Infection with hepatitis C virus (HCV) is estimated to affect 2% of the world population (90) and is a leading cause of liver-related morbidity and mortality. Human immunodeficiency virus (HIV) is also a very important global public health problem, infecting about 33 million people worldwide (129). As the transmission routes are shared by both infections, coinfection is not uncommon. In this review, we discuss the magnitude of the problem, the effect of infection with one virus on the transmission and natural history of the other, and the treatment issues unique to coinfected patients.
EPIDEMIOLOGY
The reported prevalence of HIV/HCV coinfection varies significantly among studies. Although HIV and HCV are both transmitted through parenteral, sexual, and vertical exposure, they differ in the transmission efficiencies of these routes. Thus, the risk factors of the population under study directly influence the prevalence in that particular population. Parenteral exposure modes such as intravenous drug use (IVDU) or multiple transfusions have been consistently found to be the most important risk factors for coinfection (121). In HIV-positive patients with a history of IVDU, the rate of HCV infection is reported to be 82 to 93% (61, 76, 113). On the other hand, sexual transmission of HCV is relatively inefficient, and the rate of coinfection among HIV-infected patients with a sexual risk factor is less than 10% (61). Men who have sex with men do not seem to have an overall-increased risk for coinfection (21, 61, 121), although epidemics of acute HCV have been described for HIV-infected men who have sex with men with high-risk behaviors (33). The overall burden of coinfection is estimated at 4 to 5 million people worldwide (2).
Apart from the shared routes of transmission, infection with HIV, when present in either HCV-transmitting or HCV-exposed patients, can have a direct effect on the risk of transmission of HCV. HIV-infected patients exposed to HCV are less likely to clear the acute infection (odds ratio, 0.46) (123). This scenario seems to be especially relevant to transmission via IVDU (110). On the other hand, coinfected individuals are more likely to transmit HCV. The rate of vertical transmission of HCV is increased about threefold for coinfected mothers (95) compared to that for HCV-monoinfected ones; this effect may be limited to women with low HCV RNA levels (<106 IU/ml) (69). Percutaneous exposure of health care workers to blood from coinfected patients was also shown to increase the risk of acquiring HCV (35). Although coinfected individuals have been shown to have a higher prevalence of HCV RNA in cervicovaginal secretions (85) and semen (25), sexual transmission of HCV is still rare, even to partners of coinfected patients (68).
EFFECT OF HIV/HCV COINFECTION ON THE NATURAL HISTORY OF HCV
Coinfection with HIV has a significant impact on the life cycle of HCV and on the natural history of HCV infection. A retrospective study of stored sera from multitransfused hemophiliac patients (42) and a similar study of IVDUs (15) demonstrated a significant increase in HCV RNA levels in serum after HIV seroconversion. This increase in HCV RNA levels in coinfected patients was consistently documented in other series as well (108, 124). Similar findings were reported for HCV RNA in the liver (22). This effect could be related to the acquired immunodeficiency (see below) or to a direct interaction between the viruses. In vitro, HIV was shown to increase the replication of HCV or subgenomic replicons in tissue culture. This observed increase was mediated through the interaction of HIV gp120 with CCR5/CXCR4 and is dependent on transforming growth factor β1 (63). Interestingly, following initiation of highly active antiretroviral therapy (HAART), coinfected individuals actually show a paradoxical small increase in serum levels of HCV RNA (23, 32), suggesting that immune suppression and the direct effect of HIV are not the only factors involved. It should be noted that in monoinfected patients, HCV RNA levels are not associated with disease severity, and thus it is unclear whether this biological phenomenon has any clinical significance.
The major impact of HIV/HCV coinfection on the natural history of HCV is the acceleration of liver disease progression. Coinfection is associated with a higher mortality than monoinfection with either virus alone. Prior to the HAART era, the high mortality from AIDS-related causes predominated and masked any other causes for mortality. However, as effective therapy for HIV became available and AIDS-related mortality declined, liver-associated mortality emerged as a prominent cause of death in HIV-infected patients, especially in those with coinfections. This clinical phenomenon was highlighted by the GERMIVIC (Groupe d'Etudes et de Recherche de Médecine Interne et de Maladies Infectieuses sur le Virus de l'Hépatite C) study from France, which surveyed hospital admissions and mortality of HIV patients nationwide over several time periods (26, 101, 102). In the 1995 and 1997 surveys, relative mortality rates from liver disease were low at 1.5% to 6.6% (absolute mortality rate, 0.12% to 0.13%) (26); in the subsequent surveys of 2001 (102), 2003 (101), and 2005 (103), AIDS-related mortality markedly decreased, reflecting the efficacy of HAART. Relative liver-associated mortality, however, increased to 14.3%, 12.6%, and 16.7%, respectively, to become the second-most-frequent cause of death, with virtually all cases of death from liver causes occurring in patients coinfected with HCV. Similar findings were seen in the large, prospective D:A:D (Data Collection on Adverse Events of Anti-HIV Drugs) trial (134), in which liver disease was second only to AIDS as a cause of mortality (14.5% of all deaths) and most liver-related deaths occurred in HCV-coinfected patients. Low CD4+ counts and high levels of HIV were associated with increased liver mortality. These findings were corroborated by other groups as well (6, 19, 104). It is unclear whether this increase in liver mortality is affected by the improvement in immune function following HAART. Some studies suggest that HAART actually decreases liver-related mortality (97) and that increased levels of CD4+ cells after treatment are associated with improved liver survival (93). However, in most HAART-era studies, CD4+ cell count, either on-treatment or at baseline (78), did not affect the liver-associated mortality of coinfected patients. Excessive consumption of alcohol appears to be a cofactor that increases mortality from liver disease in coinfected patients (101, 104).
Most cases of liver-associated mortality in coinfected patients are due to end-stage liver disease or hepatocellular carcinoma (105). Thus, the increased mortality of coinfected patients over that of HCV-monoinfected ones reflects accelerated progression of chronic liver disease. Several studies (for examples, see references 37, 70, and 94) demonstrated that in HCV/HIV-coinfected patients, the degree of inflammation in the liver and the rate of fibrosis progression are higher than those in HCV-monoinfected patients. Moreover, liver biopsies have shown that coinfected patients with recent-onset or acute hepatitis C can already have significant fibrosis (45). Recently, a meta-analysis (122) of 27 studies with >7,500 patients clearly demonstrated that coinfected patients are more likely to have cirrhosis, with a relative risk of 2.1 for coinfected patients compared to that for monoinfected ones. The relative risk for cirrhosis was lower for patients treated with HAART (1.72 versus 2.49), but the difference was not statistically significant. This lack of difference may be related to the specific HAART regimen. Treatment of HIV with nonnucleoside reverse transcriptase inhibitors, especially nevirapine, but not protease inhibitors, was associated with decreased progression of hepatic fibrosis (17). In the meta-analysis, the incidences of cirrhosis in coinfected patients after 20 and 30 years was estimated at 21% and 49%, respectively. Compared directly to HCV-monoinfected patients, coinfected patients seemed to develop cirrhosis 12 years earlier (16). It is unclear whether the CD4+ count is associated with liver disease progression. Various studies have reached different conclusions, while a meta-analysis found no significant association between CD4+ counts and the presence of cirrhosis or rates of fibrosis progression (122).
EFFECT OF HCV COINFECTION ON HIV-ASSOCIATED DISEASE
HCV does not seem to have a major impact on the natural history of HIV infection. In studies before the HAART era, HCV coinfection had no effect on HIV progression (40). In the HAART era, Greub et al. (48) first reported that in a cohort of 3,111 Swiss patients starting HAART, HCV seropositivity was associated with a higher risk of death or developing an AIDS-related illness. However, this finding may have been confounded by other factors, as two large American series encompassing more than 12,000 patients (118, 119) failed to confirm such an effect. It is unclear whether HIV/HCV coinfection is associated with impaired CD4+ cell recovery following HAART initiation. Individual studies reported conflicting results, while a meta-analysis (72) found only a modest effect: the increase in CD4+ cells was 33 cells/mm3 less in coinfected patients. Small studies suggested a unique genotype effect: genotype 3 seemed to be associated with HIV progression (based on a comparison of late progressors to nonprogressors) (80) and with slower recovery of CD4+ cells (3).
MECHANISMS OF HIV-HCV INTERACTION
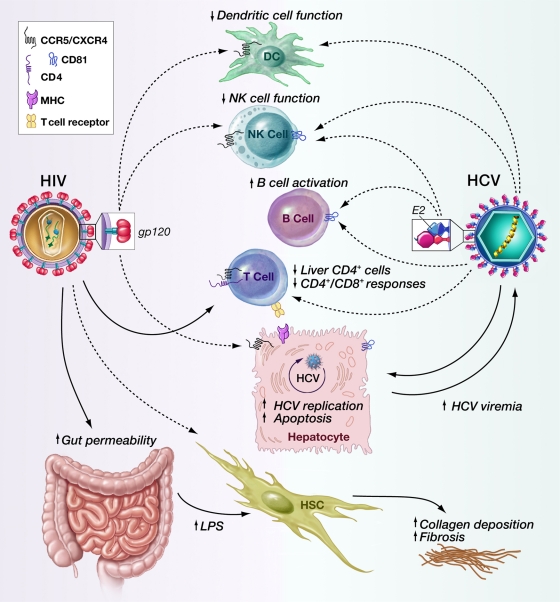
What could be the mechanism of increased HCV replication and accelerated fibrosis leading to liver mortality in HIV/HCV-coinfected patients? Potential explanations are the generalized immune suppression resulting from the loss of CD4+ T cells, an intrahepatic interaction between the viruses or their gene products (on hepatocytes or other hepatic cells), and an indirect effect on the liver secondary to HIV infection of other organs (Fig. 1).
FIG. 1.
Mechanism of HCV-HIV interaction. HIV does not infect hepatocytes but can influence the outcome of HCV infection through infection of CD4+ T cells and the effects of gp120 protein on hepatocytes and other immune cells via its interaction with chemokine receptors. HIV infection of gut-associated lymphoid tissue leads to increased LPS uptake, resulting in HSC activation and increased fibrosis. Possible HIV infection of HSCs can also lead to HSC activation. The effects of HCV on immune cells may affect the outcome of HIV infection. DC, dendritic cell; MHC, major histocompatibility complex. Dashed arrows represent the effect of viral envelope proteins or possible infection; solid arrows represent productive infection.
HIV-induced immune suppression may be a major factor. T-cell responses against HCV play an essential role in preventing progression from acute infection to chronicity (98). In HIV-infected patients that develop acute hepatitis C, HCV-specific T-cell responses are markedly diminished (34), a finding consistent with a higher rate of progression to chronicity in these patients (123). In chronic hepatitis C, T-cell responses are generally weak, coinfected patients appear to have even weaker CD4+ and CD8+ responses (29, 50), and these responses are not restored, even after CD4+ cell counts recover in response to HAART (41). Furthermore, there is evidence of decreased genetic diversity of HCV in HIV/HCV coinfection compared to that in monoinfection suggestive of reduced immune selective pressure (65, 111), though this finding is controversial (83, 107, 120). Following initiation of HAART, the genetic diversity of HCV was shown to increase (111), at least in some genomic regions (20). This increase in diversity seems to reflect an increased selective pressure, requires time to develop (7), and is seen mostly in patients with virological and CD4+ T-cell responses to HAART (112, 133).
Differences in cytokine expression and a relative decrease in the number of CD4+ T cells in the livers of HIV/HCV-coinfected patients (27) may also play a role. The importance of T-cell depletion in promoting accelerated progression of hepatitis C is not unique to HIV coinfection. Experimental depletion of CD4+ T cells in chimpanzees was associated with persistence of viremia after reinfection with HCV (47), similar to what has been observed with coinfected patients (56). In a different setting of immune suppression, recurrence of chronic hepatitis C after liver transplantation (for monoinfection) is accelerated compared to that in patients who have not received transplants. A significant risk factor is the use of steroid boluses (18) or lymphocyte-depleting agents (100) to treat acute rejections. Thus, the loss of CD4+ T cells is likely to play a major role in disease progression.
Nonetheless, these immune-related mechanisms do not fully explain the faster progression of liver fibrosis. The liver injury in coinfection could also occur independently of the immune suppression as a result of the combined effect of the two viruses on hepatocytes. Whether HIV can directly infect hepatocytes is unclear. Human hepatocytes express CXCR4 and CCR5 but do not express CD4, and thus infection is not likely. Xiao et al. (136) isolated a CD4-independent HIV strain from a patient and demonstrated in vitro infection of hepatoma cell lines and primary hepatocytes through CXCR4 and replication in them, possibly explaining early, uncorroborated reports of the detection of low levels of HIV in hepatocytes (28). Recently, Ma et al. (66) reported that a human hepatoma cell line, Huh-7.5, constitutively infected with the JFH-1 strain of HCV, expresses CD4 and can be infected by CXCR4- and CCR5-tropic HIV strains. Interestingly, HCV core antigen levels were increased in coinfected cells, consistent with the finding in vivo of a higher level of viremia. Despite these two interesting studies, convincing evidence of HIV/HCV coinfection of hepatocytes in vivo or even HIV monoinfection of hepatocytes remains to be proven and may not play a major role in the pathogenesis of disease progression. Even in the absence of hepatocyte coinfection, these cells are exposed to the effects of circulating viral proteins. The HIV envelope protein gp120 induces apoptosis of hepatocytes through CXCR4 G-protein-mediated signaling (131), and as mentioned previously (63), can also induce the expression of transforming growth factor β1, which is known to be profibrotic. Furthermore, in vitro exposure of hepatocytes to HCV E2 protein concomitantly with gp120 protein was shown to induce apoptosis via STAT1 phosphorylation and Fas ligand upregulation in a CXCR4-independent manner (9, 81).
HIV infection of liver cells other than hepatocytes may also play a role in the progression of disease in coinfected patients. Like other macrophages, Kupffer cells can be infected with HIV (53, 54), although monoinfection with HIV is not associated with significant liver pathology (62). In HIV/HCV coinfection, on the other hand, it has been postulated that the HIV-infected Kupffer cells shift to a Th2 cytokine response, in turn influencing the hepatic stellate cells (HSCs), the major mediators of collagen deposition and fibrogenesis in the liver (4). Modulation of the antigen-presenting function of the Kupffer cells by HIV in HCV coinfection may also play a role in the progression of liver damage. Hepatic sinusoidal endothelial cells express CD4 and can be infected in vitro with HIV (116). Finally, HSCs themselves may be a target of HIV. In a preliminary study, Tuyama et al. (128) demonstrated the ability of HIV to infect and replicate in HSCs in vitro. HIV infection of the cells, or even exposure to gp120, led to an induction in collagen synthesis. Whether this finding is relevant to the progression of fibrosis in coinfected patients is difficult to determine. The current limitations of robust tissue culture models for HCV and the distinct cell-specific and species-specific tropism of the two viruses limit the ability to study their interaction.
One other potential explanation for disease progression may involve the gut-liver axis. During primary HIV infection, there is a significant depletion of CD4+ T cells from gut-associated lymphoid tissue, a depletion that persists into chronic infection (49) and is associated with increased gut permeability and microbial translocation (reflected by increased lipopolysaccharide [LPS] levels), causing a systemic immune activation (24). Gut permeability and LPS-induced Kupffer cell activation are associated with liver injury in several conditions, including alcoholic liver disease, celiac sprue, gut graft-versus-host disease, and inflammatory bowel disease. Upon repeated exposure to LPS (as well as to other ligands of Toll-like receptors), monocytes and macrophages develop tolerance that limits their immune activation. In chronic hepatitis C, this tolerance to LPS is lost in peripheral monocytes and possibly in Kupffer cells due to the combined effects of gamma interferon, endotoxin, and HCV core protein (39). This finding may explain the correlation between progression of liver disease and increased LPS levels (8) that has been reported for patients with HIV/HCV coinfection.
Several abnormalities of the lymphoid system in patients with chronic hepatitis C have been described. Many HCV-infected patients exhibit evidence of polyclonal proliferation of B cells with autoantibody production, which can lead to the clinical syndromes of HCV-associated autoimmune disorders, mixed cryoglobulinemia, and non-Hodgkin's lymphoma (44). The mechanism of B-cell activation by HCV is not entirely clear; the interaction of HCV E2 glycoprotein with CD81 has been shown to lead to nonspecific B-cell activation (99). Furthermore, HCV has been shown to infect and replicate in B cells, T cells, and monocytes, though the evidence is not compelling and the clinical significance of this finding is unclear (91). Dendritic cell dysfunctions have been reported in HCV-infected patients, but this finding is controversial (1, 38, 75). HCV has also been implicated in inhibiting NK cell function by an interaction of E2 and CD81 (127). In addition to affecting the functions of T cells, HIV alters the functions and phenotypes of dendritic (92) and NK cells (43), both of which play important roles in innate and adaptive immunity and likely contribute to the diminished HCV-specific immune response in coinfected individuals. The interplay between HCV and the lymphoid system could theoretically affect, or be affected by, coinfection with HIV. More research is needed to clarify this issue.
Potential mechanisms of the effects of coinfection may be learned from patients coinfected with HIV and GB virus C (GBV-C), a flavivirus closely related to HCV (reviewed in reference 55). In studies before the HAART era, GBV-C coinfection was reported to be associated with lower levels of HIV and better survival rates, although this finding was not shared by all studies. Similarly, some, but not all, studies demonstrate better response to HAART in coinfected patients. One putative mechanism is the binding of the GBV-C envelope protein to CD81 (an important coreceptor for HCV infection) on lymphocytes, resulting in downregulation of CCR5 (82) and decreased HIV replication (135).
TREATMENT OF HCV IN COINFECTED PATIENTS
The decreased mortality of HIV-infected patients from AIDS following the widespread use of HAART emerged concomitantly with the availability of improved treatment for chronic hepatitis C. These major advances, along with the evidence for increased liver mortality in HCV/HIV-coinfected patients, prompted initiation of treatment trials for these patients.
The current treatment for chronic hepatitis C in monoinfected patients is based on the administration of pegylated interferon and ribavirin for 24 to 48 weeks, depending on the genotype, with a sustained virological response (SVR) rate of approximately 50% (46, 67). Three large randomized controlled trials, the APRICOT (AIDS Pegasys Ribavirin International Coinfection Trial) (125), RIBAVIC (30), and ACTG (AIDS Clinical Trial Group) A5071 (31) trials, compared the use of peginterferon with ribavirin to standard interferon with ribavirin in coinfected patients. All three trials demonstrated the feasibility of treating hepatitis C in HIV/HCV-coinfected individuals and the superiority of peginterferon- to standard interferon-based regimens. SVR rates were 14 to 29% in patients with HCV genotype 1 and 44 to 73% in patients with genotype 2 or 3. These rates are generally inferior to published SVR rates in monoinfected patients, but the dose of ribavirin that was used in these three trials was lower than that commonly prescribed for monoinfection. In a study where the full doses were prescribed, response rates still seemed to be lower (60).
The kinetics of serum HCV level changes during interferon-based treatment have been extensively studied and modeled. The parameters derived from the mathematical model are thought to reflect the effectiveness of interferon, the rate of elimination of infected cells, and the rate of clearance of free virions (84). In coinfected patients, the first-phase decline (representing effectiveness) and the second-phase slope (loss of infected cells) were similar to those of monoinfected patients (109), but clearance of free virions was slower. Coinfected patients became HCV RNA negative later during treatment, mainly due to higher baseline levels of the virus. The dynamics of virological response have been used to guide the duration of treatment for monoinfected patients (51). Similarly, studies of coinfected patients showed that an early virological response (HCV RNA negative or a ≥2 log decrease from baseline at week 12) predicts a SVR (89, 106). As with monoinfection, a SVR is associated with nonprogression (14) of, or even improvement (64) in, liver histology, and over long-term follow-up, significantly reduces the occurrence of hepatic decompensation or hepatocellular carcinoma (114). Furthermore, treatment of HCV in coinfected patients was shown to be cost effective (52).
Although antiviral therapy for HCV is effective in coinfected patients, it is also associated with an increased risk for complications. The interaction of ribavirin, a purine analogue, with other nucleoside reverse transcriptase inhibitors can lead to the development of symptomatic mitochondrial toxicity (59) and mortality. This syndrome is seen mostly in patients treated with didanosine (ddI) (10) and can resolve when ddI is discontinued. Hepatic decompensation is another potential complication of interferon and ribavirin treatment in coinfected patients. Although relatively rare (1.5 to 2%), it is associated with a high rate of mortality, to which preexisting cirrhosis, hyperbilirubinemia, and the use of ddI are contributing risk factors (11, 71). In fact, in a series of patients on ddI treated with interferon and ribavirin, 57% developed adverse reactions and 10% died (79). Thus, appropriate selection of treatment candidates is important. In a trial which excluded cirrhotic patients and did not allow the use of ddI, the rate of mitochondrial toxicity was less than 1% and no hepatic decompensation occurred (115). Interestingly, ribavirin appears to have a synergistic effect with ddI on the inhibition of HIV replication in vitro, perhaps through the inhibition of IMP dehydrogenase, which increases ddI phosphorylation (57). Ribavirin can interact with other antiretrovirals as well. In vitro, ribavirin antagonizes the effect of zidovudine (AZT) on HIV replication (132), while AZT use in patients receiving peginterferon and ribavirin is associated with a higher rate of anemia (13, 74, 87). When abacavir is used as the nucleoside reverse transcriptase inhibitor backbone of antiretroviral therapy, response rates to HCV treatment with peginterferon and ribavirin seem to be lower, though the mechanism is unknown (12, 73, 130).
HAART HEPATOTOXICITY
Reports of hepatotoxicity emerged soon after the advent of HAART. In a large number of series (for examples, see references 36 and 77), coinfection with HCV was persistently shown to be associated with an increased risk of HAART hepatotoxicity. Studies are heterogenous with respect to the relative prevalences of coinfected patients, HAART regimens, and definitions of hepatotoxicity. However, it is clear that most cases of enzyme elevation are not associated with clinical symptoms, resolve when treatment is modified, and may even improve when HAART is continued unchanged (36). However, serious complications can occur; in a large prospective study of 755 Italian patients (96), severe toxicity (defined as an alanine aminotransferase level greater than 10 times the upper limit of the norm or 5 times the baseline level if markedly abnormal) was seen in 26 patients (4.2 per 100 person years), all of whom had HCV infections. Seven of these 26 patients developed liver failure and died as a consequence.
The mechanisms of HAART hepatotoxicity (reviewed in reference 86) do not seem to differ between HIV monoinfection and HIV/HCV coinfection apart from the potential for the development of liver damage as a result of immune reconstitution and the worsening of immune-mediated injury to HCV-infected hepatocytes (117). Risk factors for HAART hepatotoxicity in coinfected patients include the preexisting degree of liver fibrosis (5) and infection with HCV genotype 3 (88, 126). No specific medication combination has been shown to be consistently associated with liver injury in coinfected patients, and thus, selection of HAART therapy should be based on other factors. Successful eradication of HCV infection by peginterferon and ribavirin and attainment of SVR are associated with a reduction in the risk of HAART-induced hepatotoxicity (58).
CONCLUSIONS
Coinfection with HIV and HCV is common and has a deleterious effect on the natural history of chronic hepatitis C. As control of HIV improved with HAART, liver disease gained notoriety as a major cause of mortality in coinfected patients. These patients show accelerated liver disease and are more likely to develop liver enzyme abnormalities and clinical liver toxicity when treated with HAART. Treatment of hepatitis C with peginterferon is thus indicated in most patients and has been shown to be relatively safe and effective. Treatment should not be prescribed for patients on ddI. Use of AZT should be minimized if possible. Patients with advanced cirrhosis should be treated with caution, preferably in a transplant center. Future research may enhance understanding of the interaction between the two viral infections and improve treatment options for coinfected individuals.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Albert, M. L., J. Decalf, and S. Pol. 2008. Plasmacytoid dendritic cells move down on the list of suspects: in search of the immune pathogenesis of chronic hepatitis C. J. Hepatol. 491069-1078. [DOI] [PubMed] [Google Scholar]
- 2.Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44S6-9. [DOI] [PubMed] [Google Scholar]
- 3.Antonucci, G., E. Girardi, A. Cozzi-Lepri, M. R. Capobianchi, A. De Luca, M. Puoti, E. Petrelli, G. Carnevale, G. Rizzardini, P. A. Grossi, P. Vigano, M. C. Moioli, F. Carletti, M. Solmone, G. Ippolito, and A. D. Monforte. 2005. Role of hepatitis C virus (HCV) viremia and HCV genotype in the immune recovery from highly active antiretroviral therapy in a cohort of antiretroviral-naive HIV-infected individuals. Clin. Infect. Dis. 40e101-e109. [DOI] [PubMed] [Google Scholar]
- 4.Antonucci, G., D. Goletti, S. Lanini, E. Girardi, and O. Loiacono. 2003. HIV/HCV co-infection: putting the pieces of the puzzle together. Cell Death Differ. 10S25-S26. [DOI] [PubMed] [Google Scholar]
- 5.Aranzabal, L., J. L. Casado, J. Moya, C. Quereda, S. Diz, A. Moreno, L. Moreno, A. Antela, M. J. Perez-Elias, F. Dronda, A. Marin, F. Hernandez-Ranz, A. Moreno, and S. Moreno. 2005. Influence of liver fibrosis on highly active antiretroviral therapy-associated hepatotoxicity in patients with HIV and hepatitis C virus coinfection. Clin. Infect. Dis. 40588-593. [DOI] [PubMed] [Google Scholar]
- 6.Arnold, D. M., J. A. Julian, and I. R. Walker. 2006. Mortality rates and causes of death among all HIV-positive individuals with hemophilia in Canada over 21 years of follow-up. Blood 108460-464. [DOI] [PubMed] [Google Scholar]
- 7.Babik, J. M., and M. Holodniy. 2003. Impact of highly active antiretroviral therapy and immunologic status on hepatitis C virus quasispecies diversity in human immunodeficiency virus/hepatitis C virus-coinfected patients. J. Virol. 771940-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balagopal, A., F. H. Philp, J. Astemborski, T. M. Block, A. Mehta, R. Long, G. D. Kirk, S. H. Mehta, A. L. Cox, D. L. Thomas, and S. C. Ray. 2008. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 135226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasubramanian, A., R. K. Ganju, and J. E. Groopman. 2006. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J. Infect. Dis. 194670-681. [DOI] [PubMed] [Google Scholar]
- 10.Bani-Sadr, F., F. Carrat, S. Pol, R. Hor, E. Rosenthal, C. Goujard, P. Morand, F. Lunel-Fabiani, D. Salmon-Ceron, L. Piroth, G. Pialoux, M. Bentata, P. Cacoub, and C. Perronne. 2005. Risk factors for symptomatic mitochondrial toxicity in HIV/hepatitis C virus-coinfected patients during interferon plus ribavirin-based therapy. J. Acquir. Immune Defic. Syndr. 4047-52. [DOI] [PubMed] [Google Scholar]
- 11.Bani-Sadr, F., F. Carrat, E. Rosenthal, L. Piroth, P. Morand, F. Lunel-Fabiani, M. Bonarek, N. Colin de Verdiere, G. Pialoux, P. Cacoub, S. Pol, and C. Perronne. 2005. Spontaneous hepatic decompensation in patients coinfected with HIV and hepatitis C virus during interferon-ribavirin combination treatment. Clin. Infect. Dis. 411806-1809. [DOI] [PubMed] [Google Scholar]
- 12.Bani-Sadr, F., L. Denoeud, P. Morand, F. Lunel-Fabiani, S. Pol, P. Cacoub, C. Perronne, and F. Carrat. 2007. Early virologic failure in HIV-coinfected hepatitis C patients treated with the peginterferon-ribavirin combination: does abacavir play a role? J. Acquir. Immune Defic. Syndr. 45123-125. [DOI] [PubMed] [Google Scholar]
- 13.Bani-Sadr, F., I. Goderel, C. Penalba, E. Billaud, J. Doll, Y. Welker, P. Cacoub, S. Pol, C. Perronne, and F. Carrat. 2007. Risk factors for anaemia in human immunodeficiency virus/hepatitis C virus-coinfected patients treated with interferon plus ribavirin. J. Viral Hepat. 14639-644. [DOI] [PubMed] [Google Scholar]
- 14.Barreiro, P., P. Labarga, L. Martin-Carbonero, A. Amor, A. Ruiz-Sancho, C. Castellares, J. Gonzalez-Lahoz, and V. Soriano. 2006. Sustained virological response following HCV therapy is associated with non-progression of liver fibrosis in HCV/HIV-coinfected patients. Antivir. Ther. 11869-877. [DOI] [PubMed] [Google Scholar]
- 15.Beld, M., M. Penning, V. Lukashov, M. McMorrow, M. Roos, N. Pakker, A. van den Hoek, and J. Goudsmit. 1998. Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology 244504-512. [DOI] [PubMed] [Google Scholar]
- 16.Benhamou, Y., M. Bochet, V. Di Martino, F. Charlotte, F. Azria, A. Coutellier, M. Vidaud, F. Bricaire, P. Opolon, C. Katlama, and T. Poynard. 1999. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology 301054-1058. [DOI] [PubMed] [Google Scholar]
- 17.Berenguer, J., J. M. Bellon, P. Miralles, E. Alvarez, I. Castillo, J. Cosin, J. C. Lopez, M. Sanchez Conde, B. Padilla, and S. Resino. 2008. Association between exposure to nevirapine and reduced liver fibrosis progression in patients with HIV and hepatitis C virus coinfection. Clin. Infect. Dis. 46137-143. [DOI] [PubMed] [Google Scholar]
- 18.Berenguer, M., V. Aguilera, M. Prieto, F. San Juan, J. M. Rayon, S. Benlloch, and J. Berenguer. 2006. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J. Hepatol. 44717-722. [DOI] [PubMed] [Google Scholar]
- 19.Bica, I., B. McGovern, R. Dhar, D. Stone, K. McGowan, R. Scheib, and D. R. Snydman. 2001. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin. Infect. Dis. 32492-497. [DOI] [PubMed] [Google Scholar]
- 20.Blackard, J. T., Y. Yang, P. Bordoni, K. E. Sherman, and R. T. Chung. 2004. Hepatitis C virus (HCV) diversity in HIV-HCV-coinfected subjects initiating highly active antiretroviral therapy. J. Infect. Dis. 1891472-1481. [DOI] [PubMed] [Google Scholar]
- 21.Bollepalli, S., K. Mathieson, C. Bay, A. Hillier, J. Post, D. H. Van Thiel, and A. Nadir. 2007. Prevalence of risk factors for hepatitis C virus in HIV-infected and HIV/hepatitis C virus-coinfected patients. Sex. Transm. Dis. 34367-370. [DOI] [PubMed] [Google Scholar]
- 22.Bonacini, M., S. Govindarajan, L. M. Blatt, P. Schmid, A. Conrad, and K. L. Lindsay. 1999. Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J. Viral Hepat. 6203-208. [DOI] [PubMed] [Google Scholar]
- 23.Bower, W. A., D. H. Culver, D. Castor, Y. Wu, V. N. James, H. Zheng, S. Hammer, W. L. Kuhnert, I. T. Williams, B. P. Bell, D. Vlahov, and C. S. Dezzutti. 2006. Changes in hepatitis C virus (HCV) viral load and interferon-alpha levels in HIV/HCV-coinfected patients treated with highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 42293-297. [DOI] [PubMed] [Google Scholar]
- 24.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 25.Briat, A., E. Dulioust, J. Galimand, H. Fontaine, M. L. Chaix, H. Letur-Konirsch, S. Pol, P. Jouannet, C. Rouzioux, and M. Leruez-Ville. 2005. Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS 191827-1835. [DOI] [PubMed] [Google Scholar]
- 26.Cacoub, P., L. Geffray, E. Rosenthal, C. Perronne, P. Veyssier, and G. Raguin. 2001. Mortality among human immunodeficiency virus-infected patients with cirrhosis or hepatocellular carcinoma due to hepatitis C virus in French Departments of Internal Medicine/Infectious Diseases, in 1995 and 1997. Clin. Infect. Dis. 321207-1214. [DOI] [PubMed] [Google Scholar]
- 27.Canchis, P. W., H. T. Yee, M. I. Fiel, D. T. Dieterich, R. C. Liu, L. Chiriboga, I. M. Jacobson, B. R. Edlin, and A. H. Talal. 2004. Intrahepatic CD4+ cell depletion in hepatitis C virus/HIV-coinfected patients. J. Acquir. Immune Defic. Syndr. 371125-1131. [DOI] [PubMed] [Google Scholar]
- 28.Cao, Y. Z., D. Dieterich, P. A. Thomas, Y. X. Huang, M. Mirabile, and D. D. Ho. 1992. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 665-70. [DOI] [PubMed] [Google Scholar]
- 29.Capa, L., V. Soriano, J. Garcia-Samaniego, M. Nunez, M. Romero, A. Cascajero, F. Munoz, J. Gonzalez-Lahoz, and J. M. Benito. 2007. Influence of HCV genotype and co-infection with human immunodeficiency virus on CD4+ and CD8+ T-cell responses to hepatitis C virus. J. Med. Virol. 79503-510. [DOI] [PubMed] [Google Scholar]
- 30.Carrat, F., F. Bani-Sadr, S. Pol, E. Rosenthal, F. Lunel-Fabiani, A. Benzekri, P. Morand, C. Goujard, G. Pialoux, L. Piroth, D. Salmon-Ceron, C. Degott, P. Cacoub, and C. Perronne. 2004. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 2922839-2848. [DOI] [PubMed] [Google Scholar]
- 31.Chung, R. T., J. Andersen, P. Volberding, G. K. Robbins, T. Liu, K. E. Sherman, M. G. Peters, M. J. Koziel, A. K. Bhan, B. Alston, D. Colquhoun, T. Nevin, G. Harb, and C. van der Horst. 2004. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N. Engl. J. Med. 351451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung, R. T., S. R. Evans, Y. Yang, D. Theodore, H. Valdez, R. Clark, C. Shikuma, T. Nevin, and K. E. Sherman. 2002. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS 161915-1923. [DOI] [PubMed] [Google Scholar]
- 33.Danta, M., D. Brown, S. Bhagani, O. G. Pybus, C. A. Sabin, M. Nelson, M. Fisher, A. M. Johnson, and G. M. Dusheiko. 2007. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 21983-991. [DOI] [PubMed] [Google Scholar]
- 34.Danta, M., N. Semmo, P. Fabris, D. Brown, O. G. Pybus, C. A. Sabin, S. Bhagani, V. C. Emery, G. M. Dusheiko, and P. Klenerman. 2008. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J. Infect. Dis. 1971558-1566. [DOI] [PubMed] [Google Scholar]
- 35.De Carli, G., V. Puro, and G. Ippolito. 2003. Risk of hepatitis C virus transmission following percutaneous exposure in healthcare workers. Infection 31(Suppl. 2)22-27. [PubMed] [Google Scholar]
- 36.den Brinker, M., F. W. Wit, P. M. Wertheim-van Dillen, S. Jurriaans, J. Weel, R. van Leeuwen, N. G. Pakker, P. Reiss, S. A. Danner, G. J. Weverling, and J. M. Lange. 2000. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS 142895-2902. [DOI] [PubMed] [Google Scholar]
- 37.Di Martino, V., P. Rufat, N. Boyer, P. Renard, F. Degos, M. Martinot-Peignoux, S. Matheron, V. Le Moing, F. Vachon, C. Degott, D. Valla, and P. Marcellin. 2001. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology 341193-1199. [DOI] [PubMed] [Google Scholar]
- 38.Dolganiuc, A., S. Chang, K. Kodys, P. Mandrekar, G. Bakis, M. Cormier, and G. Szabo. 2006. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-α and plasmacytoid dendritic cell loss in chronic HCV infection. J. Immunol. 1776758-6768. [DOI] [PubMed] [Google Scholar]
- 39.Dolganiuc, A., O. Norkina, K. Kodys, D. Catalano, G. Bakis, C. Marshall, P. Mandrekar, and G. Szabo. 2007. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology 1331627-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorrucci, M., P. Pezzotti, A. N. Phillips, A. C. Lepri, and G. Rezza. 1995. Coinfection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. J. Infect. Dis. 1721503-1508. [DOI] [PubMed] [Google Scholar]
- 41.Dutoit, V., D. Ciuffreda, D. Comte, J. J. Gonvers, and G. Pantaleo. 2005. Differences in HCV-specific T cell responses between chronic HCV infection and HIV/HCV co-infection. Eur. J. Immunol. 353493-3504. [DOI] [PubMed] [Google Scholar]
- 42.Eyster, M. E., M. W. Fried, A. M. Di Bisceglie, and J. J. Goedert. 1994. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Blood 841020-1023. [PubMed] [Google Scholar]
- 43.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5835-843. [DOI] [PubMed] [Google Scholar]
- 44.Ferri, C., A. Antonelli, M. T. Mascia, M. Sebastiani, P. Fallahi, D. Ferrari, S. A. Pileri, and A. L. Zignego. 2007. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig. Liver Dis. 39(Suppl. 1)S13-S21. [DOI] [PubMed] [Google Scholar]
- 45.Fierer, D. S., A. J. Uriel, D. C. Carriero, A. Klepper, D. T. Dieterich, M. P. Mullen, S. N. Thung, M. I. Fiel, and A. D. Branch. 2008. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J. Infect. Dis. 198683-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347975-982. [DOI] [PubMed] [Google Scholar]
- 47.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302659-662. [DOI] [PubMed] [Google Scholar]
- 48.Greub, G., B. Ledergerber, M. Battegay, P. Grob, L. Perrin, H. Furrer, P. Burgisser, P. Erb, K. Boggian, J. C. Piffaretti, B. Hirschel, P. Janin, P. Francioli, M. Flepp, and A. Telenti. 2000. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 3561800-1805. [DOI] [PubMed] [Google Scholar]
- 49.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harcourt, G., E. Gomperts, S. Donfield, and P. Klenerman. 2006. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut 551484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoofnagle, J. H., and L. B. Seeff. 2006. Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med. 3552444-2451. [DOI] [PubMed] [Google Scholar]
- 52.Hornberger, J., F. J. Torriani, D. T. Dieterich, N. Brau, M. S. Sulkowski, M. R. Torres, J. Green, and K. Patel. 2006. Cost-effectiveness of peginterferon alfa-2a (40kDa) plus ribavirin in patients with HIV and hepatitis C virus co-infection. J. Clin. Virol. 36283-291. [DOI] [PubMed] [Google Scholar]
- 53.Housset, C., E. Lamas, V. Courgnaud, O. Boucher, P. M. Girard, C. Marche, and C. Brechot. 1993. Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J. Hepatol. 19252-258. [DOI] [PubMed] [Google Scholar]
- 54.Hufert, F. T., J. Schmitz, M. Schreiber, H. Schmitz, P. Racz, and D. D. von Laer. 1993. Human Kupffer cells infected with HIV-1 in vivo. J. Acquir. Immune Defic. Syndr. 6772-777. [PubMed] [Google Scholar]
- 55.Kaiser, T., and H. L. Tillmann. 2005. GB virus C infection: is there a clinical relevance for patients infected with the human immunodeficiency virus? AIDS Rev. 73-12. [PubMed] [Google Scholar]
- 56.Kim, A. Y., J. Schulze zur Wiesch, T. Kuntzen, J. Timm, D. E. Kaufmann, J. E. Duncan, A. M. Jones, A. G. Wurcel, B. T. Davis, R. T. Gandhi, G. K. Robbins, T. M. Allen, R. T. Chung, G. M. Lauer, and B. D. Walker. 2006. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 3e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein, M. B., N. Campeol, R. G. Lalonde, B. Brenner, and M. A. Wainberg. 2003. Didanosine, interferon-alfa and ribavirin: a highly synergistic combination with potential activity against HIV-1 and hepatitis C virus. AIDS 171001-1008. [DOI] [PubMed] [Google Scholar]
- 58.Labarga, P., V. Soriano, M. E. Vispo, J. Pinilla, L. Martin-Carbonero, C. Castellares, R. Casado, I. Maida, P. Garcia-Gasco, and P. Barreiro. 2007. Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J. Infect. Dis. 196670-676. [DOI] [PubMed] [Google Scholar]
- 59.Lafeuillade, A., G. Hittinger, and S. Chadapaud. 2001. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 357280-281. [DOI] [PubMed] [Google Scholar]
- 60.Laguno, M., J. Murillas, J. L. Blanco, E. Martinez, R. Miquel, J. M. Sanchez-Tapias, X. Bargallo, A. Garcia-Criado, E. de Lazzari, M. Larrousse, A. Leon, M. Lonca, A. Milinkovic, J. M. Gatell, and J. Mallolas. 2004. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS 18F27-F36. [DOI] [PubMed] [Google Scholar]
- 61.Larsen, C., G. Pialoux, D. Salmon, D. Antona, Y. Le Strat, L. Piroth, S. Pol, E. Rosenthal, D. Neau, C. Semaille, and E. Delarocque Astagneau. 29 May 2008, posting date. Prevalence of hepatitis C and hepatitis B infection in the HIV-infected population of France, 2004. Euro. Surveill. 13:pii=18888. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18888. [PubMed]
- 62.Lefkowitch, J. H. 1997. The liver in AIDS. Semin. Liver Dis. 17335-344. [DOI] [PubMed] [Google Scholar]
- 63.Lin, W., E. M. Weinberg, A. W. Tai, L. F. Peng, M. A. Brockman, K. A. Kim, S. S. Kim, C. B. Borges, R. X. Shao, and R. T. Chung. 2008. HIV increases HCV replication in a TGF-β1-dependent manner. Gastroenterology 134803-811. [DOI] [PubMed] [Google Scholar]
- 64.Lissen, E., N. Clumeck, R. Sola, M. Mendes-Correa, J. Montaner, M. Nelson, J. DePamphilis, M. Pessoa, P. Buggisch, J. Main, and D. Dieterich. 2006. Histological response to pegIFNalpha-2a (40KD) plus ribavirin in HIV-hepatitis C virus co-infection. AIDS 202175-2181. [DOI] [PubMed] [Google Scholar]
- 65.López-Labrador, F. X., L. Dove, C. K. Hui, Y. Phung, M. Kim, M. Berenguer, and T. L. Wright. 2007. Trends for genetic variation of hepatitis C virus quasispecies in human immunodeficiency virus-1 coinfected patients. Virus Res. 130285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma, G., M. Moreno, W. Cardona Maya, P. Presicce, K. E. Sherman, C. Chougnet, and J. T. Blackard. 2009. HIV infection of an HCV-producing hepatocyte cell line—a model system for exploring HIV-HCV coinfection in vitro, abstr. OP-171, p. 34. 13th Int. Symp. Viral Hepat. Liver Dis., Washington, DC.
- 67.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358958-965. [DOI] [PubMed] [Google Scholar]
- 68.Marincovich, B., J. Castilla, J. del Romero, S. Garcia, V. Hernando, M. Raposo, and C. Rodriguez. 2003. Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex. Transm. Infect. 79160-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mariné-Barjoan, E., A. Berrebi, V. Giordanengo, S. F. Favre, H. Haas, M. Moreigne, J. Izopet, J. Tricoire, A. Tran, C. Pradier, and A. Bongain. 2007. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS 211811-1815. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Sierra, C., A. Arizcorreta, F. Diaz, R. Roldan, L. Martin-Herrera, E. Perez-Guzman, and J. A. Giron-Gonzalez. 2003. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin. Infect. Dis. 36491-498. [DOI] [PubMed] [Google Scholar]
- 71.Mauss, S., W. Valenti, J. DePamphilis, F. Duff, L. Cupelli, S. Passe, J. Solsky, F. J. Torriani, D. Dieterich, and D. Larrey. 2004. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS 18F21-F25. [DOI] [PubMed] [Google Scholar]
- 72.Miller, M. F., C. Haley, M. J. Koziel, and C. F. Rowley. 2005. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin. Infect. Dis. 41713-720. [DOI] [PubMed] [Google Scholar]
- 73.Mira, J. A., L. F. Lopez-Cortes, P. Barreiro, C. Tural, M. Torres-Tortosa, I. de Los Santos Gil, P. Martin-Rico, M. J. Rios-Villegas, J. J. Hernandez-Burruezo, D. Merino, M. A. Lopez-Ruz, A. Rivero, L. Munoz, M. Gonzalez-Serrano, A. Collado, J. Macias, P. Viciana, V. Soriano, and J. A. Pineda. 2008. Efficacy of pegylated interferon plus ribavirin treatment in HIV/hepatitis C virus co-infected patients receiving abacavir plus lamivudine or tenofovir plus either lamivudine or emtricitabine as nucleoside analogue backbone. J. Antimicrob. Chemother. 621365-1373. [DOI] [PubMed] [Google Scholar]
- 74.Mira, J. A., L. F. Lopez-Cortes, D. Merino, A. Arizcorreta-Yarza, A. Rivero, A. Collado, M. J. Rios-Villegas, M. Gonzalez-Serrano, M. Torres-Tortoso, J. Macias, B. Valera-Bestard, E. Fernandez-Fuertes, J. A. Giron-Gonzalez, F. Lozano, and J. A. Pineda. 2007. Predictors of severe haematological toxicity secondary to pegylated interferon plus ribavirin treatment in HIV-HCV-coinfected patients. Antivir. Ther. 121225-1235. [PubMed] [Google Scholar]
- 75.Miyazaki, M., T. Kanto, M. Inoue, I. Itose, H. Miyatake, M. Sakakibara, T. Yakushijin, N. Kakita, N. Hiramatsu, T. Takehara, A. Kasahara, and N. Hayashi. 2008. Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I. J. Med. Virol. 80980-988. [DOI] [PubMed] [Google Scholar]
- 76.Mohsen, A. H., S. Murad, and P. J. Easterbrook. 2005. Prevalence of hepatitis C in an ethnically diverse HIV-1-infected cohort in south London. HIV Med. 6206-215. [DOI] [PubMed] [Google Scholar]
- 77.Monforte Ade, A., R. Bugarini, P. Pezzotti, A. De Luca, A. Antinori, C. Mussini, G. M. Vigevani, U. Tirelli, R. Bruno, F. Gritti, M. Piazza, S. Chigiotti, A. Chirianni, C. De Stefano, E. Pizzigallo, O. Perrella, and M. Moroni. 2001. Low frequency of severe hepatotoxicity and association with HCV coinfection in HIV-positive patients treated with HAART. J. Acquir. Immune Defic. Syndr. 28114-123. [DOI] [PubMed] [Google Scholar]
- 78.Moore, D. M., R. S. Hogg, P. Braitstein, E. Wood, B. Yip, and J. S. Montaner. 2006. Risks of non-accidental mortality by baseline CD4+ T-cell strata in hepatitis-C-positive and -negative individuals initiating highly active antiretroviral therapy. Antivir. Ther. 11125-129. [PubMed] [Google Scholar]
- 79.Moreno, A., C. Quereda, L. Moreno, M. J. Perez-Elias, A. Muriel, J. L. Casado, A. Antela, F. Dronda, E. Navas, R. Barcena, and S. Moreno. 2004. High rate of didanosine-related mitochondrial toxicity in HIV/HCV-coinfected patients receiving ribavirin. Antivir. Ther. 9133-138. [PubMed] [Google Scholar]
- 80.Morsica, G., S. Bagaglio, S. Ghezzi, C. Lodrini, E. Vicenzi, E. Santagostino, A. Gringeri, M. Cusini, G. Carminati, G. Bianchi, L. Galli, A. Lazzarin, and G. Poli. 2007. Hepatitis C virus (HCV) coinfection in a cohort of HIV positive long-term non-progressors: possible protective effect of infecting HCV genotype on HIV disease progression. J. Clin. Virol. 3982-86. [DOI] [PubMed] [Google Scholar]
- 81.Munshi, N., A. Balasubramanian, M. Koziel, R. K. Ganju, and J. E. Groopman. 2003. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J. Infect. Dis. 1881192-1204. [DOI] [PubMed] [Google Scholar]
- 82.Nattermann, J., H. D. Nischalke, B. Kupfer, J. Rockstroh, L. Hess, T. Sauerbruch, and U. Spengler. 2003. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS 171457-1462. [DOI] [PubMed] [Google Scholar]
- 83.Netski, D. M., Q. Mao, S. C. Ray, and R. S. Klein. 2008. Genetic divergence of hepatitis C virus: the role of HIV-related immunosuppression. J. Acquir. Immune Defic. Syndr. 49136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282103-107. [DOI] [PubMed] [Google Scholar]
- 85.Nowicki, M. J., T. Laskus, G. Nikolopoulou, M. Radkowski, J. Wilkinson, W. B. Du, J. Rakela, and A. Kovacs. 2005. Presence of hepatitis C virus (HCV) RNA in the genital tracts of HCV/HIV-1-coinfected women. J. Infect. Dis. 1921557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nunez, M. 2006. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J. Hepatol. 44S132-S139. [DOI] [PubMed] [Google Scholar]
- 87.Núñez, M., A. Ocampo, K. Aguirrebengoa, M. Cervantes, A. Pascual, S. Echeverria, V. Asensi, P. Barreiro, J. Garcia-Samaniego, and V. Soriano. 2008. Incidence of anaemia and impact on sustained virological response in HIV/HCV-coinfected patients treated with pegylated interferon plus ribavirin. J. Viral Hepat. 15363-369. [DOI] [PubMed] [Google Scholar]
- 88.Núñez, M., P. Rios, L. Martin-Carbonero, M. Perez-Olmeda, J. Gonzalez-Lahoz, and V. Soriano. 2002. Role of hepatitis C virus genotype in the development of severe transaminase elevation after the introduction of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 3065-68. [DOI] [PubMed] [Google Scholar]
- 89.Payan, C., A. Pivert, P. Morand, S. Fafi-Kremer, F. Carrat, S. Pol, P. Cacoub, C. Perronne, and F. Lunel. 2007. Rapid and early virological response to chronic hepatitis C treatment with IFN α2b or PEG-IFN α2b plus ribavirin in HIV/HCV co-infected patients. Gut 561111-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perz, J. F., L. A. Farrington, C. Pecoraro, Y. J. Hutin, and G. L. Armstrong. 2004. Estimated global prevalence of hepatitis C virus infection, abstr. 957, p. 213. Annu. Meet. Infect. Dis. Soc. Am., Boston, MA.
- 91.Pham, T. N., and T. I. Michalak. 2008. Occult persistence and lymphotropism of hepatitis C virus infection. World J. Gastroenterol. 142789-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pineda, J. A., J. A. Garcia-Garcia, M. Aguilar-Guisado, M. J. Rios-Villegas, J. Ruiz-Morales, A. Rivero, J. del Valle, R. Luque, J. Rodriguez-Bano, M. Gonzalez-Serrano, A. Camacho, J. Macias, I. Grilo, and J. M. Gomez-Mateos. 2007. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology 46622-630. [DOI] [PubMed] [Google Scholar]
- 94.Pol, S., B. Lamorthe, N. T. Thi, V. Thiers, F. Carnot, H. Zylberberg, P. Berthelot, C. Brechot, and B. Nalpas. 1998. Retrospective analysis of the impact of HIV infection and alcohol use on chronic hepatitis C in a large cohort of drug users. J. Hepatol. 28945-950. [DOI] [PubMed] [Google Scholar]
- 95.Polis, C. B., S. N. Shah, K. E. Johnson, and A. Gupta. 2007. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clin. Infect. Dis. 441123-1131. [DOI] [PubMed] [Google Scholar]
- 96.Puoti, M., C. Torti, D. Ripamonti, F. Castelli, S. Zaltron, B. Zanini, A. Spinetti, V. Putzolu, S. Casari, L. Tomasoni, E. Quiros-Roldan, M. Favret, L. Berchich, P. Grigolato, F. Callea, and G. Carosi. 2003. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J. Acquir. Immune Defic. Syndr. 32259-267. [DOI] [PubMed] [Google Scholar]
- 97.Qurishi, N., C. Kreuzberg, G. Luchters, W. Effenberger, B. Kupfer, T. Sauerbruch, J. K. Rockstroh, and U. Spengler. 2003. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 3621708-1713. [DOI] [PubMed] [Google Scholar]
- 98.Rehermann, B. 2007. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin. Liver Dis. 27152-160. [DOI] [PubMed] [Google Scholar]
- 99.Rosa, D., G. Saletti, E. De Gregorio, F. Zorat, C. Comar, U. D'Oro, S. Nuti, M. Houghton, V. Barnaba, G. Pozzato, and S. Abrignani. 2005. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc. Natl. Acad. Sci. USA 10218544-18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosen, H. R., C. R. Shackleton, L. Higa, I. M. Gralnek, D. A. Farmer, S. V. McDiarmid, C. Holt, K. J. Lewin, R. W. Busuttil, and P. Martin. 1997. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am. J. Gastroenterol. 921453-1457. [PubMed] [Google Scholar]
- 101.Rosenthal, E., G. Pialoux, N. Bernard, C. Pradier, D. Rey, M. Bentata, C. Michelet, S. Pol, C. Perronne, and P. Cacoub. 2007. Liver-related mortality in human-immunodeficiency-virus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 study). J. Viral Hepat. 14183-188. [DOI] [PubMed] [Google Scholar]
- 102.Rosenthal, E., M. Poiree, C. Pradier, C. Perronne, D. Salmon-Ceron, L. Geffray, R. P. Myers, P. Morlat, G. Pialoux, S. Pol, and P. Cacoub. 2003. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study). AIDS 171803-1809. [DOI] [PubMed] [Google Scholar]
- 103.Rosenthal, E., D. Salmon-Ceron, C. Lewden, V. Bouteloup, G. Pialoux, F. Bonnet, M. Karmochkine, T. May, M. Francois, C. Burty, E. Jougla, D. Costagliola, P. Morlat, G. Chene, and P. Cacoub. 2009. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19). HIV Med. 10282-289. [DOI] [PubMed] [Google Scholar]
- 104.Salmon-Ceron, D., C. Lewden, P. Morlat, S. Bevilacqua, E. Jougla, F. Bonnet, L. Heripret, D. Costagliola, T. May, and G. Chene. 2005. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J. Hepatol. 42799-805. [DOI] [PubMed] [Google Scholar]
- 105.Salmon-Ceron, D., E. Rosenthal, C. Lewden, V. Bouteloup, T. May, C. Burty, F. Bonnet, D. Costagliola, E. Jougla, C. Semaille, P. Morlat, P. Cacoub, and G. Chene. 2009. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: the French national Mortalité 2005 study. J. Hepatol. 50736-745. [DOI] [PubMed] [Google Scholar]
- 106.Shea, D. O., H. Tuite, G. Farrell, M. Codd, F. Mulcahy, S. Norris, and C. Bergin. 2008. Role of rapid virological response in prediction of sustained virological response to Peg-IFN plus ribavirin in HCV/HIV co-infected individuals. J. Viral Hepat. 15482-489. [DOI] [PubMed] [Google Scholar]
- 107.Sherman, K. E., C. Andreatta, J. O'Brien, A. Gutierrez, and R. Harris. 1996. Hepatitis C in human immunodeficiency virus-coinfected patients: increased variability in the hypervariable envelope coding domain. Hepatology 23688-694. [DOI] [PubMed] [Google Scholar]
- 108.Sherman, K. E., J. O'Brien, A. G. Gutierrez, S. Harrison, M. Urdea, P. Neuwald, and J. Wilber. 1993. Quantitative evaluation of hepatitis C virus RNA in patients with concurrent human immunodeficiency virus infections. J. Clin. Microbiol. 312679-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sherman, K. E., N. J. Shire, S. D. Rouster, M. G. Peters, M. James Koziel, R. T. Chung, and P. S. Horn. 2005. Viral kinetics in hepatitis C or hepatitis C/human immunodeficiency virus-infected patients. Gastroenterology 128313-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shores, N. J., I. Maida, V. Soriano, and M. Nunez. 2008. Sexual transmission is associated with spontaneous HCV clearance in HIV-infected patients. J. Hepatol. 49323-328. [DOI] [PubMed] [Google Scholar]
- 111.Shuhart, M. C., D. G. Sullivan, K. Bekele, R. D. Harrington, M. M. Kitahata, T. L. Mathisen, L. V. Thomassen, S. S. Emerson, and D. R. Gretch. 2006. HIV infection and antiretroviral therapy: effect on hepatitis C virus quasispecies variability. J. Infect. Dis. 1931211-1218. [DOI] [PubMed] [Google Scholar]
- 112.Solmone, M., E. Girardi, E. Lalle, I. Abbate, A. D'Arminio Monforte, A. Cozzi-Lepri, A. Alessandrini, R. Piscopo, F. Ebo, L. Cosco, G. Antonucci, G. Ippolito, and M. R. Capobianchi. 2006. Evolution of HVR-1 quasispecies after 1-year treatment in HIV/HCV-coinfected patients according to the pattern of response to highly active antiretroviral therapy. Antivir. Ther. 1187-94. [PubMed] [Google Scholar]
- 113.Solomon, S. S., A. K. Srikrishnan, S. H. Mehta, C. K. Vasudevan, K. G. Murugavel, E. Thamburaj, S. Anand, M. S. Kumar, C. Latkin, S. Solomon, and D. D. Celentano. 2008. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J. Acquir. Immune Defic. Syndr. 49327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soriano, V., I. Maida, M. Nunez, J. Garcia-Samaniego, P. Barreiro, L. Martin-Carbonero, and J. Gonzalez-Lahoz. 2004. Long-term follow-up of HIV-infected patients with chronic hepatitis C virus infection treated with interferon-based therapies. Antivir. Ther. 9987-992. [DOI] [PubMed] [Google Scholar]
- 115.Soriano, V., C. Miralles, M. A. Berdun, E. Losada, K. Aguirrebengoa, A. Ocampo, P. Arazo, M. Cervantes, I. de los Santos, I. San Joaquin, S. Echeverria, M. J. Galindo, V. Asensi, P. Barreiro, J. Sola, J. J. Hernandez-Burruezo, J. Guardiola, F. Blanco, L. Martin-Carbonero, J. Garcia- Samaniego, and M. Nunez. 2007. Premature treatment discontinuation in HIV/HCV-coinfected patients receiving pegylated interferon plus weight-based ribavirin. Antivir. Ther. 12469-476. [PubMed] [Google Scholar]
- 116.Steffan, A. M., M. E. Lafon, J. L. Gendrault, C. Schweitzer, C. Royer, D. Jaeck, J. P. Arnaud, M. P. Schmitt, A. M. Aubertin, and A. Kirn. 1992. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 891582-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stone, S. F., S. Lee, N. M. Keane, P. Price, and M. A. French. 2002. Association of increased hepatitis C virus (HCV)-specific IgG and soluble CD26 dipeptidyl peptidase IV enzyme activity with hepatotoxicity after highly active antiretroviral therapy in human immunodeficiency virus-HCV-coinfected patients. J. Infect. Dis. 1861498-1502. [DOI] [PubMed] [Google Scholar]
- 118.Sulkowski, M. S., R. D. Moore, S. H. Mehta, R. E. Chaisson, and D. L. Thomas. 2002. Hepatitis C and progression of HIV disease. JAMA 288199-206. [DOI] [PubMed] [Google Scholar]
- 119.Sullivan, P. S., D. L. Hanson, E. H. Teshale, L. L. Wotring, and J. T. Brooks. 2006. Effect of hepatitis C infection on progression of HIV disease and early response to initial antiretroviral therapy. AIDS 201171-1179. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka, Y., K. Hanada, H. Hanabusa, F. Kurbanov, T. Gojobori, and M. Mizokami. 2007. Increasing genetic diversity of hepatitis C virus in haemophiliacs with human immunodeficiency virus coinfection. J. Gen. Virol. 882513-2519. [DOI] [PubMed] [Google Scholar]
- 121.Tedaldi, E. M., K. H. Hullsiek, C. D. Malvestutto, R. C. Arduino, E. J. Fisher, P. J. Gaglio, E. R. Jenny-Avital, J. P. McGowan, and G. Perez. 2003. Prevalence and characteristics of hepatitis C virus coinfection in a human immunodeficiency virus clinical trials group: the Terry Beirn Community Programs for Clinical Research on AIDS. Clin. Infect. Dis. 361313-1317. [DOI] [PubMed] [Google Scholar]
- 122.Thein, H. H., Q. Yi, G. J. Dore, and M. D. Krahn. 2008. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 221979-1991. [DOI] [PubMed] [Google Scholar]
- 123.Thomas, D. L., J. Astemborski, R. M. Rai, F. A. Anania, M. Schaeffer, N. Galai, K. Nolt, K. E. Nelson, S. A. Strathdee, L. Johnson, O. Laeyendecker, J. Boitnott, L. E. Wilson, and D. Vlahov. 2000. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 284450-456. [DOI] [PubMed] [Google Scholar]
- 124.Thomas, D. L., J. Astemborski, D. Vlahov, S. A. Strathdee, S. C. Ray, K. E. Nelson, N. Galai, K. R. Nolt, O. Laeyendecker, and J. A. Todd. 2000. Determinants of the quantity of hepatitis C virus RNA. J. Infect. Dis. 181844-851. [DOI] [PubMed] [Google Scholar]
- 125.Torriani, F. J., M. Rodriguez-Torres, J. K. Rockstroh, E. Lissen, J. Gonzalez-Garcia, A. Lazzarin, G. Carosi, J. Sasadeusz, C. Katlama, J. Montaner, H. Sette, Jr., S. Passe, J. De Pamphilis, F. Duff, U. M. Schrenk, and D. T. Dieterich. 2004. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 351438-450. [DOI] [PubMed] [Google Scholar]
- 126.Torti, C., G. Lapadula, M. Puoti, S. Casari, M. C. Uccelli, G. Cristini, D. Bella, G. Pastore, N. Ladisa, L. Minoli, G. Sotgiu, S. L. Caputo, S. Bonora, and G. Carosi. 2006. Influence of genotype 3 hepatitis C coinfection on liver enzyme elevation in HIV-1-positive patients after commencement of a new highly active antiretroviral regimen: results from the EPOKA-MASTER cohort. J. Acquir. Immune Defic. Syndr. 41180-185. [DOI] [PubMed] [Google Scholar]
- 127.Tseng, C. T., and G. R. Klimpel. 2002. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 19543-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuyama, A., F. Hong, A. Mosoian, P. Chen, B. Chen, A. Shecter, M. Klotman, and M. Bansal. 2007. HIV entry and replication in stellate cells promotes cellular activation and fibrogenesis: implications for hepatic fibrosis in HIV/HCV coinfection. Hepatology 4680A. [Google Scholar]
- 129.UNAIDS. 2008. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland.
- 130.Vispo, E., P. Barreiro, J. A. Pineda, J. A. Mira, I. Maida, L. Martin-Carbonero, S. Rodriguez-Novoa, I. Santos, L. F. Lopez-Cortes, D. Merino, A. Rivero, and V. Soriano. 2008. Low response to pegylated interferon plus ribavirin in HIV-infected patients with chronic hepatitis C treated with abacavir. Antivir. Ther. 13429-437. [PubMed] [Google Scholar]
- 131.Vlahakis, S. R., A. Villasis-Keever, T. S. Gomez, G. D. Bren, and C. V. Paya. 2003. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J. Infect. Dis. 1881455-1460. [DOI] [PubMed] [Google Scholar]
- 132.Vogt, M. W., K. L. Hartshorn, P. A. Furman, T. C. Chou, J. A. Fyfe, L. A. Coleman, C. Crumpacker, R. T. Schooley, and M. S. Hirsch. 1987. Ribavirin antagonizes the effect of azidothymidine on HIV replication. Science 2351376-1379. [DOI] [PubMed] [Google Scholar]
- 133.Wang, X. P., L. Goodwin, P. Kahn, C. Gawel, C. B. Cunha, B. Laser, B. Sahn, and M. H. Kaplan. 2006. Influence of increased CD4 cell counts on the genetic variability of hepatitis C virus in patients co-infected with human immunodeficiency virus I. J. Biomol. Tech. 17228-239. [PMC free article] [PubMed] [Google Scholar]
- 134.Weber, R., C. A. Sabin, N. Friis-Moller, P. Reiss, W. M. El-Sadr, O. Kirk, F. Dabis, M. G. Law, C. Pradier, S. De Wit, B. Akerlund, G. Calvo, A. Monforte, M. Rickenbach, B. Ledergerber, A. N. Phillips, and J. D. Lundgren. 2006. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch. Intern. Med. 1661632-1641. [DOI] [PubMed] [Google Scholar]
- 135.Xiang, J., S. L. George, S. Wunschmann, Q. Chang, D. Klinzman, and J. T. Stapleton. 2004. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet 3632040-2046. [DOI] [PubMed] [Google Scholar]
- 136.Xiao, P., O. Usami, Y. Suzuki, H. Ling, N. Shimizu, H. Hoshino, M. Zhuang, Y. Ashino, H. Gu, and T. Hattori. 2008. Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS 221749-1757. [DOI] [PubMed] [Google Scholar]