains was successful with some combinations but not for others. In no case did infection by tissue transfer correlate with the presence of the ospC gene in the infecting spirochetes (Table 4). Although some of the mouse strains had different major histocompatibility locus (H-2) alleles, this does not appear to be the reason for failure to transfer infection in some cases, since transfer in one direction (e.g., C57BL/6 to C3H/HeN) was successful, whereas transfer in the other direction (e.g., C3H/HeN to C57BL/6) was not. Rather, it seems possible that another characteristic of the strain background makes some strains more permissive for B. burgdorferi infection by tissue transfer. Although we did not quantitate spirochete genome copies in the ear punch biopsies from this experiment, a previous study showed that these two strains of mice have similar bacterial loads in their ear tissue (36), so the transfer of vastly different numbers of spirochetes is unlikely to be the explanation for successful infection in one case and unsuccessful infection in the other. C3H/HeN and C57BL/6 mice also differ in Lyme disease severity (6, 35), highlighting genetic differences between the strains in their immune responses to B. burgdorferi that also may affect the efficiency of tissue transfer-mediated infection. Although these results appear to be clear, they were not statistically significant (P = 0.1 using Fisher's exact test). Also, the inconsistency of our ability to infect RML mice by tissue transfer (52) (Tables 3 and 4) means that the negative results may be a consequence of inefficient infection by this means. Although the efficiency of infection by tissue transfer is variable, these experiments clearly demonstrate that OspC is not required for infection by this method. Statistical analysis using Fisher's exact test found no significant difference between infection by wild-type and ospC mutant spirochetes in any experiment.
Production of OspC by B. burgdorferi-infected tissue recipients.
Since we knew that OspC production is not required for infection by tissue transfer, we asked whether OspC is nevertheless produced when wild-type bacteria capable of making OspC are transferred into naïve recipients. Although we did not directly examine protein production, we used the serological response of recipient mice as an indicator of OspC production by infecting bacteria. In every case (Fig. 2A and B), mice infected by tissue transfer with wild-type spirochetes seroconverted to OspC, indicating that at least some of the infecting bacteria produced this protein. In contrast, none of the mice infected by tissue transfer with ospCK1* seroconverted to OspC (Fig. 2A and B), further confirming that infection was established by spirochetes lacking the ospC gene.
We attempted to quantitate the OspC responses of donor and recipient mice using an ELISA with purified OspC protein as the antigen (Materials and Methods), but the assay did not distinguish between infected and uninfected mouse sera in these experiments. A polyclonal rabbit anti-OspC antiserum (51) was highly reactive in this ELISA, confirming that OspC was bound to the Ni-NTA plates (data not shown). These findings suggest that anti-OspC antibodies are not a prominent component of the immune response to B. burgdorferi in these infected mice, although immunoblot analysis reveals their presence (Fig. 2). Alternatively, the purified OspC bound to the plate may be recognized by the rabbit anti-OspC antisera but not by the antibodies produced by infected mice.
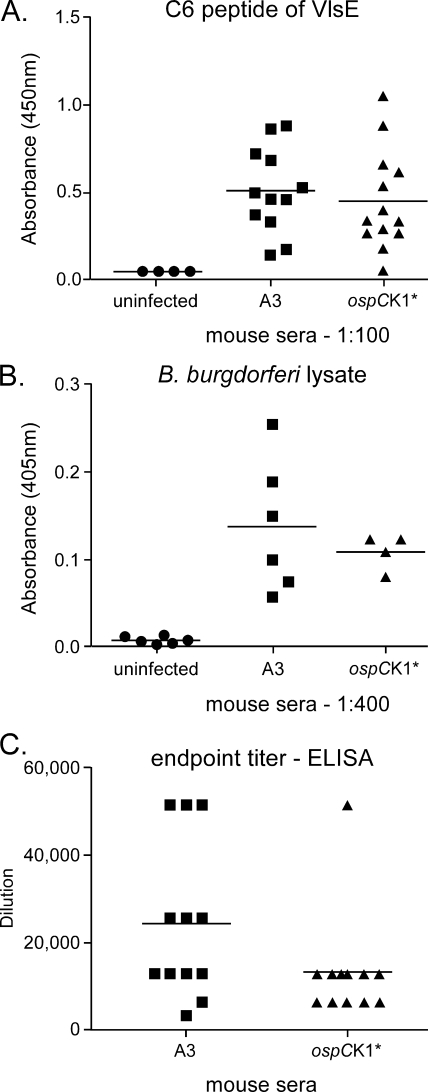
VlsE is a surface-exposed lipoprotein whose production by B. burgdorferi increases early in mammalian infection, when OspC production is waning, and continues throughout (9, 11, 24). The VlsE sequence varies as a consequence of probable gene conversion between an expression locus and several silent cassettes (10, 58, 59), allowing continual VlsE production in the face of a robust immune response. An ELISA using a conserved peptide of VlsE as the antigen is the basis for a diagnostic test for Lyme disease (33). We have used this test to assay for antibodies against VlsE in the sera from recipient mice described in Tables 3 and 4. The results (Fig. 4A) indicate that sera from wild-type- and ospCK1*-infected animals react similarly in the VlsE ELISA.
FIG. 4.
ELISAs of recipient mouse sera. (A) VlsE ELISA results. Symbols represent absorbance at 450 nm for individual sera using C6 peptide ELISA (Immunetics), with a 1:100 dilution of sera and peroxidase-conjugated goat anti-mouse secondary antibody. Absorbances for both sets of infected mouse sera were significantly greater than those for the negative controls, as assessed by one-way analysis of variance followed by Tukey's post test (P < 0.05). (B) B. burgdorferi lysate ELISA results. Symbols represent average absorbance of duplicate serum samples diluted 1:400. Absorbances for both sets of infected mouse sera were significantly higher than those for the uninfected control sera, as assessed by one-way analysis of variance followed by Tukey's post test (P < 0.05). (C) B. burgdorferi lysate ELISA titers. Symbols represent ELISA titers of individual sera, defined as the last of serial twofold serum dilutions (from 1:400 to 1:51,200) at which the absorbance at 405 nm of the diluted serum was significantly greater than the absorbance for serum from uninfected animals diluted the same amount. The antigen was whole B. burgdorferi A3 lysate, prepared as described in Materials and Methods. The ELISA titer means for A3 recipients were not significantly different from those for ospCK1* recipients, as determined by a two-tailed t test, using GraphPad Prism 5.
As a third way of assessing B. burgdorferi infection after tissue transfer, we looked for differences in the global serological responses of mice infected with wild-type versus ospC mutant spirochetes, using an ELISA with B. burgdorferi lysate as antigen. Several sera (derived from the RML mice infected by tissue transfer described in Table 3) were assayed at a dilution of 1:400 to determine if the immune responses of infected and uninfected animals differed significantly (Fig. 4B). At this dilution, absorbances for sera from mice infected with wild-type bacteria did not differ from those for mice infected with ospCK1* spirochetes, but both were significantly greater than absorbances for sera from uninfected mice (P < 0.05). To more carefully quantitate the murine immune response to infection by tissue transfer, we determined the ELISA titers for recipient sera (from mice described in Tables 3 and 4), again using whole B. burgdorferi lysate as the antigen. In this case also (Fig. 4C), the ELISA titers of A3-infected mice were not significantly different from those for ospCK1*-infected mice, although the recipient mouse responses to the transfer of tissue containing wild-type spirochetes were somewhat more robust and variable than the responses of recipients of ospCK1*-infected tissue. These findings suggest that the progress of infection after tissue transfer with ospCK1* is similar to that with wild-type spirochetes.
Spirochete burden in various tissues.
Although the function of OspC is unknown, the protein is surface exposed (53). OspC has been proposed to be an adhesin, since clones encoding fragments of OspC were selected when screening a phage display library for clones conferring adhesion to tissues in live animals (3). These authors suggested that adhesion by OspC-producing bacteria facilitates the infection and, in particular, colonization of deeper tissues, such as the heart. Other studies found higher ospC transcript levels in heart tissue (24, 34), again suggesting that OspC performs a specific function in heart tissue. Furthermore, a recent study found altered dissemination kinetics and heart colonization when other lipoproteins replaced OspC (55). In the present study, we reisolated ospCK1* from all recipient tissues tested (ear, bladder, and ankle joint), with the exception of one ear, demonstrating that dissemination occurred in the absence of OspC production (Tables 2 to 4). We were able to further test the hypothesis that OspC plays a unique role in dissemination and tissue localization, because we could use quantitative PCR to assess the spirochete burden in some tissues (including hearts, which were not cultured) that were saved from the animals described in Table 2, which were infected by tissue transfer with wild-type and ospCK1* spirochetes. We extracted DNA from infected mouse tissues and used TaqMan analysis to measure the number of copies of the B. burgdorferi flaB gene relative to the number of mouse nidogen gene copies. Although the sample size was small, comparable levels of ospCK1* and A3 spirochetal DNA were detected in ears, joints, and hearts of infected recipient C3H/HeN mice (Fig. 3B to D). The relative copy numbers of both wild-type and ospC mutant spirochete genomes were higher in recipient SCID mice, but no ospC-dependent differences were observed (Fig. 3B to D). Since the number of mice from which tissues were saved is small, statistical analysis is problematic, but these findings suggest that OspC plays no unique role in tissue colonization or dissemination.
DISCUSSION
Although OspC is absolutely required in infections initiated by tick bite or needle inoculation, this study demonstrates that B. burgdorferi ospC mutant spirochetes can infect mice when a piece of skin from a persistently infected animal is implanted beneath the skin of a recipient mouse. Three weeks after infection by tissue transfer, the distribution of ospC mutant spirochetes within mice was indistinguishable from that found after a similar infection with wild-type spirochetes. These results suggest that a product produced by B. burgdorferi during persistent infection can perform the early essential function normally performed by OspC. Although we still do not know what that early function is, we hypothesize that the function is essential throughout infection and that another protein normally takes over that function during persistence and, therefore, can fulfill that function after tissue transfer. ospC mutant spirochetes are unable to infect by tick transmission or needle inoculation of cultured bacteria, implying either that the OspC surrogate is repressed when bacteria are growing in ticks or culture medium or that it is rapidly downregulated when the bacteria enter a mammal. Functions that might be required throughout infection include binding a component that shields the bacteria from phagocytosis, blocking signaling by a host cell, or directly preventing killing by a host cell or other host component.
Alternatively, OspC may perform a unique function, such as sensing the host environment and triggering a critical aspect of host adaptation. For this second hypothesis to be consistent with our results, host-adapted bacteria in transferred tissue would have to not need this unique function. However, the processes required for infection by the tissue transfer of host-adapted spirochetes, such as replication, dissemination, and the colonization of distant tissues in the face of the innate and acquired immune defenses of a naïve mouse, closely resemble those found during infection by needle inoculation or tick transmission. In fact, it is difficult to conceive of a unique protective role for OspC that would not also be required after tissue transfer to a naïve animal. Because host-adapted bacteria derived from a persistently infected mouse can carry out the functions mentioned above in the absence of OspC, it seems more likely that another protein being produced by those bacteria can fulfill an essential protective function normally carried out by OspC after the infection of a naïve host.
If OspC performs a function during the initiation of infection that is fulfilled by another protein during the later stages, why not have a single protein perform the same function throughout infection? One possibility is that OspC performs the function optimally for the early stages of infection, but that the antigenic nature of OspC means that its continued production leads to an antibody response that clears the infection if production continues (57). A recent intriguing study (55) showed that four unrelated lipoproteins (OspA, OspE, DbpA, and VlsE), if appropriately produced, could fulfill the early protective role of OspC, allowing ospC mutant spirochetes to successfully infect SCID mice, in which OspC otherwise still is required (22, 52). These authors suggest that any lipoprotein could substitute for OspC at later times, if properly regulated. The protein substituting for OspC also would have to be a poor antigen or antigenically variable, so it could be continuously produced in a normal, immunocompetent host without disadvantage to the bacteria. The number of spirochetes transmitted by a tick normally may be so small that B. burgdorferi has optimized the critical first step by using a specialized protein (OspC) to maximize the chance of successful mammalian infection. Optimal protection at this early time also could lead to optimal proliferation, allowing the spirochete to disseminate and colonize distant sites earlier, thus facilitating acquisition by ticks feeding upon the newly infected animal.
Although spirochetes lacking lp28-1, the plasmid encoding VlsE, only transiently infect normal mice (41), a recent study demonstrated higher and more sustained OspC production by spirochetes lacking lp28-1 during this abbreviated infection, during SCID mouse infection, and during bacterial growth in DMCs (20). These authors hypothesized that ospC gene expression is downregulated during mammalian infection by a repressor encoded on lp28-1 and that the absence of the repressor in bacteria lacking lp28-1 led to constitutive OspC production (20). Another possibility is that lp28-1-encoded VlsE normally takes over an essential function from OspC, and that spirochetes unable to produce VlsE can survive in a mammal only if they continue to produce OspC. These results, combined with the data from Xu et al. (55) showing that constitutive VlsE production allows ospC mutant spirochetes to infect SCID mice, suggest that VlsE is the OspC surrogate produced during persistent infection. VlsE and OspC share some general structural features (16, 17, 29), which might be expected if they were to fulfill the same function. When we tested for VlsE production in recipient mice by assaying their sera with the commercially available C6 peptide ELISA, we found that sera from mice that were recipients of either A3- or ospCK1*-infected tissue recognize VlsE after transfer (Fig. 4A), with no significant difference between the mean responses at a single dilution, demonstrating that spirochetes produced VlsE during the infection.
Infection by tissue transfer is of variable efficiency (Tables 2 to 4). For example, our previously published tissue transfer experiment (52) suggested a requirement for OspC after infection by tissue transfer. We also carried out a recent tissue transfer study (unpublished results) in which very few of the recipient animals were infected with either wild-type or ospC mutant spirochetes. The basis for such experimental variability is unknown, but it may lead to the observed range in immune responses of recipient animals (Fig. 4C). Despite the variability of infection by tissue transfer, every successful tissue transfer is significant, because it demonstrates the feasibility of infection in the particular combination of conditions tested, especially in contrast to the absolute inability of the ospC mutant to infect by tick bite or needle inoculation (22, 50, 52).
Since the experiments in this study show that host-adapted ospC mutant spirochetes derived from infected mice are able to infect naïve animals, another question remaining is why host-adapted ospC mutant spirochetes derived by growth in DMCs implanted in rats were unable to infect mice by needle inoculation (50). Our data suggested that host adaptation under these conditions is inadequate to allow the bacteria to bypass the ospC requirement. One indication of incomplete host adaptation was that some OspA still was produced by DMC-grown spirochetes, as assessed by the silver staining of bacterial lysates separated on one- and two-dimensional gels (50). Other studies also have noted differences between the proteins produced by DMC-grown spirochetes and those produced during mouse infection (1). In addition to potentially inadequate host adaptation, the number of mice inoculated with DMC-grown ospC mutant spirochetes was low (three mice per strain). Finally, direct interaction between spirochetes and mammalian cells or a host component may be required for full host adaptation (1, 25). Additional experiments with DMC-derived spirochetes are needed to determine if OspC remains essential for infection with this source of host-adapted spirochetes.
Although OspC was not required for infection by tissue transfer, we also provided evidence that OspC is produced by enough wild-type spirochetes infecting by this method to stimulate an immune response in recipient animals (Fig. 2). While it is possible that OspC production facilitates infection by tissue transfer, we have observed no reproducible difference in the efficiency of infection by tissue transfer of wild-type and ospC mutant spirochetes. Since ospC gene regulation appears to have a stochastic component (23, 37, 49), the immunological response may just be the consequence of sporadic OspC production by infecting bacteria in the mammalian host, which can persist when B. burgdorferi infects mice that are unable to mount an acquired immune response (31). We attempted to use an ELISA with OspC as the antigen to analyze the responses of needle-inoculated and tissue-transferred animals, but the reactivity of infected mouse sera from these experiments was not sufficiently higher than that of uninfected sera, so we could not draw quantitative conclusions about possible differences in response. We were, however, successful at comparing the overall immunological response to tissue transfer with wild-type and ospC mutant bacteria, using an ELISA with total B. burgdorferi lysate as the antigen. In this case, we found that recipient mice responded similarly to infection by tissue transfer with A3 and ospCK1* spirochetes (Fig. 4B and C). The lower trend of the ELISA titers of sera from ospCK1*-infected mice is not a consequence of their lack of response to OspC that is present in the lysate, since OspC ELISA titers were low for all recipient sera, regardless of the ospC genotype of the infecting spirochetes. Also, we compared ELISAs for a limited number of sera using B. burgdorferi lysates prepared from wild-type, ospCK1, and ospCK1/pBSV2G-flaBpospC and saw no significant difference in the results, confirming that antibodies to OspC are not major contributors to ELISA reactivity. Overall, tissue transfer-mediated infection with wild-type and ospCK1* spirochetes resulted in statistically indistinguishable immune responses in recipient mice.
Finally, we found that efficient dissemination to distant sites and efficient tissue colonization can occur in the absence of ospC, since we could reisolate ospCK1* spirochetes from all but two recipient mouse tissues tested (20 infected mice, with 4 tissues per mouse; Tables 2 to 4) and because wild-type and mutant spirochete genome levels in DNA derived from ears, joints, and hearts (saved from animals described in Table 2) were not significantly different (Fig. 3). The study by Xu et al. (55) also addressed the question of a potential role for OspC in bacterial dissemination and tissue colonization. These authors found a delay of up to 2 weeks in the time at which ospC mutant spirochetes complemented with heterologous lipoproteins were culturable from sites distant from the inoculation site compared to that of spirochetes complemented with a wild-type ospC gene (55). The strains producing the decorin-binding protein DbpA in lieu of OspC were never found at distant sites (55). Their interpretation of these and other data presented (reiterated in a microcommentary published in the same journal [42]) was that OspC plays a role in some aspect of dissemination and that the other lipoproteins are less able to perform that role. They hypothesized that the overproduction of DbpA, a protein that binds mammalian extracellular matrix, prevents the bacteria from leaving the inoculation site. An alternative explanation of their data is that the primary and perhaps sole role of OspC is in protection from killing at early times, and that the other lipoproteins protect less effectively. A lower number of spirochetes surviving the initial assault of the mammalian innate immune system could lead to reduced numbers of spirochetes present and a delayed time course for dissemination and colonization. Our experiments show that spirochetes lacking OspC can disseminate and efficiently colonize distant tissues within 3 weeks, but it remains possible that a presumptive OspC surrogate produced by transferred bacteria not only protects the bacteria from clearance but also fulfills any role that OspC normally performs for later stages of infection, such as dissemination. Since the Xu et al. results (55) also are consistent with OspC either playing a solely protective role or directly facilitating dissemination and colonization, further experiments are required to discriminate between these possibilities.
Despite the absolute dependence of natural infection by tick bite (and experimental infection by needle inoculation) on OspC production, we have been able to circumvent that requirement and infect naïve animals with spirochetes lacking OspC. Given that ospC mutant spirochetes normally are cleared from a mouse in less than 48 h (51), it seems likely that a protein produced during persistent infection can (and does) substitute for OspC during tissue transfer-mediated infection. The simplest reason that such a protein would exist is that normal infection requires a succession of proteins, beginning with OspC, to persist in the mammalian environment. Determining the specific protective function of OspC and identifying its successor, if it exists, should help reveal how B. burgdorferi survives the pressure from innate and acquired mammalian defenses in order to be acquired by another feeding tick.
Acknowledgments
We thank Gary Hettrick and Anita Mora for preparing the figures. We thank Janis Weis (University of Utah) and Kim Hasenkrug (RML) for helpful discussions. Merry Schrumpf and Tom Schwan (RML) provided invaluable help with protein purification and ELISA and also generously provided the rabbit anti-OspC antibody used in the immunoblot. Robert Gilmore (Centers for Disease Control, Fort Collins, CO) kindly provided the pET29b-ospC plasmid used as a source of recombinant OspC protein. Members of the RML Veterinary Branch provided expert animal care. We appreciate the helpful comments of Robert Heinzen, Chris Bosio, and members of the Rosa laboratory, especially Mollie Jewett, who also graciously assisted with one study.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Akins, D. K., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 1012240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alghaferi, M. Y., J. M. Anderson, J. Park, P. G. Auwaerter, J. N. Aucott, D. E. Norris, and J. S. Dumler. 2005. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J. Clin. Microbiol. 431879-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonara, S., R. M. Chafel, M. LaFrance, and J. Coburn. 2007. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol. Microbiol. 66262-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W. 1993. Antigenic stability of Borrelia burgdorferi during chronic infections of immunocompetent mice. Infect. Immun. 614955-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W. 1999. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect. Immun. 6736-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162133-138. [DOI] [PubMed] [Google Scholar]
- 7.Bissett, M. L., and W. Hill. 1987. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J. Clin. Microbiol. 252296-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 1822445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bykowski, T., K. Babb, K. von Lackum, S. P. Riley, S. J. Norris, and B. Stevenson. 2006. Transcriptional regulation of the Borrelia burgdorferi antigenically variable VlsE surface protein. J. Bacteriol. 1884879-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutte, L., D. J. Botkin, L. Gao, and S. J. Norris. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. 5e1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X.-Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 725063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley, H., and B. T. Huber. 2003. Host-adapted Borrelia burgdorferi in mice express OspA during inflammation. Infect. Immun. 714003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Silva, A. M., E. Fikrig, E. Hodzic, F. S. Kantor, S. R. Telford III, and S. W. Barthold. 1998. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177395-400. [DOI] [PubMed] [Google Scholar]
- 14.Dykhuizen, D. E., D. Brisson, S. Sandigursky, G. P. Wormser, J. Nowakowski, R. B. Nadelman, and I. Schwartz. 2008. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am. J. Trop. Med. Hyg. 78806-810. [PMC free article] [PubMed] [Google Scholar]
- 15.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect. Immun. 737869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eicken, C., V. Sharma, T. Klabunde, M. B. Lawrenz, J. M. Hardham, S. J. Norris, and J. C. Sacchettini. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J. Biol. Chem. 27721691-21696. [DOI] [PubMed] [Google Scholar]
- 17.Eicken, C., V. Sharma, T. Klabunde, R. T. Owens, D. S. Pikas, M. Höök, and J. C. Sacchettini. 2001. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J. Biol. Chem. 27610010-10015. [DOI] [PubMed] [Google Scholar]
- 18.Elias, A. F., J. L. Bono, J. J. Kupko, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 629-40. [DOI] [PubMed] [Google Scholar]
- 19.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 702139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Embers, M. E., X. Alvarez, T. Ooms, and M. T. Philipp. 2008. The failure of immune response evasion by linear plasmid 28-1-deficient Borrelia burgdorferi is attributable to persistent expression of an outer surface protein. Infect. Immun. 763984-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingerle, V., G. Goettner, L. Gern, B. Wilske, and U. Schulte-Spechtel. 2007. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int. J. Med. Microbiol. 29797-107. [DOI] [PubMed] [Google Scholar]
- 22.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 1013142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, M., T. Oman, H. Xu, J. Blevins, M. V. Norgard, and X. F. Yang. 2008. Abrogation of ospAB constitutively activates the Rrp2-RpoN-RpoS pathway (sigmaN-sigmaS cascade) in Borrelia burgdorferi. Mol. Microbiol. 701453-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodzic, E., S. Feng, K. J. Freet, and S. W. Barthold. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 715042-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson, C. R., J. G. Frye, F. D. Quinn, and F. C. Gherardini. 2001. Increased expression of Borrelia burgdorferi vlsE in response to human endothelial cell membranes. Mol. Microbiol. 41229-239. [DOI] [PubMed] [Google Scholar]
- 26.Jewett, M. W., R. Byram, A. Bestor, K. Tilly, K. Lawrence, M. N. Burtnick, F. Gherardini, and P. A. Rosa. 2007. Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Mol. Microbiol. 66975-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewett, M. W., K. Lawrence, A. C. Bestor, K. Tilly, D. Grimm, P. Shaw, M. VanRaden, F. Gherardini, and P. A. Rosa. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol. Microbiol. 641358-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 727147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumaran, D., S. Eswaramoorthy, B. J. Luft, S. Koide, J. J. Dunn, C. L. Lawson, and S. Swaminathan. 2001. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J. 20971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagal, V., D. Portnoi, G. Faure, D. Postic, and G. Baranton. 2006. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 8645-652. [DOI] [PubMed] [Google Scholar]
- 31.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 676702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 725759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petkov, P. M., Y. Ding, M. A. Cassell, W. Zhang, G. Wagner, E. E. Sargent, S. Asquith, V. Crew, K. A. Johnson, P. Robinson, V. E. Scott, and M. V. Wiles. 2004. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 141806-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porcella, S. F., S. J. Raffel, M. E. Schrumpf, M. E. Schriefer, D. T. Dennis, and T. G. Schwan. 2000. Serodiagnosis of Louse-Borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 383561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 9713865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radolf, J. D., and M. J. Caimano. 2008. The long strange trip of Borrelia burgdorferi outer-surface protein C. Mol. Microbiol. 691-4. [DOI] [PubMed] [Google Scholar]
- 43.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi, p. 253-259. In J. A. Nickoloff (ed.), Methods in molecular biology: electroporation protocols for microorganisms, vol. 47. Humana Press, Inc., Totowa, NJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 39382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 922909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwan, T. G., M. E. Schrumpf, R. H. Karstens, J. R. Clover, J. Wong, M. Daugherty, M. Struthers, and P. A. Rosa. 1993. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J. Clin. Microbiol. 313096-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiler, K. P., Z. Vavrin, E. Eichwald, J. B. Hibbs, Jr., and J. J. Weis. 1995. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect. Immun. 633886-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seinost, G., D. E. Dykhuizen, R. J. Dattwyler, W. T. Golde, J. J. Dunn, I. N. Wang, G. P. Wormser, M. E. Schriefer, and B. J. Luft. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 673518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava, S. Y., and A. M. de Silva. 2008. Reciprocal expression of ospA and ospC in single cells of Borrelia burgdorferi. J. Bacteriol. 1903429-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart, P. E., X. Wang, D. M. Bueschel, D. R. Clifton, D. Grimm, K. Tilly, J. A. Carroll, J. J. Weis, and P. A. Rosa. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 743547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilly, K., A. Bestor, M. W. Jewett, and P. Rosa. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 751517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 743554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilske, B., V. Preac-Mursic, G. Schierz, and K. V. Busch. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl. Bakteriol. Hyg. A 26392-102. [DOI] [PubMed] [Google Scholar]
- 54.Wilske, B., V. Preac-Mursic, G. Schierz, R. Kuhbeck, A. G. Barbour, and M. Kramer. 1988. Antigenic variability of Borrelia burgdorferi, p. 126-143. In J. L. Benach and E. M. Bosler (ed.), Lyme disease and related disorders. New York Academy of Sciences, New York, NY. [DOI] [PubMed]
- 55.Xu, Q., K. McShan, and F. T. Liang. 2008. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defenses. Mol. Microbiol. 6915-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, Q., K. McShan, and F. T. Liang. 2007. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol. Microbiol. 64220-231. [DOI] [PubMed] [Google Scholar]
- 57.Xu, Q., S. V. Seemanapalli, K. McShan, and F. T. Liang. 2006. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 745177-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89275-285. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 663689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]