Abstract
Pseudomonas aeruginosa-induced activation of NF-κB and secretion of proinflammatory cytokines by airway epithelial cells require that the bacteria express flagellin. We tested whether P. aeruginosa and human airway epithelial cells secrete factors that modulated this response. Experiments were performed with both the Calu-3 cell line and primary cultures of tracheal epithelial cells. P. aeruginosa strain PAK ΔfliC (flagellin knockout) did not activate NF-κB or interleukin-8 (IL-8) but inhibited flagellin-activated NF-κB by 40 to 50% and IL-8 secretion by 20 to 25%. PAK ΔfliC also inhibited NF-κB induced by IL-1β and Toll-like receptor 2 agonist Pam3CSK4. Similar inhibitions were observed with strains PAK, PAO1, and PA14. The inhibitory factor was present in conditioned medium isolated from PAK ΔfliC or Calu-3 plus PAK ΔfliC, but it was not present in conditioned medium isolated from Calu-3 cells alone or from PAK ΔfliC that had been heat treated. Inhibition by PAK ΔfliC-conditioned medium was exerted from either the apical or the basolateral side of the epithelium, was enhanced in simple Ringer's solution over that in tissue culture medium, and did not result from altered pH or depletion of glucose. The inhibitory effect of conditioned medium was abolished by boiling and appeared from filtration studies to result from effects of a factor with a molecular mass of <3 kDa. These and further studies with isogenic mutants led to the conclusion that the NF-κB and IL-8 response of airway epithelial cells to P. aeruginosa results from a balance of proinflammatory effects of flagellin and antiinflammatory effects of a small (<3-kDa), heat-sensitive factor(s) that is not lipopolysaccharide, C12 homoserine lactone, alginate, CIF, or exotoxin A, S, T, U, or Y.
The gram-negative bacterium Pseudomonas aeruginosa commonly infects the lungs of cystic fibrosis patients and contributes to triggering the excessive inflammation in the lungs of these patients. This inflammatory response is initiated by the airway epithelial cells responding to the bacteria by activating cell signaling (including activation of the key transcription factor NF-κB) and secretion of proinflammatory cytokines and chemokines (e.g., interleukin 8 [IL-8]) that recruit neutrophils to the infected region (18, 33). Although airway epithelial cells express many Toll-like receptors (TLR) (1, 22) and could therefore be activated by many bacterial products, previous experiments have focused on the important roles of flagellin and TLR5. Activation of the airway epithelial innate host defense response by P. aeruginosa is dependent on expression of flagellin in the bacteria (1, 10, 30, 35, 36) and TLR5 in the epithelial cells (5, 8, 19, 26).
The magnitude of this response could potentially be regulated by other factors released by the bacteria and epithelial cells. For example, flagellin triggers release of ATP from human airway epithelial cells (NCIH292) (20), and ATP synergizes with ATP in activating proinflammatory responses (6). Epithelial or neutrophil-secreted lipoxins may inhibit proinflammatory responses of these cells (13). There may also be antiinflammatory regulatory roles for bacterium-produced products. Various beneficial effects of probiotic bacteria in the intestine (9) and stomach (3) may be mediated through effects to reduce inflammation. The gram-positive bacteria Bifidobacterium breve and Streptococcus thermophilus, as well as intestinal commensal bacteria, release low-molecular-mass (<3-kDa) metabolites that reduce lipopolysaccharide (LPS)-stimulated release of tumor necrosis factor alpha by human intestinal epithelial cells (HT29-19A) (21). Recent experiments showed that P. aeruginosa-produced homoserine lactone (C12) inhibits LPS-stimulated NF-κB signaling and activation of macrophages (14).
The present experiments were designed to test whether, in addition to producing and releasing proinflammatory flagellin, P. aeruginosa may also produce other pro- or antiinflammatory products. Our initial approach was to treat the human airway epithelial cell line Calu-3 with flagellin and other factors that activate proinflammatory NF-κB signaling and release of IL-8 and then also in the presence of P. aeruginosa strain PAK ΔfliC, which is defective in producing the flagellin structural gene and is therefore ineffective at activating NF-κB and IL-8 (10, 31). Flagellin-stimulated NF-κB and IL-8 were inhibited by PAK ΔfliC. We tested for the specificity of this inhibitory effect by determining whether PAK ΔfliC inhibited NF-κB stimulated by IL-1β and Pam3CSK4 (TLR2 agonist). Different strains and isogenic mutants of P. aeruginosa were used to test whether the inhibitory effect is mediated by the type III secretion system (TTSS) and TTSS toxins (32), C12 homoserine lactone (14), exotoxin A (4, 34), CIF (an epoxide hydrolase; see reference 17), and alginate (23). We also tested whether the TLR4 agonist LPS modulates flagellin-stimulated activation. Finally, we tested whether P. aeruginosa or the epithelial cells were producing and secreting the inhibitory factor by testing heat-killed bacteria and supernatants or filtrates of supernatants isolated from bacteria or from epithelial cells exposed to the bacteria.
MATERIALS AND METHODS
Tissue culture.
Calu-3 cells, a human gland epithelial cell line homozygous for the wild-type cystic fibrosis transmembrane conductance regulator (16, 29), was cultured in ATCC Eagle's minimum essential medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. For most experiments, cells were passaged at a 1:2 dilution and the remaining cell suspension was seeded directly onto a 24- or 12-well tissue culture plate (BD Falcon, Bedford, MA). In some experiments, cells were passaged onto either 1.0- or 4.2-cm2 Transwell membranes (0.4- or 1-μm pore size; BD Falcon) and then grown until the cells formed confluent monolayers. Cells grow equivalently on either of these filters (15). In other experiments, Calu-3 cells were grown in typical tissue culture medium and then incubated during experiments in Ringer's solution containing 145 mM NaCl, 2 mM KCl, 1.5 mM K2HPO4, 1 mM MgSO4, 10 mM HEPES, 2 mM CaCl2, and 10 mM glucose.
Human bronchial primary cultures were kindly provided by Walter E. Finkbeiner, Department of Pathology, University of California, San Francisco, San Francisco General Hospital, and cultured as previously described (31). In brief, strips of epithelium were removed from the underlying tissue and treated with protease overnight. Cells were plated on permeable filter supports (Snapwell 3407 0.4-μm-pore-size polycarbonate membrane; Corning Costar, Cambridge, MA) precoated with human placental collagen (15 μg/cm2) at a density of ∼106/cm2. Cells were grown in Dulbecco modified Eagle medium/F12 culture medium supplemented with 2% Ultroser G (Biotechnics, Paris, France).
Bacterial culture and preparation of bacterial-cell-conditioned and bacterial- and epithelial-cell-conditioned solutions.
The strains of P. aeruginosa used were PAK, PAK ΔfliC, PAK ΔexoA, PAK ΔalgC, PAO1, PAO1 ΔexoSTY, PAO1 ΔpopB, PAO1 ΔlasI, PA14, and PA14 Δcif (Table 1). All P. aeruginosa strains were grown overnight in Luria-Bertani culture medium at 37°C with shaking. We prepared these bacterial suspensions for addition to epithelial cells by washing them twice with phosphate-buffered saline (by pelleting them at ∼16,000 × g and then resuspending them in the same volume of phosphate-buffered saline) and then resuspending them in tissue culture medium minus antibiotics or in Ringer's solution (145 mM NaCl, 1.2 mM MgSO4, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 10 mM HEPES, 10 mM glucose, pH 7.4) for addition to Calu-3 cells. The bacterial concentration was adjusted to an optical density at 600 nm of 1 (109 CFU/ml), and then the bacteria were diluted to 106 to 108 CFU/ml as needed. In some experiments, 10% FBS was added to Ringer's solution, while in others, glucose-free Ringer's solution was used. For experiments with heat-killed bacteria, a suspension was made of bacteria at 108 CFU/ml in antibiotic-free medium as described above and then heated at 85°C for 1 h, cooled to 37°C, and used with or without flagellin.
TABLE 1.
P. aeruginosa strains used in this study
| Strain | Relevant characteristic(s) | Source or reference(s) |
|---|---|---|
| PAO1 | P. aeruginosa laboratory strain, wild type | ATCC |
| PAO1 ΔexoSTY | PAO1 ΔexoS ΔexoT ΔexoY, exotoxins S, T, and Y | 32 |
| PAO1 ΔpopB | PAO1 ΔpopB, type III secretion apparatus | 32 |
| PAO1 ΔlasI | PAO1 ΔlasI, enzyme for producing autoinducer | 24 |
| PAK | P. aeruginosa laboratory strain, wild type | S. Lory, 10 |
| PAK ΔfliC | PAK ΔfliC, flagellin structural gene | S. Lory, 10 |
| PAK ΔexoA | PAK ΔexoA, exotoxin A | 4 |
| PAK ΔalgC | PAK ΔalgC, phosphomannomutase required for production of alginate and LPS | 7, 23 |
| PA14 | P. aeruginosa laboratory strain, wild type | 17 |
| PA14 Δcif | PA14 Δcif, epoxide hydrolase | 17 |
Bacterial-cell-conditioned solutions were used to test effects of bacterial-cell-secreted factors on Calu-3 cells. For experiments testing P. aeruginosa-conditioned solutions on Calu-3 cells, PAK ΔfliC cells (108 CFU/ml) were suspended in medium or Ringer's solution (as described above), incubated at 37°C for 4 or 18 h, and then centrifuged at 16,000 × g for 2 min. The supernatant was collected and run through a 0.2-μm-pore-size syringe filter (for further clearance of bacteria) and then added to Calu-3 cells. For experiments testing P. aeruginosa-epithelial-cell-conditioned solutions, Calu-3 cells were grown to confluence on 24-well plates in typical tissue culture medium. Antibiotic-free tissue culture medium or a suspension of PAK ΔfliC (108 CFU/ml) in the same medium was placed on Calu-3 cells and incubated at 37°C for 4 h. After this 4-h incubation, the medium or bacterial suspension was collected, combined to minimize variation, and centrifuged and filtered as described above. For the preparation of boiled supernatant, the bacterial-cell-epithelial-cell-conditioned solution was kept in a sealed tube, placed in a boiling water bath for 15 min, and cooled to 37°C at use.
For experiments in which molecular-weight-based separation of the bacterial supernatant was performed, bacterial-cell-conditioned solution was generated by incubating PAK ΔfliC (108 CFU/ml) in Ringer's solution for 18 h at 37°C and then centrifuging and filtering it as described above to remove whole bacteria. This solution then was run through molecular mass cutoff (Microcon YM-3 for 3 kDa and YM-30 for 30 kDa) filters (Millipore, Bedford, MA). Due to the volume limitation of the filters, the bacterial supernatant was separated into aliquots and filtered separately.
The filtrate (containing molecules smaller than the cutoff size) and retentate (left over on filters containing molecules larger than the cutoff size) were collected and combined. The volume of the filtrate (flowthrough) was close to that of the original solution and was then used for treatment directly with or without flagellin. The retentate (left on the filter) was diluted in Ringer's solution to a volume equal to half the original volume (to compensate for the volume lost during filtration) and used for treatment of Calu-3 cells.
Flagellin and LPS.
P. aeruginosa or Salmonella enterica serovar Typhimurium flagellin (10−4 g/ml in water; Invivogen, San Diego, CA) was stored at −80°C and diluted from the stock in the incubation medium at the stated concentrations. This solution was vortexed vigorously and heated to 37°C before being added to the solutions to ensure dispersal as monomers. Previous experiments showed that LPS contamination of this preparation is small and cannot account for the activating effects of flagellin on NF-κB and IL-8 secretion. P. aeruginosa LPS (Sigma Chemical Co., St. Louis, MO) was used for some experiments by being dissolved in the medium at 10−6 g/ml as previously done (31).
NF-κB activation: luciferase adenovirus and immunoblot analysis.
A recombinant adenoviral vector expressing a luciferase reporter gene driven by transcriptional activation (adv-NF-κB-luc) (27) was used for studies to determine effects on NF-κB activation as described previously. This vector contained the luciferase gene driven by four tandem copies of the NF-κB consensus sequence and was stored in 10 mM Tris with 20% glycerol at −80°C. The virus was added to Calu-3 cells at a multiplicity of infection (MOI) of 100, and they were returned to the incubator for 24 h. Cells were then washed to remove viruses and left to grow for another day. Previous experiments showed that the adenovirus elicits expression in >75% of the cells (28).
For experiments to measure NF-κB activation and IL-8 secretion, luciferase-expressing cells were incubated in either antibiotic-free tissue culture medium (Eagle's minimum essential medium; Mediatech) or Ringer's solution as described. Treatments were performed by diluting stock solutions of reagents in corresponding medium. Normal experiments involved exposing epithelial cells to the various agonists for 4 h. Cells were then washed and processed with the luciferase assay system by using Reporter Lysis Buffer (Promega, Madison, WI) to measure NF-κB-mediated transcriptional induction according to the manufacturer's protocol. Measurements of luciferase activity (relative light units) were performed in triplicate for each sample and normalized to the protein concentration (Bradford assay). Averages were then expressed relative to the average control value in the epithelial cells, which was set equal to 1.0. In experiments in which responses were somewhat variable from day to day, responses were normalized to the maximal response to the stimulant elicited in the experiment, usually 10−6 g/ml flagellin.
NF-κB signaling activity was also assayed by immunoblot analysis of NF-κB p65, IκΒα, and IKKα and phosphorylated NFκB p65, IκΒα, and IKKα/β. Calu-3 cells were grown in wells; incubated in Ringer's solution; treated with flagellin (10−6 g/ml), PAK ΔfliC (108 CFU/ml, MOI of 100), or flagellin plus PAK ΔfliC for up to 4 h; and then lysed in M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) containing 5 μg/ml leupeptin, 5 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride, and 50 nM calyculin A. Protein concentrations were determined with Bradford reagent (Bio-Rad, Hercules, CA). Immunoblot analysis was performed by first separating protein (10 to 50 μg/lane) electrophoretically by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferring it to polyvinylidene difluoride membranes. Membranes were blocked (5% nonfat dried milk) in 20 mM Tris-HCl (pH 7.5)-150 mM NaCl-0.05% Tween 20 and then incubated with specific antibodies. Primary antibodies (diluted 1:1,000) for NF-κB p65, phospho-p65 (serine 536), IκΒα, phospho-IκΒα (serine 32), IKKα, and phospho-IKKα/β (serine176/180) were purchased from Cell Signaling (Danvers, MA). Blots were first probed for phosphorylated proteins. The membranes were then stripped and probed with antibodies for nonphosphorylated proteins. Immunostaining of β-actin was performed to control for the amount of protein in each sample. Binding of primary antibodies was visualized by enhanced chemiluminescence with horseradish peroxidase-conjugated secondary antibodies (1:2,000) and Renaissance Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences). Quantitation was performed with NIH Image on blots with the background subtracted and normalization to maximal intensities measured during the experiment.
Enzyme-linked immunosorbent assay of IL-8 secretion.
Samples of incubation medium were collected from plastic wells in which epithelial cells were grown. Samples from control or treated cells were collected, cleared of any P. aeruginosa or cellular debris by centrifugation (5 min, 1,000 × g), stored at −20°C until use, thawed, diluted 1:100 or 1:200 in 100 μl of Assay Diluent (BD Pharmingen, San Diego, CA), run in triplicate in accordance with the manufacturer's protocol (OptEIA Human IL-8 Set; BD PharMingen), and read at 450 nm with an ELX808 Ultra Microplate Reader (Bio-Tek Instruments, Winooski, VT). Averages of the triplicates are reported.
Statistics.
Quantitative comparisons were made with a t test for paired or unpaired samples, as appropriate. Differences were considered statistically significant at P < 0.05.
RESULTS
PAK and PAK ΔfliC inhibit flagellin-, IL-1β-, and Pam3CSK4-activated NF-κB and IL-8.
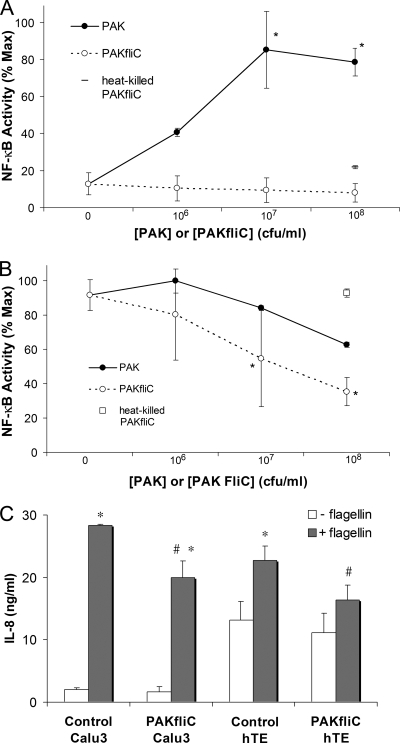
Calu-3 cells were grown on plastic plates and left untreated or treated overnight with adenovirus to express NF-κB-regulated luciferase. Cells expressing NF-κB-regulated luciferase were treated with different combinations of PAK, PAK ΔfliC, and flagellin and then processed for luciferase activity measurement. Medium from some of these cells was sampled at the start of the experiment and then after 4 h for measurement of IL-8 by enzyme-linked immunosorbent assay. Results from experiments testing the dose-dependent effects of PAK, PAK ΔfliC, and heat-killed PAK ΔfliC on Calu-3 cell NF-κB-luc activity under control conditions and during simultaneous exposure to flagellin are shown in Fig. 1A and B. PAK activated NF-κB of Calu-3 cells at 106 CFU/ml, and there was further, apparently maximal, activation of NF-κB at 107 and 108 CFU/ml. PAK ΔfliC at 106 to 108 CFU/ml or heat-killed PAK ΔfliC at 108 CFU/ml had little effect on NF-κB of Calu-3 cells (Fig. 1A). Calu-3 cells that had been treated with 10−7 g/ml flagellin (intermediate concentration for activating TLR5 [references 6 and 30]) exhibited sixfold activation of NF-κB (Fig. 1B). In the presence of flagellin, adding PAK caused a slight activation of NF-κB at 106 CFU/ml but reductions in NF-κB at 107 and 108 CFU/ml (Fig. 1B). PAK ΔfliC caused only reductions in the NF-κB activity of flagellin-treated Calu-3 cells (Fig. 1B). At 108 CFU/ml, PAK ΔfliC reduced flagellin-stimulated NF-κB activation by about 60%. This dose of PAK ΔfliC reduced flagellin-stimulated IL-8 secretion by both Calu-3 cells and primary cultures of human tracheal epithelial cells by about 30% (Fig. 1C). In contrast, heat-killed PAK ΔfliC (108 CFU/ml) did not alter flagellin-stimulated NF-κB activation of Calu-3 cells (Fig. 1B). These results indicated that PAK and PAK ΔfliC both inhibited flagellin-stimulated NF-κB activity and IL-8 secretion by airway epithelial cells, and this inhibitory effect was abolished by heating the bacteria.
FIG. 1.
Stimulatory and inhibitory effects of P. aeruginosa on NF-κB in Calu-3 cells. PAK, PAK ΔfliC, and heat-killed (80°C, 1 h) PAK ΔfliC at the doses shown were added to NF-κB-luc-expressing Calu-3 cells grown in medium for 4 h in a 37°C incubator. (A) Effects of PAK, PAK ΔfliC, and heat-killed PAK ΔfliC. Luciferase activity was expressed relative to the maximal activity attained during treatment with PAK, which averaged a 6.2-fold ± 0.4-fold increase compared to that of untreated control Calu-3 cells. The symbols * and # indicate significant difference from the value measured for untreated control cells. n = 3 to 6 for all data. (B) Effects of PAK, PAK ΔfliC, and heat-killed PAK ΔfliC on NF-κB in flagellin-stimulated Calu-3 cells. Luciferase activity was expressed relative to the maximal activity attained during treatment with flagellin (10−7 g/ml) or flagellin plus PAK. The response to flagellin averaged a 6.1-fold ± 0.6-fold increase compared to that of untreated control Calu-3 cells. In the presence of flagellin, PAK inhibited NF-κB compared to flagellin alone, and PAK ΔfliC caused even larger inhibitions of flagellin-stimulated NF-κB activity. Heat-killed PAK ΔfliC (open square) did not inhibit the response to flagellin. Data are averages ± standard deviations (n = 3). The symbol * indicates significant difference (P < 0.05) from flagellin-treated cells. (C) Inhibitory effect of PAK ΔfliC on flagellin-stimulated IL-8 secretion. Calu-3 cells or primary human tracheal epithelial cells (hTE) were left untreated or were treated with flagellin (10−7 g/ml), PAK ΔfliC (108 CFU/ml), or flagellin plus PAK ΔfliC for 4 h. Bathing media were sampled at t = 0 and after 4 h, and IL-8 was measured. PAK ΔfliC had no effect on IL-8 secretion by control cells but reduced the response to flagellin. Results are averages ± standard deviations (n = 3). The symbol * indicates significant difference from flagellin-treated cells.
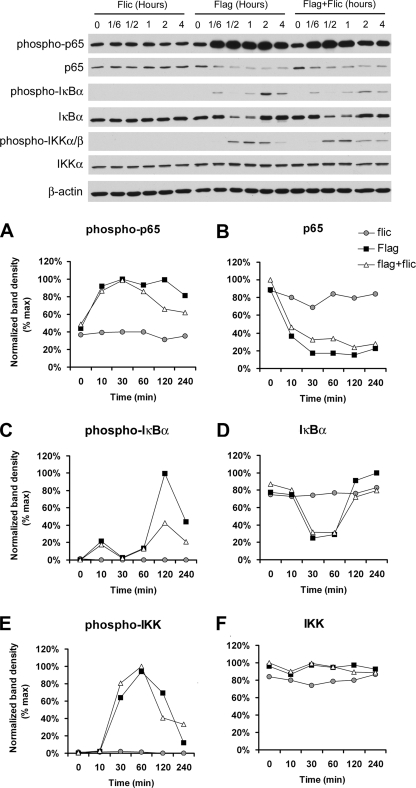
Further analysis of NF-κB signaling was performed by Western blotting of NF-κB (p65), IκBα, and IKK and the phosphorylated versions of these proteins during the treatment of Calu-3 cells with flagellin, PAK ΔfliC, or flagellin plus PAK ΔfliC. Typical blots are shown in Fig. 2, and quantitation of the changes is shown in Fig. 2A to F. Flagellin, but not PAK ΔfliC, stimulated the phosphorylation of p65 (Fig. 2A), IκBα (Fig. 2C), and IKK (Fig. 2E), beginning within 10 min. There were also reductions in the levels of p65 (Fig. 2B), likely resulting from migration into the nucleus that occurred within the first 30 min. Levels of IκBα decreased over 10 to 30 min but then recovered, likely resulting from its degradation and resynthesis (Fig. 2D). These results were consistent with rapid activation of NF-κB signaling by flagellin but not by PAK ΔfliC. Adding flagellin plus PAK ΔfliC together elicited effects that were intermediate between those of flagellin and PAK ΔfliC. The most dramatic effects of PAK ΔfliC were to inhibit the second-phase increase in IκBα phosphorylation and resynthesis of IκBα that occurred at 120 and 240 min (Fig. 2C and D). PAK ΔfliC also inhibited p65 phosphorylation at 120 and 240 min (Fig. 2A). These effects were consistent with PAK ΔfliC-induced reduction of NF-κB-luciferase activity (Fig. 1).
FIG. 2.
Western blot assay of effects of flagellin and PAK ΔfliC on Calu-3 NF-κB signaling. Calu-3 cells were incubated in Ringer's solution and treated with flagellin (10−6 g/ml), PAK ΔfliC (108 CFU/ml, MOI of 100), or flagellin plus PAK ΔfliC for the times shown, lysed, and then processed for immunoblot analysis of NF-κB p65, IκΒα, phospho-IκΒα (serine 32), IKKα, and phospho-IKKα/β (serine176/180). Binding of primary antibodies was visualized with horseradish peroxidase-conjugated secondary antibodies and quantitated (A to F) with NIH Image, normalized to maximal intensities measured during the experiment. See the text for details. The results shown are typical of three similar experiments.
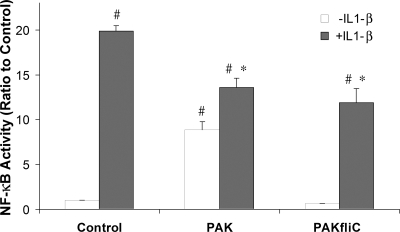
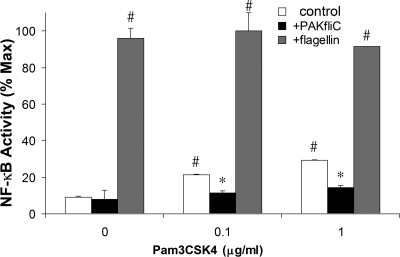
We also tested PAK and PAK ΔfliC on NF-κB-luc activated by IL-1β (2) and the TLR2 agonist Pam3CSK4. As summarized in Fig. 3, IL-1β (50 ng/ml) increased NF-κB-activated luciferase of Calu-3 cells by 20-fold, while PAK (108 CFU/ml) caused 10-fold activation and PAK ΔfliC (108 CFU/ml) did not activate NF-κB. IL-1β-induced NF-κB-luciferase activity was reduced by 32% by PAK and by 40% by PAK ΔfliC. As summarized in Fig. 4, Pam3CSK4 (0.1 and 1.0 μg/ml) elicited two- to threefold increases in NF-κB-regulated luciferase activity and simultaneous exposure to PAK ΔfliC (108 CFU/ml) reduced the response to Pam3CSK4 by >50%. Thus, both IL-1β and Pam3CSK4 activated NF-κB, though to different extents, and PAK ΔfliC reduced this activation.
FIG. 3.
PAK and PAK ΔfliC inhibit IL-1β-activated NF-κB activation. NF-κB-luc-expressing Calu-3 cells were left untreated (control) or incubated for 4 h with 50 ng/ml IL-1β, 108 CFU/ml PAK, and/or 108 CFU/ml PAK ΔfliC and then processed for luciferase activity measurement. Average luciferase ratios with respect to the control (± standard deviations) were plotted. IL-1β caused 20-fold stimulation over the control value (untreated cells). PAK reduced NF-κB activation in the presence of IL-1β by 32%. PAK ΔfliC reduced NF-κB activation in the presence of IL-1β by 40%. The symbol # indicates significant difference from control cells. The symbol * indicates significant difference from IL-1β-treated cells. n = 3 for all data.
FIG. 4.
PAK ΔfliC inhibits Pam3CSK4-stimulated NF-κB activation. NF-κB-luc-expressing Calu-3 cells were incubated for 4 h with 10−6 g/ml flagellin or 0.1 or 1.0 μg/ml Pam3CSK4 and/or 108 CFU/ml PAK ΔfliC in the combinations shown. Luciferase activation was expressed as a percentage of the maximal (Max) stimulation caused by flagellin in any experiment, and average percentages (± standard deviations) were plotted. n = 3 for all data. The symbol # indicates significant difference from the value measured for cells without treatment. The symbol * indicates significant difference in comparison to treatment with Pam3CSK4-treated versus Pam3CSK4-plus-PAK ΔfliC-treated cells. Values for flagellin versus flagellin plus either concentration of PAM3CSK4 were not statistically significantly different from each other.
Are type III secretion and bacterial-cell-epithelium contact required for the inhibitory effect?
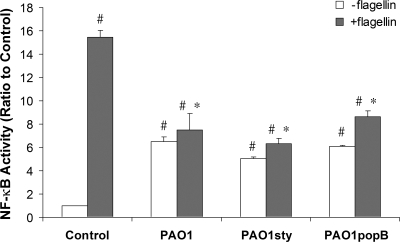
P. aeruginosa could have mediated the inhibitory effect through the TTSS injection of exotoxin S, T, or Y into the epithelial cells. PAK does not produce ExoU, so this exotoxin was not involved. The TTSS requires bacterial contact with epithelial basolateral membranes (15). The roles of ExoS, -T, and -Y and of the TTSS apparatus were tested with strain PAO1, which, like PAK, does not produce ExoU. PAO1 and mutants that do not express exoS, exoT, or exoY (PAO1 ΔexoSTY) or the TTSS apparatus itself (PAO1 ΔpopB; 108 CFU/ml) (32) caused five- to sixfold increases in Calu-3 cell NF-κB activity, while PAO1, PAO1 ΔexoSTY, or PAO1 ΔpopB inhibited flagellin-stimulated NF-κB responses by about 50% (Fig. 5). Thus, PAO1 on its own activated NF-κB in Calu-3 cells but inhibited flagellin-stimulated NF-κB. This inhibition did not require ExoS, -T, -U, or -Y or the TTSS apparatus.
FIG. 5.
P. aeruginosa inhibition of flagellin-stimulated NF-κB activation: roles for TTSS, ExoS, ExoT, and ExoY? NF-κB-luc-expressing Calu-3 cells were incubated for 4 h without treatment or with 10−6 g/ml flagellin and/or 108 CFU/ml PAO1, PAO1 ΔexoSTY, or PAO1 ΔpopB. The symbol # indicates significant difference from the value measured in control cells. The symbol * indicates significant difference from the value measured in flagellin-treated cells. n = 3 for all data.
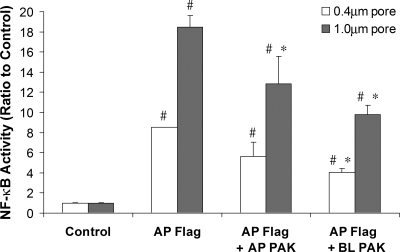
Tests of the role of bacterial contact with the epithelium were performed by growing NF-κB-luc-expressing Calu-3 cells on filters with 0.4-μm (prevents bacterial passage) or 1.0-μm (allows bacterial passage) pores (15) and exposing cells to flagellin (10−6 g/ml) on the apical surface and also to PAK (108 CFU/ml) on the apical or basolateral surface. As shown in Fig. 6, Calu-3 cells on both 0.4- and 1.0-μm-pore-size filters responded to apical flagellin with increases in NF-κB activity. The larger luciferase responses from cells grown on 1.0- versus 0.4-μm-pore-size filters may have resulted from greater access of the adenovirus to the cells through the larger pores and consequently higher luciferase expression. Flagellin-stimulated NF-κB activity was reduced by about 33% when PAK was added to the apical surface of these monolayers, and there was an even larger inhibition when PAK was added to the basolateral surface. This inhibitory effect was similar for Calu-3 cells grown on 0.4- and 1.0-μm-pore-size filters. These results showed that PAK inhibited flagellin activation of NF-κB during exposure to either the apical or the basolateral surface of the epithelium, and contact of the bacteria with the epithelium was not required for this inhibitory effect.
FIG. 6.
P. aeruginosa inhibition of flagellin-stimulated NF-κB activation: role for bacterial-cell-epithelium contact? Calu-3 cells grown to confluence on 1.0- or 0.4-μm-pore-size filters were infected with NF-κB-luc and then left untreated (control) or exposed to 10−6 g/ml flagellin with or without 108 CFU/ml PAK on the apical or basolateral surface for 4 h. Luciferase activities were expressed relative to that of cells grown on 0.4- or 1.0-μm-pore-size filters. The symbol # indicates significant difference from each control (on a 0.4- or 1.0-μm-pore-size filter). The symbol * indicates significant difference from flagellin-treated cells (on a 0.4- or 1.0-μm-pore-size filter). n = 3 for all data.
Do bacteria secrete an antiinflammatory factor(s) into the medium?
Recent experiments showed for macrophages that the quorum-sensing molecule C12 homoserine lactone was responsible for inhibiting LPS/TLR4-induced activation of NF-κB and tumor necrosis factor alpha secretion (14). We therefore compared responses to flagellin, PAO1, and/or PAO1 ΔlasI (lasI encodes the enzyme responsible for the synthesis of C12). As summarized in Table 2, flagellin (10−6 g/ml) elicited 9.5-fold activation of NF-κB by Calu-3 cells, while PAO1 and PAO1 ΔlasI (each at 108 CFU/ml) activated NF-κB only 2.0- to 2.5-fold. PAO1 and PAO1 ΔlasI both reduced flagellin-stimulated NF-κB activity by approximately 50%, showing that C12 was unlikely to explain the P. aeruginosa-induced inhibition of flagellin-stimulated NF-κB signaling in Calu-3 cells.
TABLE 2.
Role for LasI, ExoA, AlgC, CIF, or LPS in P. aeruginosa inhibition of flagellin-stimulated NF-κB activation?a
| Treatment | Avg luciferase expression ± SD
|
nd | |
|---|---|---|---|
| Without flagellin | With flagellin | ||
| Control | 1.00 ± 0.02 | 9.50 ± 0.58 | 9 |
| PAO1 | 2.27 ± 0.27b | 4.25 ± 0.29c | 3 |
| PAO1 ΔlasI | 2.12 ± 0.07b | 4.15 ± 0.43c | 3 |
| PAK | 5.57 ± 0.32b | 7.13 ± 0.39c | 3 |
| PAK ΔexoA | 6.09 ± 0.18b | 7.05 ± 0.03c | 3 |
| PAK ΔalgC | 1.35 ± 0.22b | 1.63 ± 0.05c | 3 |
| PA14 | 2.39 ± 0.12b | 4.57 ± 1.48c | 3 |
| PA14 Δcif | 2.36 ± 0.17b | 4.22 ± 0.45c | 3 |
NF-κB-luc-expressing Calu-3 cells were incubated for 4 h without treatment or with flagellin (10−6 g/ml) and/or different strains of P. aeruginosa (108 CFU/ml). The luciferase values shown are relative to those of untreated control cells for different comparisons. Isogenic comparisons were made for PAO1 versus PAO1 ΔlasI, PAK versus PAK ΔexoA and PAK ΔalgC, and PA14 versus PA14 Δcif.
Significantly different (P < 0.05) from controls.
Significantly different (P < 0.05) from flagellin-treated cells.
n is the number of different comparisons.
Similar experiments were performed to test other factors that could have been secreted or released to reduce flagellin-triggered proinflammatory signaling, including CIF, which has epoxide hydrolase activity (17) and could have altered regulatory factors secreted by the epithelial cells. As summarized in Table 2, PAK and exotoxin A mutant strain PAK ΔexoA (108 CFU/ml) both activated NF-κB sixfold and reduced flagellin-stimulated NF-κB activity by about 25%. Alginate mutant PAK ΔalgC activated NF-κB by only 50% and reduced the flagellin-stimulated response by about 85%. PA14 and the CIF mutant PA14 Δcif activated NF-κB by 2.5-fold and inhibited flagellin-activated NF-κB by about 60%. LPS (10−6 g/ml, activates TLR4) had no effect on NF-κB activation when added alone to Calu-3 cells (data not shown), consistent with previous data showing no effects of LPS on inflammatory responses of primary human airway epithelial cells (31), and there was similar activation of NF-κB by flagellin (10−6 g/ml) and flagellin plus LPS (data not shown). These experiments showed that ExoA, alginate, CIF, and LPS were not responsible for P. aeruginosa inhibition of flagellin-stimulated inflammatory signaling.
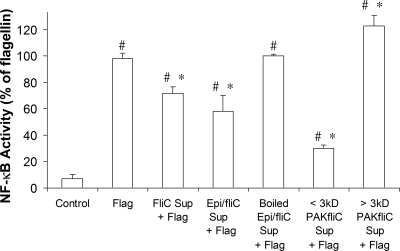
We tested whether the inhibitory effects of P. aeruginosa on Calu-3 cells are elicited by bacterium- or epithelium-derived products and whether these products are sensitive to boiling and filtration. Flagellin elicited similar activation of NF-κB in Calu-3 cells incubated in fresh medium or in medium that had been exposed to the cells for 4 h, indicating that the epithelial cells alone were not producing an inhibitory factor (data not shown). As summarized in Fig. 7, supernatant isolated from PAK ΔfliC or from epithelial cells exposed to PAK ΔfliC inhibited flagellin-stimulated NF-κB responses of Calu-3 cells by 30 to 40%, similar to the 60% inhibition elicited by intact PAK ΔfliC. Inhibitory effects of epithelium-plus-PAK ΔfliC-conditioned medium were abolished by boiling. These results were consistent with the idea that PAK ΔfliC secreted a heat-sensitive factor into the medium that was inhibitory to flagellin activation of NF-κB in Calu-3 cells.
FIG. 7.
P. aeruginosa secretes a small (<3-kDa), heat sensitive factor that inhibits flagellin-stimulated NF-κB activation. NF-κB-luc-expressing Calu-3 cells were incubated in tissue culture medium for 4 h without treatment (control) or with flagellin (10−6 g/ml) or flagellin plus supernatant isolated from PAK ΔfliC (108 CFU/ml) (FliC Sup + Flag); supernatant isolated from Calu-3 cells exposed to PAK ΔfliC (108 CFU/ml) for 4 h (Epi/fliC Sup + Flag); supernatant isolated from Calu-3 cells exposed to PAK ΔfliC (108 CFU/ml) for 4 h, boiled for 15 min, and then cooled to 37°C (Boiled Epi/fliC Sup + Flag); the same supernatant that had been filtered through a 3-kDa-cutoff filter (filtrate) (< 3kD PAKfliC Sup + Flag); or the resulting retentate (> 3kD PAKfliC Sup + Flag). Luciferase activities were measured and expressed relative to the magnitude of the responses to flagellin. Averages ± standard deviations are shown. n = 3 to 9 for each condition. The symbol # indicates significant difference from control cells. The symbol * indicates significant difference from flagellin-treated cells.
Further filtration tests were performed to determine the rough molecular size of the PAK ΔfliC-secreted inhibitory factor. PAK ΔfliC was incubated in Ringer's solution for 18 h, and the supernatant was isolated and added to Calu-3 cells directly or filtered through a 3- or 30-kDa-cutoff filter before addition to Calu-3 cells. As summarized in Fig. 7, the <3-kDa filtrate potently inhibited flagellin-stimulated NF-κB-activity, while the >3-kDa retentate caused no significant inhibition of flagellin-activated NF-κB. Similar results were obtained with the 30-kDa-cutoff filter (n = 3; data not shown). These data showed that the molecular mass of the PAK ΔfliC-secreted inhibitory factor was lower than 3 kDa.
We finally tested whether the inhibitory effect of P. aeruginosa was dependent on the composition of the epithelial incubation medium. NF-κB-luc-expressing Calu-3 cells were incubated for 4 h in tissue culture medium, in Ringer's solution, or in Ringer's solution without glucose or supplemented with serum and treated with flagellin, PAK ΔfliC, and/or supernatants from PAK ΔfliC-exposed (108 CFU/ml) Calu-3 cells. Flagellin-stimulated NF-κB luciferase responses (compared to those of untreated control cells) were larger for Calu-3 cells grown in tissue culture medium (19.2 ± 0.8) than responses in Ringer's solution (11.0 ± 0.4), but there was similar (though somewhat more pronounced) PAK ΔfliC inhibition of Calu-3 cells in Ringer's solution (62% inhibition) versus those in tissue culture medium (31%) (data not shown; n = 3). Further, supernatants isolated from Calu-3 cells incubated with PAK ΔfliC (108 CFU/ml) inhibited the flagellin-activated NF-κB activity of Calu-3 cells incubated in glucose-free (86% inhibition), glucose-containing (77% inhibition), or glucose-plus-FBS-containing (36% inhibition) Ringer's solution (data not shown; n = 3 for each comparison). These results showed that the effect of the PAK ΔfliC-secreted inhibitory factor did not require the presence of glucose or FBS, and this inhibitory effect of PAK ΔfliC was similar in tissue culture medium and Ringer's solution.
DISCUSSION
A major conclusion from these studies was that multiple strains of P. aeruginosa elicited both stimulatory (proinflammatory) and inhibitory (antiinflammatory) effects on both Calu-3 and primary cultures of human airway epithelial cells: PAK, PAO1, and PA14 each activated NF-κB and/or IL-8 secretion by Calu-3 cells but inhibited flagellin-stimulated NF-κB/IL-8 activation. Based on the lack of stimulation by PAK ΔfliC (Fig. 1) (10, 31, 36), it seems likely that the stimulatory effect of these different P. aeruginosa strains was mediated through their production and release of flagellin onto the Calu-3 cells. The smaller activation of NF-κB and IL-8 by intact bacteria (up to 108 CFU/ml) than by 10−7 or 10−6 g/ml flagellin could have resulted from the bacteria releasing flagellin at concentrations below 10−7 or 10−6 g/ml. However, the responses to 10−6 g/ml flagellin were larger than the responses to 10−6 g/ml flagellin plus 108 CFU/ml P. aeruginosa, indicating that P. aeruginosa elicited effects that decreased flagellin/TLR5-mediated activation of NF-κB. This antiinflammatory effect was eliminated by heating the bacteria, indicating that the bacteria needed to be living to elicit their effects or that the factor was heat sensitive. Previous experiments monitoring changes in epithelial cell gene expression in response to heat-killed P. aeruginosa will have missed these antiinflammatory effects (25).
PAK- or PAK ΔfliC-induced inhibition of inflammatory responses also occurred during treatment with IL-1β and the TLR2 agonist Pam3CSK4, indicating that the antiinflammatory effect of P. aeruginosa was exerted at a level besides interference with epithelial cell receptor function. It is possible that the inhibitory effects were exerted at postreceptor steps in the signaling pathways, e.g., MyD88, TRAF, etc. Further work is required to determine such mechanisms. The inhibitory effect of PAK ΔfliC on flagellin-stimulated IL-8 secretion was smaller than that on NF-κB, indicating that although there was potent inhibition of NF-κB, there may have been compensatory effects of PAK ΔfliC on other signaling pathways leading to the activation of other transcription factors (AP-1 and NF-IL-6) that are stimulatory to IL-8 secretion. Western blot assays (Fig. 2) indicated that inhibition by PAK ΔfliC may have been elicited by reducing NF-κB migration into the nucleus, though further experiments are required to confirm this interpretation.
Experiments performed with epithelial cells grown on large- versus small-pore filters showed that the inhibitory effect did not require contact of the bacteria with the epithelium. This effect of P. aeruginosa was therefore different from the inhibitory effects of Escherichia coli on interferon-activated signal transducer and activator of transcription 1 signaling, which required bacterial contact with the epithelial cells (12).
Instead, the present studies showed that P. aeruginosa secreted the inhibitory factor into the medium. Although the molecular identity of the factor has not been determined, studies with isogenic mutants showed that inhibitory effects did not require TTSS or TTSS-secreted exotoxin S, T, Y, or U and also did not require exotoxin A, alginate, or CIF. Filtration studies showed that the molecular mass of the bacterial-cell-secreted inhibitory factor is lower than 3 kDa. The inhibitory effect is mediated by addition to either side of the Calu-3 monolayer, indicating that the bacterial-cell-produced inhibitor may be able to permeate epithelial-cell membranes. Supernatant isolated from either PAK ΔfliC or epithelial cells plus PAK ΔfliC elicited similar inhibition of flagellin-stimulated NF-κB, indicating that PAK ΔfliC alone was sufficient to generate this effect. Many of these results obtained with P. aeruginosa and Calu-3 cells were similar to those elicited by lactic acid bacteria (21) or butyrate (11) in intestinal epithelial cell lines. Butyrate is unlikely to be the low-molecular-weight inhibitor observed here because the P. aeruginosa-secreted factor was inactivated by boiling. The low-molecular-weight (i.e., 297), membrane-permeant, P. aeruginosa-produced quorum-sensing molecule C12 homoserine lactone inhibits NF-κB in airway epithelial cells (14) and could therefore have been the antiinflammatory factor. However, PAO1 and PAO1 ΔlasI elicited similar inhibition of flagellin-induced activation of NF-κB, indicating that C12 was also unlikely to be the still-to-be identified inhibitor.
An interesting implication of our study is that the overall epithelial inflammatory response induced by P. aeruginosa may result from the different release or secretion of flagellin and also from the different production or secretion of the inhibitory factor(s). Thus, different inflammatory responses to different strains of P. aeruginosa (e.g., Table 2) may result from different amounts of flagellin and/or from different amounts of the inhibitory factor(s). Such effects may also explain the apparently higher potency of the P. aeruginosa-produced inhibitory factor when the bacteria and epithelial cells were incubated in Ringer's solution than when they were incubated in tissue culture medium. Another implication is that in intact lung airways, the composition of the airway surface liquid may affect epithelial inflammatory responses through effects on P. aeruginosa production of both flagellin and the inhibitory factor(s). The cystic fibrosis transmembrane conductance regulator plays an important role in controlling airway surface liquid composition and could therefore control proinflammatory signaling by altering the bacterial expression of both proinflammatory factors like flagellin and antiinflammatory factors that remain to be identified.
Acknowledgments
This work was supported by grants from the NIH (DK51799 and PN2-EY018241), the CF Foundation, and Cystic Fibrosis Research, Inc.
We thank Dan Portnoy, Greg Barton, and Russell Vance for advice; Russell Vance Joanne Engel, Bruce Stanton, Steve Lory, and Pete Greenberg for different strains of P. aeruginosa; and Walt Finkbeiner for primary cultures of human tracheal epithelial cells.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Adamo, R., S. Sokol, G. Soong, M. I. Gomez, and A. Prince. 2004. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30627-634. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 3.Canducci, F., F. Cremonini, A. Armuzzi, S. Di Caro, M. Gabrielli, L. Santarelli, E. Nista, A. Lupascu, D. De Martini, and A. Gasbarrini. 2002. Probiotics and Helicobacter pylori eradication. Dig. Liver Dis. 34(Suppl. 2)S81-S83. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary, V. K., Y. Jinno, M. G. Gallo, D. FitzGerald, and I. Pastan. 1990. Mutagenesis of Pseudomonas exotoxin in identification of sequences responsible for the animal toxicity. J. Biol. Chem. 26516306-16310. [PubMed] [Google Scholar]
- 5.Feuillet, V., S. Medjane, I. Mondor, O. Demaria, P. P. Pagni, J. E. Galán, R. A. Flavell, and L. Alexopoulou. 2006. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. USA 10312487-12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu, Z., K. Bettega, S. Carroll, K. Buchholz, and T. E. Machen. 2007. Role of Ca2+ in responses of airway epithelia to P. aeruginosa, flagellin, ATP and thapsigargin. Am. J. Physiol. Lung Cell Mol. Physiol. 292L353-L364. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg, J. B., K. Hatano, and G. B. Pier. 1993. Synthesis of lipopolysaccharide O side chains by Pseudomonas aeruginosa PAO1 requires the enzyme phosphomannomutase. J. Bacteriol. 1751605-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi., D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 9.Heyman, M., and S. Ménard. 2002. Probiotic microorganisms: how they affect intestinal pathophysiology. Cell. Mol. Life Sci. 591151-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hybiske, K., J. Ichikawa, V. Huang, S. J. Lory, and T. E. Machen. 2004. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to P. aeruginosa. Cell. Microbiol. 649-62. [DOI] [PubMed] [Google Scholar]
- 11.Inan, M. S., R. J. Rasoulpour, L. Yin, A. K. Hubbard, D. W. Rosenberg, and C. Giardina. 2000. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 118724-734. [DOI] [PubMed] [Google Scholar]
- 12.Jandu, N., P. J. Ceponis, S. Kato, J. D. Riff, D. M. McKay, and P. M. Sherman. 2006. Conditioned medium from enterohemorrhagic Escherichia coli-infected T84 cells inhibits signal transducer and activator of transcription 1 activation by gamma interferon. Infect. Immun. 741809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karp, C. L., L. M. Flick, K. W. Park, S. Softic, T. M. Greer, R. Keledjian, R. Yang, J. Uddin, W. B. Guggino, S. F. Atabani, Y. Belkaid, Y. Xu, J. A. Whitsett, F. J. Accurso, M. Wills-Karp, and N. A. Petasis. 2004. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 5388-392. [DOI] [PubMed] [Google Scholar]
- 14.Kravchenko, V. V., G. F. Kaufmann, J. C. Mathison, D. A. Scott, A. Z. Katz, D. C. Grauer, M. Lehmann, M. M. Meijler, K. D. Janda, and R. J. Ulevitch. 2008. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science 321259-263. [DOI] [PubMed] [Google Scholar]
- 15.Lee, A., D. Chow, B. Haus, W. Tseng, D. Evans, S. Fleiszig, G. Chandy, and T. Machen. 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277L204-L217. [DOI] [PubMed] [Google Scholar]
- 16.Lee, M. C., C. M. Penland, J. H. Widdicombe, and J. J. Wine. 1998. Evidence that Calu-3 human airway cells secrete bicarbonate. Am. J. Physiol. 274L450-K453. [DOI] [PubMed] [Google Scholar]
- 17.MacEachran, D. P., B. A. Stanton, and G. A. O'Toole. 2008. Cif is negatively regulated by the TetR family repressor CifR. Infect. Immun. 763197-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machen, T. E. 2006. Innate immune response in CF airway epithelia: hyperinflammatory? Am. J. Physiol. Cell Physiol. 291C218-C230. [DOI] [PubMed] [Google Scholar]
- 19.McNamara, N., and C. Basbaum. 2002. Mechanism by which bacterial flagellin stimulates host mucin production. Adv. Exp. Med. Biol. 506269-273. [DOI] [PubMed] [Google Scholar]
- 20.McNamara, N., M. Gallup, A. Sucher, I. Maltseva, D. McKemy, and C. Basbaum. 2006. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate ERK1/2. Am. J. Respir. Cell Mol. Biol. 34653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ménard, S., C. Candalh, J. C. Bambou, K. Terpend, N. Cerf-Bensussan, and M. Heyman. 2004. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muir, A., G. Soong, S. Sokol, B. Reddy, M. I. Gomez, A. Van Heeckeren, and A. Prince. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30777-783. [DOI] [PubMed] [Google Scholar]
- 23.Olvera, C., J. B. Goldberg, R. Sánchez, and G. Soberón-Chávez. 1999. The Pseudomonas aeruginosa algC gene product participates in rhamnolipid biosynthesis. FEMS Microbiol. Lett. 17985-90. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez, A., and P. B. Davis. 2004. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J. Struct. Funct. Genomics 5179-194. [DOI] [PubMed] [Google Scholar]
- 26.Prince, A. 2006. Flagellar activation of epithelial signaling. Am. J. Respir. Cell Mol. Biol. 34548-551. [DOI] [PubMed] [Google Scholar]
- 27.Sanlioglu, S., C. M. Williams, L. Samavati, N. S. Butler, G. Wang, P. B. McCray, Jr., T. C. Ritchie, G. W. Hunninghake, E. Zandi, and J. F. Engelhardt. 2001. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates TNFα secretion through IKK regulation of NF-κB. J. Biol. Chem. 27630188-30198. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzer, C., B. Illek, J. H. Suh, S. J. Remington, H. Fischer, and T. E. Machen. 2007. Organelle redox of CF and CFTR-corrected airway epithelia. Free Radic. Biol. Med. 43300-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, B. Q., W. E. Finkbeiner, J. J. Wine, R. J. Mrsny, and J. H. Widdicombe. 1994. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl secretion. Am. J. Physiol. 266L493-L501. [DOI] [PubMed] [Google Scholar]
- 30.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 41247-1253. [DOI] [PubMed] [Google Scholar]
- 31.Tseng, J., Do, J., J. H. Widdicombe, and T. E. Machen. 2006. Innate immune responses of human tracheal epithelium to Pseudomonas aeruginosa flagellin, TNF-α, and IL-1β. Am. J. Physiol. Cell Physiol. 290C678-C690. [DOI] [PubMed] [Google Scholar]
- 32.Vance, R. E., A. Rietsch, and J. J. Mekalanos. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect. Immun. 731706-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber, A. J., G. Soong, R. Bryan, S. Saba, and A. Prince. 2001. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 281L71-L78. [DOI] [PubMed] [Google Scholar]
- 34.Yates, S. P., R. Jørgensen, G. R. Andersen, and A. R. Merrill. 2006. Stealth and mimicry by deadly bacterial toxins. Trends Biochem. Sci. 31123-133. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Z., J. P. Louboutin, D. J. Weiner, J. B. Goldberg, and J. M. Wilson. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 737151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Z., W. Reenstra, D. J. Weiner, J. P. Louboutin, and J. M. Wilson. 2007. The p38 mitogen-activated protein kinase signaling pathway is coupled to Toll-like receptor 5 to mediate gene regulation in response to Pseudomonas aeruginosa infection in human airway epithelial cells. Infect. Immun. 755985-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]