In the struggle between host and pathogen, competition for resources is often a key point in determining who will be the ultimate winner. The goal of the pathogen is to secure the necessary resources, often nutrients, from the host, while the goal of the host is to sequester the utilizable resources from the pathogen to help prevent infection. Among the key nutrients necessary to virtually all forms of life is iron. Iron plays an essential role in a diverse number of cellular processes. For instance, it serves as an enzymatic cofactor in metabolism and for electron transport. Thus, obtaining sufficient amounts of iron and maintaining iron stores are critical functions for both pathogen and host. However, having too much iron can be detrimental, as excess iron can lead to the formation of hydroxyl radicals through Fenton chemistry, which in turn may lead to cellular and DNA damage.
Based on this yin and yang relationship, it is perhaps no surprise that the human host has several mechanisms for sequestering iron. In the body, iron is stored primarily in ferritin and hemosiderin while the majority of functional iron is found in hemoglobin (4). This being said, there are several other iron storage molecules, like lactoferrin and transferrin, which sequester iron at the mucosal surfaces and within the circulatory system, respectively, and have been found to be iron sources for some pathogens (95). Global sequestration of free iron prevents possible oxidative damage as well as easy acquisition of free iron by pathogenic microbes. Additionally, in a further attempt to limit iron availability to pathogens during infection, the host decreases iron absorption from the gut, increases production of iron storage molecules, and shifts iron from the plasma into the storage molecules (123). Also, iron storage molecules are positioned in areas that are likely to be sites of infection. Thus, the host is immediately able to remove iron from those sites if a pathogen is detected (123). This process of removing free iron and other nutrients from the body and containing them in various storage molecules is termed “nutritional immunity” (124).
Despite these well-orchestrated defenses, bacterial pathogens have evolved mechanisms to breach the iron stores as well as to compete with the host for free iron. Proof of their success can be found by the strong connection between host iron overload and increased susceptibility to several bacterial infections. Indeed, in a recent review of 67 years of medical literature, Khan et al. found an increased association between infection with bacterial pathogens, such as Escherichia coli, Listeria monocytogenes, and Vibrio vulnificus, and hemochromatosis (84). In addition, iron overload in hemodialysis patients is associated with an increased number of bacterial infections as well as an increase in septicemic episodes (118). The importance of iron and infection in humans has also been validated for multiple pathogens by use of animal models. For instance, in a murine model of V. vulnificus infection, there is a drastic decrease in the 50% lethal dose, from 106 to 1.1 bacterial cells, in mice injected with extra iron (127). Also, L. monocytogenes exhibits increased growth in vivo and displays a decreased 50% lethal dose in mice given additional iron (116). Finally, when excess iron is introduced in murine infection models for both Neisseria meningitidis (76) and Salmonella enterica serovar Typhimurium (85), infection is enhanced. While this is by no means an exhaustive list, it is clear that excess iron in the host helps to create a more hospitable environment for opportunistic pathogens. This is likely due to an increase in available free iron and potentially a decrease in antibacterial leukocyte activities (123).
In the midst of the mounting evidence for the connection between iron availability and increased susceptibility to bacterial infection, a mutant of S. Typhimurium that showed constitutive high-level expression of the iron-enterochelin and ferrichrome iron uptake systems was isolated (46). This strain was named the fur mutant, for iron (Fe) uptake regulation (46), and today represents what we know as the ferric uptake regulator. The first fur mutants of E. coli were identified in 1981 and showed constitutive expression of cir, fhuA, and fecA, three iron uptake systems that are typically upregulated when available iron is low (71). Within 1 year, E. coli fur complementation studies showed that fur carried on an F′ lac plasmid restored the wild-type phenotype in a strain bearing a chromosomal fur mutation (70). E. coli fur was successfully cloned in 1984 (69), and the gene sequence was derived shortly thereafter (112). Sequence and biochemical analyses went on to show that Fur is conserved across a wide range of bacterial species and is a small regulatory protein (15 to 17 kDa, approximately 150 amino acids) that functions as a dimer, is cofactored by Fe(II), and is usually autoregulatory.
A greater understanding of the mechanism of Fur regulation came with the first description of a DNA binding consensus sequence for E. coli Fur (39). This 19-bp consensus sequence, GATAATGATAATCATTATC (39), became the gold standard for comparison of types of Fur regulation across bacterial species and facilitated the understanding of exactly how Fur functions as a regulator. When iron is readily available in the bacterial cell, Fur binds iron and dimerizes, and the iron-bound Fur dimers bind to the consensus sequence in target promoters. Binding of Fur at the promoters prevents the binding of RNA polymerase; thus, transcription of the target gene is prevented. While Fur was first characterized as a transcriptional repressor under iron-abundant conditions, it has subsequently been shown to function as an activator and even to repress certain genes in the absence of the iron cofactor; these diverse types of Fur regulation are discussed in further detail below (see Fig. 1). While fur regulation continued to be studied for E. coli and for a wide variety of bacteria, it would take 25 years before what was arguably the next big breakthrough in the study of this regulator occurred—the crystallization of Pseudomonas aeruginosa Fur (106). Having the crystal structure of this important regulatory protein has enabled researchers to begin to make connections between what is known from genetic studies and the actual structure of the protein.
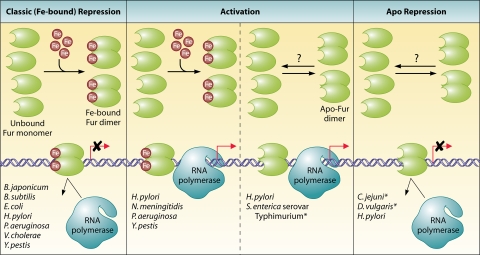
FIG. 1.
Basic features of Fe-Fur repression, apo-Fur repression, and Fur activation. Characteristic features of each type of Fur regulation are shown as they interact with a target DNA promoter. (Left) Classical iron-bound Fur repression. As iron becomes increasingly available in the bacterial cell, the Fe(II) cofactor binds to apo-Fur monomers, and these now-iron-bound monomers dimerize. The iron-bound Fur dimers repress transcription by binding to the Fur box in their target promoters and block the binding of RNA polymerase. (Center) Iron-bound Fur and apo-Fur activation. On the left, iron-bound Fur dimers are formed under conditions of iron abundance, and these dimers bind to Fur boxes in their respective target promoters and activate gene transcription. On the right, apo-Fur dimers form under low-iron conditions. These apo-Fur dimers bind to Fur boxes in their target promoters and activate gene transcription. (Right) apo-Fur repression. Under iron depletion conditions, Fur is in its apo form, and apo-Fur binds to the Fur boxes of its target promoters. This binding blocks the binding of RNA polymerase; hence, transcription is repressed. For the sake of simplicity, apo-Fur repression and activation are depicted as being mediated through an apo-Fur dimer, although it is not known whether apo-Fur functions as a monomer or a dimer. Abbreviated lists of organisms that utilize each type of Fur regulation are listed in each panel. An asterisk indicates organisms for which apo-Fur regulation has been suggested but direct interaction has not yet been determined.
Fur, IRON HOMEOSTASIS, AND BACTERIAL SURVIVAL
Since iron is an essential nutrient for nearly all bacterial life but deadly in excess quantities, Fur's regulation of iron uptake and storage genes plays a significant role in the lives of the diverse number of bacteria that utilize it. As E. coli Fur is among the best studied, there have been numerous publications detailing Fur regulation of iron uptake systems in this model organism. These include the ferric citrate transport system (fecABCDE), the ferrichrome-iron receptor (fhuA), the colicin I receptor (cir) (63, 69, 71), the regulator of the fecABCDE operon (fecIR) (3), the ferrienterochelin receptor (fepA), the ferric ion uptake gene (fiu) (69), the aerobactin (iucA) operon (5, 39, 47, 50), and the divergent operons of the ferrienterochelin receptor-ferric enterobactin esterase (fepA-fes) (49, 79). Additionally, recent macroarray analyses of the iron-dependent and Fur-dependent regulons in E. coli have confirmed the previously characterized Fur regulatory targets as well as identified several new Fur targets (92).
The large number of iron uptake genes that have been found to be controlled by Fur regulation is not restricted to gram-negative bacteria. Indeed, iron uptake is also regulated by Fur in the model gram-positive organism Bacillus subtilis. The best characterized of these systems include the catecholate siderophore dhb and a gene involved in ferrihydroximate transport, fhuD (19). Interestingly, despite the fact that in vivo repression of dhb is seen only in the presence of iron, in vitro Fur binds to the dhb promoter even in the absence of its iron cofactor, though with slightly lower affinity than when iron is present (18). Like with E. coli, global microarray analysis identified as many as 20 Fur-regulated operons, the majority of which are involved in iron acquisition (7).
Countless studies have gone on to show that, like with E. coli and B. subtilis, Fur plays an essential role in iron acquisition systems and many other homeostatic processes for numerous bacterial pathogens. While these are too numerous to discuss exhaustively here, a few key examples that illustrate genes involved in siderophore production, iron acquisition from heme, and iron storage are detailed. A more extensive list of pathogens that utilize Fur and their Fur-regulated genes is summarized in Table 1. However, once again, due to space limitations and the large volume of research on this important regulator, this table is by no means an exhaustive list.
TABLE 1.
Diverse Fur-regulated genes from model organisms and bacterial pathogensa
| Organism | Gene name(s) and description | Type of Fur regulation | Reference(s) |
|---|---|---|---|
| B. subtilis | dhb, catecholate siderophore | Fe-Fur repression | 18, 19 |
| fhuD, ferri-hydroximate transport | Fe-Fur repression | 19 | |
| B. japonicum | irr, heme biosynthetic pathway regulator | Fe-Fur repression | 56, 68 |
| C. jejuni | Cj0859c, hypothetical | Suspected apo-Fur repression | 77 |
| Cj1364, fumarate hydratase | Suspected apo-Fur repression | 77 | |
| E. coli | bfr, bacterioferritin | Indirect Fur activation | 91 |
| cfaB, CFA/I fimbrial subunit gene | Fe-Fur repression | 82 | |
| cir, colicin I receptor | Fe-Fur repression | 63, 69, 71 | |
| fecABCDE, ferric citrate transport | Fe-Fur repression | 3, 69, 71 | |
| fecIR, regulator of fecABCDE operon | Fe-Fur repression | 3 | |
| fepA, ferrienterochelin receptor | Fe-Fur repression | 49, 69, 79 | |
| fes, ferric enterobactin esterase | Fe-Fur repression | 49, 79 | |
| fhuA, ferrichrome-iron receptor | Fe-Fur repression | 69, 71 | |
| fiu, ferric ion uptake | Fe-Fur repression | 69 | |
| fur, ferric uptake regulator | Fe-Fur repression | 38 | |
| hly, hemolysin | Fe-Fur repression | 55 | |
| iha, IrgA homolog adhesin | Fe-Fur repression | 110 | |
| iucA, aerobactin | Fe-Fur repression | 5, 39, 47, 50 | |
| sltA and sltB, Shiga toxins (Shiga-like toxins) | Fe-Fur repression | 21 | |
| sodA, Mn-containing superoxide dismutase | Fe-Fur repression | 11, 27, 72, 98, 108, 117 | |
| sodB, Fe-containing superoxide dismutase | Indirect Fur activation | 41, 42, 98 | |
| H. ducreyi | hgbA, hemoglobin binding protein | Fe-Fur repression | 24 |
| H. pylori | amiE, aliphatic amidase | Fe-Fur repression | 23, 119 |
| ceuE, periplasmic iron binding protein | Fe-Fur repression | 120 | |
| exbB, biopolymer transport protein | Fe-Fur repression | 32 | |
| fecA, ferric citrate transport | Fe-Fur repression | 120 | |
| feoB, ferrous iron transport | Fe-Fur repression | 120 | |
| frpB, iron uptake system | Fe-Fur repression | 33, 37, 120 | |
| fur, ferric uptake regulator | Fe-Fur repression, apo-Fur activation | 35, 36 | |
| nifS, Fe-S cluster synthesis protein | Fe-Fur activation | 2 | |
| pfr, prokaryotic ferritin | apo-Fur repression | 14, 23, 37 | |
| sodB, Fe-containing superoxide dismutase | apo-Fur repression | 23a, 45 | |
| vacA, vacuolating cytotoxin | Indirect Fur repression | 58 | |
| L. monocytogenes | fri, ferritin-like protein | Fe-Fur repression | 52 |
| fur, ferric uptake regulator | Fur regulation | 86 | |
| svpA-srtB, iron uptake locus | Fe-Fur repression | 97 | |
| M. smegmatis | kat, catalase-peroxidase | Fe-Fur repression | 109, 132 |
| M. tuberculosis | kat, catalase-peroxidase | Fe-Fur repression | 109, 132 |
| N. gonorrhoeae | fbpA, periplasmic binding protein | Fe-Fur repression | 40 |
| fur, ferric uptake regulator | Fe-Fur repression | 113 | |
| opaA, opaB, opaC, opaD, opaF, opaG, opaJ, opaK, opaE, opaH, and opaI, opacity proteins | Fe-Fur repression | 113 | |
| sodB, superoxide dismutase | Fur activation | 113 | |
| tbpA and tbpB, transferrin receptors | Fe-Fur repression | 1 | |
| tonB, receptor | Fe-Fur repression | 113 | |
| N. meningitidis | norB, nitric oxide reductase | Fe-Fur activation | 34 |
| nuoA, NADH dehydrogenase I chain A | Fe-Fur activation | 34 | |
| pan1 (now referred to as aniA), anaerobically induced outer membrane protein | Fe-Fur activation | 34 | |
| P. aeruginosa | bfr, bacterioferritin | Fe-Fur activation | 126 |
| fhuA, ferrichrome-iron receptor | Fe-Fur repression | 99 | |
| katB, catalase | Indirect Fur repression | 74 | |
| pchR, pyochelin siderophore | Fe-Fur repression | 99 | |
| pfeR, enterobactin receptor regulator | Fe-Fur repression | 100 | |
| pvdS, alternate sigma factor | Fe-Fur repression | 99 | |
| sdh, succinate dehydrogenase | Indirect Fur activation | 126 | |
| sodA, Mn-containing superoxide dismutase | Fe-Fur repression | 73, 74 | |
| sodB, Fe-containing superoxide dismutase | Indirect Fur activation | 126 | |
| tonB, receptor | Fe-Fur repression | 100 | |
| toxA, exotoxin A | Indirect Fur repression | 99, 107 | |
| S. aureus | fhuCBD, ferrichrome-iron receptor | Fe-Fur repression | 78, 130 |
| kat, catalase-peroxidase | Fur activationb | 78 | |
| sirABC, siderophore transport system | Fe-Fur repression | 78, 130 | |
| S. coelicolor | catC, catalase-peroxidase | Fe-Fur repression | 66 |
| fur, ferric uptake regulator | Fe-Fur repression | 66 | |
| S. enterica serovar | hmp, flavohemoglobin | Fe-Fur repression | 29 |
| Typhimurium | iro-28, iron-regulated protein | apo-Fur activation | 54, 67 |
| mntH, bacterial homolog of mammalian natural-resistance-associated macrophage protein 1 | Fe-Fur repression | 80, 83 | |
| rfrA and rfrB, sRNA | Fe-Fur repression | 43 | |
| sodB, Fe-containing superoxide dismutase | Indirect Fur activation | 43 | |
| V. cholerae | hly, hemolysin | Fe-Fur repression | 114 |
| irgA, outer membrane protein | Fe-Fur repression | 60, 90 | |
| V. vulnificus | fur, ferric uptake regulator | apo-Fur activation | 87 |
| hupA, heme utilization gene | Fe-Fur repression | 89 | |
| vuuA, vulnibactin receptor | Fe-Fur repression | 122 | |
| Y. pestis | bfr, bacterioferritin | Fe-Fur repression | 59 |
| fhuCDB, ferrichrome-iron receptor | Fe-Fur repression | 59 | |
| feoAB, ferrous iron transport | Fe-Fur repression | 59 | |
| fepB, ferrienterochelin receptor | Fe-Fur repression | 59 | |
| ftnA, iron storage protein | Fe-Fur activation | 59 | |
| iucA, aerobactin biosynthesis protein | Fe-Fur repression | 59 | |
| katA, catalase | apo-Fur activation | 59 | |
| napF, ferredoxin-type protein | Fe-Fur activation | 59 | |
| tonB, receptor | Fe-Fur repression | 59 |
Due to the large volume of research on Fur, this table does not represent an exhaustive list of Fur-regulated genes.
Not determined whether Fur activation is mediated through Fe-Fur or apo-Fur.
Siderophores are iron-binding proteins secreted by bacteria to acquire iron from the environment. In P. aeruginosa, Fur regulates the production of the siderophores pyoverdin and pyochelin (107). In fact, pyoverdin has been found in the sputum of cystic fibrosis patients infected with P. aeruginosa (64), and isolates from patients produce both siderophores (65). A siderophore transport system (sir) and the ferrichrome uptake operon (fhu) are also regulated by Fur in Staphylococcus aureus (78, 130). Next, Fur regulates genes involved in the acquisition of iron from unique host sources, like heme and transferrin. V. vulnificus Fur regulates hupA, a heme utilization gene (89), while the causative agent of human chancroid, Haemophilus ducreyi, utilizes Fur to control the expression of hgbA, which encodes a protein involved in hemoglobin binding (24). It is interesting to note that even though pathogenic Neisseria species produce transferrin receptors (tbpA and tbpB) to bind host iron sources, rather than producing siderophores to scavenge iron directly, the host may be attempting to limit iron availability and to decrease colonization by producing antibodies to these receptors during infection (1). Finally, Fur regulation of iron storage molecules is also important for bacterial pathogenesis to help ensure that once iron is acquired from the host, it is stored for use by the bacteria and contained to prevent the toxic effects of excess free iron. fri, the only identified ferritin-like protein in L. monocytogenes, is Fur regulated (52), as is a seven-gene locus, svpA-srtB, that is likely involved in iron uptake (97). Additionally, Helicobacter pylori Fur has been shown to regulate genes involved in iron acquisition and storage. The pfr gene, which encodes a prokaryotic ferritin molecule, is repressed by Fur in the absence of iron in what is termed apo-Fur regulation (14, 37), while iron uptake systems encoded by frpB (33, 37, 120), fecA (120), ceuE (120), feoB (120), and exbB (32) have all been found to be repressed by Fur in the presence of iron.
Not only does Fur play a role in iron acquisition in animal pathogens, but it has been found to be important for plant pathogens as well. In Pseudomonas syringae, Fur represses siderophore production (25), and in Bradyrhizobium japonicum, Fur regulates irr, the regulator of the heme biosynthetic pathway (56, 68). Even from this limited list of pathogens, it is clear that Fur is critical for iron acquisition and storage in a wide variety of bacterial species.
Fur AND THE LINK TO VIRULENCE
Not only is Fur involved in regulating iron homeostasis, it is also more directly involved in colonization and virulence. fur mutants of H. pylori are less efficient at colonization than the wild type in a murine model of infection (20) and are easily outcompeted by wild-type bacteria in in vivo competition assays with a Mongolian gerbil model of infection (58). Thus, while it is not an essential gene in H. pylori, Fur certainly provides an advantage in establishing colonization. In addition, fur mutants of several pathogens exhibit decreased virulence in animal models. A murine skin abscess model of S. aureus infection shows that fur mutants are attenuated (78). Likewise, fur mutants of L. monocytogenes (111) and Campylobacter jejuni (103) show reduced virulence in murine and chick models of infection, respectively, as do Edwardsiella tarda fur mutants in fish (121). Even in plant pathogens, like P. syringae, Fur mutants show decreased virulence (25).
In addition to playing a role in colonization, Fur regulates numerous genes that are important for bacterial pathogenesis. For instance, in P. aeruginosa, Fur has been shown to be involved in toxin production, biofilm formation, and quorum sensing. Fur is believed to be indirectly involved in toxA expression, since it does not interact with the promoter of either toxA or its regulator, regAB (99, 107). Although the role Fur plays in biofilm formation in P. aeruginosa is not well characterized, it has been shown that a fur mutant forms more-mature biofilms than does the wild type under iron-limited conditions (8). Finally, Fur is indirectly involved in the regulation of quorum sensing in P. aeruginosa through the regulation of two small, noncoding RNAs (sRNAs), prrF1 and prrF2, which in turn regulate degradation enzymes for the precursor molecule to the Pseudomonas quinolone signal (101).
In Vibrio cholerae, Fur negatively regulates hemolysin production (114) and an outer membrane virulence determinant, irgA (60, 90), while in Neisseria gonorrhoeae, Fur has been shown to interact directly with the promoters of all 11 opa genes, which encode outer membrane proteins that aid in adherence to and invasion of host cells (113). In N. meningitidis, Fur is implicated in the regulation of several genes associated with virulence (62), and heat shock proteins are deregulated in a fur mutant of N. meningitidis independently of the iron-Fur regulon (31). In E. coli, the Shiga toxins (Shiga-like toxins) SltA and SltB (21) and hemolysin (55) are repressed by Fur. Additionally, in uropathogenic and enterohemorrhagic strains of E. coli, Fur negatively regulates the IrgA homolog adhesin (iha) (110), while in enterotoxigenic E. coli, expression of the fimbrial adhesin CFA/I is repressed by Fur (82). Finally, the vacuolating cytotoxin (vacA) of H. pylori has been shown to be indirectly regulated by Fur (58). Thus, Fur plays a role in colonization and virulence in a diverse number of pathogens.
Fur AND LOW PH
In addition to its role in the regulation of virulence factors, Fur is important for the regulation of processes that are necessary for survival in vivo and thus are linked to virulence. For instance, Fur is an important regulator of genes involved in the acid resistance response. Arguably, this is best exemplified in S. Typhimurium, where Fur is involved in the acid tolerance response (ATR). Specifically, an S. Typhimurium fur mutant is unable to mount an effective ATR at pH 5.8 (53). Therefore, fur mutants are more sensitive to acid (pH 3.3) than the wild type, and several ATR genes are not induced at pH 5.8 in the absence of fur (53). Intriguingly, Fur's role in acid resistance appears to be independent of iron and its role in iron acquisition (67), as iron availability does not affect the ATR and “iron-blind” fur mutants still display an acid resistance phenotype (67). Further work has shown that Fur is involved primarily in helping S. Typhimurium combat organic acid stress but plays only a minor role in inorganic acid stress (10). Fur in H. pylori has also been implicated in regulating genes involved in fighting acid stress (17, 58, 93); in fact, when the organism is exposed to low pH, the number of genes in the Fur regulon is significantly increased (58). These genes include gluP, encoding a predicted glucose/galactose transporter; ruvC, encoding a predicted Holliday junction endodeoxyribonuclease; fliP, encoding a flagellar biosynthetic protein; and amiE, encoding the aliphatic amidase (58). AmiE helps counteract acid stress through the production of ammonia as a by-product of the hydrolysis of aliphatic amides (119). While Fur in these organisms is not solely responsible for acid resistance, it certainly plays a significant role in helping the bacteria adapt and adjust to acidic conditions that would be encountered within the host.
Fur AND OXIDATIVE STRESS
Another survival mechanism in which Fur has been found to play a role in pathogenesis is fighting oxidative stress via regulation of genes, like catalase and superoxide dismutase, that help to combat toxic oxygen products. Catalases and hydroperoxidases convert peroxides into water and oxygen, and superoxide dismutases convert superoxide radicals into oxygen and peroxide. For instance, in several organisms, the catalase (kat) gene, which encodes the catalase enzyme, is regulated by Fur. Fur represses katG, a combined catalase-peroxidase, in both Mycobacterium tuberculosis and Mycobacterium smegmatis, and this regulation is predicted to be universal in all Mycobacterium species (109, 132). Some bacterial species, like Yersinia pestis (59), S. aureus (78), and P. aeruginosa (74), also utilize Fur to activate kat expression. E. coli fur mutants are more susceptible to UVA irradiation oxidative damage due to decreased production of the hydroperoxidases (HPI and HPII) (75). The neutrophil activating protein (napA), which helps to protect H. pylori from oxidative damage, is suspected to be under the control of Fur (28, 94, 102). In addition, S. Typhimurium Fur helps the bacterium counteract the effects of nitric oxide stress through the regulation of hmp, a flavohemoglobin (29). Also of interest for this organism is the Fur regulation of mntH, a gene that encodes a bacterial homolog of mammalian natural-resistance-associated macrophage protein 1 (80, 83). MntH is thought to help S. Typhimurium fight hydrogen peroxide-related injury upon entrance into macrophages (83).
Another oxidative survival gene that is commonly regulated by Fur is superoxide dismutase (sod). Superoxide dismutases are classified based on their metal cofactor: SodA, SodB, and SodC utilize Mn(II), Fe(II), and Cu(II) or Zn(II), respectively. The type of Sod varies with the bacterial species. In N. gonorrhoeae, Fur directly binds to the sod promoter, which results in sod activation (113), while in E. coli, sodA is directly repressed by Fur under iron-abundant conditions (11, 27, 72, 98, 108, 117). In comparison, the iron superoxide dismutase (sodB) in E. coli is indirectly activated by Fur (41, 42, 98). As is seen here, methods of Fur regulation of sod genes are exceptionally diverse, and to add to this mixture of direct or indirect activation and direct repression, there is one more manner in which Fur has been shown to regulate sod. In H. pylori, sodB is directly repressed by Fur in the absence of iron, i.e., in its apo form (45).
It is evident that Fur is a global regulator that is involved in bacterial pathogenesis as well as in many aspects of bacterial life (even some not described here, e.g., Fur regulation of metabolic genes). As mentioned, classical Fur regulation involves the binding of iron-bound Fur dimers to the promoter region of target genes to occlude the RNA polymerase binding site; however, as also mentioned above, recent studies have shown instances where Fur functions as an activator or as a repressor in the absence of its iron cofactor. Presently, only one organism utilizes Fur in all of these different ways—H. pylori. While iron-bound Fur repression in this organism is well understood, apo-Fur repression and Fur activation comparatively remain in the proverbial “black box.” In the remainder of this review, we highlight and compare the complexities of Fur regulation in this important human pathogen.
H. PYLORI AND IRON-ASSOCIATED DISEASE
Interestingly, H. pylori infections are often associated with development of an iron deficiency anemia (IDA) that is usually unresponsive to iron replacement therapies (9). Two recent epidemiological studies highlighted this link by looking at adolescents and pregnant women, two groups of people who are at increased risk for IDA. During the adolescent years, an increased amount of iron is needed to support the rapid growth of the child, and similarly, during pregnancy, women need more iron due to increased blood volume and the iron needs of the developing fetus. In the first study, three adolescent children with IDA were unresponsive to iron supplementation (22). After the teens were found to be infected with H. pylori and the infections were eradicated, the anemia resolved and iron levels returned to normal by 3 months posttreatment (22). In the second study, a link between IDA in pregnancy and H. pylori infection was made. Out of 117 pregnant women, 27 had anemia; 18 were classified as suffering from IDA (96). All 27 of the anemic patients were shown to be H. pylori infected (96). The close association between H. pylori infection and IDA prompted Cardamone et al. to suggest that in cases of refractory IDA in teens, H. pylori infection should be considered as a diagnosis even in the absence of gastric symptoms (22).
While there is a strong epidemiological association between H. pylori and IDA, the mechanism by which the bacterium causes IDA is not known—does the bacterium directly remove iron from the host, or is the severe inflammation associated with the infection the source of the iron loss? Several studies of H. pylori strains isolated from patients with IDA have attempted to explain the epidemiological association. Proteomic analysis of 15 strains (7 from IDA patients and 8 from non-IDA patients) revealed that IDA strains phylogenetically clustered together and separately from the non-IDA strains (105). Additionally, in a study of IDA strain isolates compared to non-IDA isolates, the strains from IDA patients showed increased uptake of both Fe(II) and Fe(III) (131); while the reason for this increased iron uptake is not known, certain polymorphisms in feoB, a ferrous iron transporter, have been shown to occur in IDA-derived strains of H. pylori (81). Even though the exact mechanism by which H. pylori and IDA are linked is not well understood, it is highly likely that Fur plays some role in this process, as it is the primary regulator of iron uptake and storage genes in this organism.
IRON-BOUND Fur REPRESSION
Fur was first identified in H. pylori in 1998 (16), is conserved (over 95% identical at the DNA and amino acid levels) among H. pylori strains (15), and is 34% identical and 56% similar to E. coli Fur. Although Fur is nonessential in H. pylori (26), as discussed above, it has been shown to be important for efficient colonization (20, 58). Based on the strong similarity between H. pylori Fur and E. coli Fur, it is likely no surprise that aspects of Fur regulation are similar for H. pylori and E. coli. As mentioned above, the best-described means of Fur regulation is classically hallmarked by iron-bound Fur dimers binding to specific regions in iron-regulated promoters called Fur boxes (Fig. 1, left). Fur binding blocks the binding of RNA polymerase, thus preventing transcription of these target genes (47, 49). In E. coli, the Fur box is a 19-bp region, GATAATGATAATCATTATC, that is highly conserved in this organism (39). The E. coli Fur box has also been reevaluated as three repeats of GATAAT, with the second and third repeats separated by a single nucleotide and the last repeat inverted (48). Although the Fur box of E. coli is used as the standard to which other Fur binding sequences are compared, it is not clearly conserved in all organisms that exhibit Fur regulation. For example, in B. subtilis, the Fur box is a 15-bp inverted repeat in a 7-1-7 configuration (6). Two of these motifs ([7-1-7]2) may overlap to form the classic 19-bp E. coli sequence (6). In Y. pestis, the Fur box consists of two inverted repeats of AATGATAAT separated by a single nucleotide (59). One common feature among Fur boxes is the high number of A/T nucleotides relative to C/G nucleotides. Thus, for H. pylori it is perhaps no surprise that the definition of a consensus Fur box is somewhat hindered by the fact that it is a highly A/T-rich organism (approximately 60%). Based on an alignment of several Fur-regulated genes, the consensus Fur box in H. pylori is NNNNNAATAATNNTNANN (94). This consensus sequence is significantly different from that for E. coli and is certainly less conserved, even among H. pylori Fur-regulated genes, than the E. coli sequence. While it is currently unclear, it may be that the requirement for Fur binding is less reliant on a recognition sequence and more related to the overall structural configuration of the target promoter sequence in H. pylori. This notion is further supported by the fact that H. pylori Fur is only partially able to complement an E. coli fur mutant (14) and that an E. coli Fur titration assay (FURTA-Ec) was not very successful at identifying Fur-regulated genes in H. pylori (12, 15, 16) until the system was modified to allow H. pylori Fur expression (51).
Even though all of the specifics are not known, iron-bound Fur repression in H. pylori has been well documented, and binding to several gene targets has been confirmed through DNase footprinting analysis. Indeed, the predicted Fur regulon in H. pylori is quite extensive (30, 44, 94). The regulon includes iron uptake genes, like frpB and exbB (32, 33, 37) among others, and amiE, as well as other genes involved in functions like acid resistance (23, 119). Generally, iron-bound Fur-regulated genes in H. pylori have one to three Fur binding sites within their promoters (32, 33, 37). The sites with the highest affinity span the −10 and/or −35 promoter element; the lower-affinity Fur binding sites are located further upstream from the primary Fur box (32, 33, 37). This high-affinity orientation supports the current hypothesis of Fur competing with RNA polymerase for binding to target promoters. Indeed, what we know about Fe-bound Fur regulation in H. pylori agrees with what is seen for many other organisms and is the most common mechanism of Fur regulation.
apo-Fur REGULATION
Currently unique to H. pylori is the utilization of Fur as a repressor even in the absence of its Fe(II) cofactor. This phenomenon is termed apo-Fur regulation. It occurs under conditions of low iron availability and involves iron-free Fur binding to target promoters to prevent the binding of RNA polymerase. The apo-Fur regulon consists of an entirely different set of genes than the Fe-bound Fur regulon and is predicted to contain approximately 16 genes (44), though few genes have definitively been shown to be regulated in this manner. Expression of the iron storage molecule Pfr is regulated by apo-Fur (14, 23, 37); pfr expression is repressed under conditions of low iron but is constitutively expressed in a fur mutant (14). DNase I footprinting analysis of the pfr promoter using iron-free Fur revealed that there were three regions of protection (37). As with iron-bound Fur repression, the region with the highest affinity for Fur covered the region to which RNA polymerase would bind (in this case, both the −10 and −35 promoter elements) (Fig. 1, right) (37). The other two regions were further upstream from the transcriptional start site (37). From a bacterial standpoint, repression of pfr under iron-limited conditions makes biological sense, as producing a storage molecule when the molecule to be stored is not available would be a waste of energy and resources.
Another confirmed apo-Fur target is sodB. Binding of Fur to sodB in the absence of iron was shown via DNase I footprinting analysis and electrophoretic mobility shift assays (EMSAs) (45). Unlike pfr, the sodB promoter has only one Fur binding region, which spans the −10 and −35 promoter elements (45). Interestingly, comparison of the three pfr Fur boxes and the sodB Fur box shows very little sequence homology between them. Additionally, there is little homology with the known iron-bound H. pylori Fur boxes and even less homology with the E. coli consensus Fur binding sequence (37, 45). Recent work from our group suggests that there are strain-specific nucleotide differences in the recognition sites in apo-Fur-regulated promoters and that these differences may alter the affinity of apo-Fur for these promoters; a single nucleotide difference in the sodB Fur box in strain G27 results in the loss of sodB regulation (23a).
Even with the direct binding data provided by DNase I footprinting and EMSA of the pfr and sodB promoters, the concept of apo-Fur regulation remains widely debated in the Fur field. The debate centers around whether or not Fur could actually be found unbound to iron in vitro. Is it possible to strip Fur of all of its iron cofactor in the laboratory? Some argue that the DNase footprinting data are artificial because it is impossible to create apo-Fur in vitro; however, it is clear from mutational and transcriptional analyses that genes in the “apo-Fur” regulon are repressed in the absence of iron and constitutively expressed in a fur mutant, regardless of iron availability. One possibility is that the existence of Fur-regulated sRNAs, which control apo targets, could explain the in vivo data. sRNAs are a subclass of natural antisense transcripts that base pair with complementary mRNA transcripts and thus can alter the stability of the mRNA or its ability to be translated (115). Up until very recently, there were no identified sRNAs in H. pylori, but recently four have been identified (128, 129). Two of the natural antisense transcripts in H. pylori, NAT-39 and NAT-67, were found to be complementary to frpB and ceuE, respectively (129). Both of these genes are members of the iron-bound Fur regulon (94). While it has been shown that NAT-39 and NAT-67 bind to their respective targets, it has not yet been determined what regulatory role this plays in gene expression and in iron homeostasis (129). The only other sRNAs identified in H. pylori, IG-443 and IG-524, are predicted to regulate the flagellar motor switch gene (fliM) and fumarase (fumC), respectively (128). Interestingly, IG-443 is encoded in the intergenic region between fur and HP1033 (128). Given that the existence of sRNAs in H. pylori is a very recent discovery, the possibility of an sRNA that could regulate genes in the apo-Fur regulon cannot be ruled out; however, to date there is no strong evidence of this being the case. Regardless, regulatory sRNA cannot account for the direct in vitro binding data demonstrated for apo-Fur and the pfr and sodB promoters.
Interestingly, there is some evidence that apo-Fur regulation may be found in other bacterial species. Microarray analysis of C. jejuni revealed that Cj1364, fumarate hydratase, and Cj0859c, a hypothetical protein, had reduced expression under iron-replete conditions and had increased expression in the fur mutant (77). More recently, microarray analysis of the nonpathogen Desulfovibrio vulgaris Hildenborough predicted that there are nine genes which are repressed by iron-free Fur (13). More-specific studies need to be performed to determine whether apo-Fur regulation actually occurs in these organisms.
AUTOREGULATION OF Fur
While some organisms have additional regulatory proteins to regulate Fur expression, like the catabolite activator protein in E. coli (38), RpoS in V. vulnificus (88), and NikR in H. pylori (32), autoregulation of Fur is the most conserved mechanism of fur regulation. Fur represses its own expression under iron-replete conditions. Biologically speaking, it makes sense to link the expression of Fur to the level of available iron, given the dangers of iron toxicity. Fur can be thought of as a rheostat that senses the available iron and responds by regulating its own expression accordingly (35, 36). It was determined early on that E. coli Fur was autoregulatory (38). Similarly, Fur from E. tarda (121) and Fur from N. gonorrhoeae (113) are autoregulatory, and Fur from Streptomyces coelicolor is predicted to be autoregulatory (66). In all of these instances, Fur autoregulation is the straightforward classical iron-bound Fur repression. However, in some organisms, Fur autoregulation appears to be more complex. For example, for L. monocytogenes, Northern blot analysis reveals that fur is upregulated under iron-limited conditions, yet in vitro DNase I footprinting analysis shows that Fur is able to bind to and protect the Fur box region of the fur promoter in the absence of the metal cofactor (86). The authors suggest that these results indicate that Fur binding is also dependent on an as-yet-unidentified factor (86). In contrast to this and iron-bound Fur autoregulation, V. vulnificus Fur has been shown to bind to and activate fur expression in the absence of iron (87).
Fur autoregulation in H. pylori may very well be the most complex Fur autoregulatory circuit known to date, since it combines both the classical iron-bound Fur repression and the apo-Fur activation that is exhibited in V. vulnificus. Initial studies by Delany et al. revealed that there were three Fur binding regions in the H. pylori fur promoter. In order from highest to lowest affinity for Fur, operator I spans nucleotides −34 to −66, operator II spans nucleotides +19 to −13, and operator III spans nucleotides −87 to −104 (35). The first two operators are likely to be involved in repression of the fur promoter, as they encompass both the −10 and −35 promoter elements, but the role of the third and farthest-upstream operator was initially unclear (35). In their subsequent work, Delany et al. showed that the third operator region was indeed important for Fur autoregulation and that it functions as a site for apo-Fur activation (similarly to the type for V. vulnificus). Additionally, operator I is involved in both iron-bound Fur repression and apo-Fur activation of expression through binding Fur in its respective forms (36). Which form binds is driven by the prevalence of iron, as both forms bind to this operator with equal affinities. The current model of H. pylori Fur autoregulation also suggests that if the concentration of Fur dips below a certain level, then Fur binding to operator I is lost, allowing this site to act as an UP element for RNA polymerase (36). Given that this organism utilizes Fur in both its iron-bound and apo forms, it is perhaps not surprising that Fur autoregulation in H. pylori is a complex mixture of iron-bound Fur repression and apo-Fur activation. Additionally, with few regulatory proteins relative to its genome size, H. pylori would likely have evolved to utilize every regulatory mechanism it has to ensure proper homeostasis.
Fur ACTIVATION
The complexity of fur autoregulation in H. pylori points to yet another regulatory function of Fur; Fur can act as a positive regulator. The first indication that Fur may act as a positive regulator came from microarray analyses where a number of genes were suggested to be Fur induced (30, 44). Another gene, oorD, a ferredoxin-like protein, is suspected of being activated by Fe-Fur, as its expression is decreased in the absence of iron, and EMSA shows that Fe-bound Fur binds to its promoter (58).
Despite this circumstantial evidence, the process of Fur activation in H. pylori is currently poorly understood, except with nifS. NifS is a Fe-S cluster synthesis protein that has been shown to be activated by iron-bound Fur (2). EMSA analysis shows Fur binding to the nifS promoter in the presence of the Fe(II) substitute Mn, and nifS expression is increased in the presence of iron (2). Interestingly, the two predicted Fur boxes for nifS are located far upstream of the transcriptional start site in the nifS promoter (2), similarly to the apo-Fur activation site within the fur promoter. It appears from the examples of fur, nifS, and possibly oorD that both iron-bound Fur and apo-Fur can act as transcriptional activators in addition to acting as repressors.
While there is clearly much to be learned about Fur activation in H. pylori, Fur activation in other organisms is better understood. For N. meningitidis, microarray analysis suggested that Fur activates multiple genes in the presence of iron (31, 62). Moreover, direct iron-bound activation of the NMB1436-NMB1438 operon (61, 62) and the aniA, norB, and nuoA promoters (34) has been shown. As with H. pylori, the Fur boxes for the Fur-activated genes in N. meningitidis are located further upstream in the promoters (34, 61). S. Typhimurium utilizes both iron-bound Fur and apo-Fur to activate a subset of genes, although whether this is direct or indirect activation remains unclear (54). Additionally, the iron-regulated protein IRO-28 appears to be activated by apo-Fur under iron-limited conditions in S. Typhimurium (54, 67). In P. aeruginosa, direct iron-bound Fur activation has been identified for the bacterioferritin gene bfrB (126).
It is clear from these examples that Fur activation does not occur in the same manner as Fur repression (Fig. 1, center). For both iron-bound Fur and apo-Fur repression, the Fur boxes are located near the transcriptional start site and usually span at least one of the key promoter elements. Binding at this location blocks the binding of RNA polymerase. In contrast, the Fur boxes for Fur-activated genes are all located far upstream from the transcriptional start site; thus, binding of RNA polymerase is not hindered. Perhaps by binding further upstream within the promoter, Fur is able to change the overall structure of the DNA, enabling better binding of the RNA polymerase to help facilitate transcription.
CONCLUSIONS AND FUTURE DIRECTIONS
More than 30 years since the first fur mutant was identified, Fur has gone from being thought of as a simple repressor of iron uptake genes to being considered a global regulator with multiple functions. This protein is still the source of much research and interest within the scientific community, which has gone beyond the classic field of regulation. For instance, with the increase in the field of bioinformatics Fur has been used as a model system to look at the identification of new targets (104). In addition, Fur has even been used to help identify whether fleas are infected with Y. pestis (57), indicating that this important regulatory protein could be used as a genetic marker in the diagnosis of other bacterial infections. Finally, because it is an important global regulator, Fur could even be the future target of antibiotics or other antibacterial treatments (125).
As discussed here, Fur can act as either a repressor or an activator and function with or without its iron cofactor. For H. pylori, we find all of these different modes of Fur utilized within a single species. Based on this, it is perhaps not surprising that we also see that the most debate about Fur regulation stems from this organism. Although apo-Fur has been demonstrated to bind directly to target promoters in vitro, and in vivo transcriptional data indicate repression only in the absence of iron, as demonstrated by several groups (14, 23, 37, 45), to this day, apo-Fur repression remains a highly debated mechanism of action, and the possibility of apo-Fur actually existing in vitro remains a point of contention.
All debate aside, there are still several unanswered questions about Fur regulation in H. pylori. For instance, what does Fur recognize in its target promoters? There is no strong Fur box consensus sequence in this organism for any type of Fur regulation (iron-bound repression, apo repression, or activation). This may indicate that, in H. pylori, Fur recognizes more than just a specific DNA sequence. Perhaps there is something important about the overall three-dimensional structure of the DNA. This may be true of other organisms as well; B. japonicum Fur represses irr, and there is no clear Fur box within the irr promoter (56). The challenge to answering the question of what Fur recognizes in H. pylori promoters, at least for iron-bound Fur and apo-Fur repression, is that these Fur boxes commonly overlap/encompass the −10 and −35 promoter elements, making promoter mutation analysis virtually impossible.
For H. pylori, very little is understood about Fur activation and the mechanism of action for Fur with the activated promoter. While microarrays are able to globally analyze the Fur regulon, they are not capable of determining the specifics of Fur regulation; therefore, it is not clear from these types of studies whether Fur directly activates the identified genes or whether Fur indirectly regulates these targets. Further studies using EMSAs and/or DNase I footprinting need to be conducted on potential Fur targets to better determine the role of Fur in their activation. Also, how does Fur binding contribute to upregulating transcription? Does the binding of Fur alter the structure of the DNA to allow for the binding of RNA polymerase, or does Fur binding alter the binding site of a negative regulator of the target gene, thereby preventing the repressor from binding?
While the mechanism of iron-bound Fur regulation is well understood, comparatively little is known about apo-Fur regulation. For instance, it is not known whether apo-Fur functions as a monomer or a dimer. If it functions as a dimer, how does it compare to the structure of the iron-bound dimer? Another interesting question is what apo-Fur recognizes in its target promoters, since the current data do not suggest a conserved binding sequence. These questions, as well as the continued determination of the biological existence of apo-Fur, warrant further exploration. It will also be interesting to learn whether other species have apo-Fur repression or whether this type of regulation is specific to the evolution of H. pylori. Are there amino acid residues that are particularly important for one type of regulation or another? Structure-function analysis has not really been applied to Fur of H. pylori, and yet this organism utilizes Fur as a repressor and an activator in both its iron-bound and apo forms, which is not known to occur in any other bacterial species. Because of the diverse manners in which Fur impacts pathogenesis (through regulation of iron uptake systems, virulence factors, and oxidative and pH-mediated stress responses, etc.), understanding Fur regulation in H. pylori will enrich and broaden our understanding of the role Fur plays in the larger realm of bacterial pathogenesis.
Acknowledgments
Research in the laboratory of D. Scott Merrell is made possible by grants R073LA from USUHS and AI065529 from the NIAID.
Contents of the manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or the DOD.
Biography
 Beth M. (Uccellini) Carpenter was raised in western Pennsylvania in the small town of Indiana. She left Indiana to pursue a degree in biochemistry at The Catholic University of America and to live in one of her favorite cities, Washington, DC. She currently lives in northern Virginia with her husband and is pursuing her Ph.D. in Emerging Infectious Diseases at Uniformed Services University in Bethesda, MD. At the university, she has been a Dean's Special Fellow and was recently awarded the Val G. Hemming Fellowship to support her endeavors. Her work on Helicobacter pylori's ferric uptake regulator stems from her biochemistry background and her curiosity about how this bacterium can “do so much” with one small protein. Her interest in science is not surprising given the fact that two of her favorite childhood questions were “What's that?” and “Why?”
Beth M. (Uccellini) Carpenter was raised in western Pennsylvania in the small town of Indiana. She left Indiana to pursue a degree in biochemistry at The Catholic University of America and to live in one of her favorite cities, Washington, DC. She currently lives in northern Virginia with her husband and is pursuing her Ph.D. in Emerging Infectious Diseases at Uniformed Services University in Bethesda, MD. At the university, she has been a Dean's Special Fellow and was recently awarded the Val G. Hemming Fellowship to support her endeavors. Her work on Helicobacter pylori's ferric uptake regulator stems from her biochemistry background and her curiosity about how this bacterium can “do so much” with one small protein. Her interest in science is not surprising given the fact that two of her favorite childhood questions were “What's that?” and “Why?”
 Jeannette M. Whitmire was born in Valparaiso, IN, and moved to northern Virginia while in elementary school. She obtained her degree in biology from Mary Washington College in the historic town of Fredericksburg, VA. After graduation, she began working at Uniformed Services University in Bethesda, MD, where she has held various positions, including Laboratory Technician, Research Biologist, and her current position as Microbiologist. While working at USUHS, she completed her Master's degree in Biotechnology and Bioinformatics from The Johns Hopkins University in Baltimore, MD. She is fascinated by Helicobacter pylori's ability to colonize a seemingly uninhabitable environment and its classification as a carcinogen. She currently resides in Manassas, VA, with her husband and daughter. During rare escapes from the metropolitan Washington, DC, area with her family, her love of science is reflected in her enjoyment of the beauty and complexity of nature.
Jeannette M. Whitmire was born in Valparaiso, IN, and moved to northern Virginia while in elementary school. She obtained her degree in biology from Mary Washington College in the historic town of Fredericksburg, VA. After graduation, she began working at Uniformed Services University in Bethesda, MD, where she has held various positions, including Laboratory Technician, Research Biologist, and her current position as Microbiologist. While working at USUHS, she completed her Master's degree in Biotechnology and Bioinformatics from The Johns Hopkins University in Baltimore, MD. She is fascinated by Helicobacter pylori's ability to colonize a seemingly uninhabitable environment and its classification as a carcinogen. She currently resides in Manassas, VA, with her husband and daughter. During rare escapes from the metropolitan Washington, DC, area with her family, her love of science is reflected in her enjoyment of the beauty and complexity of nature.
 D. Scott Merrell (Scotty) was born and raised in rural Bald Knob, AR, but left the South to complete his Ph.D. studies at Tufts Medical School, followed by postdoctoral training at Stanford University. He is currently an Assistant Professor in the Department of Microbiology and Immunology at Uniformed Services University in Bethesda, MD. Intrigued by bacteria that manage to colonize in inhospitable environments, Scotty's research group studies colonization and virulence factors of the gastric pathogen Helicobacter pylori. Scotty is a former Applied Genomics of Infectious Diseases ID Training Fellow and a Damon Runyon-Walter Winchell Cancer Fund Postdoctoral Fellow and received a Merck Irving S. Sigal Memorial Award for excellence in basic research in medical microbiology and infectious diseases in 2008. Despite his many years away from Arkansas, Scotty still confesses a secret passion for Southern-fried catfish, hush puppies, and pickled green tomatoes.
D. Scott Merrell (Scotty) was born and raised in rural Bald Knob, AR, but left the South to complete his Ph.D. studies at Tufts Medical School, followed by postdoctoral training at Stanford University. He is currently an Assistant Professor in the Department of Microbiology and Immunology at Uniformed Services University in Bethesda, MD. Intrigued by bacteria that manage to colonize in inhospitable environments, Scotty's research group studies colonization and virulence factors of the gastric pathogen Helicobacter pylori. Scotty is a former Applied Genomics of Infectious Diseases ID Training Fellow and a Damon Runyon-Walter Winchell Cancer Fund Postdoctoral Fellow and received a Merck Irving S. Sigal Memorial Award for excellence in basic research in medical microbiology and infectious diseases in 2008. Despite his many years away from Arkansas, Scotty still confesses a secret passion for Southern-fried catfish, hush puppies, and pickled green tomatoes.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Agarwal, S., C. A. King, E. K. Klein, D. E. Soper, P. A. Rice, L. M. Wetzler, and C. A. Genco. 2005. The gonococcal Fur-regulated tbpA and tbpB genes are expressed during natural mucosal gonococcal infection. Infect. Immun. 734281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamuri, P., N. Mehta, A. Burk, and R. J. Maier. 2006. Regulation of the Helicobacter pylori Fe-S cluster synthesis protein NifS by iron, oxidative stress conditions, and Fur. J. Bacteriol. 1885325-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169483-490. [DOI] [PubMed] [Google Scholar]
- 4.Aster, J. C. 2005. Red blood cell and bleeding disorders, p. 619-659. In V. Kumar, A. K. Abbas, and N. Fausto (ed.), Pathologic basis of disease, 7th ed. Elsevier Saunders, Philadelphia, PA.
- 5.Bagg, A., and J. B. Neilands. 1987. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 265471-5477. [DOI] [PubMed] [Google Scholar]
- 6.Baichoo, N., and J. D. Helmann. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 1845826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baichoo, N., T. Wang, R. Ye, and J. D. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 451613-1629. [DOI] [PubMed] [Google Scholar]
- 8.Banin, E., M. L. Vasil, and E. P. Greenberg. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 10211076-11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barabino, A. 2002. Helicobacter pylori-related iron deficiency anemia: a review. Helicobacter 771-75. [DOI] [PubMed] [Google Scholar]
- 10.Bearson, B. L., L. Wilson, and J. W. Foster. 1998. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J. Bacteriol. 1802409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaumont, M. D., and H. M. Hassan. 1993. Characterization of regulatory mutations causing anaerobic derepression of the sodA gene in Escherichia coli K12: cooperation between cis- and trans-acting regulatory loci. J. Gen. Microbiol. 1392677-2684. [DOI] [PubMed] [Google Scholar]
- 12.Beier, D., G. Spohn, R. Rappuoli, and V. Scarlato. 1997. Identification and characterization of an operon of Helicobacter pylori that is involved in motility and stress adaptation. J. Bacteriol. 1794676-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender, K. S., H. C. Yen, C. L. Hemme, Z. Yang, Z. He, Q. He, J. Zhou, K. H. Huang, E. J. Alm, T. C. Hazen, A. P. Arkin, and J. D. Wall. 2007. Analysis of a ferric uptake regulator (Fur) mutant of Desulfovibrio vulgaris Hildenborough. Appl. Environ. Microbiol. 735389-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bereswill, S., S. Greiner, A. H. van Vliet, B. Waidner, F. Fassbinder, E. Schiltz, J. G. Kusters, and M. Kist. 2000. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J. Bacteriol. 1825948-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bereswill, S., F. Lichte, S. Greiner, B. Waidner, F. Fassbinder, and M. Kist. 1999. The ferric uptake regulator (Fur) homologue of Helicobacter pylori: functional analysis of the coding gene and controlled production of the recombinant protein in Escherichia coli. Med. Microbiol. Immunol. 18831-40. [DOI] [PubMed] [Google Scholar]
- 16.Bereswill, S., F. Lichte, T. Vey, F. Fassbinder, and M. Kist. 1998. Cloning and characterization of the fur gene from Helicobacter pylori. FEMS Microbiol. Lett. 159193-200. [DOI] [PubMed] [Google Scholar]
- 17.Bijlsma, J. J., B. Waidner, A. H. Vliet, N. J. Hughes, S. Hag, S. Bereswill, D. J. Kelly, C. M. Vandenbroucke-Grauls, M. Kist, and J. G. Kusters. 2002. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect. Immun. 70606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bsat, N., and J. D. Helmann. 1999. Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo. J. Bacteriol. 1814299-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29189-198. [DOI] [PubMed] [Google Scholar]
- 20.Bury-Mone, S., J. M. Thiberge, M. Contreras, A. Maitournam, A. Labigne, and H. De Reuse. 2004. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53623-638. [DOI] [PubMed] [Google Scholar]
- 21.Calderwood, S. B., and J. J. Mekalanos. 1987. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J. Bacteriol. 1694759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardamone, M., G. Alex, M. D. Harari, W. P. Moss, and M. R. Oliver. 2008. Severe iron-deficiency anaemia in adolescents: consider Helicobacter pylori infection. J. Paediatr. Child Health 44647-650. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter, B. M., T. K. McDaniel, J. M. Whitmire, H. Gancz, S. Guidotti, S. Censini, and D. S. Merrell. 2007. Expanding the Helicobacter pylori genetic toolbox: modification of an endogenous plasmid for use as a transcriptional reporter and complementation vector. Appl. Environ. Microbiol. 737506-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Carpenter, B. M., H. Gancz, R. P. Gonzalez-Nieves, A. L. West, J. M. Whitmire, S. L. Michel, and D. S. Merrell. 2009. A single nucleotide change affects Fur-dependent regulation of sodB in H. pylori. PLoS ONE 4e5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson, S. D., C. E. Thomas, and C. Elkins. 1996. Cloning and sequencing of a Haemophilus ducreyi fur homolog. Gene 176125-129. [DOI] [PubMed] [Google Scholar]
- 25.Cha, J. Y., J. S. Lee, J. I. Oh, J. W. Choi, and H. S. Baik. 2008. Functional analysis of the role of Fur in the virulence of Pseudomonas syringae pv. tabaci 11528: Fur controls expression of genes involved in quorum-sensing. Biochem. Biophys. Res. Commun. 366281-287. [DOI] [PubMed] [Google Scholar]
- 26.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 1831259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Compan, I., and D. Touati. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 1751687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooksley, C., P. J. Jenks, A. Green, A. Cockayne, R. P. Logan, and K. R. Hardie. 2003. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J. Med. Microbiol. 52461-469. [DOI] [PubMed] [Google Scholar]
- 29.Crawford, M. J., and D. E. Goldberg. 1998. Regulation of the Salmonella typhimurium flavohemoglobin gene. A new pathway for bacterial gene expression in response to nitric oxide. J. Biol. Chem. 27334028-34032. [DOI] [PubMed] [Google Scholar]
- 30.Danielli, A., D. Roncarati, I. Delany, V. Chiarini, R. Rappuoli, and V. Scarlato. 2006. In vivo dissection of the Helicobacter pylori Fur regulatory circuit by genome-wide location analysis. J. Bacteriol. 1884654-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delany, I., R. Grifantini, E. Bartolini, R. Rappuoli, and V. Scarlato. 2006. Effect of Neisseria meningitidis Fur mutations on global control of gene transcription. J. Bacteriol. 1882483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delany, I., R. Ieva, A. Soragni, M. Hilleringmann, R. Rappuoli, and V. Scarlato. 2005. In vitro analysis of protein-operator interactions of the NikR and Fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J. Bacteriol. 1877703-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delany, I., A. B. Pacheco, G. Spohn, R. Rappuoli, and V. Scarlato. 2001. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J. Bacteriol. 1834932-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 521081-1090. [DOI] [PubMed] [Google Scholar]
- 35.Delany, I., G. Spohn, A. B. Pacheco, R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2002. Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol. Microbiol. 461107-1122. [DOI] [PubMed] [Google Scholar]
- 36.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2003. An anti-repression Fur operator upstream of the promoter is required for iron-mediated transcriptional autoregulation in Helicobacter pylori. Mol. Microbiol. 501329-1338. [DOI] [PubMed] [Google Scholar]
- 37.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 421297-1309. [DOI] [PubMed] [Google Scholar]
- 38.De Lorenzo, V., M. Herrero, F. Giovannini, and J. B. Neilands. 1988. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur. J. Biochem. 173537-546. [DOI] [PubMed] [Google Scholar]
- 39.de Lorenzo, V., S. Wee, M. Herrero, and J. B. Neilands. 1987. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (Fur) repressor. J. Bacteriol. 1692624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai, P. J., A. Angerer, and C. A. Genco. 1996. Analysis of Fur binding to operator sequences within the Neisseria gonorrhoeae fbpA promoter. J. Bacteriol. 1785020-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubrac, S., and D. Touati. 2002. Fur-mediated transcriptional and post-transcriptional regulation of FeSOD expression in Escherichia coli. Microbiology 148147-156. [DOI] [PubMed] [Google Scholar]
- 42.Dubrac, S., and D. Touati. 2000. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 1823802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellermeier, J. R., and J. M. Slauch. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst, F. D., S. Bereswill, B. Waidner, J. Stoof, U. Mader, J. G. Kusters, E. J. Kuipers, M. Kist, A. H. van Vliet, and G. Homuth. 2005. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology 151533-546. [DOI] [PubMed] [Google Scholar]
- 45.Ernst, F. D., G. Homuth, J. Stoof, U. Mader, B. Waidner, E. J. Kuipers, M. Kist, J. G. Kusters, S. Bereswill, and A. H. van Vliet. 2005. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J. Bacteriol. 1873687-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ernst, J. F., R. L. Bennett, and L. I. Rothfield. 1978. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J. Bacteriol. 135928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escolar, L., V. de Lorenzo, and J. Perez-Martin. 1997. Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein. Mol. Microbiol. 26799-808. [DOI] [PubMed] [Google Scholar]
- 48.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1998. Binding of the fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J. Mol. Biol. 283537-547. [DOI] [PubMed] [Google Scholar]
- 49.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1998. Coordinated repression in vitro of the divergent fepA-fes promoters of Escherichia coli by the iron uptake regulation (Fur) protein. J. Bacteriol. 1802579-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 2000. Evidence of an unusually long operator for the Fur repressor in the aerobactin promoter of Escherichia coli. J. Biol. Chem. 27524709-24714. [DOI] [PubMed] [Google Scholar]
- 51.Fassbinder, F., A. H. van Vliet, V. Gimmel, J. G. Kusters, M. Kist, and S. Bereswill. 2000. Identification of iron-regulated genes of Helicobacter pylori by a modified fur titration assay (FURTA-Hp). FEMS Microbiol. Lett. 184225-229. [DOI] [PubMed] [Google Scholar]
- 52.Fiorini, F., S. Stefanini, P. Valenti, E. Chiancone, and D. De Biase. 2008. Transcription of the Listeria monocytogenes fri gene is growth-phase dependent and is repressed directly by Fur, the ferric uptake regulator. Gene 410113-121. [DOI] [PubMed] [Google Scholar]
- 53.Foster, J. W. 1991. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J. Bacteriol. 1736896-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster, J. W., and H. K. Hall. 1992. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J. Bacteriol. 1744317-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frechon, D., and E. Le Cam. 1994. Fur (ferric uptake regulation) protein interaction with target DNA: comparison of gel retardation, footprinting and electron microscopy analyses. Biochem. Biophys. Res. Commun. 201346-355. [DOI] [PubMed] [Google Scholar]
- 56.Friedman, Y. E., and M. R. O'Brian. 2003. A novel DNA-binding site for the ferric uptake regulator (Fur) protein from Bradyrhizobium japonicum. J. Biol. Chem. 27838395-38401. [DOI] [PubMed] [Google Scholar]
- 57.Gabitzsch, E. S., R. Vera-Tudela, R. J. Eisen, S. W. Bearden, K. L. Gage, and N. S. Zeidner. 2008. Development of a real-time quantitative PCR assay to enumerate Yersinia pestis in fleas. Am. J. Trop. Med. Hyg. 7999-101. [PubMed] [Google Scholar]
- 58.Gancz, H., S. Censini, and D. S. Merrell. 2006. Iron and pH homeostasis intersect at the level of Fur regulation in the gastric pathogen Helicobacter pylori. Infect. Immun. 74602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao, H., D. Zhou, Y. Li, Z. Guo, Y. Han, Y. Song, J. Zhai, Z. Du, X. Wang, J. Lu, and R. Yang. 2008. The iron-responsive Fur regulon in Yersinia pestis. J. Bacteriol. 1903063-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldberg, M. B., S. A. Boyko, and S. B. Calderwood. 1991. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 881125-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grifantini, R., E. Frigimelica, I. Delany, E. Bartolini, S. Giovinazzi, S. Balloni, S. Agarwal, G. Galli, C. Genco, and G. Grandi. 2004. Characterization of a novel Neisseria meningitidis Fur and iron-regulated operon required for protection from oxidative stress: utility of DNA microarray in the assignment of the biological role of hypothetical genes. Mol. Microbiol. 54962-979. [DOI] [PubMed] [Google Scholar]
- 62.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 1009542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griggs, D. W., and J. Konisky. 1989. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of Fur protein to the promoters. J. Bacteriol. 1711048-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas, B., J. Kraut, J. Marks, S. C. Zanker, and D. Castignetti. 1991. Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 593997-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haas, B., E. Murphy, and D. Castignetti. 1991. Siderophore synthesis by mucoid Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Can. J. Microbiol. 37654-657. [DOI] [PubMed] [Google Scholar]
- 66.Hahn, J. S., S. Y. Oh, and J. H. Roe. 2000. Regulation of the furA and catC operon, encoding a ferric uptake regulator homologue and catalase-peroxidase, respectively, in Streptomyces coelicolor A3(2). J. Bacteriol. 1823767-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall, H. K., and J. W. Foster. 1996. The role of Fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquisition. J. Bacteriol. 1785683-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamza, I., Z. Qi, N. D. King, and M. R. O'Brian. 2000. Fur-independent regulation of iron metabolism by Irr in Bradyrhizobium japonicum. Microbiology 146669-676. [DOI] [PubMed] [Google Scholar]
- 69.Hantke, K. 1984. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol. Gen. Genet. 197337-341. [DOI] [PubMed] [Google Scholar]
- 70.Hantke, K. 1982. Negative control of iron uptake systems in Escherichia coli. FEMS Microbiol. Lett. 1583-86. [Google Scholar]
- 71.Hantke, K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182288-292. [DOI] [PubMed] [Google Scholar]
- 72.Hassan, H. M., and H. C. Sun. 1992. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc. Natl. Acad. Sci. USA 893217-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassett, D. J., M. L. Howell, U. A. Ochsner, M. L. Vasil, Z. Johnson, and G. E. Dean. 1997. An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J. Bacteriol. 1791452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hassett, D. J., P. A. Sokol, M. L. Howell, J. F. Ma, H. T. Schweizer, U. Ochsner, and M. L. Vasil. 1996. Ferric uptake regulator (Fur) mutants of Pseudomonas aeruginosa demonstrate defective siderophore-mediated iron uptake, altered aerobic growth, and decreased superoxide dismutase and catalase activities. J. Bacteriol. 1783996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoerter, J. D., A. A. Arnold, C. S. Ward, M. Sauer, S. Johnson, T. Fleming, and A. Eisenstark. 2005. Reduced hydroperoxidase (HPI and HPII) activity in the Δfur mutant contributes to increased sensitivity to UVA radiation in Escherichia coli. J. Photochem. Photobiol. B 79151-157. [DOI] [PubMed] [Google Scholar]
- 76.Holbein, B. E., K. W. Jericho, and G. C. Likes. 1979. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect. Immun. 24545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes, K., F. Mulholland, B. M. Pearson, C. Pin, J. McNicholl-Kennedy, J. M. Ketley, and J. M. Wells. 2005. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology 151243-257. [DOI] [PubMed] [Google Scholar]
- 78.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunt, M. D., G. S. Pettis, and M. A. McIntosh. 1994. Promoter and operator determinants for fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. J. Bacteriol. 1763944-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeda, J. S., A. Janakiraman, D. G. Kehres, M. E. Maguire, and J. M. Slauch. 2005. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J. Bacteriol. 187912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeon, B. H., Y. J. Oh, N. G. Lee, and Y. H. Choe. 2004. Polymorphism of the Helicobacter pylori feoB gene in Korea: a possible relation with iron-deficiency anemia? Helicobacter 9330-334. [DOI] [PubMed] [Google Scholar]
- 82.Karjalainen, T. K., D. G. Evans, D. J. Evans, Jr., D. Y. Graham, and C. H. Lee. 1991. Iron represses the expression of CFA/I fimbriae of enterotoxigenic E. coli. Microb. Pathog. 11317-323. [DOI] [PubMed] [Google Scholar]
- 83.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J. Bacteriol. 1843151-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan, F. A., M. A. Fisher, and R. A. Khakoo. 2007. Association of hemochromatosis with infectious diseases: expanding spectrum. Int. J. Infect. Dis. 11482-487. [DOI] [PubMed] [Google Scholar]
- 85.Kochan, I., J. Wasynczuk, and M. A. McCabe. 1978. Effects of injected iron and siderophores on infections in normal and immune mice. Infect. Immun. 22560-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ledala, N., S. L. Pearson, B. J. Wilkinson, and R. K. Jayaswal. 2007. Molecular characterization of the Fur protein of Listeria monocytogenes. Microbiology 1531103-1111. [DOI] [PubMed] [Google Scholar]
- 87.Lee, H. J., S. H. Bang, K. H. Lee, and S. J. Park. 2007. Positive regulation of fur gene expression via direct interaction of Fur in a pathogenic bacterium, Vibrio vulnificus. J. Bacteriol. 1892629-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee, H. J., K. J. Park, A. Y. Lee, S. G. Park, B. C. Park, K. H. Lee, and S. J. Park. 2003. Regulation of fur expression by RpoS and Fur in Vibrio vulnificus. J. Bacteriol. 1855891-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Litwin, C. M., and B. L. Byrne. 1998. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect. Immun. 663134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Litwin, C. M., and S. B. Calderwood. 1994. Analysis of the complexity of gene regulation by Fur in Vibrio cholerae. J. Bacteriol. 176240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 994620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McHugh, J. P., F. Rodriguez-Quinones, H. Abdul-Tehrani, D. A. Svistunenko, R. K. Poole, C. E. Cooper, and S. C. Andrews. 2003. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. J. Biol. Chem. 27829478-29486. [DOI] [PubMed] [Google Scholar]
- 93.Merrell, D. S., M. L. Goodrich, G. Otto, L. S. Tompkins, and S. Falkow. 2003. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect. Immun. 713529-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merrell, D. S., L. J. Thompson, C. C. Kim, H. Mitchell, L. S. Tompkins, A. Lee, and S. Falkow. 2003. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 716510-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miethke, M., and M. A. Marahiel. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71413-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulayim, B., N. Y. Celik, and F. F. Yanik. 2008. Helicobacter pylori infection detected by 14C-urea breath test is associated with iron deficiency anemia in pregnant women. J. Obstet. Gynaecol. Res. 34980-985. [DOI] [PubMed] [Google Scholar]
- 97.Newton, S. M., P. E. Klebba, C. Raynaud, Y. Shao, X. Jiang, I. Dubail, C. Archer, C. Frehel, and A. Charbit. 2005. The svpA-srtB locus of Listeria monocytogenes: Fur-mediated iron regulation and effect on virulence. Mol. Microbiol. 55927-940. [DOI] [PubMed] [Google Scholar]
- 98.Niederhoffer, E. C., C. M. Naranjo, K. L. Bradley, and J. A. Fee. 1990. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J. Bacteriol. 1721930-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ochsner, U. A., A. I. Vasil, and M. L. Vasil. 1995. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J. Bacteriol. 1777194-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ochsner, U. A., and M. L. Vasil. 1996. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc. Natl. Acad. Sci. USA 934409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oglesby, A. G., J. M. Farrow III, J. H. Lee, A. P. Tomaras, E. P. Greenberg, E. C. Pesci, and M. L. Vasil. 2008. The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J. Biol. Chem. 28315558-15567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olczak, A. A., G. Wang, and R. J. Maier. 2005. Up-expression of NapA and other oxidative stress proteins is a compensatory response to loss of major Helicobacter pylori stress resistance factors. Free Radic. Res. 391173-1182. [DOI] [PubMed] [Google Scholar]
- 103.Palyada, K., D. Threadgill, and A. Stintzi. 2004. Iron acquisition and regulation in Campylobacter jejuni. J. Bacteriol. 1864714-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Panina, E. M., A. A. Mironov, and M. S. Gelfand. 2001. Comparative analysis of FUR regulons in gamma-proteobacteria. Nucleic Acids Res. 295195-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park, S. A., H. W. Lee, M. H. Hong, Y. W. Choi, Y. H. Choe, B. Y. Ahn, Y. J. Cho, D. S. Kim, and N. G. Lee. 2006. Comparative proteomic analysis of Helicobacter pylori strains associated with iron deficiency anemia. Proteomics 61319-1328. [DOI] [PubMed] [Google Scholar]
- 106.Pohl, E., J. C. Haller, A. Mijovilovich, W. Meyer-Klaucke, E. Garman, and M. L. Vasil. 2003. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol. Microbiol. 47903-915. [DOI] [PubMed] [Google Scholar]
- 107.Prince, R. W., C. D. Cox, and M. L. Vasil. 1993. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J. Bacteriol. 1752589-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Privalle, C. T., and I. Fridovich. 1993. Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J. Biol. Chem. 2685178-5181. [PubMed] [Google Scholar]
- 109.Pym, A. S., P. Domenech, N. Honore, J. Song, V. Deretic, and S. T. Cole. 2001. Regulation of catalase-peroxidase (KatG) expression, isoniazid sensitivity and virulence by furA of Mycobacterium tuberculosis. Mol. Microbiol. 40879-889. [DOI] [PubMed] [Google Scholar]
- 110.Rashid, R. A., P. I. Tarr, and S. L. Moseley. 2006. Expression of the Escherichia coli IrgA homolog adhesin is regulated by the ferric uptake regulation protein. Microb. Pathog. 41207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rea, R. B., C. G. Gahan, and C. Hill. 2004. Disruption of putative regulatory loci in Listeria monocytogenes demonstrates a significant role for Fur and PerR in virulence. Infect. Immun. 72717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schaffer, S., K. Hantke, and V. Braun. 1985. Nucleotide sequence of the iron regulatory gene fur. Mol. Gen. Genet. 200110-113. [DOI] [PubMed] [Google Scholar]
- 113.Sebastian, S., S. Agarwal, J. R. Murphy, and C. A. Genco. 2002. The gonococcal Fur regulon: identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 1843965-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stoebner, J. A., and S. M. Payne. 1988. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect. Immun. 562891-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Storz, G., J. A. Opdyke, and A. Zhang. 2004. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 7140-144. [DOI] [PubMed] [Google Scholar]
- 116.Sword, C. P. 1966. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J. Bacteriol. 92536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tardat, B., and D. Touati. 1993. Iron and oxygen regulation of Escherichia coli MnSOD expression: competition between the global regulators Fur and ArcA for binding to DNA. Mol. Microbiol. 953-63. [DOI] [PubMed] [Google Scholar]
- 118.Tielemans, C. L., C. M. Lenclud, R. Wens, F. E. Collart, and M. Dratwa. 1989. Critical role of iron overload in the increased susceptibility of haemodialysis patients to bacterial infections. Beneficial effects of desferrioxamine. Nephrol. Dial. Transplant. 4883-887. [DOI] [PubMed] [Google Scholar]
- 119.van Vliet, A. H., J. Stoof, S. W. Poppelaars, S. Bereswill, G. Homuth, M. Kist, E. J. Kuipers, and J. G. Kusters. 2003. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori Fur repressor. J. Biol. Chem. 2789052-9057. [DOI] [PubMed] [Google Scholar]
- 120.van Vliet, A. H., J. Stoof, R. Vlasblom, S. A. Wainwright, N. J. Hughes, D. J. Kelly, S. Bereswill, J. J. Bijlsma, T. Hoogenboezem, C. M. Vandenbroucke-Grauls, M. Kist, E. J. Kuipers, and J. G. Kusters. 2002. The role of the ferric uptake regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter 7237-244. [DOI] [PubMed] [Google Scholar]
- 121.Wang, F., S. Cheng, K. Sun, and L. Sun. 2008. Molecular analysis of the fur (ferric uptake regulator) gene of a pathogenic Edwardsiella tarda strain. J. Microbiol. 46350-355. [DOI] [PubMed] [Google Scholar]
- 122.Webster, A. C., and C. M. Litwin. 2000. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 68526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weinberg, E. D. 1978. Iron and infection. Microbiol. Rev. 4245-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weinberg, E. D. 1974. Iron and susceptibility to infectious disease. Science 184952-956. [DOI] [PubMed] [Google Scholar]
- 125.Whitmire, J. M., H. Gancz, and D. S. Merrell. 2007. Balancing the double-edged sword: metal ion homeostasis and the ulcer bug. Curr. Med. Chem. 14469-478. [DOI] [PubMed] [Google Scholar]
- 126.Wilderman, P. J., N. A. Sowa, D. J. FitzGerald, P. C. FitzGerald, S. Gottesman, U. A. Ochsner, and M. L. Vasil. 2004. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA 1019792-9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao, B., W. Li, G. Guo, B. Li, Z. Liu, K. Jia, Y. Guo, X. Mao, and Q. Zou. 2009. Identification of small noncoding RNAs in Helicobacter pylori by a bioinformatics-based approach. Curr. Microbiol. 58258-263. [DOI] [PubMed] [Google Scholar]
- 129.Xiao, B., W. Li, G. Guo, B. S. Li, Z. Liu, B. Tang, X. H. Mao, and Q. M. Zou. 23 December 2008. Screening and identification of natural antisense transcripts in Helicobacter pylori by a novel approach based on RNase I protection assay. Mol. Biol. Rep. [Epub ahead of print.] doi: 10.1007/s11033-008-9390-5. [DOI] [PubMed]
- 130.Xiong, A., V. K. Singh, G. Cabrera, and R. K. Jayaswal. 2000. Molecular characterization of the ferric-uptake regulator, Fur, from Staphylococcus aureus. Microbiology 146659-668. [DOI] [PubMed] [Google Scholar]
- 131.Yokota, S., M. Konno, E. Mino, K. Sato, M. Takahashi, and N. Fujii. 2008. Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin. Infect. Dis. 46e31-e33. [DOI] [PubMed] [Google Scholar]
- 132.Zahrt, T. C., J. Song, J. Siple, and V. Deretic. 2001. Mycobacterial FurA is a negative regulator of catalase-peroxidase gene katG. Mol. Microbiol. 391174-1185. [DOI] [PubMed] [Google Scholar]