Abstract
Paratuberculosis is a chronic infectious disorder and a major problem in farmed ruminants. This disease is caused by Mycobacterium avium subsp. paratuberculosis. M. avium subsp. paratuberculosis is an important pathogen that causes Johne's disease in animals and also has been implicated as a possible cause of Crohn's disease in humans, but little is known about the protective immune responses to this microorganism. Fibronectin attachment protein (FAP) is a member of a family of fibronectin-binding proteins produced by several species of mycobacteria which is important in the pathogenesis of M. avium. Addition of recombinant FAP to human respiratory tract organ cultures inhibits M. avium binding to areas where there is epithelial damage. We characterized the role of FAP in promoting adaptive and innate immune responses. FAP functionally activated dendritic cells by augmenting the expression of CD80, CD86, major histocompatibility complex class I, and major histocompatibility complex class II. Moreover, FAP induced the allogeneic immunostimulatory capacity of dendritic cells by stimulating dendritic cell production of Th1-promoting interleukin-12. FAP also increased the production of gamma interferon by T cells in mixed-lymphocyte reactions, which would be expected to contribute to the Th1 polarization of the immune response. The expression of surface markers and cytokine production in dendritic cells was mediated by both mitogen-activated protein kinases and NF-κB pathways. These results show that FAP modulates the adaptive immune responses to M. avium subsp. paratuberculosis by inducing maturation and activation of dendritic cells, which drives Th1 polarization.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that play key roles in the regulation of immune responses to a variety of antigens (26, 34). Immature DCs are distributed mainly in tissues interfacing with the external environment, where they capture and process antigens with high efficiency (3, 7). Immature DCs in peripheral tissues capture and process exogenous agents which trigger DC maturation (35). Maturing DCs then migrate into the lymphoid organs, where they activate naïve T cells by stimulating antigenic peptide-presenting major histocompatibility complex (MHC) type I and II receptors and their costimulatory molecules (27). Stimulation of these receptors activates mitogen-activated protein kinases (MAPKs) and transcription nuclear factor κB (NF-κB), which regulates the maturation and differentiation of the DCs (14). In this manner, as part of the innate immune response, when DCs encounter microbes and their products in the tissues that interface with the external environment, they produce proinflammatory cytokines that activate lymphocytes to directly kill infected cells (19, 33). Thus, DCs provide a link between the innate and adaptive immune responses (4).
Paratuberculosis, or Johne's disease, is a chronic granulomatous enteritis of domestic and wild ruminants that is caused by Mycobacterium avium subsp. paratuberculosis (31). This slow-growing, acid-fast bacillus invades macrophages in lymphoid tissue of the ileum, where it induces recruitment of inflammatory cells, suppresses phagosome maturation, and results in granulomatous enteritis (30). The granulomas cause the intestinal villi to become distorted and lose absorptive surface area. M. avium subsp. paratuberculosis enters intestinal tissue via M cells, which are found in the dome covering Peyer's patches (21). Attachment and internalization of M. avium subsp. paratuberculosis by epithelial cells have been shown to be dependent on the interaction between fibronectin attachment proteins (FAPs) and fibronectin (28, 29). The FAPs are a family of fibronectin-binding proteins that are present in several species of mycobacteria (36). Addition of recombinant FAP inhibits M. avium binding to extracellular matrix in areas where there is epithelial damage, demonstrating the importance of FAP in M. avium pathogenesis (30). Recently, it was reported that human DCs infected with virulent M. avium exhibited limited and reversible upregulation of maturation markers, including CD80, CD86, and CD40. M. avium-infected DCs induced low levels of allogeneic lymphocyte proliferation, CD8+ T-cell activation, and memory CD4+ T-cell activation compared with in vitro mature DCs (8, 15). The level of identity between the FAPs of M. avium and M. avium subsp. paratuberculosis was 96%.
However, it is not known if hosts mount protective immune responses against FAP of M. avium subsp. paratuberculosis. Understanding how the immune system responds to FAP is critical for the development of an effective M. avium subsp. paratuberculosis vaccine.
In this study, we investigated the effects of a noncytotoxic concentration of FAP on the maturation and function of DCs. Our findings demonstrated for the first time that exposure to FAP induces phenotypic and functional maturation of DCs. Exposure to FAP also induced the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 MAPK and NF-κB nuclear translocation, similar to exposure to lipopolysaccharide (LPS). Our data also suggest that FAP induced activation of DCs and initiated the adaptive immune response by polarizing T-cell development to a Th1 response. The critical role of FAP in the activation of these professional APCs and in the immune response to M. avium subsp. paratuberculosis suggests that FAP may be exploited for development of an M. avium subsp. paratuberculosis vaccine.
MATERIALS AND METHODS
Animals.
Male 8- to 12-week-old C57BL/6 (H-2Kb and I-Ab) and BALB/c (H-2Kd and I-Ad) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, South Korea). They were housed in a pathogen-free environment in our animal facility for at least 1 week before use.
Reagents and antibodies.
Recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant mouse interleukin-4 (IL-4) were purchased from R&D Systems (Minneapolis, MN). Dextran-fluorescein isothiocyanate (FITC) (molecular mass, 40,000 Da) and LPS (from Escherichia coli O55:B5) were obtained from Sigma-Aldrich (St. Louis, MO). An endotoxin filter (END-X) and an endotoxin removal resin (END-X B15) were acquired from Associates of Cape Cod (East Falmouth, MA). Cytokine enzyme-linked immunosorbent assay (ELISA) kits for murine IL-12p70, IL-4, and gamma interferon (IFN-γ) were purchased from BD PharMingen (San Jose, CA). FITC- or phycoerythrin (PE)-conjugated monoclonal antibodies (MAbs) were used for flow cytometry to detect antibodies (Table 1). To determine protein levels by Western blotting, anti-phospho-ERK, anti-ERK, anti-phospho-p38, and anti-p38 antibodies were obtained from Cell Signaling (Danvers, MA). Anti-Topo I antibody was obtained from Santa Cruz Biotechnology, (Santa Cruz, CA). Anti-p65 antibody was obtained from Abcam (Cambridge, MA).
TABLE 1.
Antibodies used in this study
| Antibody | Host animal | Dilution | Companya |
|---|---|---|---|
| Annexin V-FITC | Mouse (apoptosis marker) | 1:100 | BD Pharmingen |
| Anti-CD11c (N418) | Mouse (DC marker) | 1:100 | BD Pharmingen |
| CD11c (HL3)-FITC | Mouse (DC marker) | 1:100 | BD Pharmingen |
| CD11c (HL3)-PE | Mouse (DC marker) | 1:100 | BD Pharmingen |
| CD4 (L3T4)-FITC | Mouse (T-cell marker) | 1:100 | BD Pharmingen |
| CD40 (1C10)-PE | Mouse (DC activation marker) | 1:100 | BD Pharmingen |
| CD80 (16-10A1)-PE | Mouse (DC activation marker) | 1:100 | BD Pharmingen |
| CD86 (GL1)-PE | Mouse (DC activation marker) | 1:100 | BD Pharmingen |
| IAb β-chain (AF-120.1)-PE | Mouse (DC activation marker) | 1:100 | BD Pharmingen |
| IL-10 (JESS-16E3)-FITC | Mouse (Th2 marker) | 1:100 | BD Pharmingen |
| IL-12p40/p70 (C15.6)-PE | Mouse (Th1 marker) | 1:100 | BD Pharmingen |
| H2Kb (AF6-88.5)-PE | Mouse (DC activation marker) | 1:100 | BD Pharmingen |
| p-ERK1/2 | Mouse (signal marker) | 1:1,000 | Cell Signaling |
| ERK1/2 | Mouse (signal marker) | 1:2,000 | Cell Signaling |
| p-p38 | Mouse (signal marker) | 1:1,000 | Cell Signaling |
| p38 | Mouse (signal marker) | 1:2,000 | Cell Signaling |
| Topo I (C-15) | Mouse (nucleus protein marker) | 1:2,000 | Santa Cruz |
| p-65 | Mouse (signal marker) | 1:500 | Abcam |
BD Pharmingen, BD Pharmingen, San Jose, CA; Cell Signaling, Cell Signaling, Danvers, MA; Santa Cruz, Santa Cruz Biotechnology, Santa Cruz, CA; Abcam, Abcam, Cambridge, MA.
Generation and culture of DCs.
DCs were generated from murine whole bone marrow cells. Briefly, bone marrow was flushed from the tibiae and femurs of C57BL/6 mice, and red blood cells were depleted with ammonium chloride. The cells were plated in six-well culture plates (106 cells/ml, 3 ml/well) and cultured at 37°C in the presence of 5% CO2 using OptiMEM (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 × 10−5 M β-mercaptoethanol, 10 mM HEPES (pH 7.4), 20 ng/ml recombinant mouse GM-CSF, and 20 ng/ml recombinant mouse IL-4. On day 3 of culture, floating cells were gently removed, and fresh medium was added. On day 6 or 7 of culture, nonadherent cells and loosely adherent proliferating DC aggregates were harvested for analysis or stimulation, or in some experiments they were replated into 60-mm dishes (106 cells/ml, 5 ml/dish). On day 6, 80% or more of the nonadherent cells expressed CD11c. To obtain highly purified populations for subsequent analyses, the DCs were labeled with bead-conjugated anti-CD11c MAb (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by positive selection on paramagnetic columns (LS columns; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the cell fraction selected was >95%.
Bacterial strains and culture conditions.
M. avium subsp. paratuberculosis JTC303 was grown at 37°C in modified 7H9 broth supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (Becton Dickinson, Sparks, MD) and 2 μg/ml of mycobactin J (Allied Monitor, Fayette, MO) for 1 month as previously described. Both E. coli BL21(DE3), purchased from Novagen (Madison, WI), and E. coli DH5α were grown at 37°C in Luria broth (FisherBiotech, Fair Lawn, NJ). After plasmid transformation, E. coli was cultured in Luria broth containing ampicillin (100 μg/ml).
Plasmid and vector construction.
The cloning vector plasmid pGEM-T Easy was purchased from Promega (Madison, WI), and the expression vector pET-22b(+) was purchased from Novagen (Madison, WI). Both plasmids contained an ampicillin resistance marker. The expression vector contained a T7 promoter and a C-terminal six-His tag-encoding sequence.
Expression and purification of recombinant FAP.
Preparation of recombinant FAP has been described previously (5). Briefly, genomic DNA was isolated from the M. avium subsp. paratuberculosis JTC303 strain, and the DNA encoding FAP (MAP1569) (http://www.ncbi.nlm.nih.gov) lacking the signal sequence was amplified by PCR. After purification of the PCR product, it was ligated into the pET-22b(+) vector digested with the NdeI and XhoI enzymes. Expression of FAP in transformed BL21(DE3) cells was induced with isopropyl-beta-d-thiogalactopyranoside (IPTG) (Promega, Madison, WI). The soluble FAP was extracted after cell disruption by sonication. FAP containing a C-terminal histidine tag was purified using Ni-nitrilotriacetic acid (NTA) resin (Qiagen, Chatsworth, CA). The FAP was dialyzed five times in 10 mM phosphate-buffered saline (PBS) (pH 7.2) using a Slide-A-Lyzer dialysis cassette with a 3-kDa cutoff (Pierce, Rockford, IL). After dialysis, contaminating endotoxin was removed using Detoxi-Gel Affinity Pak columns (Pierce, Rockford, IL). FAP was incubated with endotoxin removal resin overnight to remove LPS and concentrated with a Centricon device (2,000-Da cutoff; Millipore). Also, endotoxin was assayed under endotoxin-free experimental conditions using a Limulus amebocyte lysate pyrogen kit (Biowhittaker, Walkersville, MD). The experiments were conducted according to the manufacturer's protocol. The quantity of endotoxin in the FAP was ≤0.01 ng/mg. The final concentration of purified FAP was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Stimulation of DC by FAP.
FAP was dissolved in culture medium and was added to cultures of isolated DCs in six-well plates (106 cells/ml, 2 ml/well). For analysis of apoptosis, DCs were stimulated with LPS in medium or with medium alone, and apoptosis was analyzed over time by staining surface-exposed phosphatidylserine with FITC-annexin V in combination with propidium iodine (PI) (BD PharMingen, San Jose, CA) according to the manufacturer's instructions.
Flow cytometric analysis.
On day 6, bone marrow DCs were harvested, washed with PBS, and resuspended in fluorescence-activated cell sorter washing buffer (2% FBS and 0.1% sodium azide in PBS). Cells were first blocked with 10% (vol/vol) normal goat serum for 15 min at 4°C and stained with PE-conjugated anti-H-2Kb (MHC class I), anti-I-Ab (MHC class II), anti-CD40, anti-CD80, and anti-CD86 along with FITC-conjugated anti-CD11c (PharMingen, San Diego, CA) for 30 min at 4°C. The stained cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Quantitation of antigen uptake.
Endocytosis was quantitated as described by Lutz et al. (17). In brief, 2 × 105 cells were equilibrated at 37°C or 4°C for 45 min and then pulsed with fluorescein-conjugated dextran (molecular mass, 40,000 Da; Sigma-Aldrich) at a concentration of 1 mg/ml. Cold staining buffer was added to stop the reaction. The cells were washed three times, stained with PE-conjugated anti-CD11c antibodies, and then analyzed with the FACSCalibur. Nonspecific binding of dextran to DCs was determined by incubation of DCs with FITC-conjugated dextran at 4°C, and the resulting background value was subtracted from the specific binding values. The medium used for the cultures with FAP stimulation was supplemented with GM-CSF, which is required for the capture of antigens by DCs.
Cytokine assays.
Cells were first blocked with 10% (vol/vol) normal goat serum for 15 min at 4°C and then stained with FITC-conjugated CD11c+ antibody for 30 min at 4°C. Cells stained with the appropriate isotype-matched immunoglobulin (Ig) were used as negative controls. The cells were fixed and permeabilized with a Cytofix/Cytoperm kit (PharMingen, San Jose, CA) used according to the manufacturer's instructions. Intracellular IL-12p40/p70, IL-10, and IFN-γ were detected with fluorescein R-PE-conjugated antibodies (PharMingen) in a permeation buffer. The cells were analyzed with a FACSCalibur flow cytometer using the CellQuest program. The presence of murine IL-12p70, IL-4, and IFN-γ in DCs was determined using an ELISA kit (PharMingen, San Jose, CA) according to the manufacturer's instructions.
Mixed-lymphocyte reaction.
Responder T cells, which participate in allogeneic T-cell reactions, were isolated using a MACS column from total mononuclear cells prepared from BALB/c mice (Miltenyi Biotec, Bergisch Gladbach, Germany). Staining with FITC-conjugated anti-CD3 antibodies (BD PharMingen, San Diego, CA) revealed that the preparation consisted mainly of CD3+ cells (>95%). The lymphocyte population (95% CD3+ cells) was then washed twice in PBS and labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE) in PBS as previously described (19). After they were shaken for 8 min at room temperature, the cells were washed once in FBS and twice in PBS with 10% FBS. Unstimulated DCs (1 × 104 cells) or DCs exposed to FAP (200 ng/ml) or LPS (200 ng/ml) for 24 h were cocultured with 1 × 105 allogeneic CFSE-labeled T lymphocytes in 96-well, U-bottom plates (Nunc, Thermo Fisher Scientific, Rochester, NY). A negative control (CD3+ lymphocytes alone) and a positive control (CD3+ lymphocytes with 5 μg of concanavalin A) were included in each experiment. After 4 days, the cells were harvested and washed in PBS. Lymphocytes optically gated by CFSE dilution were assessed by flow cytometry.
Nuclear and cytoplasmic extracts and Western blotting.
The cells were pretreated with 200 ng/ml FAP. Then following 15 or 30 min of incubation at 37°C, cells were washed twice with cold PBS and lysed with modified RIPA buffer (1.0% NP-40, 1.0% sodium deoxycholate, 150 nM NaCl, 10 mM Tris-HCl [pH 7.5], 5.0 mM sodium pyrophosphate, 1.0 mM NaVO4, 5.0 mM NaF, 10 mM/ml leupeptin, 0.1 mM phenylmethylsulfonyl fluoride) for 15 min at 4°C. Lysates were cleared by centrifugation at 14,000 × g for 20 min at 4°C. The protein content of cell lysates was determined using a micro bicinchoninic acid assay kit (Pierce, Rockford, IL). Equivalent amounts of proteins were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting using anti-phospho-ERK1/2, anti-phospho-c-Jun N-terminal kinase, or anti-phospho-p38 MAPK MAb for 3 h, as described by the manufacturers. Following three washes with Tris-buffered saline with Tween, membranes were incubated with secondary horseradish peroxidase-conjugated anti-mouse IgG for 1 h. After the blots were washed, they were developed using the enhanced chemiluminescence system by following the manufacturer's instructions. DC nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL) according to the manufacturer's instructions. NF-κB p-p65 subunits in the nuclear extracts were detected by Western blot analysis with an anti-NF-κB p-p65 subunit antibody.
Statistics.
All results were expressed as means ± standard deviations. Statistical significance was determined using a Student t test for unpaired observations, and the differences were compared to determine statistical significance by using one-way analysis of variance, followed by Bonferroni's post hoc test.
RESULTS
Purification of recombinant FAP and cytotoxicity.
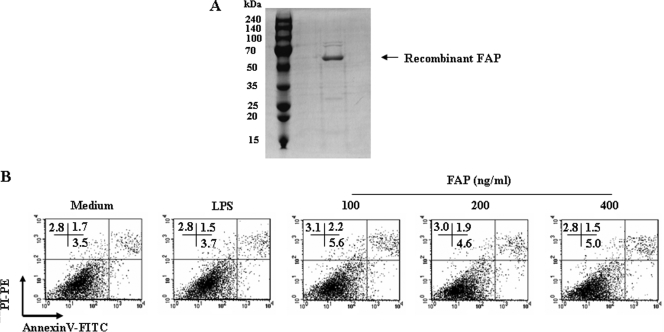
Soluble recombinant FAP was first produced and characterized for use in this study. Recombinant FAP was extracted after cell disruption by sonication and was purified using Ni- NTA resin. After dialysis, the endotoxin contaminating FAP was removed, which was confirmed by SDS-PAGE. The purified FAP had a molecular mass of approximate 55 kDa as assessed by SDS-PAGE (Fig. 1A). The molecular mass of FAP that was higher than the predicted molecular mass (∼32 kDa) may have been the result of the high proline content (5, 30).
FIG. 1.
FAP is not cytotoxic to DCs. (A) Recombinant FAP was produced in BL21 cells, purified with NTA resin, and subjected to SDS-PAGE. (B) Bone marrow-derived DCs were analyzed by flow cytometry. FAP (100 to 400 ng/ml) was added on day 6, and cultures were harvested 24 h later. The DCs were stained with anti-CD11c, annexin V, and PI. The percentage of positive cells (annexin V- and PI-stained cells) in each quadrant is indicated. The results are representative of the results of three experiments.
There were no statistically significant differences in the percentages of dead cells in DC cultures exposed to up to 400 μg/ml of FAP when cell death was detected by annexin V-PI staining (Fig. 1B). This indicated that our recombinant FAP was not cytotoxic to DCs and did not contain significant amounts of endotoxin that would interfere with our studies using concentrations below 400 μg/ml.
FAP induces maturation of DCs.
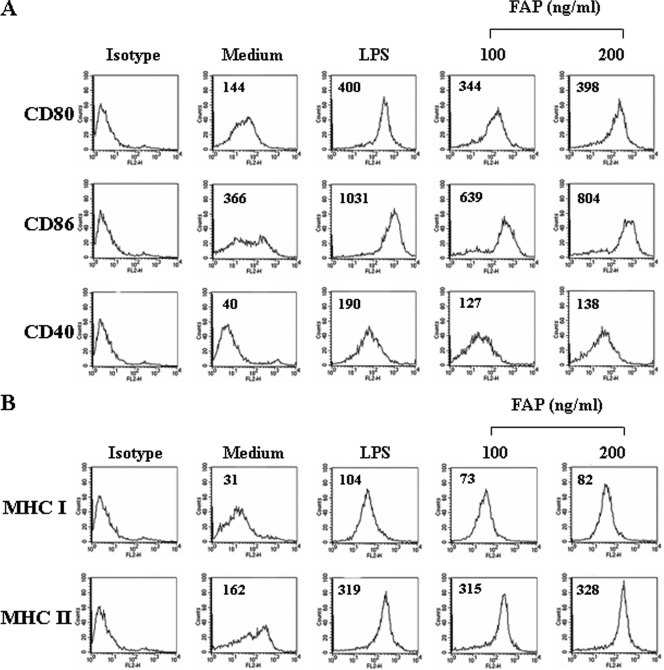
We hypothesized that DCs are the first cells that respond to FAP during an immune response to M. avium. To determine if FAP affects the maturation of sentinel DCs to effector DCs, bone marrow-derived DCs were cultured for 6 days in OptiMEM supplemented with GM-CSF and IL-4 under standard conditions, followed by 1 day of growth in the presence of 100 or 200 ng/ml of FAP. LPS were used as a positive control. The resulting populations of DCs were analyzed by flow cytometry to determine expression of surface molecules involved in helper T-cell activation. As shown in Fig. 2A and 2B, FAP-treated DCs showed increased expression of surface markers, including CD80, CD86, CD40, MHC class I, and MHC class II. The expression of MHC class II by FAP-treated DCs was greater than that by LPS-treated DCs (Fig. 2B). The significant upregulation of the costimulatory and MHC class molecules was FAP dose dependent.
FIG. 2.
FAP induces the expression of costimulatory and MHC class molecules on DCs in a dose-dependent manner. DCs were cultured for 24 h in the presence of 100 or 200 ng/ml FAP and analyzed by two-color flow cytometry. The cells were gated to exclude CD11c+ cells. Medium, untreated control; LPS, positive control (200 ng/ml LPS). DCs were stained with anti-CD80, anti-CD86, anti-CD40, anti-MHC class I, or anti-MHC class II. The results are representative of the results of three experiments.
FAP induces IL-12 secretion and does not influence IL-10 production during DC maturation.
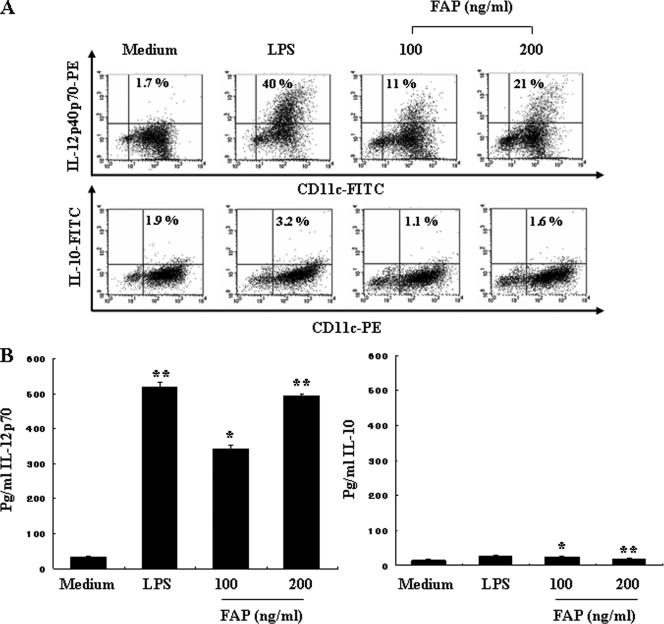
Next, we determined if FAP-stimulated DCs could produce proinflammatory cytokines that were needed to stimulate a Th response. The primary factors that drive the development of Th1 and Th2 cells are IL-12 from APCs and IFN-γ or IL-4 from T cells, respectively. IL-12 production was previously determined to be a specific marker of DC activation (20). Thus, we hypothesized that FAP-activated DCs produce IL-12. We analyzed the production of both intracellular IL-12p40/p70 and bioactive IL-12p70 in FAP-treated DCs using flow cytometry. As shown in Fig. 3A, FAP treatment of DCs increased the percentage of IL-12-positive cells compared to the results obtained for untreated DCs. We also investigated the production of IL-10, a pleoiotropic cytokine known to have inhibitory effects on the accessory functions of DCs and thereby to prevent Th responses. As expected, the levels of expression of IL-10 were comparable for FAP-treated and untreated control DCs (Fig. 3A). These results were confirmed by performing an ELISA of the culture supernatants, which revealed high levels of IL-12p70 produced by DCs stimulated with FAP for 24 h (473 ± 12.2 pg/ml), whereas the concentration of IL-10 remained at baseline levels (Fig. 3B). These results indicated that exposure to FAP induces the functional maturation of DCs that produce high levels of IL-12.
FIG. 3.
Cytokine production by FAP-treated DCs. DCs were generated by stimulating immature DCs with 200 ng/ml LPS, 100 ng/ml FAP, or 200 ng/ml FAP for 24 h. (A) Analysis of IL-12p40/p70 and IL-10 expression in CD11c+ DCs by intracellular cytokine staining. The percentage of positive cells is indicated for each condition and is representative of the results of four separate experiments. (B) Analysis of IL-12p70 and IL-10 production by magnetic bead-purified DCs (1 × 106 cells) using ELISA. The data are the means and standard deviations of three experiments. *, P < 0.5 for a comparison with untreated DCs; **, P < 0.01 for a comparison with untreated DCs. Medium, chemically untreated control group.
FAP reduces the endocytic activity of DCs.
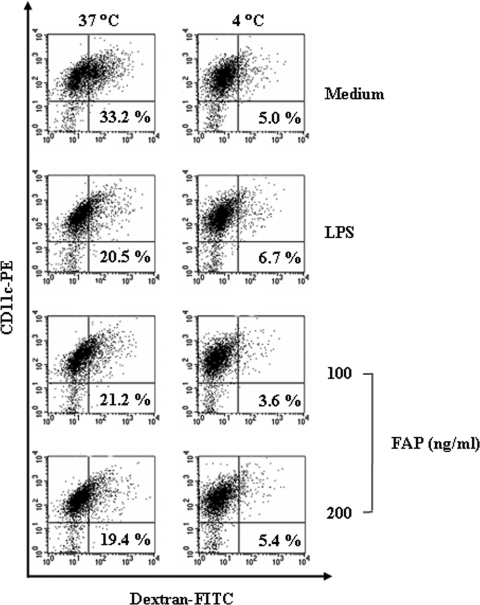
The expression of surface molecules on DCs and the observed changes in IL-12 production show that exposure to FAP leads to profound induction of the phenotypic maturation of DCs. We also investigated whether FAP-stimulated DCs had the reduced endocytic activity characteristic of functionally mature DCs. We exposed the DCs to FAP in the presence of dextran-FITC and assessed the percentage of double-positive cells (CD11c+ and dextran-FITC positive) by flow cytometry. The percentage of double-positive cells was lower in the LPS-treated DC cultures than in the untreated DCs cultures (Fig. 4). Similarly, the percentage of double-positive cells was lower in the FAP-treated DC cultures than in the unstimulated DC cultures (Fig. 4). Thus, the FAP-treated DCs had reduced endocytic activity, which indicates functional maturity. Repetition of these experiments at 4°C showed that the uptake of dextran-FITC by DCs was inhibited at low temperatures.
FIG. 4.
FAP-treated DCs have reduced endocytic capacity. DCs were treated with 200 ng/ml FAP or LPS for 24 h and stained with a PE-conjugated anti-CD11c+ antibody. Endocytic activity at 37°C or 4°C was assessed by flow cytometry analysis of dextran-FITC uptake. The percentages of dextran-FITC-positive CD11c+-positive cells are indicated. The results are representative of the results of four experiments.
FAP-treated DCs stimulate a Th1-type response.
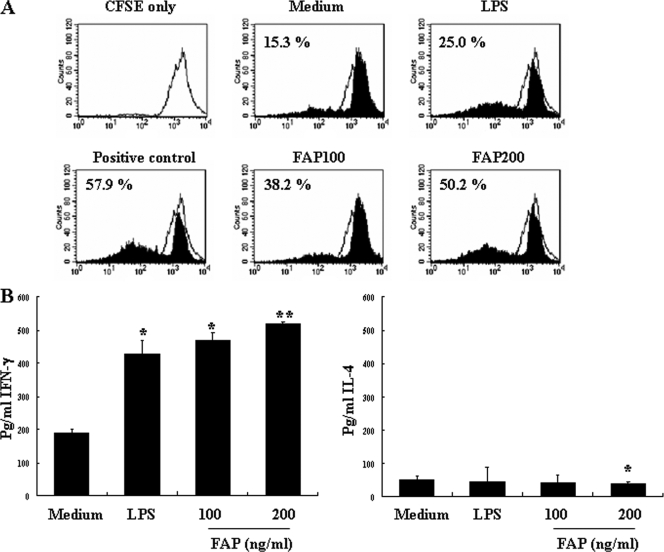
DCs have the unique ability to induce a primary immune response by stimulating naïve T cells to differentiate into active Th1 or Th2 cells. The increased expression of costimulatory and MHC molecules by FAP-stimulated DCs enhances the presentation of antigen to T cells and therefore should also enhance T-cell activation. In order to determine if FAP stimulated naïve CD4+ T-cell activation by DCs, we exposed DCs to FAP for 24 h and performed an allogeneic mixed-lymphocyte reaction analysis. CD4+ splenic T cells from BALB/c mice were cocultured with FAP-treated DCs derived from C57BL/6 mice. As shown in Fig. 5A, the FAP-treated DCs elicited a higher rate of T-cell proliferation than the untreated control DCs elicited. Interestingly, the maturation induced by FAP stimulation profoundly increased the allostimulatory capacity of the DCs. These results demonstrate that FAP enhances the immunostimulatory capacity of DCs to stimulate T cells. Furthermore, we investigated the cytokine production profile of the CD4+ T cells stimulated by FAP-treated DCs and LPS-treated DCs. As shown in Fig. 5B, allogeneic T cells primed with FPA-treated DCs produced a Th1 cytokine profile that included high levels of IFN-γ and low levels of IL-4.
FIG. 5.
FAP-treated DCs induce proliferation of CD4+ T cells and a Th1 response. The DCs were incubated for 24 h in medium with phorbol myristate acetate (positive control), 100 ng/ml FAP, 200 ng/ml FAP, or 200 ng/ml LPS. The DCs were then washed and cocultured with allogeneic T cells for 3 days. (A) Percentage of T-cell proliferation as determined by CFSE flow cytometry. (B) Results for cells examined for cytokine release after 48 h. IL-4 and IFN-γ concentrations in culture supernatants were measured by ELISA. The data are expressed as pg/ml/106 cells (means and standard deviations for triplicate cultures). *, P < 0.05, and **, P < 0.01, for a comparison with T cells primed with untreated DCs. Medium, chemically untreated control group.
FAP activates TLR4 and MAPKs in DCs.
Toll-like receptors (TLRs) link the innate and adaptive immune responses (25). TLR ligands include glycolipids, peptidoglycans, and LPS, which are produced by a wide variety of microorganisms. Ligand binding to the TLRs activates MAPK signaling pathways and the production of IL-12 by monocyte/macrophage lineage cells and DCs (22). The activation of MAPKs, including ERK1/2, c-Jun N-terminal kinases, p38 MAPK, and NF-κB, is critical for maturation of DCs (26). Recent studies suggested that the three MAPK signaling pathways differentially regulate all aspects of DC phenotype maturation and cytokine production (14, 37). In order to investigate the effects of FAP on the MAPK and NF-κB signaling pathways, DCs were treated with 200 ng/ml of FAP, and kinase activation was assessed. ERK1/2 and p38 MAPK activities were measured by phospho-specific Western blotting, and NF-κB activation was indicated by nuclear translocation of the NF-κB p65 subunit detected by Western blotting. FAP induced the phosphorylation of ERK1/2 and p38 MAPK in DCs (Fig. 6A). In addition, nuclear translocation of the p65 subunit was observed in FAP-treated DCs (Fig. 6B). Activation of these pathways is consistent with TLR stimulation by FAP. Several bacterial proteins have been suggested to enhance to the expression of TLR4 by DCs (12, 24). Therefore, we next determined if FAP enhanced TLR4 expression by DCs using real-time quantitative reverse transcription-PCR. The levels of TLR4 mRNA were significantly higher in FAP-treated DCs than in untreated control DCs (Fig. 6C). These data indicate that FAP induced DC TLR4 expression and activation of MAPK and NF-κB pathways.
FIG. 6.
FAP enhanced TLR4 expression, MAPK activation, and NF-κB nuclear translocation. DCs were treated with 200 ng/ml FAP for 15, 30, or 45 min. (A) Cell lysates were prepared and blotted with anti-phospho-ERK1/2, anti-ERK1/2, anti-phosopho-p38, and anti-p38 antibody. (B) Nuclear extracts were blotted with anti-phosphop-p65 antibody. A signal was detected with biotinylated goat anti-rabbit IgG and visualized using enhanced chemiluminescence. (C) Total RNA was extracted, and quantitative real-time PCR was performed using sequence-specific primers for TLR4 and glyceraldehyde-3-phosphate dehydrogenase. Glyceraldehyde-3-phosphate dehydrogenase was included as an internal control. Medium, untreated DC group. For each sample, ΔΔCT (ddCT; crossing point) values were calculated as the CT for the target gene minus the CT for the glyceraldehyde-3-phosphate dehydrogenase gene. Gene expression was derived according to the equation 2−ΔΔCT; changes in gene expression were expressed relative to basal levels. **, P < 0.01 for the comparison with the medium control. The data are the means and standard deviations of three experiments.
DISCUSSION
Holsti et al. (11) previously demonstrated that FAP-A-inoculated mice showed stimulation of a strong T-cell response in vivo. However, this group did not determine the effect of FAP-A on the detailed mechanism of the T-cell response in vitro. Here we report for the first time that M. avium subsp. paratuberculosis FAP is a highly stimulatory protein that induces a Th1 response by stimulating DC maturation and IL-12 production.
We also provide evidence that FAP activates TLR signaling and induces the expression of TLR by DCs. The recognition of antigen by TLRs leads to activation of MAPK and NF-κB pathways in DCs (6, 10, 13, 16). Consistent with this finding, FAP was able to activate both MAPK and NF-κB pathways in DCs. NF-κB activation is essential for the expression of a variety of the proinflammatory cytokines (9). Therefore, the activation of NF-κB by FAP is a possible mechanism underlying the increased expression of IL-12 by DCs. Together, our results suggest that FAP binds to a TLR on DCs and elicits MAPK- and NF-κB-mediated IL-12 production. This IL-12 production is then responsible for activating CD4-positive T cells to differentiate into Th1 cells that produce IFN-γ. IL-23, a more recently discovered member of the IL-12 family, also promotes a Th1 response, but it has functions distinct from those of IL-12. IL-23 is a heterodimeric cytokine composed of the p19 and p40 subunits. While the p19 subunit is unique to IL-23, p40 is shared with IL-12 (23). IL-23 is required for the generation of effector memory T cells and is also needed for generation of IL-17-producing T cells, which play an important role in the inflammatory response (1, 23). Through these activities, IL-23 plays a critical role in chronic inflammatory diseases.
Recently, Lei et al. (15) suggested that M. avium can infect bovine DCs and does not induce strong DC antigen presentation. In addition, M. avium-infected DCs produce a small amount of the IL-12 cytokine but a large amount of the IL-10 cytokine. The greater IL-10 cytokine production may inhibit not only DC maturation and initiation of Th1 polarization but also bystander macrophage activation at the infection site (15).
In this way, FAP forms a link between the innate DC response and the adaptive Th1 immune response to M. avium. The nature of the bacterial pathogens that are encountered by the adaptive immune response tailors the balance of the Th1/Th2 response to a particular pathogen. Our results show that M. avium FAP activates DCs and specifically produces a Th1 immune response ex vivo. FAP induces Th1 polarization by increasing IL-12 production by DCs and IFN-γ production by T cells. However, it is possible that FAP, like the chlamydial major outer membrane protein antigen (MOMP), may elicit a different T-cell response in vivo. MOMP also induces CD4+ Th1 polarization in vivo, but DCs pulsed ex vivo with MOMP and adoptively transferred into naïve mice generate a Th2 immune response (32). Another bacterial protein, PorA of Neisseria meningitidis, also directs differentiation of CD4+ T cells toward a Th2-type response (2). Together, previous studies of the role of FAP in immune responses to mycobacteria have shown that the presentation of FAP peptide by M. avium-infected macrophages to T cells contributes to the development of protective immunity to this pathogen (11). Thus, it is critical to extend the study of FAP antigenicity to in vivo studies to fully understand the immune response to M. avium.
In summary, we demonstrated that FAP is a potent antigen and initiates a specific Th1 immune response in vitro. Therefore, FAP, in addition to the known M. avium antigen LPS, may play key roles in determining the nature of the immune response against M. avium subsp. paratuberculosis. Further understanding of the mechanism by which FAP modulates DC function may lead to development of effective M. avium subsp. paratuberculosis vaccines and an effective immunotherapy adjuvant for other infectious diseases.
Acknowledgments
This work was supported in part by Korea Research Foundation grant KRF-2008-314-E00195 funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) and by the Korea Science and Engineering Foundation through National Research Laboratory Program grant R0A-2005-000-10008-0).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2781910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Al-Bader, T., K. A. Jolley, H. E. Humphries, J. Holloway, J. E. Heckels, A. E. Semper, P. S. Friedmann, and M. Christodoulides. 2004. Activation of human dendritic cells by the PorA protein of Neisseria meningitidis. Cell. Microbiol. 6651-662. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18767-811. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 5.Cho, D., S. J. Shin, A. M. Talaat, and M. T. Collins. 2007. Cloning, expression, purification and serodiagnostic evaluation of fourteen Mycobacterium paratuberculosis proteins. Protein Expr. Purif. 53411-420. [DOI] [PubMed] [Google Scholar]
- 6.Didierlaurent, A., B. Brissoni, D. Velin, N. Aebi, A. Tardivel, E. Kaslin, J. C. Sirard, G. Angelov, J. Tschopp, and K. Burns. 2006. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol. Cell. Biol. 26735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dustin, M. L., and A. C. Chan. 2000. Signaling takes shape in the immune system. Cell 103283-294. [DOI] [PubMed] [Google Scholar]
- 8.Hanekom, W. A., M. Mendillo, C. Manca, P. A. Haslett, M. R. Siddiqui, C. Barry III, and G. Kaplan. 2003. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 188257-266. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 10.Hirata, N., Y. Yanagawa, T. Ebihara, T. Seya, S. Uematsu, S. Akira, F. Hayashi, K. Iwabuchi, and K. Onoe. 2008. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol. Immunol. 452734-2742. [DOI] [PubMed] [Google Scholar]
- 11.Holsti, M. A., J. S. Schorey, E. J. Brown, and P. M. Allen. 1998. Identification of epitopes of fibronectin attachment protein (FAP-A) of Mycobacterium avium which stimulate strong T-cell responses in mice. Infect. Immun. 661261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeannin, P., T. Renno, L. Goetsch, I. Miconnet, J. P. Aubry, Y. Delneste, N. Herbault, T. Baussant, G. Magistrelli, C. Soulas, P. Romero, J. C. Cerottini, and J. Y. Bonnefoy. 2000. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat. Immunol. 1502-509. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, Y., G. Chen, Y. Zheng, L. Lu, C. Wu, Y. Zhang, Q. Liu, and X. Cao. 2008. TLR4 signaling induces functional nerve growth factor receptor p75NTR on mouse dendritic cells via p38MAPK and NF-kappa B pathways. Mol. Immunol. 451557-1566. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. S., I. D. Jung, Y. I. Jeong, C. M. Lee, Y. K. Shin, S. Y. Lee, D. S. Suh, M. S. Yoon, K. S. Lee, Y. H. Choi, H. Y. Chung, and Y. M. Park. 2007. d-Pinitol inhibits Th1 polarization via the suppression of dendritic cells. Int. Immunopharmacol. 7791-804. [DOI] [PubMed] [Google Scholar]
- 15.Lei, L., and J. M. Hostetter. 2007. Limited phenotypic and functional maturation of bovine monocyte-derived dendritic cells following Mycobacterium avium subspecies paratuberculosis infection in vitro. Vet. Immunol. Immunopathol. 120177-186. [DOI] [PubMed] [Google Scholar]
- 16.Lin, Y. L., Y. C. Liang, S. S. Lee, and B. L. Chiang. 2005. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 78533-543. [DOI] [PubMed] [Google Scholar]
- 17.Lutz, M. B., C. U. Assmann, G. Girolomoni, and P. Ricciardi-Castagnoli. 1996. Different cytokines regulate antigen uptake and presentation of a precursor dendritic cell line. Eur. J. Immunol. 26586-594. [DOI] [PubMed] [Google Scholar]
- 18.Lyons, A. B. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods 243147-154. [DOI] [PubMed] [Google Scholar]
- 19.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1545071-5079. [PubMed] [Google Scholar]
- 20.Miro, F., C. Nobile, N. Blanchard, M. Lind, O. Filipe-Santos, C. Fieschi, A. Chapgier, G. Vogt, L. de Beaucoudrey, D. S. Kumararatne, F. Le Deist, J. L. Casanova, S. Amigorena, and C. Hivroz. 2006. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J. Immunol. 1773625-3634. [DOI] [PubMed] [Google Scholar]
- 21.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25131-137. [DOI] [PubMed] [Google Scholar]
- 22.Netea, M. G., C. van der Graaf, J. W. Van der Meer, and B. J. Kullberg. 2004. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75749-755. [DOI] [PubMed] [Google Scholar]
- 23.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13715-725. [DOI] [PubMed] [Google Scholar]
- 24.Palmer, C. D., B. E. Mutch, S. Workman, J. P. McDaid, N. J. Horwood, and B. M. Foxwell. 2008. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkappaB activity. Blood 1111781-1788. [DOI] [PubMed] [Google Scholar]
- 25.Pennini, M. E., R. K. Pai, D. C. Schultz, W. H. Boom, and C. V. Harding. 2006. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 1764323-4330. [DOI] [PubMed] [Google Scholar]
- 26.Rescigno, M., M. Martino, C. L. Sutherland, M. R. Gold, and P. Ricciardi-Castagnoli. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1882175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock, K. L., and K. Clark. 1996. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J. Immunol. 1563721-3726. [PubMed] [Google Scholar]
- 28.Schorey, J. S., M. A. Holsti, T. L. Ratliff, P. M. Allen, and E. J. Brown. 1996. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol. Microbiol. 21321-329. [DOI] [PubMed] [Google Scholar]
- 29.Schorey, J. S., Q. Li, D. W. McCourt, M. Bong-Mastek, J. E. Clark-Curtiss, T. L. Ratliff, and E. J. Brown. 1995. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 632652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Secott, T. E., T. L. Lin, and C. C. Wu. 2001. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 692075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Secott, T. E., T. L. Lin, and C. C. Wu. 2004. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect. Immun. 723724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw, J., V. Grund, L. Durling, D. Crane, and H. D. Caldwell. 2002. Dendritic cells pulsed with a recombinant chlamydial major outer membrane protein antigen elicit a CD4+ type 2 rather than type 1 immune response that is not protective. Infect. Immun. 701097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu, U., M. Kiniwa, C. Y. Wu, C. Maliszewski, N. Vezzio, J. Hakimi, M. Gately, and G. Delespesse. 1995. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol. 251125-1128. [DOI] [PubMed] [Google Scholar]
- 34.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9271-296. [DOI] [PubMed] [Google Scholar]
- 35.Young, L. J., N. S. Wilson, P. Schnorrer, A. Mount, R. J. Lundie, N. L. La Gruta, B. S. Crabb, G. T. Belz, W. R. Heath, and J. A. Villadangos. 2007. Dendritic cell preactivation impairs MHC class II presentation of vaccines and endogenous viral antigens. Proc. Natl. Acad. Sci. USA 10417753-17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, W., J. S. Schorey, R. Groger, P. M. Allen, E. J. Brown, and T. L. Ratliff. 1999. Characterization of the fibronectin binding motif for a unique mycobacterial fibronectin attachment protein, FAP. J. Biol. Chem. 2744521-4526. [DOI] [PubMed] [Google Scholar]
- 37.Zinkernagel, R. M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156199-209. [DOI] [PubMed] [Google Scholar]