Abstract
Werner syndrome is a premature aging disorder characterized by cancer predisposition that is caused by loss of the Werner syndrome protein (WRN) helicase/exonuclease DNA repair protein. Hexavalent chromium is an environmental carcinogen and genotoxicant that is associated with respiratory cancers and induces several forms of DNA damage, including lesions that interfere with DNA replication. Based on the evidence that WRN protein facilitates repair of stalled and collapsed replication forks, we hypothesized that WRN functions in the cellular response to and recovery from Cr(VI)-induced genotoxicity and genomic instability. Here we report that human cells deficient in WRN protein are hypersensitive to Cr(VI) toxicity, and exhibit a delayed reduction in DNA breaks and stalled replication forks, indicated by γH2AX foci, during recovery from Cr(VI) exposure. Cr(VI)-induced WRN protein translocation from the nucleoli into nucleoplasmic foci in S-phase cells, and these foci colocalized with γH2AX foci indicating WRN responds to replication-associated DNA damage. As further evidence that Cr(VI) triggers stalled DNA replication, we observed Cr(VI) treatment induced an accumulation of cells in S-phase that exhibited high levels of γH2AX foci. Therefore, these data demonstrate a novel role for WRN protein in cellular protection against the environmental genotoxicant Cr(VI) and further provide evidence that Cr(VI) induces DNA replicative stress which has implications for aging and cancer.
Keywords: Werner syndrome protein, chromium, DNA damage, replication stress, DNA repair
The Werner syndrome protein (WRN) is a member of the RecQ DNA helicase family that functions in DNA repair pathways to preserve genome integrity. WRN is the only human RecQ helicase that has 3′–5′ exonuclease activity in addition to the helicase activity, rendering it capable of processing a distinct subset of DNA structures (Opresko et al., 2004). Loss of WRN leads to Werner syndrome (WS), a rare disorder characterized by features of premature aging and cancer predisposition (Wu and Hickson, 2006). WRN's role in tumorigenesis and cancer has been well documented. The WRN gene is epigenetically inactivated in a variety of human tumors, suggesting a role as a tumor suppressor (Agrelo et al., 2006). Conversely, WRN protein promotes the growth of some rapidly proliferating tumor cell lines (Opresko et al., 2007). The protection against genomic instability and cancer, as well as the facilitation of cell proliferation, is attributable to WRN's function in DNA replication, recombination, and repair (Opresko, 2008; Opresko et al., 2004).
Roles for WRN in facilitating DNA replication after DNA damage are supported by several lines of evidence. First, WS cells normally exhibit a prolonged S-phase and impaired replication fork progression, suggesting a role for WRN in replication past natural impediments or endogenous damage (Dhillon et al., 2007). Second, WRN-deficient cells exhibit a reduced rate of replication fork progression after exposure to exogenous DNA damage or dideoxynucleotide triphosphate depletion (Sidorova et al., 2008). Third, WRN-deficient cells are hypersensitive to select chemotherapeutic DNA damaging agents, particularly those that interfere with DNA replication (Dhillon et al., 2007; Rodriguez-Lopez et al., 2007). Finally, DNA cross-linking agents induce more γH2AX foci in WRN-deficient cells than in wild-type cells, indicative of replication-dependent DNA double-strand breaks (DSBs) (Cheng et al., 2008).
Hexavalent chromium Cr(VI) is a human carcinogen and environmental pollutant that is generated in the stainless steel, chrome plating, and other industrial processes, and is present in the majority of National Priority List toxic waste sites (Wise et al., 2008). Cr(VI) exposure is a well-established risk factor for developing respiratory diseases and cancers primarily in the occupational setting (Ha et al., 2004). Extensive studies indicate that Cr(VI) carcinogenesis is likely attributable to its genotoxicity, however an understanding of cellular pathways that are required for resistance to Cr(VI) induced DNA damage is limited. Cr(VI) is readily taken up by the cell and is ultimately reduced to Cr(III) (O'Brien et al., 2002). Cr(III) reacts with DNA in divergent manners generating Cr-DNA binary adducts, amino acid/glutathione/ascorbate/protein-Cr-DNA ternary adducts, and DNA-Cr-DNA interstrand cross-links (ICLs) (O'Brien et al., 2002; Xu et al., 1996), which are presumed to induce single-strand breaks (SSBs) and DSBs (Ha et al., 2004; Xu et al., 1996). Cr(III)-induced lesions, particularly ICLs, impede the progression of a variety of DNA polymerases in vitro (Bridgewater et al., 1998; O'Brien et al., 2002). Furthermore, cellular Cr(VI) exposure induces S-phase dependent DSBs that can be detected by γH2AX foci and pulse field gel electrophoresis (Ha et al., 2004; Reynolds et al., 2009), suggesting that Cr-DNA complexes trigger replication fork collapse to DSBs. Therefore, Cr(VI) cytotoxicity is attributed partly to the inhibition of DNA replication.
Based on the evidence that WRN facilitates repair of stalled replication forks (Cheng et al. 2008; Dhillon et al. 2007; Pichierri et al., 2001; Sidorova, 2008), we hypothesized that WRN functions in the cellular recovery from Cr(VI)-induced genotoxic stress. Here we report that WRN-deficient cells are more sensitive to Cr(VI) toxicity and that stalled replication forks and DSBs, indicated by γH2AX foci, persist longer in WRN-deficient cells compared with controls. Cr(VI) exposure induced WRN protein translocation from the nucleoli to nucleoplasmic γH2AX foci in S-phase cells, supporting a role for WRN in responding to replication-associated DNA breaks and stress. Consistent with this, Cr(VI) exposure induced an accumulation of cells in S-phase that exhibited high levels of nucleoplasmic γH2AX foci indicative of stalled DNA replication. Collectively, our results indicate that Cr(VI) induces replicative stress and that WRN functions in the repair of and recovery from stalled or collapsed replication forks caused by Cr(VI) exposure. This is the first molecular evidence that WRN protein plays a role in protecting cells from exposure to the environmental pollutant Cr(VI).
MATERIALS AND METHODS
Cell culture and treatment.
Human U2OS osteosarcoma cells were obtained from ATCC (Manassas, VA), and human WI-38 fetal lung fibroblasts from the Coriell Cell Repositories (Camden, NJ). The telomerase-immortalized normal fibroblast cell line (hTERT GM01604) was described previously (Ouellette et al., 2000). The telomerase-immortalized WS fibroblast cell line (hTERT AMIE15010) was a kind gift of Dr Junko Oshima (University of Washington). U2OS cell lines stably expressing an short hairpin RNA (shRNA) against WRN (shWRN) or a scrambled (shRNA) control (shCtrl), or stably expressing WRN with an EYFP fluorescent tag (EYFP-WRN U2OS) were described previously (Harrigan et al., 2006; Opresko et al., 2004). To maintain selective pressure for the shRNAs and EYFP-WRN expression, the cells were cultured in the presence of hygromycin (200 μg/ml, Calbiochem) and geneticin G418 (200 μg/ml, Invitrogen), respectively. Cells were cultured in Dulbecco's Modified Eagle Media supplemented with 10% fetal bovine serum in humidified chambers with 5% CO2 at 37°C. 15% fetal bovine serum was used for fibroblast cultures. Cells were exposed to potassium dichromate (Sigma-Aldrich Chemical Co., MO) in a series of experiments (time course, concentration-response and recovery analysis). Concentrations given in the results and figure legends are the concentrations of Cr(VI) ions and not the formula of the compound (e.g., 2μM potassium dichromate = 4μM Cr(VI)).
Cellular toxicity.
The cellular toxicity of Cr (VI) was determined with a cell proliferation assay using (3,4,5-dimehtylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in 96-well plates and cell a viability assay (CVA). Cells were exposed to Cr (VI), and the toxicity was evaluated with a Cultrex TACS MTT Cell Proliferation Assay kit (Travigen, MD) according to the manufacturer's instruction. In cell viability assays, cells were seeded at a density of 1 × 105 cells per well in six-well dishes and incubated overnight. Cells were exposed to various concentrations of Cr(VI) for 24 h. Subsequently, cells were subcultured by seeding 8 × 103 cells from each well into three 10-cm culture dishes followed by incubation in Cr(VI)-free medium for 8 days. Cell number was counted at the end of the experiment using a Z1 Coulter Particle Counter (Beckman Coulter, aperture 100 μm). Results were expressed as the cell number in the treated wells relative to cells in the untreated wells (fraction of control).
Western blotting analysis.
To confirm WRN depletion by the shRNA against WRN, the cell lysates were immunoblotted with rabbit anti-WRN polyclonal antibody (Novus, Littleton, CO). For analysis of chromatin associated proteins, the protein in the soluble and insoluble fractions (pellet) of the cell lysates were prepared as described previously (Cheng et al., 2003) and probed with antibodies (mouse anti-WRN 195C/G6 monoclonal, Opresko et al., 2007; mouse anti-glyceraldehyde-3-phosphate dehydrogenase monoclonal, Santa Cruz, CA; mouse anti-Lamin B1 polyclonal, MBL). Secondary antibodies were detected with enhanced chemiluminescent plus (Amersham Biosciences, NJ).
Immunofluorescence.
EYFP-WRN U2OS cells (2 × 105) were seeded in 35-mm glass bottom culture dishes (MatTek Corp., MA). Cells were fixed with 2% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 15 min, and then blocked in 2% BSA for 1 h. For γH2AX detection, cells were incubated with mouse anti-γH2AX monoclonal antibody (1:500; Upstate, Billerica, MA) and then with Cy5-conjugated goat anti-mouse secondary antibody (1:400; JIR Laboratories, Inc., West Grove, PA). For the quantitation of WRN and γH2AX foci colocalization, those foci that were adjacent but not clearly overlapping were not considered to colocalize and were excluded from analysis.
To indentify S-phase cells, the cells were pulse-labeled with 50μM bromodeoxyuridine (BrdU) (Sigma-Aldrich) in fresh Cr(VI)-free media for 1 h, which is the time required for DNA replication in U2OS cells (Major et al. 2004). Duplex DNA was denatured following the permeabilization of cells as described previously (Esin et al., 1999). BrdU was detected with mouse anti-BrdU monoclonal anitbody (BD Pharminagen, 1:50) and Cy5-conjugated goat anti-mouse secondary antibody (JIR laboratories, Inc., 1:400). BrdU incorporation under nondenaturing conditions (Bischof et al., 2001) was conducted to identify regions of ssDNA (Xu et al., 1996).
Detection of colocalization of γ-H2AX with BrdU incorporation was conducted with double-immunostaining. The BrdU labeling and immunostaining of γ-H2AX were conducted as stated above, and then the samples were fixed again with 2% paraformaldehyde. After DNA denaturation, the cells were blocked in 2% BSA and then immunostained with sheep anti-BrdU antibody (1:100; Genetex, Irvine, CA), followed by incubation with a fluorescein-5-isothiocyanate-conjugated anti-sheep IgG antibody (JIR Laboratories, Inc., 1:100). Nuclei were counterstained with 10 μg/ml 4′,6-diamidino-2-phenylindole (DAPI).
Laser scanning confocal microscopy.
Individual image planes (1024 × 1024 pixel resolution) were acquired as single scans on an Olympus FluoView 1000 confocal microscope (Olympus America, Inc., NY) equipped with a PlanApo N 60x/1.42 oil immersion objective with Fluoview software. Images obtained with different fluorescence filter sets were merged using the Fluoview software and then processed using Adobe Photoshop. At least 10 random fields were obtained from each individual treatment. All images from the same experiments were taken with the same exposure settings to ensure consistent quality for quantitative analyses. For nucleoplasmic WRN foci and γH2AX foci analysis, only distinct foci were quantified.
Statistical analysis.
All statistical analyses were performed using SAS software (SAS, version 9.2, NC). The data analysis was conducted using one-way ANOVA followed by Duncan's multiple comparison. To examine the correlation among different types of markers, Pearson correlation analyses were conducted. The statistically significant level was set at p ≤ 0.05.
RESULTS
WRN Deficiency Leads to Increased Cellular Sensitivity to Cr(VI) Toxicity
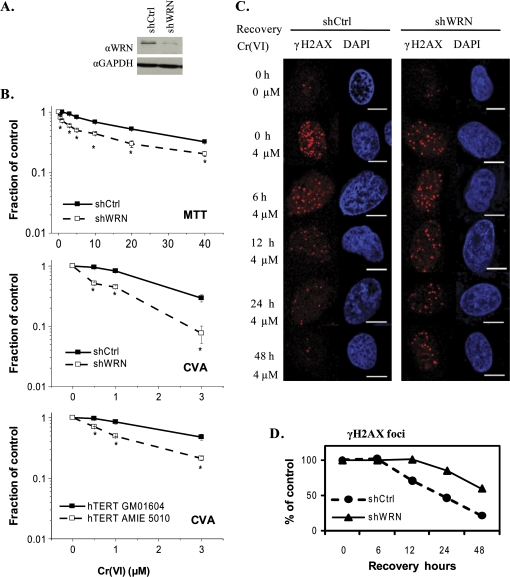
WS cells are hypersensitive to genotoxic chemotherapeutic agents that induce DNA replication fork stalling such as DNA cross-linkers and topoisomerase poisons (Pichierri et al., 2001). However, limited information is known about WRN's role in responding to environmental genotoxicants. To test whether WRN functions in protecting cells from Cr(VI) toxicity, we used U2OS cell lines that stably express either a short hairpin (sh) RNA targeting WRN mRNA or a control shRNA (Fig. 1A). These are well characterized model cell lines that were shown to respond similarly to WS cells with regard to hypersensitivity to DNA damaging agents (Cheng et al., 2006, 2008; Harrigan et al., 2006). Cells were treated with 0.5–40μM Cr(VI) for 72 h and analyzed for toxicity by the metabolic MTT assay. WRN depleted cells exhibited a significant increase in sensitivity to Cr(VI) toxicity, compared with the control shRNA cells (Fig. 1B). Exposure to 0.5μM Cr(VI) induced the same level of toxicity in the WRN-deficient cells as 5μM Cr(VI) in the control (Supplemental Table S1), revealing that WRN depletion caused a 10-fold increase in sensitivity to Cr(VI). Furthermore, WRN depleted cells were significantly more sensitive to Cr(VI) induced reproductive cell death as determined by a cell viability assay that detects both cell death and irreversible cell cycle arrest (see Material and Methods) (Fig. 1). Because the cell viability assay is more sensitive than the metabolic assay, the exposures were limited to 24 h and lower Cr(VI) concentrations were tested. Increased Cr(VI) sensitivity was also observed with telomerase-immortalized fibroblasts from a WS patient (hTERT AMIE 5010), when compare to fibroblasts from a normal patient (hTERT GM01604) (Fig. 1). Together, the results from the U2OS and fibroblast cell lines indicate that loss of WRN protein function results in increased sensitivity to Cr(VI)-induced toxicity.
FIG. 1.
WRN-deficient cells are hypersensitive to Cr(VI) toxicity and exhibit delayed recovery from Cr(VI)-induced DNA damage. (A)Western blot showing WRN expression levels in U2OS cells stably expressing either the control or WRN shRNAs. (B) Cellular toxicity of Cr(VI). U2OS cells were exposed to the indicated Cr(VI) concentrations for 72 h and cell proliferation was evaluated by the MTT assay. In the cell viability assay (CVA), U2OS cells, and telomerase-immortalized wild-type (GM01604) and WS (AMIE5010) fibroblasts were exposed to the indicated Cr(VI) concentrations for 24 h. Then a subpopulation of cells were cultured in Cr(VI)-free medium for 8 days and the final cell number was counted. Data represent the mean ± SE from two independent experiments. Asterisks indicate significant difference between the two cell lines, p < 0.05. (C, D) γH2AX kinetics after cell exposures to 0 or 4μM Cr(VI) for 24 h, followed by culturing in Cr(VI)-free medium for the indicated recovery times. Cells were immunostained for γH2AX (red). Bars, 10 μm. (D) The average number of γH2AX foci per cell, based on at least 50 randomly chosen cells for each time point in each group. Values represent the percent relative to the corresponding untreated group. GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase.
We hypothesized that WRN-deficient cells are hypersensitive to Cr(VI) due to a decreased ability to recover from Cr(VI)-induced DNA damage or replication stalling. Phosphorylation of histone H2AX (γH2AX) is a biomarker of DNA DSBs and replicative stress (Davalos and Campisi, 2003). γH2AX foci formation indicates that exposure to an agent triggered a cellular DNA damage response, and is a marker for genotoxicity but not necessarily cytotoxicity if the cells can recover from the DNA damage. To examine the kinetics of Cr(VI)-induced DNA damage and repair, cells were exposed to 4μM Cr(VI) for 24 h, and then cultured in Cr(VI)-free medium. After Cr(VI) treatment similar numbers of γH2AX foci were observed in both wild-type and WRN depleted cell lines (average number per cell of 21 and 18, respectively) (Fig. 1C). Subsequent culturing in Cr(VI)-free medium resulted in a progressive reduction in γH2AX foci number that began after 6 h of recovery in control cells, but not until after 12 h in WRN depleted cells (Fig. 1D). After 48 h of recovery, the number of γH2AX foci returned to background levels in control cells, whereas numerous γH2AX foci persisted in the WRN-deficient (an average of 11 γH2AX foci versus 4.4 in control cells). Therefore, WRN depletion results in the longer persistance of γH2AX foci induced by Cr(VI) exposure, and the much slower recovery from Cr(VI)-induced DSBs and/or stalled replication forks.
WRN Translocates from the Nucleolus into Nucleoplasmic Foci in Response to Cr(VI)
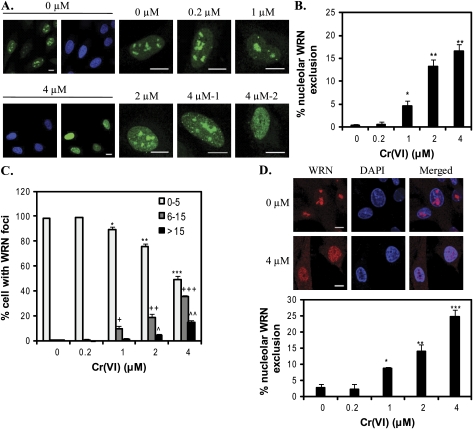
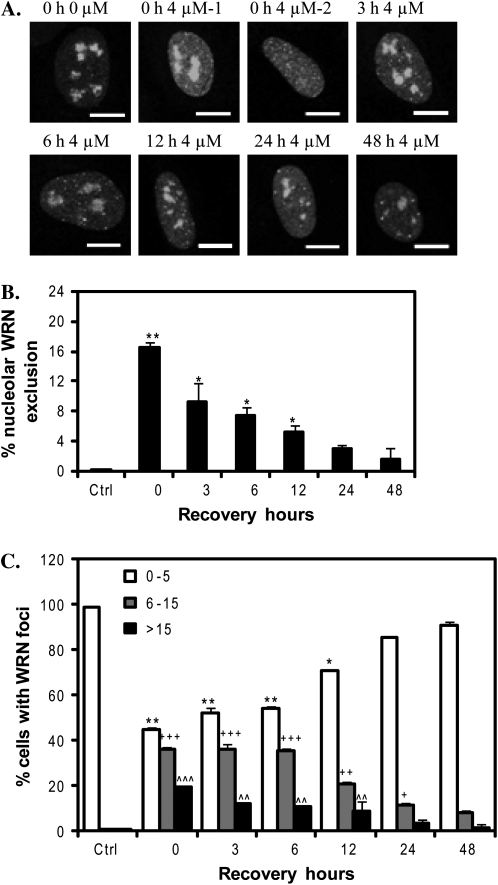
Because WRN depleted cells are hypersensitive to Cr(VI) toxicity and exhibit a delayed reduction in γH2AX foci, we predicted that WRN has a direct role in responding to Cr(VI)-mediated DSBs and stalled forks. WRN responds to DNA damage primarily through alterations in subnuclear localization directed by posttranslational modifications (Blander et al., 2002; Karmakar and Bohr, 2005; Muftuoglu et al., 2008). WRN mainly localizes to the nucleolus in most of the human cell lines tested (Blander et al., 2002; Karmakar and Bohr, 2005; Suzuki et al., 2001). Exposure to chemicals that generate DSBs, ICLs, base damage, and replication fork arrest, induces WRN translocation to nucleoplasic foci (Blander et al., 2002; Karmakar and Bohr, 2005). We investigated whether Cr(VI) exposure alters WRN subcellular localization, by employing a human U2OS cell line that stably expresses an EYFP-WRN fusion protein as previously described (Opresko et al., 2004). The EYFP tag does not alter WRN localization, but enables the detection of clear distinct foci for quantitation (Suzuki et al., 2001). WRN resides mainly in the nucleolus in untreated cells, but moves into the nucleoplasm upon Cr(VI) exposure (Fig. 2A). The percent of cells that exhibited no nucleolar WRN, indicative of complete WRN mobilization (Fig. 2A, 4μM-2), increased significantly from an average of 0.3% to as high as 16.5% after 4μM Cr(VI) treatment (Fig. 2B). We classified the WRN nucleoplasmic localization patterns into three categories: (1) 0–5 WRN foci (background levels, observed in 98% of untreated cells); (2) 6–15 foci; and (3) > 15 foci which includes diffuse WRN staining across the nucleoplasm (multiple poorly defined tiny foci) (Fig. 2A, 4μM-2). The average percent of cells decreased in group 1, and increased in groups 2 and 3 as a function of Cr(VI) concentration (Fig. 2C). Thus, Cr(VI) exposure induced the mobilization of WRN protein, and both the extent and pattern of WRN mobilization was dependent on Cr(VI) concentration.
FIG. 2.
Cr(VI) induces WRN translocation. (A) Representative confocal images of EYFP-WRN U2OS cells (green). U2OS cells were exposed to various Cr(VI) concentrations for 24 h. Bars, 10 μm. (B) The percent of U2OS cells exhibiting WRN nucleolar exclusion. (C) The distribution of U2OS cells showing WRN nucleoplasmic foci. Cells were categorized into three groups, as indicated, according to the nucleoplasmic WRN foci number. In (B) and (C), data represent the mean ± SE from five independent experiments, based on at least 50 randomly chosen cells from each Cr(VI) treatment. (D) Representative confocal images of WI38 cells exposed to 0 or 4μM Cr(VI) for 24 h. Cells were immunostained for WRN. Bars, 10 μm. The percent of WI38 cells exhibiting nucleolar WRN exclusion based on at least 120 randomly chosen cells from each Cr(VI) treatment. Data represent the mean ± SE from two independent experiments. Symbol(s) above a bar indicates significantly different from the control (p < 0.05). Bars with a different number of symbols are significantly different (p < 0.05) from each other within each category.
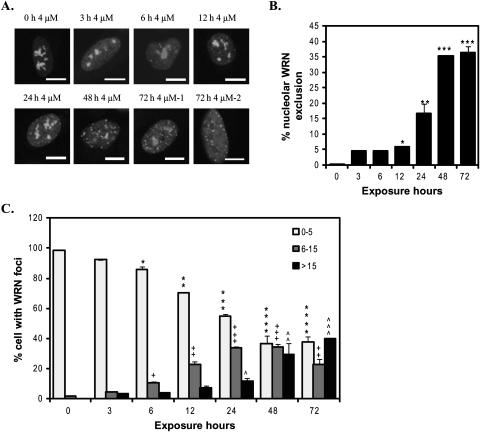
WRN mobilization depended not only on the Cr(VI) concentration, but also on the duration of Cr(VI) exposure. EYFP-WRN movement in U2OS cells was observed as early as 3 h after 4μM Cr(VI) treatment and increased with time (Fig. 3). The average percent of cells with WRN nucleolar exclusion increased significantly starting from 6% at 12 h and reached a plateau of 35% after 48 h (Fig. 3B). Similarly, the percent of cells with few WRN nucleoplasmic foci (0–5) decreased during the time course and reached a plateau at 48 h, whereas the percent of cells with multiple WRN foci (greater than 15) continued to increase with exposure time (Fig. 3C). Thus, WRN responds rapidly to Cr(VI) treatment and the response increases and persists throughout the Cr(VI) exposure.
FIG. 3.
WRN translocation to nucleoplasmic foci in response to Cr(VI) treatment is time dependent. (A) Confocal images of EYFP-WRN U2OS cells exposed to 4μM Cr(VI) for the indicated times. Cells were analyzed for nucleoplasmic WRN foci and nucleolar WRN exclusion. Bars, 10 μm. (B) The percent of the cells exhibiting WRN nucleolar exclusion. Data are presented as mean ± SE from two independent experiments. (C) The percent of cells with various WRN nucleoplasmic foci number, as indicated. Quantitation analysis was conducted as in Figure 2C. Data are presented as mean ± SE from two independent experiments. At least 50 cells were randomly chosen from each Cr(VI) treatment. Symbol(s) above a bar indicates significantly different from the control (p < 0.05). Bars with a different number of symbols above are significantly different (p < 0.05) from each other within each category.
To ensure that Cr(VI) also induces the translocation of endogenous WRN in a nontransformed cell line, we exposed WI-38 diploid human lung fibroblasts which represent relevant targeted cells for Cr(VI) exposure by inhalation. In the untreated cells, WRN localized to the nucleolus as expected (Fig. 2D). In contrast, Cr(VI) exposure increased the percent of the cells showing WRN nucleolar exclusion from an average of 2.8% at 0.2μM to 25% at 4μM Cr(VI), in a concentration-dependent manner (Fig. 2D and Supplemental Fig. S1). Nearly 100% of the WI38 cells exhibited WRN nucleolar exclusion at 8μM Cr(VI) (data not shown). This indicates that endogenous WRN in nontransformed lung fibroblasts rapidly responds to Cr(VI) exposure, as observed for EYFP-WRN in the U2OS cell line. These data also provide evidence that endogenous WRN plays an important role in protecting normal cells from Cr(VI)-induced toxicity.
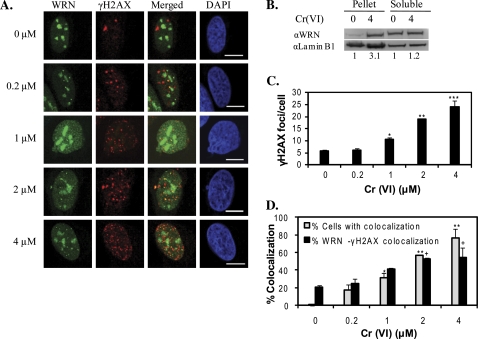
WRN Localizes to Sites of DNA Damage Induced by Cr(VI) Exposure
Next, we investigated whether WRN is recruited to Cr(VI)-induced sites of DSBs or stalled replication forks. Exposure of the cells to 4μM Cr(VI) induced a threefold increase in WRN colocalization with chromatin DNA (Fig. 4B). Cr(VI) exposures above 0.2μM caused a concentration dependent increase in the number of γH2AX foci per cell from an average of 6.1–24 foci at the highest concentration. A significant correlation was observed between the increases in nucleoplasmic WRN foci and the number of γH2AX foci after Cr(VI) treatment (Supplemental Table S2), indicating that WRN protein mobilization is determined by the level of Cr(VI) induced DSBs and stressed replication forks. To measure directly whether WRN translocates to γH2AX foci after Cr(VI) treatment we evaluated two parameters: (1) the percent of cells exhibiting colocalized foci and (2) the percent of WRN foci that colocalized with γH2AX foci. The average percent of cells showing WRN and γH2AX colocalization increased significantly starting from 1μM Cr(VI) treatment and greater, compared with untreated cells, and was as high as 76% at 4μM Cr(VI) (Fig. 4D). The average percent of WRN foci that colocalized with γH2AX foci also increased significantly as a function of Cr(VI) concentration, up to 55% at 4μM Cr(VI). Notably, cells exhibiting colocalization of WRN and γH2AX in untreated cells was rare (0.1%), but in these cells about 20% of the WRN foci localized to γH2AX sites (Fig. 4D), indicating that WRN is also involved in the repair of spontaneous DNA damage.
FIG. 4.
WRN localizes to sites of Cr(VI) induced DNA damage. (A) Confocal images of EYFP-WRN U2OS cells exposed to various Cr(VI) concentrations for 24 h. Cells were immunostained for γH2AX foci. The EYFP-WRN (green) and γH2AX (red) images were merged to detect colocalized foci (yellow). Bars, 10 μm. (B) Western blot shows an increase in WRN protein levels in the insoluble chromatin (pellet), but not the soluble, lysate fraction after exposure of U2OS cells to 4μM Cr(VI) for 24 h. The numbers below represent the fold increase in WRN relative to the untreated group after normalization to the loading control (Lamin B1). (C) Average number of γH2AX foci per cell. (D) The percent of cells showing colocalized WRN and γH2AX foci and the percent of WRN foci colocalizing with γH2AX foci. In (C) and (D), the data represent the mean ± SE from two independent experiments, based on at least 50 randomly chosen cells for each Cr(VI) treatment. Symbol(s) above a bar indicates significantly different from the control (p < 0.05). Bars with a different number of symbols are significantly different (p < 0.05) from each other within each category.
The Kinetics of WRN Relocalization to the Nucleoli after Cr(VI) Exposure Correlates with Recovery from DNA Damage
To investigate WRN response to Cr(VI) post exposure, we exposed EYFP-WRN U2OS cells to 4μM Cr(VI) for 24 h and then cultured the cells in Cr(VI)-free medium for the indicated recovery times (Fig. 5A). As reported in Figure 2, Cr(VI)-induced WRN mobilization into numerous nucleoplasmic foci in which cells either retained some WRN in the nucleoli (Fig. 5A, 4μM-1) or exhibited complete mobilization and nucleolar exclusion (Fig. 5A, 4μM-2). We found that Cr(VI)-induced WRN mobilization was reversible in a time-dependent fashion (Fig. 5A). Consistent with this, the percent of cells showing nucleolar WRN exclusion and the average WRN foci number per cell decreased to full basal levels by 24 h and 48 h, respectively (Figs. 5B and 5C). Because all of the U2OS cell lines had similar levels of Cr(VI)-induced γH2AX foci at 24 h (data not shown), we compared the kinetics of WRN relocalization (Fig. 5) to the reduction in γH2AX foci (Fig. 1D) during recovery from Cr(VI) exposure. The reduction in the numbers of WRN foci and γH2AX foci was highly and significantly correlated (r = 0.97, p = 0.002), indicating that WRN translocation back to the nucleoli coincides with the repair of DSBs and recovery from stalled forks.
FIG. 5.
WRN relocalizes to the nucleoli post Cr(VI) exposure. (A) Confocal images of EYFP-WRN U2OS cells exposed to 4μM Cr(VI) for 24 h, followed by incubation in Cr(VI)-free medium for various times. Bars, 10 μm. (B) The percent of the cells exhibiting WRN nucleolar exclusion. (C) The distribution of cells with various WRN nucleoplasmic foci number. Quantitative analysis was conducted as in Figure 2C. Data in (B) and (C) represent the mean ± SE from two independent experiments. Symbol(s) above a bar indicates significantly different from the control (p < 0.05). Bars with a different number of symbols above are significantly different (p < 0.05) from each other within each category.
Cr(VI) Induction of WRN Mobilization to Nucleoplasmic Foci Occurs in S-Phase Cells
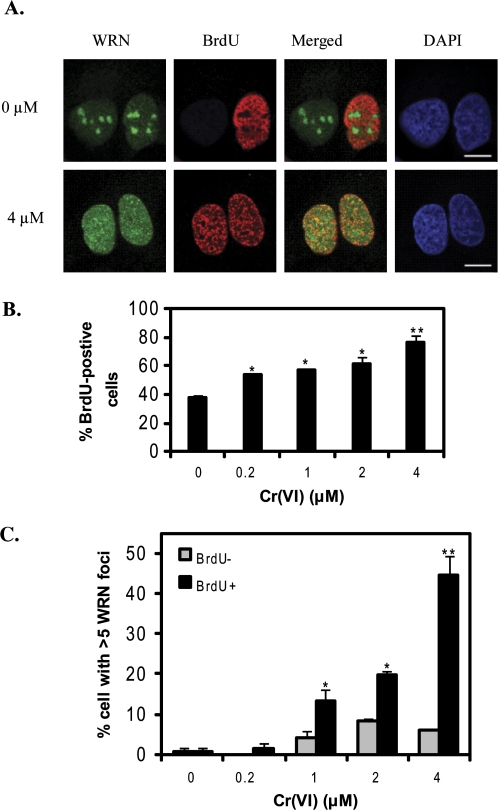
Because Cr(VI) induces lesions that cause replication fork blocks (Bridgewater et al., 1998; Ha et al., 2004), we predicted that WRN may respond to Cr(VI)-induced DNA damage during S-phase. To identify S-phase cells we assayed for incorporation of the nucleotide analog BrdU which is widely used as a marker of cells undergoing DNA replication (Bischof et al., 2001). BrdU was not detected by immunostaining under nondenaturing conditions, indicating that 4μM Cr(VI) does not induce large regions of single-stranded DNA (Bischof et al., 2001; Xu et al., 1996). In contrast, BrdU staining was detected in a significant fraction of the cells under denaturing conditions reflecting the incorporation of BrdU in duplex DNA (Figs. 6A, B). Approximately 38% of the untreated cells were in S-phase reflecting the short population doubling time (0.7–0.8 days) of this cell line (Graat et al., 2006). Cr(VI) exposures as low as 0.2μM increased the fraction of S-phase cells more than 15%, and 4μM Cr(VI) doubled the fraction of S-phase cells (Fig. 6B). These data indicate that Cr(VI) induces a significant accumulation of cells in S-phase, consistent with replicative stress.
FIG. 6.
Cr(VI) induces an accumulation of S-phase cells that exhibit increased nucleoplasmic WRN foci. (A) Confocal images of EYFP-WRN U2OS cells exposed to various Cr (VI) concentrations for 24 h. Cells were pulse-labeled with BrdU and immunostained for BrdU incorporation (red). Bars, 10 μm. (B) The percent of cells with BrdU incorporation. (C) The percent of the BrdU-positive and negative cells that exhibited more than five nucleoplasmic WRN foci. Data in (B) and (C) represent the mean ± SE from two independent experiments. Symbol(s) above a bar indicates significantly different from the control (p < 0.05). Bars with a different number of symbols above are significantly different (p < 0.05) from each other within each category.
Next we examined WRN staining in the S-phase population. Because an average of 0–5 WRN nucleoplasmic foci per cell represents background levels (Fig. 2C), we calculated the percent of BrdU-positive cells showing more than five WRN foci (Fig. 6C). This percent doubled even with 0.2μM Cr(VI) and increased as a function of Cr(VI) concentration up to an average of 77% (Fig. 6C). In contrast, there was no concentration-dependent increase in WRN foci number in non-S phase cells (Fig. 6C). Furthermore, a significant correlation existed between WRN nucleoplasmic foci number and the percent of BrdU-positive cells across Cr(VI) concentrations (Supplemental Table S2). Thus, we found that WRN nucleoplasmic foci induced by Cr(VI) were primarily in S-phase cells. These data demonstrate that WRN responds to Cr(VI) induced DNA damage primarily in S-phase of the cell cycle, providing evidence that Cr(VI) induces replicative stress.
Cr(VI) Induces γH2AX Foci in S-Phase Cells Indicative of Replication Fork Stress
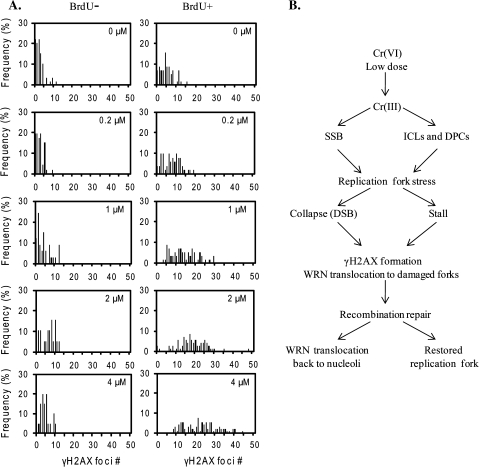
To confirm that Cr(VI) exposure induces DNA replicative stress we examined the formation of γH2AX foci in cells undergoing DNA replication, which is a biomarker for stalled and damaged replication forks (Davalos and Campisi, 2003). Cr(VI) exposure induced an increase in the fraction of S-phase cells with levels of γH2AX foci that are above background, from an average of 11% in untreated cells up to 82% after 4μM Cr(VI) exposure (Fig. 7). Consistent with this, a significant correlation existed between the percent of cells in S phase and γH2AX foci number (Supplemental Table S2). We plotted the percent distribution of BrdU-positive and -negative cells against γH2AX foci number (Fig. 7A). Most of the BrdU-negative cells exhibited low background levels of γH2AX foci (0–10) across all Cr(VI) concentrations. In contrast, the percent distribution shifted to the right (increased foci number) as a function of increased Cr(VI) concentration in the BrdU-positive cells. These data further support a role for Cr(VI) exposure in causing replication stress.
FIG. 7.
DNA damage occurs primarily in S-phase cells after Cr(VI) exposure. (A) The frequency distribution of BrdU-negative and -positive cells is plotted against the number of γH2AX foci in the cells, based on approximately 100 randomly chosen cells from each Cr(VI) treatment. (B) Model for WRN's role in protection against Cr(VI)-induced genotoxicity. See the text for details.
DISCUSSION
Emerging evidence strongly supports a role for WRN in the recovery from, and repair of, replication-associated DSBs induced by endogenous and exogenous sources (Dhillon et al., 2007; Otterlei et al., 2006; Rodriguez-Lopez et al., 2007; Sidorova, 2008). DSB formation by Cr(VI) may result from replication inhibition through the production of DNA blocking lesions, such as ICLs (Ha et al., 2004; Xu et al., 1996). Here we report the first molecular evidence that WRN functions in responding to, and recovery from, Cr(VI)-induced DNA damage and replication stress. WRN deficiency resulted in hypersensitivity to Cr(VI) toxicity in U2OS and fibroblast cell lines, and delayed repair of induced DSBs and stalled forks. WRN localized to DNA damage sites in response to Cr(VI)-induced replication stress, which is indicated by WRN mobilization to nucleoplasmic foci in S-phase cells, that colocalized with γH2AX foci. The kinetics of WRN mobilization back to the nucleolus during recovery from Cr(VI) treatment coincided with the kinetics of recovery from DSBs and stalled forks. The observation that Cr(VI) induced an accumulation of cells in S-phase that exhibited increased γH2AX foci provides further evidence the Cr(VI) triggers replicative stress. To our knowledge, this is the first demonstration that WRN functions in protecting cells from replicative stress induced by the environmental pollutant Cr(VI).
Previous reports indicate that WRN-deficient cells are hypersensitive to toxicity induced by select DNA damaging agents that cause replicative stress (Dhillon et al., 2007; Rodriguez-Lopez et al., 2007). Thus, we hypothesized that the hypersensitivity of WRN-deficient cells to Cr(VI) toxicity reported here was also caused by replicative stress (Fig. 1B). Consistent with this, we observed that WRN depleted cells responded similarly to Cr(VI) exposure as cells that are deficient in other proteins implicated in the recovery from stalled forks, namely FA-A (Fanconi anemia complementation group A) and MUS81. FA-A is part of the Fanconi anemia pathway that repairs ICLs (Vilcheck et al., 2002), and MUS81 is an endonuclease proposed to cleave stalled replication forks for repair (Franchitto et al., 2008). Similar to WRN (Fig. 4C), FA-A or MUS81 deficiencies also increases sensitivity to Cr(VI) toxicity and γH2AX foci accumulation, compared with controls (Tamblyn et al., 2008; Vilcheck et al., 2002). In line with our results (Fig. 7), the induced γH2AX foci occurred primarily in S-phase cells (Tamblyn et al., 2008; Vilcheck et al., 2002) suggesting they resulted from replication fork stalling and damage. Furthermore, we found that WRN does not prevent DSB formation in response to Cr(VI), but rather facilitates their repair. The numbers of γH2AX foci were similar in WRN depleted and control cells immediately after Cr(VI) exposure (Fig. 1C), but persisted longer in the WRN depleted cells during recovery (Fig. 1D). Similar results were reported for WRN-deficient fibroblasts when replication fork stress was induced by nucleotide depletion (Dhillon et al. 2007). In summary, our data suggest that WRN-deficient cells exhibit increased sensitivity to Cr(VI) toxicity due to an accumulation of DSBs and stalled replication forks.
WRN trafficking between the nucleolus and nucleoplasm upon replication stress has been well documented (Blander et al., 2002; Karmakar and Bohr, 2005; Sakamoto et al., 2001). The WRN localization studies reported here support a direct role for WRN in recovery from Cr(VI) induced DNA damage. WRN mobilization from the nucleoli into discrete nucleoplasmic foci was dependent on both Cr(VI) concentration and exposure duration, and the mobilization was reversed upon removal of Cr(VI) from the media (Figs. 2, 3, 5, and Fig. S1). Importantly, Cr(VI) induced mobilization of endogenous WRN in primary lung fibroblasts (Fig. 2D), and lung represents a relevant target for Cr(VI) exposure by inhalation and subsequent respiratory disease. Notably, the percent of cells with nucleolar WRN exclusion remained at maximal levels between 48 and 72 h Cr(VI) exposure (Fig. 3B), possibly because WRN-independent pathways are initiated or upregulated during extended Cr(VI) exposures. Cr(VI) induction of WRN movement to nucleoplasmic γH2AX foci strongly supports a role for WRN in responding to and/or repairing the DNA damage (Fig. 4A). Although the majority of WRN and γH2AX foci colocalized, the colocalization was not 100% potentially due to WRN roles in repair of other non-DSB DNA lesions (Muftuoglu et al. 2008) and other WRN-independent pathways for DSB repair (Sidorova et al., 2008). Nonetheless, the WRN trafficking kinetics are in good agreement with the kinetics of γH2AX foci formation during Cr(VI) exposure (Fig. 1D, Figs. 2A–C, Figs. 4A, B). Upon recovery from Cr(VI) exposure, cells showed a parallel reduction in WRN and γH2AX foci number (Figs. 1D and 5), which further supports a role for WRN in recovery from Cr(VI)-induced DNA damage and replication fork stalling.
Our data suggest that WRN responds to Cr(VI)-induced DSBs that are formed indirectly during replication. Upon replicative stress, cells arrest in S-phase, allowing time for DNA repair and replication fork re-initiation (Wu and Hickson, 2006). We observed an accumulation of cells in S-phase after Cr(VI) exposure in agreement with previous reports (Fig. 6; Bakke et al., 1984; Xie et al., 2005; Xu et al., 1996). Indeed, the accumulation of cells in S-phase is induced by other chemicals that inhibit DNA replication and trigger replicative stress, which also correlate with an increase in WRN nucleoplasmic foci (Sakamoto et al., 2001). Similarly, we found that the Cr(VI)-induced WRN nucleoplasmic foci were primarily in S-phase cells (Fig. 6). WRN is proposed to play important roles in both normal DNA replication by resolving secondary structures such as G4-quadraplex, and in replication after DNA damage by promoting the restoration of stalled replication forks through repair of subsequent DSBs (Dhillon et al., 2007; Rodriguez-Lopez et al., 2007). Our finding that after Cr(VI) exposure the majority of cells (> 87%) with increased γH2AX foci were in S-phase (Fig. 7A), agrees with previous reports that Cr(VI) induces γH2AX foci specifically in S-phase cells or cells in G2 that passed through S-phase (Ha et al., 2004; Reynolds et al., 2007; Tamblyn et al., 2008; Vilcheck et al., 2002). Together these studies suggest that Cr(VI) induction of DSBs depends on DNA replication.
Based on data from the current and previous studies, we propose a model for WRN in responding to Cr(VI)-induced DNA damage to protect against toxicity (Fig. 7B). Treatment with low concentrations of Cr(VI) can produce SSBs, that can cause replication fork collapse into DSBs (Ha et al., 2004). Alternatively, ICLs and bulky protein-Cr-DNA cross-links block replication fork progression (Ha et al., 2004), and endonucleases MUS81-EME1 or XPF-ERCC1 nick the template strand, forming DSBs amenable to repair by homologous recombination (Motycka et al., 2004; Tamblyn et al., 2008). Presumably, the generation of DSBs activates checkpoints resulting in the accumulation of cells in S-phase (Bakke et al., 1984; Ha et al., 2004; Xie et al., 2005). We propose WRN is mobilized to facilitate DSB repair and recovery from stalled forks. It is possible that WRN translocation may occur prior to S-phase arrest, because WRN collaborates with ATM in an intra S-phase checkpoint response to collapsed replication forks (Cheng et al., 2008). DSBs from collapsed replication forks during S-phase are mainly repaired through HR in human somatic cells (Wu and Hickson, 2006). WRN roles in HR repair of Cr(VI) induced replication-dependent DSBs is supported by several lines of evidence. (1) WRN interacts with many HR proteins (Otterlei et al., 2006; Sidorova, 2008) and functions in the HR repair of replication-associated DSBs, including those induced by ICLs (Cheng et al., 2006; Otterlei et al., 2006) and Cr(VI) induces ICLs formation (Xu et al., 1996). (2) HR deficient cell lines are more sensitive to Cr(VI) toxicity (O'Brien et al., 2002), and Cr(VI) induces RAD51 foci formation (Tamblyn et al., 2008). Following replication fork recovery, indicated by a reduction in γH2AX foci, WRN relocalizes back to the nucleoli.
Our study provides further evidence that Cr(VI) induces replicative stress and replication-associated DNA breaks. Furthermore, we show that decreased repair of Cr(VI) replication-dependent DNA damage, as in WRN-deficient cells, leads to increased toxicity in both U2OS and fibroblasts cells. The failure to properly restore damaged replication forks can lead to DSBs, chromosomal rearrangements and increased cancer as observed in WS patients (Opresko et al., 2007). WRN mobilization in Cr(VI)-targeted cells (lung fibroblasts) upon Cr(VI) exposure supports a role for WRN in protecting against Cr(VI)-induced genotoxicity and carcinogenicity. Risk assessment studies indicate that Cr(VI) exposure below the permissible limit may still pose a significant threat and cause an additional 45 cancer deaths per 1000 workers (OSHA, 2006; Reynolds et al., 2009). Thus, a complete understanding the mechanisms of Cr(VI)-induced genotoxicity and carcinogenicity, and the cellular pathways that protect against the potential mutagenic consequences of Cr(VI) exposure is critical.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Health and National Institute of Environmental Health Sciences (ES0515052); and the Ellison Medical Foundation to P.L.O.
Acknowledgments
We thank Gregory A. Gibson for his assistance in the confocal microscope in the Center for Biological Imaging (University of Pittsburgh). We also thank Dr Laura J. Niedernhofer and the Opresko lab for critical reading of the manuscript.
References
- Agrelo R, Cheng WH, Setien F, Ropero S, Espada J, Fraga MF, Herranz M, Paz MF, Sanchez-Cespedes M, Artiga MJ, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke O, Jakobsen K, Eik-Nes KB. Concentration-dependent effects of potassium dichromate on the cell cycle. Cytometry. 1984;5:482–486. doi: 10.1002/cyto.990050508. [DOI] [PubMed] [Google Scholar]
- Bischof O, Kim SH, Irving J, Beresten S, Ellis NA, Campisi J. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell. Biol. 2001;153:367–380. doi: 10.1083/jcb.153.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Zalle N, Daniely Y, Taplick J, Gray MD, Oren M. DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J. Biol. Chem. 2002;277:50934–50940. doi: 10.1074/jbc.M210479200. [DOI] [PubMed] [Google Scholar]
- Bridgewater LC, Manning FC, Patierno SR. Arrest of replication by mammalian DNA polymerases alpha and beta caused by chromium-DNA lesions. Mol. Carcinog. 1998;23:201–206. [PubMed] [Google Scholar]
- Cheng WH, Kusumoto R, Opresko PL, Sui X, Huang S, Nicolette ML, Paull TT, Campisi J, Seidman M, Bohr VA. Collaboration of Werner syndrome protein and BRCA1 in cellular responses to DNA interstrand cross-links. Nucleic Acids Res. 2006;34:2751–2760. doi: 10.1093/nar/gkl362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Muftic D, Muftuoglu M, Dawut L, Morris C, Helleday T, Shiloh Y, Bohr VA. WRN is required for ATM activation and the S-phase checkpoint in response to interstrand cross-link-induced DNA double-strand breaks. Mol. Biol. Cell. 2008;19:3923–3933. doi: 10.1091/mbc.E07-07-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, von Kobbe C, Opresko PL, Fields KM, Ren J, Kufe D, Bohr VA. Werner syndrome protein phosphorylation by abl tyrosine kinase regulates its activity and distribution. Mol. Cell. Biol. 2003;23:6385–6395. doi: 10.1128/MCB.23.18.6385-6395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Campisi J. Bloom syndrome cells undergo p53-dependent apoptosis and delayed assembly of BRCA1 and NBS1 repair complexes at stalled replication forks. J. Cell. Biol. 2003;162:1197–1209. doi: 10.1083/jcb.200304016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon KK, Sidorova J, Saintigny Y, Poot M, Gollahon K, Rabinovitch PS, Monnat RJ., Jr Functional role of the Werner syndrome RecQ helicase in human fibroblasts. Aging Cell. 2007;6:53–61. doi: 10.1111/j.1474-9726.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Esin S, Batoni G, Saruhan-Direskeneli G, Harris RA, Grunewald J, Pardini M, Svenson SB, Campa M, Wigzell H. In vitro expansion of T-cell-receptor Valpha2.3(+) CD4(+) T lymphocytes in HLA-DR17(3), DQ2(+) individuals upon stimulation with Mycobacterium tuberculosis. Infect. Immun. 1999;67:3800–3809. doi: 10.1128/iai.67.8.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchitto A, Pirzio LM, Prosperi E, Sapora O, Bignami M, Pichierri P. Replication fork stalling in WRN-deficient cells is overcome by prompt activation of a MUS81-dependent pathway. J. Cell. Biol. 2008;183:241–252. doi: 10.1083/jcb.200803173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, Schaap GR, Gerritsen WR, Wuisman PI, van Beusechem VW. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br. J. Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: Involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Wilson DM, 3rd, Prasad R, Opresko PL, Beck G, May A, Wilson SH, Bohr VA. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase beta. Nucleic Acids Res. 2006;34:745–754. doi: 10.1093/nar/gkj475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar P, Bohr VA. Cellular dynamics and modulation of WRN protein is DNA damage specific. Mech. Ageing Dev. 2005;126:1146–1158. doi: 10.1016/j.mad.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol. Cell. Biol. 2004;24:2649–2661. doi: 10.1128/MCB.24.7.2649-2661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motycka TA, Bessho T, Post SM, Sung P, Tomkinson AE. Physical and functional interaction between the XPF/ERCC1 endonuclease and hRad52. J. Biol. Chem. 2004;279:13634–13639. doi: 10.1074/jbc.M313779200. [DOI] [PubMed] [Google Scholar]
- Muftuoglu M, Kusumoto R, Speina E, Beck G, Cheng WH, Bohr VA. Acetylation regulates WRN catalytic activities and affects base excision DNA repair. PLoS ONE. 2008;3:e1918. doi: 10.1371/journal.pone.0001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T, Mandel HG, Pritchard DE, Patierno SR. Critical role of chromium (Cr)-DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry. 2002;41:12529–12537. doi: 10.1021/bi020452j. [DOI] [PubMed] [Google Scholar]
- Opresko PL. Telomere ResQue and preservation—Roles for the Werner syndrome protein and other RecQ helicases. Mech. Ageing Dev. 2008;129:79–90. doi: 10.1016/j.mad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Calvo JP, von Kobbe C. Role for the Werner syndrome protein in the promotion of tumor cell growth. Mech. Ageing Dev. 2007;128:423–436. doi: 10.1016/j.mad.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Otterlei M, Graakjaer J, Bruheim P, Dawut L, Kolvraa S, May A, Seidman MM, Bohr VA. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell. 2004;14:763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- OSHA(Occupational Safety and Health Administration) Department of Labor. Occupational exposure to hexavalent chromium. Final rule. Fed. Regist. 2006;71:10099–10385. [PubMed] [Google Scholar]
- Otterlei M, Bruheim P, Ahn B, Bussen W, Karmakar P, Baynton K, Bohr VA. Werner syndrome protein participates in a complex with RAD51, RAD54, RAD54B and ATR in response to ICL-induced replication arrest. J. Cell. Sci. 2006;119:5137–5146. doi: 10.1242/jcs.03291. [DOI] [PubMed] [Google Scholar]
- Ouellette MM, McDaniel LD, Wright WE, Shay JW, Schultz RA. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum. Mol. Genet. 2000;9:403–411. doi: 10.1093/hmg/9.3.403. [DOI] [PubMed] [Google Scholar]
- Pichierri P, Franchitto A, Mosesso P, Palitti F. Werner's syndrome protein is required for correct recovery after replication arrest and DNA damage induced in S-phase of cell cycle. Mol. Biol. Cell. 2001;12:2412–2421. doi: 10.1091/mbc.12.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MF, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: Linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MF, Peterson-Roth EC, Bespalov IA, Johnston T, Gurel VM, Menard HL, Zhitkovich A. Rapid DNA double-strand breaks resulting from processing of Cr-DNA cross-links by both MutS dimers. Cancer Res. 2009;69:1071–1079. doi: 10.1158/0008-5472.CAN-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lopez AM, Whitby MC, Borer CM, Bachler MA, Cox LS. Correction of proliferation and drug sensitivity defects in the progeroid Werner's Syndrome by Holliday junction resolution. Rejuvenation Res. 2007;10:27–40. doi: 10.1089/rej.2006.0503. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Nishikawa K, Heo SJ, Goto M, Furuichi Y, Shimamoto A. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells. 2001;6:421–430. doi: 10.1046/j.1365-2443.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- Sidorova JM. Roles of the Werner syndrome RecQ helicase in DNA replication. DNA Repair (Amst.) 2008;7:1776–1786. doi: 10.1016/j.dnarep.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Li N, Folch A, Monnat RJ., Jr The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle. 2008;7:796–807. doi: 10.4161/cc.7.6.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Shiratori M, Furuichi Y, Matsumoto T. Diverged nuclear localization of Werner helicase in human and mouse cells. Oncogene. 2001;20:2551–2558. doi: 10.1038/sj.onc.1204344. [DOI] [PubMed] [Google Scholar]
- Tamblyn L, Li E, Sarras H, Srikanth P, Hande MP, McPherson JP. A role for Mus81 in the repair of chromium-induced DNA damage. Mutat. Res. 2008;660:57–65. doi: 10.1016/j.mrfmmm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Vilcheck SK, O'Brien TJ, Pritchard DE, Ha L, Ceryak S, Fornsaglio JL, Patierno SR. Fanconi anemia complementation group A cells are hypersensitive to chromium(VI)-induced toxicity. Environ. Health Perspect. 2002;110(Suppl. 5):773–777. doi: 10.1289/ehp.02110s5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- Wu L, Hickson ID. DNA helicases required for homologous recombination and repair of damaged replication forks. Annu. Rev. Genet. 2006;40:279–306. doi: 10.1146/annurev.genet.40.110405.090636. [DOI] [PubMed] [Google Scholar]
- Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise JP., Sr Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat. Res. 2005;586:160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bubley GJ, Detrick B, Blankenship LJ, Patierno SR. Chromium(VI) treatment of normal human lung cells results in guanine-specific DNA polymerase arrest, DNA-DNA cross-links and S-phase blockade of cell cycle. Carcinogenesis. 1996;17:1511–1517. doi: 10.1093/carcin/17.7.1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.