Abstract
Salmonella enterica serotype Typhimurium elicits acute neutrophil influx in the human intestinal mucosa within 1 or 2 days after infection, resulting in inflammatory diarrhea. In contrast, no overt symptoms are observed within the first 1 or 2 weeks after infection with S. enterica serotype Typhi. Here we show that introduction of the capsule-encoding viaB locus of serotype Typhi reduced the ability of serotype Typhimurium to elicit acute intestinal inflammation in a streptomycin-pretreated mouse model. Serotype Typhimurium requires a functional invasion-associated type III secretion system (type III secretion system 1 [T3SS-1]) to elicit cecal inflammation within 48 h after infection of streptomycin-pretreated mice, and the presence of the viaB locus reduced its invasiveness for human intestinal epithelial cells in vitro. However, a reduced activity of T3SS-1 could not account for the ability of the viaB locus to attenuate cecal inflammation, because introduction of the viaB locus into an invasion-deficient serotype Typhimurium strain (invA mutant) resulted in a significant reduction of pathology and inflammatory cytokine expression in the cecum 5 days after infection of mice. We conclude that a T3SS-1-independent mechanism contributes to the ability of the viaB locus to reduce intestinal inflammation.
Salmonella enterica serotype Typhimurium causes acute gastroenteritis, characterized by exudative inflammatory infiltrates in the intestine that are dominated by neutrophils (34). Exudative inflammation and diarrhea develop within 48 h after ingesting the organisms. The orchestration of these rapid intestinal inflammatory responses can be studied using the streptomycin-pretreated mouse model (11). In this model, motility and chemotaxis contribute to the induction of inflammatory responses in the cecum at early time points, between 10 and 24 h after infection, but become dispensable at later stages, between 48 and 120 h after infection (32). Mutants that lack a functional invasion-associated type III secretion system (type III secretion system 1 [T3SS-1]) are unable to elicit cecal inflammation within the first 48 h after infection, but marked inflammatory changes with neutrophil recruitment still develop in the cecal mucosa between 72 and 120 h after infection (5). These data suggest that flagella and T3SS-1 are important for eliciting early inflammatory changes in the ceca of mice. Induction of T3SS-1-independent inflammatory changes at later stages of infection (72 to 120 h after inoculation) require the presence of a functional myeloid differentiation primary response protein 88 (MyD88), an intracellular adaptor required for signaling through bacterium-specific Toll-like receptors (TLRs) (13).
In contrast to serotype Typhimurium, S. enterica serotype Typhi does not elicit neutrophil recruitment in the intestinal mucosa but instead is associated with a systemic infection termed typhoid fever (34). This disease has an incubation period of approximately 2 weeks (24), which suggests that the pathogen initially prevents the generation of marked host responses. Severe interstitial inflammation in the intestine may develop late, 2 to 3 weeks after the onset of symptoms, and may result in hemorrhage, ulceration, and intestinal perforation at areas of Peyer's patches (4). However, neutrophils remain scarce in intestinal infiltrates of typhoid fever patients (18, 22, 23, 31). The clinical presentation of typhoid fever suggests that serotype Typhi can prevent the rapid recruitment of neutrophils that characterizes the exudative inflammatory changes observed during serotype Typhimurium-induced gastroenteritis (27).
Recently, the viaB locus has been implicated in reducing the amount of neutrophil chemoattractants (CXC chemokines) produced by cultured human epithelial cells, macrophages, or colonic tissue explants in response to serotype Typhi infection (26, 38, 39). Introduction of the cloned viaB locus into serotype Typhimurium reduces neutrophil recruitment and CXC chemokine expression in bovine ligated ileal loops (28). The viaB locus contains genes involved in the regulation (tviA), biosynthesis (tviBCDE), and export (vexABCDE) of the serotype Typhi Vi capsular polysaccharide (36). The TviA regulatory protein represses expression of flagella (39), and the presence of the viaB locus reduces T3SS-1-mediated invasion (1, 41), two factors important for inducing early intestinal inflammatory responses in the streptomycin-pretreated mouse model (10, 32) and in bovine ligated ileal loops (30, 40). Flagella contribute to inflammation in the ceca of streptomycin-pretreated mice largely by conferring motility and chemotaxis (32), which in turn promotes invasion of the intestinal epithelium by increasing bacterial contact with host cells (14, 15). Collectively, these observations raise the possibility that the viaB locus may reduce intestinal inflammation in vivo because the efficiency of T3SS-1-mediated invasion is reduced. However, this idea has not been tested experimentally, in part because no animal model is available for the strictly human-adapted serotype Typhi.
Here we constructed a serotype Typhimurium strain stably expressing the Vi capsular antigen to test the role of the viaB locus in attenuating cecal inflammation in the streptomycin-pretreated mouse model. Our results suggest a mechanism for viaB-mediated attenuation of inflammatory responses in vivo that is independent of the serotype Typhimurium T3SS-1.
MATERIALS AND METHODS
Bacterial strains, cell lines, and culture conditions.
Bacterial strains used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl) or LB agar containing the appropriate antibiotics at the following concentrations: carbenicillin (100 μg/ml), chloramphenicol (30 μg/ml), nalidixic acid (50 μg/ml), kanamycin (50 μg/ml), tetracycline (10 μg/ml), or streptomycin (50 μg/ml). To assess growth at different salt concentrations, LB broth containing 10 mM or 300 mM NaCl was used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description or relevant genotypic or phenotypic characteristic(s)a | Reference or source |
|---|---|---|
| Bacterial strains | ||
| S. enterica serotype Typhimurium strains | ||
| IR715 | Nalidixic acid-resistant derivative of wild-type isolate ATCC 14028 | 33 |
| AJB715 | IR715 phoN::Kmr | 17 |
| AJB75 | IR715 invA::TnphoA | 3 |
| AJB634 | IR715 invA::pEP185.2 | 35 |
| EHW26 | IR715 fliC::Tn10 fljB5001::MudJ | 26 |
| TH3730 | LT2 PflhDC::Tn10dTc[del-25](T-POP) | 16 |
| SW258 | IR715 PflhDC::Tn10dTc[del-25](T-POP) | This study |
| TH140 | IR715 phoN::VR3 | This study |
| TH141 | IR715 phoN::VR2VR3 | This study |
| TH164 | IR715 phoN::VR1BR2R3 | This study |
| TH170 | IR715 phoN::viaB | This study |
| TH167 | IR715 invA::pEP185.2 phoN::viaB | This study |
| TH172 | IR715 rpoS::Cmr | This study |
| TH177 | IR715 rpoS::CmrphoN::viaB | This study |
| S. enterica serotype Typhi strains | ||
| Ty2 | ATCC 19430 rpoS | ATCC |
| STY2 | Ty2 viaB::Kmr | 26 |
| SW74 | Ty2 ΔtviB-vexE | 39 |
| Plasmids | ||
| pHP45Ω | oripMB1; Ampr Strepr | 25 |
| pGP704 | oriR6K mobRP4; Ampr | 20 |
| pUC4-KIXX | Kmr cassette | Pharmacia |
| pCMXX | Cmr cassette | 3 |
| pTVIA2 | tviA cloned in pWSK30 | 38 |
| pDC5 | viaB cloned in pWSK29 | 28 |
| pSW84 | phoN flanking region 2 (FR2) cloned in pGP704 | This study |
| pSW85 | FR1 and FR2 cloned in pGP704 | This study |
| pSW93 | Cmr cassette cloned in pSW85 | This study |
| pTH122 | Kmr cassette cloned in pSW84 | This study |
| pTH124 | Cmr cassette cloned in pSW84 | This study |
| pTH160 | viaB VR1A cloned in pTH122 | This study |
| pTH161 | viaB VR1B cloned in pTH124 | This study |
| pTH132 | viaB VR2 cloned in pTH122 | This study |
| pTH133 | viaB VR3 cloned in pSW93 | This study |
Ampr, ampicillin resistant; Strepr, streptomycin resistant.
The colorectal carcinoma cell line T84 (ATCC CCL-248) was obtained from the American Type Culture Collection. T84 cells were routinely cultured in Dulbecco's modified Eagle medium (DMEM)-F12 medium (Gibco), containing 1.2 g/liter sodium bicarbonate, 2.5 mM l-glutamine, 15 mM HEPES, and 0.5 mM sodium pyruvate (Gibco), supplemented with 10% fetal calf serum.
Construction of plasmids and mutants.
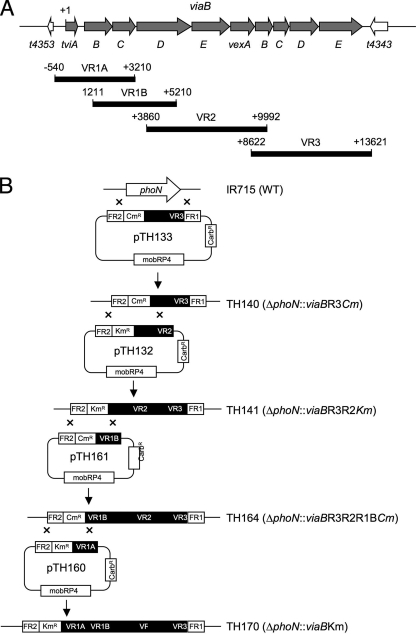
An S. enterica serotype Typhimurium strain carrying the viaB locus inserted in the phoN gene was constructed by allelic exchange. The flanking regions of phoN (phoN flanking region 1 [FR1] and FR2) were amplified by PCR using the primers listed in Table 2 and cloned into suicide vector pGP704 (20), giving rise to plasmid pSW85. The chloramphenicol resistance (Cmr) cassette from plasmid pCMXX (3) was cloned into the SmaI restriction site between the two flanking regions, giving rise to plasmid pSW93. DNA regions (viaB region 1A [VR1A], VR1B, VR2, and VR3) comprising parts of the viaB locus were amplified by PCR using the primer listed in Table 2. The VR3 DNA region was cloned into pSW93 to give rise to plasmid pTH133. The phoN FR2 fragment was cloned into a pGP704 to give rise to plasmid pSW84. Plasmids pTH122 and pTH124 were constructed by cloning a kanamycin resistance (Kmr) cassette from pUC4-KIXX and a Cmr cassette, respectively, into the SmaI restriction site of plasmid pSW84. The VR2 and VR1A DNA regions were cloned into plasmid pHT122 to give rise to plasmids pTH132 and pTH160, respectively. The VR1B DNA region was cloned into plasmid pHT124 to give rise to plasmid pTH161.
TABLE 2.
Nucleotide primers used for cloning
| Purpose | Primer | Nucleotide sequencea |
|---|---|---|
| Amplification of FR1 (phoN flanking region 1) | SW85 | 5′-GCAGATCTACTGGCCTATTACTCG-3′ |
| SW86 | 5′-GTCGACGAGTAAAGAAGATCACCC-3′ | |
| Amplification of FR2 | SW83 | 5′-CCCGGGTCTTTAACATATTCCAGG-3′ |
| SW84 | 5′-GAATTCGCTGCTCCAGTTGAAGG-3′ | |
| Amplification of VR1A (viaB region 1A) | TH38 | 5′-CGGGAGCTCTATTAATGGGTGACTCTAAT-3′ |
| TH39 | 5′-GGGGTACCGTTTTACCGGACTCACAATA-3′ | |
| Amplification of VR1B | TH40 | 5′-GGGGTACCTTCAACCAGGATTTCTATGT-3′ |
| TH41 | 5′-GAAGATCTTTTCTCAAACAACTTGTTCA-3′ | |
| Amplification of VR2 | TH35 | 5′-CGGGAGCTCAGCCCTGGAAAAAAATGGCGA-3′ |
| TH36 | 5′-GGGGTACCGGAAAATGCCAGAGGGTAAA-3′ | |
| Amplification of VR3 | TH32 | 5′-GCTCTAGACGTACCACTGGTATGGATATC-3′ |
| TH33 | 5′-ACGCGTCGACCGAATAAGGATCCCCCTGGT-3′ | |
| Deletion of rpoS | TH56 | 5′-AAGGCCAGTCGACAGACTGGCCTTTTTTTGACAAGGGTACGTGTAGGCTGGAGCTGCTTC-3′ |
| TH57 | 5′-TTGCTAGTTCCGTCAAGGGATCACGGGTAGGAGCCACCTTCATATGAATATCCTCCTTAG-3′ |
The engineered sequences not present in the target gene are underlined.
Suicide plasmid pTH133 was conjugated into serotype Typhimurium strain IR175, and a chloramphenicol-resistant, carbenicillin-sensitive exconjugant (TH140) carrying a phoN::VR3 insertion was isolated. Suicide plasmid pTH132 was conjugated into serotype Typhimurium strain TH140, and a kanamycin-resistant, chloramphenicol-sensitive, carbenicillin-sensitive exconjugant carrying a phoN::VR2VR3 insertion was named TH141. Suicide plasmid pTH161 was conjugated into serotype Typhimurium strain TH141, and a kanamycin-sensitive, chloramphenicol-resistant, carbenicillin-sensitive exconjugant carrying a phoN::VR1BVR2VR3 insertion was named TH164. Suicide plasmid pTH160 was conjugated into serotype Typhimurium strain TH164, and a kanamycin-resistant, chloramphenicol-sensitive, carbenicillin-sensitive exconjugant carrying a phoN::viaB insertion was isolated. Finally, the phoN::viaB insertion was introduced into new serotype Typhimurium wild-type strain background (IR175) by P22 phage transduction to give rise to strain TH170, and the insertion was verified by PCR.
A deletion of rpoS was constructed by the PCR-based λ Red recombinase system (6). A DNA fragment was amplified by PCR with TH56 and TH57 primers using pKD3 as a template. The PCR product was introduced into S. enterica serotype Typhimurium IR715 carrying plasmid pKD46. The rpoS mutation was transferred by phage P22-mediated transduction into the S. enterica serotype Typhimurium strains IR715 and TH170 to give rise to strains TH172 and TH177, respectively, and the insertions were verified by PCR.
A serotype Typhimurium LT2 strain derivative carrying a Ptet::flhDC insertion (i.e., the T-POP transposon inserted upstream of flhDC) has been described previously (16). The Ptet::flhDC insertion was introduced into serotype Typhimurium strain IR715 by P22 phage-mediated transduction to give rise to strain SW258.
Detection of capsular polysaccharide expression.
Serum agglutination was performed by resuspending 1.5 ml of a bacterial culture adjusted to an optical density at 600 nm of 1.0 in 10 μl of sterile distilled water. A volume of 5 μl of the bacterial suspension was mixed with 10 μl of rabbit antiserum against the Vi antigen, O4 antigen, or O9 antigen (BD) on a glass slide. Agglutination was read after rocking the glass for 1 min.
Detection of Vi expression by flow cytometry was performed as described previously (28). DNA was labeled with propidium iodide, and Vi antigen was detected by labeling cells with rabbit anti-Vi serum (1:250 dilution; BD) and goat anti-rabbit fluorescein isothiocyanate (FITC) conjugate (1:250 dilution; Jackson ImmunoLabs). After gating for propidium iodide-positive counts (bacteria), the FITC fluorescence (Vi expression) intensity was determined for each particle (FACSCalibur; Becton Dickinson).
Tissue culture experiments.
Tissue culture experiments using the human colorectal carcinoma cell line T84 were performed as described previously (39). In brief, T84 cells were seeded in 24-well plates at a density of 1 × 105 cells per well and incubated for 48 h. Serotype Typhimurium strains were used to infect tissue culture cells at a multiplicity of infection of 5 for 1 h. Cells were washed four times with Dulbecco's phosphate-buffered saline (Gibco), and medium containing 0.1 mg/ml gentamicin (Gibco) was added. After 90 min, the cells were washed four times with Dulbecco's phosphate-buffered saline, and intracellular bacteria were quantified by spreading serial 10-fold dilutions of T84 cell lysates (1% Triton X-100) on LB agar plates to determine the number of CFU. Each experiment was repeated three times.
Animal experiments.
We used 8- to 10-week-old C57BL/6 mice (Jackson Laboratories) for our studies. The streptomycin-pretreated mouse model has been described previously (2). Mice were inoculated intragastrically with 0.1 ml of streptomycin (200-mg/ml solution in phosphate-buffered saline). Mice were inoculated intragastrically 24 h later either with 0.1 ml of sterile LB broth or 1 × 109 CFU of S. enterica serotype Typhimurium strains carrying plasmid pHP45Ω (25) to confer streptomycin resistance. At 1, 3, or 5 days after infection, groups of five mice were euthanized. The ceca were collected and divided into tip (histopathological analysis), center, and proximal part (RNA extraction). Samples from colon contents, Peyer's patches, mesenteric lymph nodes, liver, and spleen were homogenized in phosphate-buffered saline, and 10-fold serial dilutions were plated on LB agar plates containing the appropriate antibiotics to determine bacterial numbers.
RNA extraction and quantitative real-time PCR.
RNA was extracted from mouse cecum using Tri reagent (Molecular Research Center) as described previously (28). Real-time PCR was carried out using TaqMan reverse transcription reagent (Applied Biosystems) with 1 μg of RNA from each sample in a 50-μl volume, and 4 μl of cDNA was used as the template for each reverse transcription-PCR in a 25-μl volume. Real-time reverse transcription-PCR was performed with the primer pairs listed in Table 3 using SYBR green (Applied Biosystems) and the 7900HT fast real-time PCR system. The data were analyzed using the comparative threshold cycle method (Applied Biosystems).
TABLE 3.
Nucleotide primers for quantitative real-time PCR
| Target gene | Nucleotide sequence |
|---|---|
| Gapdh | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| 5′-AGGTCGGTGTGAACGGATTTG-3′ | |
| Ifng | 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ |
| 5′-TGGCTCTGCAGGATTTTCATG-3′ | |
| Il22 | 5′-GGCCAGCCTTGCAGATAACA-3′ |
| 5′-GCTGATGTGACAGGAGCTGA-3′ | |
| Tnfa | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ |
| 5′-TGGGAGTAGACAAGGTACAACCC-3′ | |
| Il6 | 5′-GAGGATACCACTCCCAACAGACC-3′ |
| 5′-AAGTGCATCATCGTTGTTCATACA-3′ | |
| Kc | 5′-TGCACCCAAACCGAAGTCAT-3′ |
| 5′-TTGTCAGAAGCCAGCGTTCAC-3′ | |
| Mip2 | 5′-AGTGAACTGCGCTGTCAATGC-3′ |
| 5′-AGGCAAACTTTTTGACCGCC-3′ | |
| Mcp1 | 5′-CTTCTGGGCCTGCTGTTCA-3′ |
| 5′-CCAGCCTACTCATTGGGATCA-3′ | |
| Nos2 | 5′-TTGGGTCTTGTTCACTCCACGG-3′ |
| 5′-CCTCTTTCAGGTCACTTTGGTAGG-3′ | |
| Lcn2 | 5′-ACATTTGTTCCAAGCTCCAGGGC-3′ |
| 5′-CATGGCGAACTGGTTGTAGTCCG-3′ |
Histopathology.
Tissue samples were fixed in formalin, processed according to standard procedures for paraffin embedding, cut into 5-μm sections, and stained with hematoxylin and eosin. The following pathological changes were scored by blinded examination (examination by an individual unaware of which mice were in which group): (i) edema, (ii) epithelial erosion, (iii) neutrophil recruitment, (iv) recruitment of mononuclear cells, and (v) inflammatory exudate. The pathological changes were scored by blinded examination on a scale from 0 to 3 as follows: 0, absence of lesions; 1, mild; 2, moderate; 3, severe. A total of 10 microscopic fields per animal were evaluated and then averaged within each score.
Statistical analysis.
For tissue culture experiments, the values, which were percentages, were transformed logarithmically prior to statistical analysis using Student's t test. Cytokine expression and histopathology scores were compared by a nonparametric Mann-Whitney U test. P values of <0.05 were considered statistically significant.
RESULTS
The viaB locus reduces invasiveness of serotype Typhimurium for epithelial cells.
The viaB locus has been implicated in reducing the invasiveness of S. enterica serotype Typhi (1, 41). We first wanted to determine whether this effect is also observed in S. enterica serotype Typhimurium. A serotype Typhimurium wild-type strain (IR715) carrying the cloned viaB region on a low-copy-number plasmid (pDC5) has been described recently (28). We compared the invasiveness of serotype Typhimurium IR715(pDC5) to that of a nonflagellated serotype Typhimurium mutant (a fliC fljB mutant, strain EHW26) (26) and a noninvasive serotype Typhimurium strain carrying a mutation in invA (strain AJB75), a gene encoding a structural component of the T3SS-1 (Fig. 1). Introduction of the viaB locus reduced invasiveness of serotype Typhimurium for human colonic epithelial (T84) cells significantly (P < 0.05). As expected, mutations in invA (AJB75) or in fliC fljB (EHW26) also reduced invasiveness (Fig. 1A).
FIG. 1.
The cloned viaB locus reduces serotype Typhimurium invasion. (A) Effect of the cloned viaB locus (pDC5) on invasion of human colonic epithelial (T84) cells by serotype Typhimurium. Bars represent geometric means ± standard errors (error bars) from three independent experiments. The genotype (wild type [wt]) and plasmid content (−, no plasmid) of serotype Typhimurium strains and whether the bacteria were grown in the absence (−) or presence (+) of tetracycline prior to invasion assays are indicated below the graph. Values that are statistically significantly different are indicated by brackets and P values. NS, not significant. (B) Model for TviA-mediated regulation of capsule and invasion gene expression.
We reasoned that reduced invasiveness could be due to the production of the Vi capsular antigen, since the invasion-associated T3SS of Shigella flexneri is sensitive to the length of polysaccharide chains present on the bacterial cell surface (37). Alternatively, reduced invasiveness could be explained by a TviA-mediated repression of flhDC (39), two genes encoding a regulator controlling expression of flagellin genes and T3SS-1 invasion genes in serotype Typhimurium (7) (Fig. 1B). To distinguish between these possibilities, we used a serotype Typhimurium mutant in which expression of the flhDC genes was under the control of a tetracycline-inducible promoter (strain SW258). As expected, this strain was noninvasive when grown in the absence of tetracycline, but it invaded T84 cells at wild-type levels when expression of flhDC was induced in the presence of tetracycline. Introduction of the viaB locus (pDC5) or the cloned tviA gene (pTVIA2) did not reduce invasiveness of strain SW258 (Ptet::flhDC) in the absence of tetracycline. When expression of flhDC was induced in strain SW258 (Ptet::flhDC) by adding tetracycline, introduction of the cloned viaB locus (pDC5) did not result in reduced invasion (Fig. 1A). We conclude that the viaB locus reduced invasiveness of serotype Typhimurium largely because TviA is a potent repressor of flhDC. Constitutive expression of flhDC from the Ptet promoter restored invasiveness of a serotype Typhimurium strain expressing Vi antigen, which suggested that the Vi capsule did not affect invasiveness in this model. Furthermore, these data suggested that TviA-mediated regulation of genes other than flhDC did not significantly alter invasiveness of serotype Typhimurium.
A serotype Typhimurium isolate carrying pDC5 is not well suited for studies of the role of the viaB locus in vivo because plasmid instability was observed during infection of mice (28) and because transcription of the tviABCDE vexABCDE genes on pDC5 is driven by the promoter of the Escherichia coli lac operon (Plac), which results in constitutive expression. A virulent serotype Typhimurium strain (C5.507) stably expressing a chromosomally encoded Vi antigen has been described recently (9). However, this strain was generated by conjugational transfer of a DNA region of undefined size from serotype Typhi into serotype Typhimurium, presumably containing, at a minimum, the entire 134-kb Salmonella pathogenicity island 7 (SPI7). Transfer of such a large genomic region raises the possibility that phenotypic changes in this strain may be attributable to the transfer of serotype Typhi genes located outside of the 14-kb viaB locus. Thus, to study the role of the viaB locus in vivo, we constructed a serotype Typhimurium strain (TH170) carrying the tviABCDE vexABCDE genes inserted in the phoN gene (phoN::viaB), hereafter referred to as the Vi+ serotype Typhimurium strain (Fig. 2).
FIG. 2.
Cloning strategy for generating a serotype Typhimurium mutant carrying a chromosomally inserted viaB locus. (A) Genetic map of genes within the viaB locus (gray arrows) and genes flanking the viaB locus (white arrows). The positions and lengths of PCR products (VR1A, VR1B, VR2, and VR3) used for construction of suicide plasmids are indicated below relative to the first nucleotide of the tviA start codon (+1). (B) Schematic drawing of a series of mutants (strains TH140, TH141, TH164, and TH160) generated by allelic exchange with a series of suicide plasmids (pTH133, pTH132, pTH161, and pTH160) to introduce the viaB locus into the phoN locus of the serotype Typhimurium wild-type (WT) strain IR715. Carbr, carbenicillin resistant.
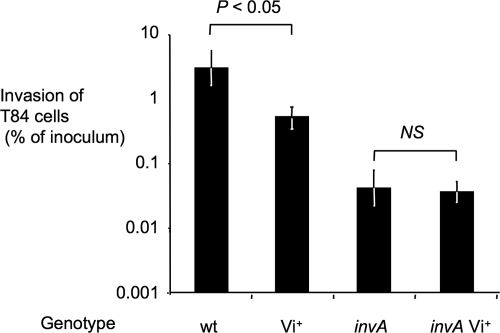
An initial in vitro characterization of the Vi+ serotype Typhimurium strain (TH170) was performed by studying its invasiveness for human colonic epithelial cells. The invasiveness of the Vi+ serotype Typhimurium strain (TH170) for T84 cells was reduced compared to that of the serotype Typhimurium wild-type strain (IR715) (Fig. 3). Invasiveness of an invA Vi+ serotype Typhimurium strain (TH167) was not significantly different (P > 0.05) from that of a strain carrying a mutation in invA (AJB634).
FIG. 3.
Effect of a chromosomal insertion of the viaB locus (Vi+) on invasion of human colonic epithelial (T84) cells by serotype Typhimurium. Bars represent geometric means ± standard errors (error bars) from three independent experiments. The genotype of serotype Typhimurium strains is indicated below the graph (wild type [wt]). Values that are statistically significantly different are indicated by brackets and P value. NS, not significant.
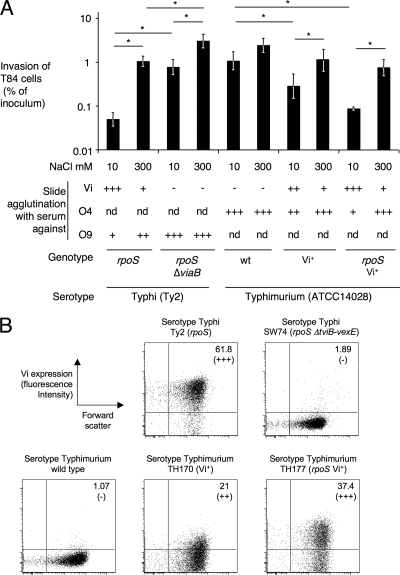
To study the effect that a chromosomally located viaB locus transcribed from its native promoter exerts on invasiveness, the Vi+ serotype Typhimurium strain (TH170) was grown under conditions known to induce Vi antigen expression (low osmolarity) or conditions known to repress Vi antigen expression (high osmolarity) prior to invasion of T84 cells. The Vi+ serotype Typhimurium strain (TH170) was less invasive for T84 cells when it was grown in medium containing 10 mM NaCl (Vi antigen-inducing conditions) than when it was grown in medium containing 300 mM NaCl (Vi antigen-repressing conditions) (Fig. 4). However, differences in invasiveness caused by growth under low- or high-osmolarity conditions were more pronounced for serotype Typhi wild-type isolate Ty2 than for the Vi+ serotype Typhimurium strain (TH170). Under inducing conditions, expression of the Vi antigen was higher in serotype Typhi strain Ty2 than in the Vi+ serotype Typhimurium strain (TH170), as indicated by slide agglutination. Serotype Typhi strain Ty2 carries a mutation in rpoS, encoding the stationary-phase sigma factor σS, which results in increased expression of the Vi antigen under inducing conditions (29). To investigate whether differences in the invasiveness of Vi antigen-expressing serotype Typhi and the Vi+ serotype Typhimurium strain were due to σS, a rpoS mutation was introduced into strain TH170. Expression of the Vi antigen and invasiveness of the resulting serotype Typhimurium strain (TH177) grown under low- or high-osmolarity conditions was similar to that of serotype Typhi strain Ty2 (Fig. 4A). Consistent with previous reports (38), high expression of the Vi antigen resulted in partial inhibition of agglutination with serum against the lipopolysaccharide O antigen in both serotypes. Expression of the Vi capsular polysaccharide on the cell surface was quantified by flow cytometry to confirm results from slide agglutination (Fig. 4B). Collectively, these data suggested that a chromosomally inserted viaB locus exerted similar effects on epithelial cell invasion in serotypes Typhi and Typhimurium.
FIG. 4.
Expression of the Vi antigen in serotype Typhimurium. (A) Effect of high-osmolarity (300 mM NaCl) and low-osmolarity (10 mM NaCl) growth conditions on invasion of human colonic epithelial (T84) cells by serotypes Typhi and Typhimurium. Bars represent average values of the percent invasion ± standard errors (error bars) from three independent experiments. Values that are statistically significantly different (P < 0.05) are indicated by the black horizontal lines and asterisks. Results from slide agglutination tests performed on inoculum cultures using serum against the Vi antigen, O4 antigen, or O9 antigen are indicated below the graph as follows: +++, strong agglutination; ++, medium agglutination; +, weak agglutination; −, no agglutination; nd, not determined. The genotypes of serotype Typhi and serotype Typhimurium strains are indicated below the slide agglutination data (wild type [wt]). (B) Expression of the Vi antigen was detected on the surfaces of serotype Typhi (top panels) or serotype Typhimurium strains (bottom panels) by flow cytometry. Bacteria were grown under Vi-inducing conditions (10 mM NaCl) and labeled with rabbit anti-Vi serum and goat anti-rabbit FITC conjugate, and fluorescence intensities (y axis) were determined for 10,000 particles. Positive counts (as percentages) are indicated in the right upper corner of each graph, and results from Vi slide agglutination are given below in parentheses.
The viaB locus reduces intestinal inflammation during serotype Typhimurium infection of streptomycin-pretreated mice.
The streptomycin-pretreated mouse model was used to investigate the effect of the viaB locus on the induction of acute intestinal inflammation during serotype Typhimurium infection. Inactivation of the phoN gene (strain AJB715, phoN::Kmr) had no effect on the ability of serotype Typhimurium to induce inflammation and inflammatory cytokine expression in the cecum (data not shown). In contrast, the presence of the viaB locus (strain TH170) significantly reduced the severity of inflammatory changes observed in the cecum by 72 h after infection with serotype Typhimurium as indicated by histopathological analysis (Fig. 5).
FIG. 5.
The viaB locus reduces histopathology but does not affect bacterial recovery during serotype Typhimurium infection of streptomycin-pretreated mice. (A to C) Histopathological appearance of the cecum of a mock-infected mouse (A), a mouse infected with the serotype Typhimurium wild-type strain (B), or a mouse infected with a Vi+ serotype Typhimurium strain (C) 72 h after infection. (D to F) Histopathological appearance of the cecum of a mock-infected mouse (D), a mouse infected with a serotype Typhimurium invA mutant (E), or a mouse infected with a Vi+ serotype Typhimurium invA mutant (F) 120 h after infection. All images were taken from hematoxylin-and-eosin-stained cecal sections at the same magnification (original magnification, ×100). (G) Histopathology score determined by blinded examination of cecal sections collected at the indicated time points. Each bar represents an individual animal. Values that are statistically significantly different between the different groups are indicated by brackets and P values. (H) Bacterial numbers recovered from the colon contents, mesenteric lymph nodes, and spleens of animals 72 h after infection. (I) Bacterial numbers recovered from the colon contents, mesenteric lymph nodes, and spleens of animals 120 h after infection. NS, not significant.
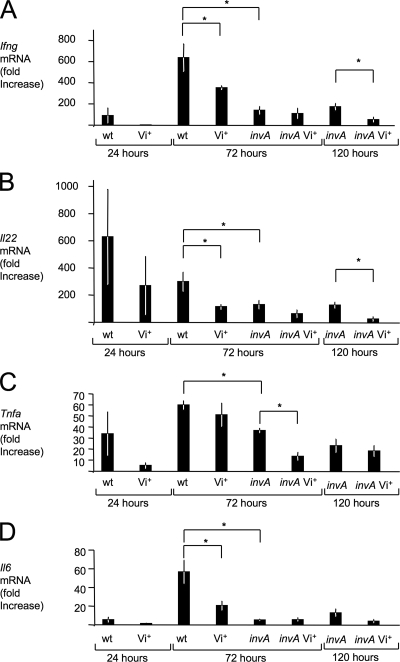
To further characterize the inflammatory changes observed during infection, mRNA levels of genes encoding proinflammatory cytokines, including gamma interferon (IFN-γ, encoded by the Ifng gene), interleukin 22 (IL-22, encoded by the Il22 gene), tumor necrosis factor alpha (TNF-α, encoded by the Tnfa gene), and IL-6 (encoded by the Il6 gene) were determined by quantitative real-time PCR. These cytokines are among the ones whose mRNA levels are most prominently induced in the cecal mucosa in response to serotype Typhimurium infection (8). Compared to the serotype Typhimurium wild-type strain (IR715), the Vi+ serotype Typhimurium strain (TH170) elicited reduced levels of Ifng, Il22, Tnfa, and Il6 at 24 h after infection, but these differences were not statistically significant (Fig. 6). At 72 h after infection, the serotype Typhimurium wild-type strain (IR715) elicited significantly (P < 0.05) higher levels of Ifng, Il22, and Il6 in the cecal mucosa than the Vi+ serotype Typhimurium strain (TH170). To further analyze the development of inflammatory changes in tissue, mRNA levels were determined for genes encoding chemokines, including keratinocyte-derived cytokine (Kc, encoded by the Kc gene), macrophage inflammatory protein 2 (Mip-2, encoded by the Mip2 gene), and macrophage chemotactic protein 1 (Mcp-1, encoded by the Mcp1 gene) (Fig. 7). In addition, we determined mRNA levels of genes encoding the antimicrobial proteins inducible nitric oxide synthase (iNOS, encoded by the Nos2 gene) and lipocalin-2 (Lcn-2, encoded by the Lcn2 gene) (Fig. 8). Lower expression levels of these genes were observed 24 h after infection with the Vi+ serotype Typhimurium strain (TH170) compared to the serotype Typhimurium wild-type strain (IR715), but these differences were not statistically significant. By 72 h after infection, the serotype Typhimurium wild-type strain (IR715) elicited significantly (P < 0.05) higher levels of Kc, Mip2, Mcp1, and Lcn2 mRNA in the cecal mucosa than the Vi+ serotype Typhimurium strain (TH170) (Fig. 7 and 8), while bacteria were recovered at similar numbers from intestinal contents and tissue from each group (Fig. 5).
FIG. 6.
Cytokine responses elicited in the ceca of streptomycin-pretreated mice after serotype Typhimurium infection. Transcript levels of Ifng (A), Il22 (B), Tnfa (C), and Il6 (D) at the indicated time points after infection were measured by quantitative real-time PCR. Bars represent changes (geometric means) in mRNA levels compared to a group of mock-infected mice from the same time point ± standard errors (error bars). Values that are statistically significantly different (P < 0.05) are indicated by brackets and asterisks. wt, wild type.
FIG. 7.
Chemokine responses elicited in the ceca of streptomycin-pretreated mice after serotype Typhimurium infection. Transcript levels of Kc (A), Mip2 (B), and Mcp1 (C) at the indicated time points after infection were measured by quantitative real-time PCR. Bars represent changes (geometric means) in mRNA levels compared to a group of mock-infected mice from the same time point ± standard errors (error bars). Values that are statistically significantly different (P < 0.05) are indicated by brackets and asterisks. wt, wild type.
FIG. 8.
Antimicrobial responses elicited in the ceca of streptomycin-pretreated mice after serotype Typhimurium infection. Transcript levels of Nos2 (A) and Lcn2 (B) at the indicated time points after infection were measured by quantitative real-time PCR. Bars represent changes (geometric means) in mRNA levels compared to a group of mock-infected mice from the same time point ± standard errors (error bars). Values that are statistically significantly different (P < 0.05) are indicated by brackets and asterisks. wt, wild type.
Collectively, these data suggested that the presence of the viaB locus markedly reduced the ability of serotype Typhimurium to induce intestinal inflammation 72 h after infection of streptomycin-pretreated mice.
The viaB locus attenuates intestinal inflammatory responses elicited by a serotype Typhimurium invA mutant.
While motility and chemotaxis are not required for eliciting inflammation 72 h after infection of streptomycin-pretreated mice (32), T3SS-1 is a major player in eliciting host responses at this time point (5, 13). Data from our in vitro model suggested that the presence of the viaB locus reduced T3SS-1-mediated invasion of epithelial cells (Fig. 3 and 4), which may account for an attenuation of intestinal inflammation. We therefore wanted to determine whether inactivation of T3SS-1 would eliminate the ability of the viaB locus to attenuate intestinal inflammatory responses in vivo. To this end, we compared intestinal inflammation elicited by a serotype Typhimurium invA mutant (strain AJB634) with that elicited by a Vi+ serotype Typhimurium invA mutant (strain TH167). No statistically significant differences in cytokine expression had been detected at 24 h after infection in our previous experiment, and we therefore chose the 72-hour time point for this analysis. Compared to the serotype Typhimurium wild-type strain, the invA mutant elicited significantly (P < 0.05) lower mRNA levels of Ifng, Il22, Tnfa, Il6, Kc, Mip2, Nos2, and Lcn2 in the cecal mucosa (Fig. 6, 7, and 8). These data were consistent with previous data, implicating T3SS-1 in eliciting cecal inflammation at this time point (5, 13). Introduction of the viaB locus into the invA mutant (TH167) caused a significant (P < 0.05) further reduction in mRNA levels of Tnfa, Mcp1, and Nos2, while bacteria were recovered at similar numbers from intestinal contents and tissue (data not shown). These data suggested that the mechanism by which the viaB locus attenuates intestinal inflammation was at least in part T3SS-1 independent.
Previous studies show that a contribution to intestinal inflammatory responses of T3SS-1-independent mechanisms becomes more pronounced at later time points after infection (5, 13). We therefore selected a 120-hour time point to more thoroughly characterize the effect of the viaB locus on intestinal inflammation elicited by a serotype Typhimurium invA mutant. Comparison of histopathological changes elicited by the Vi+ serotype Typhimurium invA mutant (TH167) with those observed for the invA mutant (AJB634) revealed that the viaB locus significantly (P < 0.05) reduced the severity of inflammatory responses observed in the cecal mucosa 120 h after infection (Fig. 5). This finding was further supported by an analysis of changes in mRNA levels of proinflammatory genes. Compared to the serotype Typhimurium invA mutant (AJB634), infection with the Vi+ serotype Typhimurium invA mutant (TH167) was associated with significantly (P < 0.05) lower mRNA levels of Ifng, Il22, Kc, Mip2, Nos2, and Lcn2 in the cecal mucosa (Fig. 6, 7, and 8), while bacteria were recovered in similar numbers from intestinal contents and tissue (Fig. 5). Collectively, these data suggested that the viaB locus causes a significant attenuation of intestinal inflammatory responses by an invA-independent mechanism.
DISCUSSION
While symptoms of gastroenteritis typically develop within 12 to 72 h after ingestion of serotype Typhimurium, typhoid fever has an incubation period of approximately 2 weeks (24). These clinical observations suggest that unlike serotype Typhimurium, serotype Typhi can prevent the generation of overt host responses during the initial phase of infection. The viaB locus has recently been implicated in attenuating intestinal inflammatory responses to serotype Typhi in a bovine ligated ileal loop model (28). However, streptomycin-pretreated mice do not develop intestinal inflammation when infected orally with the strictly human-adapted serotype Typhi wild-type strain or a viaB deletion mutant (28). To further analyze the role of the viaB locus in preventing the generation of host responses in vivo, we characterized a Vi+ serotype Typhimurium strain using the streptomycin-pretreated mouse model. Our results support the idea that the viaB locus enables bacteria to attenuate intestinal inflammatory responses during the initial phase, between 72 and 120 h after infection.
Deletion of the viaB locus (21) or repression of Vi antigen biosynthesis under conditions of high osmolarity (41) increases the invasiveness of serotype Typhi for intestinal epithelial cells. Consistent with these observations, we found that introduction of the viaB locus into serotype Typhimurium reduced its invasiveness for human colonic epithelial cells in vitro. The reduced invasiveness of a serotype Typhimurium strain carrying the viaB locus was not due to expression of the Vi antigen but was mediated through FlhDC, a regulator of flagellar and invasion gene expression. The TviA regulatory protein encoded within the viaB locus represses flhDC (39). It is possible that reduced FlhDC levels result in decreased invasion because FlhDC activates expression of fliZ, which encodes a positive regulator of T3SS-1 gene expression (19). However, additional experiments are needed to rigorously test this hypothesis.
The picture emerging from this and other studies is that by repressing flhDC, the TviA regulatory protein may reduce expression of T3SS-1 and flagella, two virulence factors contributing to the initiation of inflammatory responses in streptomycin-pretreated mice (5, 32). We did not observe that the viaB locus significantly reduced inflammatory responses at 24 h after infection, a time point at which flagella are required for fully inducing this host response (32). By 72 h after infection, the presence of flagella is no longer required for inducing pathological changes, while a functional T3SS-1 remains essential for eliciting severe inflammatory changes (5, 32). At this time point, the presence of the viaB locus significantly reduced the ability of serotype Typhimurium to elicit intestinal inflammatory responses. While our data did not rule out the possibility that reduced T3SS-1 expression may contribute to an attenuation of host responses in a Vi+ serotype Typhimurium strain, they suggested that this was not the only mechanism, because the viaB locus further reduced inflammatory responses elicited by a T3SS-1-deficient serotype Typhimurium mutant at both 72 h and 120 h after infection.
A remaining question is by which mechanism the viaB locus attenuates intestinal inflammation. T3SS-1-independent inflammatory changes require the presence of MyD88, an adaptor molecule for bacterium-specific TLRs, such as TLR4, TLR5, and TLR2 (13). However, inflammatory responses elicited by a T3SS-1-deficient serotype Typhimurium mutant are not attenuated in TLR4-, TLR5-, or TLR2-deficient mice, and it has been proposed that MyD88-dependent responses may be mediated through a combination of TLRs (12). The Vi capsular antigen prevents TLR4 recognition of serotype Typhimurium by murine bone marrow-derived macrophages and in a mouse model for serotype Typhimurium bacteremia (38). Furthermore, by repressing flagellin expression, TviA reduces the ability of epithelial cells to detect serotype Typhimurium through TLR5 (39). These data suggest that the viaB locus enables serotype Typhi to evade recognition by multiple TLRs. This interference with innate immune recognition by a combination of TLRs represents a possible mechanism by which the viaB locus may attenuate MyD88-dependent, T3SS-1-independent intestinal inflammatory responses.
Acknowledgments
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant C06 RR12088-01 from the National Center for Research Resources, National Institutes of Health. Work in A.J.B.'s laboratory was supported by Public Health Service grants AI040124, AI044170, and AI079173. C.T. is supported by Scientist Development grant 0835248N from the American Heart Association.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Arricau, N., D. Hermant, H. Waxin, C. Ecobichon, P. S. Duffey, and M. Y. Popoff. 1998. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol. Microbiol. 29835-850. [DOI] [PubMed] [Google Scholar]
- 2.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Rüssmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäumler, A. J., R. M. Tsolis, P. J. Valentine, T. A. Ficht, and F. Heffron. 1997. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect. Immun. 652254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitar, R., and J. Tarpley. 1985. Intestinal perforation in typhoid fever: a historical and state-of-the-art review. Rev. Infect. Dis. 7257-271. [DOI] [PubMed] [Google Scholar]
- 5.Coburn, B., Y. Li, D. Owen, B. A. Vallance, and B. B. Finlay. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 733219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichelberg, K., and J. E. Galán. 2000. The flagellar sigma factor FliA (σ28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect. Immun. 682735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godinez, I., T. Haneda, M. Raffatellu, M. D. George, T. A. Paixao, H. G. Rolan, R. L. Santos, S. Dandekar, R. M. Tsolis, and A. J. Bäumler. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 762008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale, C., F. Bowe, D. Pickard, S. Clare, J. F. Haeuw, U. Powers, N. Menager, P. Mastroeni, and G. Dougan. 2006. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine 244312-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hapfelmeier, S., K. Ehrbar, B. Stecher, M. Barthel, M. Kremer, and W. D. Hardt. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72795-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hapfelmeier, S., and W. D. Hardt. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13497-503. [DOI] [PubMed] [Google Scholar]
- 12.Hapfelmeier, S., A. J. Muller, B. Stecher, P. Kaiser, M. Barthel, K. Endt, M. Eberhard, R. Robbiani, C. A. Jacobi, M. Heikenwalder, C. Kirschning, S. Jung, T. Stallmach, M. Kremer, and W. D. Hardt. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in ΔinvG S. Typhimurium colitis. J. Exp. Med. 205437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hapfelmeier, S., B. Stecher, M. Barthel, M. Kremer, A. J. Muller, M. Heikenwalder, T. Stallmach, M. Hensel, K. Pfeffer, S. Akira, and W. D. Hardt. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 1741675-1685. [DOI] [PubMed] [Google Scholar]
- 14.Jones, B. D., C. A. Lee, and S. Falkow. 1992. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect. Immun. 602475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, G. W., L. A. Richardson, and D. Uhlman. 1981. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J. Gen. Microbiol. 127351-360. [DOI] [PubMed] [Google Scholar]
- 16.Karlinsey, J. E., S. Tanaka, V. Bettenworth, S. Yamaguchi, W. Boos, S. I. Aizawa, and K. T. Hughes. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol. Microbiol. 371220-1231. [DOI] [PubMed] [Google Scholar]
- 17.Kingsley, R. A., A. D. Humphries, E. H. Weening, M. R. De Zoete, S. Winter, A. Papaconstantinopoulou, G. Dougan, and A. J. Bäumler. 2003. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: identification of intestinal colonization and persistence determinants. Infect. Immun. 71629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus, M. D., B. Amatya, and Y. Kimula. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12949-955. [PubMed] [Google Scholar]
- 19.Lin, D., C. V. Rao, and J. M. Slauch. 2008. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J. Bacteriol. 19087-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake, M., L. Zhao, T. Ezaki, K. Hirose, A. Q. Khan, Y. Kawamura, R. Shima, M. Kamijo, T. Masuzawa, and Y. Yanagihara. 1998. Vi-deficient and nonfimbriated mutants of Salmonella typhi agglutinate human blood type antigens and are hyperinvasive. FEMS Microbiol. Lett. 16175-82. [DOI] [PubMed] [Google Scholar]
- 22.Mukawi, T. J. 1978. Histopathological study of typhoid perforation of the small intestines. Southeast Asian J. Trop. Med. Public Health 9252-255. [PubMed] [Google Scholar]
- 23.Nguyen, Q. C., P. Everest, T. K. Tran, D. House, S. Murch, C. Parry, P. Connerton, V. B. Phan, S. D. To, P. Mastroeni, N. J. White, T. H. Tran, V. H. Vo, G. Dougan, J. J. Farrar, and J. Wain. 2004. A clinical, microbiological, and pathological study of intestinal perforation associated with typhoid fever. Clin. Infect. Dis. 3961-67. [DOI] [PubMed] [Google Scholar]
- 24.Olsen, S. J., S. C. Bleasdale, A. R. Magnano, C. Landrigan, B. H. Holland, R. V. Tauxe, E. D. Mintz, and S. Luby. 2003. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol. Infect. 13013-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29303-313. [DOI] [PubMed] [Google Scholar]
- 26.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Bäumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 733367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffatellu, M., D. Chessa, R. P. Wilson, C. Tukel, M. Akcelik, and A. J. Bäumler. 2006. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 7419-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. E. Winter, C. A. Rossetti, S. D. Lawhon, H. Chu, T. Lau, C. L. Bevins, L. G. Adams, and A. J. Bäumler. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 754342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santander, J., S. Y. Wanda, C. A. Nickerson, and R. Curtiss III. 2007. Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect. Immun. 751382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 695619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprinz, H., E. J. Gangarosa, M. Williams, R. B. Hornick, and T. E. Woodward. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am. J. Dig. Dis. 11615-624. [DOI] [PubMed] [Google Scholar]
- 32.Stecher, B., S. Hapfelmeier, C. Muller, M. Kremer, T. Stallmach, and W. D. Hardt. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 724138-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stojiljkovic, I., A. J. Bäumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 1771357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsolis, R. M., G. M. Young, J. V. Solnick, and A. J. Bäumler. 2008. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 6883-892. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401804-808. [DOI] [PubMed] [Google Scholar]
- 36.Virlogeux, I., H. Waxin, C. Ecobichon, and M. Y. Popoff. 1995. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology 1413039-3047. [DOI] [PubMed] [Google Scholar]
- 37.West, N. P., P. Sansonetti, J. Mounier, R. M. Exley, C. Parsot, S. Guadagnini, M. C. Prevost, A. Prochnicka-Chalufour, M. Delepierre, M. Tanguy, and C. M. Tang. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 3071313-1317. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, R. P., M. Raffatellu, D. Chessa, S. E. Winter, C. Tükel, and A. J. Bäumler. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol. 10876-890. [DOI] [PubMed] [Google Scholar]
- 39.Winter, S. E., M. Raffatellu, R. P. Wilson, H. Rüssmann, and A. J. Bäumler. 2008. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 10247-261. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 703843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, L., T. Ezak, Z. Y. Li, Y. Kawamura, K. Hirose, and H. Watanabe. 2001. Vi-suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer's patches. Microbiol. Immunol. 45149-158. [DOI] [PubMed] [Google Scholar]