Abstract
Receptors (FcγRs) for the constant region of immunoglobulin G (IgG) are an important link between humoral immunity and cellular immunity. To help define the role of FcγRs in determining the fate of human immunodeficiency virus type 1 (HIV-1) immune complexes, cDNAs for the four major human Fcγ receptors (FcγRI, FcγRIIa, FcγRIIb, and FcγRIIIa) were stably expressed by lentiviral transduction in a cell line (TZM-bl) commonly used for standardized assessments of HIV-1 neutralization. Individual cell lines, each expressing a different FcγR, bound human IgG, as evidence that the physical properties of the receptors were preserved. In assays with a HIV-1 multisubtype panel, the neutralizing activities of two monoclonal antibodies (2F5 and 4E10) that target the membrane-proximal external region (MPER) of gp41 were potentiated by FcγRI and, to a lesser extent, by FcγRIIb. Moreover, the neutralizing activity of an HIV-1-positive plasma sample known to contain gp41 MPER-specific antibodies was potentiated by FcγRI. The neutralizing activities of monoclonal antibodies b12 and 2G12 and other HIV-1-positive plasma samples were rarely affected by any of the four FcγRs. Effects with gp41 MPER-specific antibodies were moderately stronger for IgG1 than for IgG3 and were ineffective for Fab. We conclude that FcγRI and FcγRIIb facilitate antibody-mediated neutralization of HIV-1 by a mechanism that is dependent on the Fc region, IgG subclass, and epitope specificity of antibody. The FcγR effects seen here suggests that the MPER of gp41 could have greater value for vaccines than previously recognized.
Fc receptors (FcRs) are differentially expressed on a variety of cells of hematopoietic lineage, where they bind the constant region of antibody (Ab) and provide a link between humoral and cellular immunity. Humans possess two classes of FcRs for the constant region of IgG (FcγRs) that, when cross-linked, are distinguished by their ability to either activate or inhibit cell signaling (69, 77, 79). The activating receptors FcγRI (CD64), FcγRIIa (CD32), and FcγRIII (CD16) signal through an immunoreceptor tyrosine-based activation motif (ITAM), whereas FcγRIIb (CD32) contains an inhibitory motif (ITIM) that counters ITAM signals and B-cell receptor signals. It has been suggested that a balance between activating and inhibitory FcγRs coexpressed on the same cells plays an important role in regulating adaptive immunity (23, 68). Moreover, the inhibitory FcγRIIb, being the sole FcγR on B cells, appears to play an important role in regulating self-tolerance (23, 68). The biologic role of FcγRs may be further influenced by differences in their affinity for immunoglobulin G (IgG); thus, FcγRI is a high-affinity receptor that binds monomeric IgG (mIgG) and IgG immune complexes (IC), whereas FcγRIIa, FcγRIIb, and FcγRIIIa are medium- to low-affinity receptors that preferentially bind IgG IC (10, 49, 78). FcγRs also exhibit differences in their relative affinity for the four IgG subclasses (10), which has been suggested to influence the balance between activating and inhibitory FcγRs (67).
In addition to their participation in acquired immunity, FcγRs can mediate several innate immune functions, including phagocytosis of opsonized pathogens, Ab-dependent cell cytotoxicity (ADCC), antigen uptake by professional antigen-presenting cells, and the production of inflammatory cytokines and chemokines (26, 35, 41, 48, 69). In some cases, interaction of Ab-coated viruses with FcγRs may be exploited by viruses as a means to facilitate entry into FcγR-expressing cells (2, 33, 47, 84). Several groups have reported FcγR-mediated Ab-dependent enhancement (ADE) of HIV-1 infection in vitro (47, 51, 58, 63, 94, 96), whereas other reports have implicated FcγRs in efficient inhibition of the virus in vitro (19, 21, 29, 44-46, 62, 98) and possibly as having beneficial effects against HIV-1 in vivo (5, 27, 28, 42). These conflicting results are further complicated by the fact that HIV-1-susceptible cells, such as monocytes and macrophages, can coexpress more than one FcγR (66, 77, 79).
HIV-1 entry requires sequential interactions between the viral surface glycoprotein, gp120, and its cellular receptor (CD4) and coreceptor (usually CCR5 or CXCR4), followed by membrane fusion that is mediated by the viral transmembrane glycoprotein gp41 (17, 106). Abs neutralize the virus by binding either gp120 or gp41 and blocking entry into cells. Several human monoclonal Abs that neutralize a broad spectrum of HIV-1 variants have attracted considerable interest for vaccine design. Epitopes for these monoclonal Abs include the receptor binding domain of gp120 in the case of b12 (71, 86), a glycan-specific epitope on gp120 in the case of 2G12 (13, 85, 86), and two adjacent epitopes in the membrane-proximal external region (MPER) of g41 in the cases of 2F5 and 4E10 (3, 11, 38, 93). At least three of these monoclonal Abs have been shown to interact with FcRs and to mediate ADCC (42, 43).
A highly standardized and validated assay for neutralizing Abs against HIV-1 that quantifies reductions in luciferase (Luc) reporter gene expression after a single round of virus infection in TZM-bl cells has been developed (60, 104). TZM-bl (also called JC53BL-13) is a CXCR4-positive HeLa cell line that was engineered to express CD4 and CCR5 and to contain integrated reporter genes for firefly Luc and Escherichia coli β-galactosidase under the control of the HIV-1 Tat-regulated promoter in the long terminal repeat terminal repeat sequence (74, 103). TZM-bl cells are permissive to infection by a wide variety of HIV-1, simian immunodeficiency virus, and human-simian immunodeficiency virus strains, including molecularly cloned Env-pseudotyped viruses. Here we report the creation and characterization of four new TZM-bl cell lines, each expressing one of the major human FcγRs. These new cell lines were used to gain a better understanding of the individual roles that FcγRs play in determining the fate of HIV-1 IC. Two FcγRs that potentiated the neutralizing activity of gp41 MPER-specific Abs were identified.
MATERIALS AND METHODS
Cells and cDNAs.
TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program (ARRRP) as contributed by John Kappes and Xiaoyun Wu. The HT1080 and 293T/17 cell lines were obtained from the American Type Culture Collection (Manassas, VA). These three cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, HEPES, and gentamicin. 293FT cells, which constitutively expresses the simian virus 40 large T antigen from the cytomegalovirus promoter (65), were obtained from Invitrogen (Carlsbad, CA) and were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, sodium pyruvate, and nonessential amino acids. cDNAs for human FcγRIIa (H131 allele), FcγRIIb, and RIIIa (F158 allele) were a gift from Jeffrey Ravetch (The Rockefeller University, NY). cDNA sequences for FcγRI and the FcɛRγ subunit were synthesized by Blue Heron Biotechnology (Bothell, WA) by using published sequences (55, 75).
Abs, myeloma proteins, and HIV-1-positive plasma samples.
Monoclonal Ab b12 (IgG1) was a gift from Dennis Burton (Scripps Research Institute, La Jolla, CA). Monoclonal Abs 2G12 (IgG1), 2F5 (IgG1 and IgG3), and 4E10 (IgG1 and IgG3) were purchased from Polymun Scientific GmbH (Vienna, Austria). Additional gp41-specific monoclonal Abs were obtained from the NIH ARRRP as kindly provided by either Susan Zolla-Pazner (126-7, 98.6, 240-D, and 246-D) (34, 107), Marshall Posner and Lisa Cavacini (F240) (16), or Polymun Scientific GmbH (5F3). The gp41-specific monoclonal Abs m43 and m47 were a gift from Dimiter Dimitrov (National Cancer Institute, Frederick, MD). The monoclonal Abs 2.2B and 7B12 were a gift from James Robinson (Tulane University). HIV immune globulin (HIVIG) is a purified IgG fraction prepared from pooled plasma samples from asymptomatic, HIV-1 Ab-positive donors with CD4+ counts of above 400 cells/μl; this product is available from the NIH ARRRP.
A Fab fragment of 4E10 IgG1 was obtained by using a standard preparation kit from Pierce (Rockford, IL). Briefly, 2 mg of the monoclonal Ab was digested with immobilized papain overnight at 37°C. The Fab fragment was separated from the Fc fragment on a protein A column and suspended in phosphate-buffered saline (PBS) to a concentration of 0.5 mg/ml.
Fluorescein isothiocyanate (FITC)-conjugated Abs for the detection of FcγRs (anti-CD16 clone 3G8, anti-CD32 clone FLI8.26, and anti-CD64 clone 10.1) and phycoerythrin-conjugated Abs for the detection of CD4 (anti-CD4 clone RPA-T4), CCR5 (anti-CD195 clone 2D7), and CXCR4 (anti-CD184 clone 12G5) were purchased from BD Biosciences/BD Pharmingen (San Diego, CA). Rabbit polyclonal Ab to the human FcɛRIγ subunit was obtained from Upstate (Cambridge, MA). Pure IgG1, IgG2, IgG3, and IgG4 human myeloma proteins were obtained from Sigma-Aldrich (St. Louis, MO). In some cases these myeloma proteins were heat aggregated at 63°C for 30 min before use.
Plasma samples from chronically HIV-1-infected blood donors were obtained from Zeptometrix Corporation (plasma samples 01648, 01652, and 01686), from the South African National Blood Services (plasma samples BB12, BB24, BB34, and BB87), and from the Beth Israel Deaconess Medical Center (plasma samples 011, 012, 013, and 014). Donors 01648, 01652, and 01686 were infected with HIV-1 subtype B, whereas donors BB12, BB24, BB34, and BB87 were infected with HIV-1 subtype C, as determined by env sequences. Demographically, donors 011, 012, 013, and 014 were likely infected with HIV-1 subtype B. Plasma samples from two healthy, HIV-1-negative individuals were obtained from Zeptometrix Corporation (plasma samples 0210 and 0211).
Lentiviral expression vectors for the FcγRs.
The cDNA sequences for the human FcγRs and the FcɛRγ chain were amplified by PCR using primers FcγRI forward (5′-CACCCTTGGAGACAACATGTGGTTC-3′) and reverse (5′-GAGCAAGTGGGCAGGGGACGGTCCAGA-3′), FcγRIIa forward (5′-CACCGTCCTTGGCTGGTTTCTGGGATG-3′) and reverse (5′-CCACTCAGCAAGCTGAGAGTATGAC-3′), FcγRIIb forward (5′-CACCTGCGGAATTCCCAATTCCGGC-3′) and reverse (5′-GCAAGACAATGGAGACTAAATACGG-3′), FcγRIIIa forward (5′-CACCGTGACTTGTCCACTCCAGTGTG-3′) and reverse (5′-TGCTGCTGCTACTGCTCTTATTAC-3′), and FcɛRγ forward (5′-CACCAGAACGGCCGATCTCCAGCC-3′) and reverse (5′-GTAAACAGCATCTGAGCATTAGTC-3′). Final PCR products were cloned in the pLenti6/V5 Directional Topo lentiviral vector (Invitrogen, Carlsbad, CA). Every vector was sequenced to confirm the correct orientation and to verify that the open reading frame for the receptors remained unaltered. Recombinant lentiviral vectors were packed into virions in 293FT cells by using the Virapower packaging mix according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). The infectious titer of each recombinant lentiviral stock was determined in HT1080 cells in the presence of the selection antibiotic blasticidin. Expression of the FcγRs was assessed by fluorescence microscopy after staining blasticidin-resistant HT1080 cells with FITC-conjugated Abs specific for each receptor.
Transduction and expression of FcγR in TZM-bl cells.
TZM-bl cells were seeded at a density of 106 cells/10 ml growth medium in 100-mm culture plates and incubated overnight at 37°C. The medium was replaced with 4 ml of medium containing 2 to 3 transducing units of recombinant lentivirus per cell and the cultures incubated overnight at 37°C. Virus-containing medium was replaced with 10 ml of fresh growth medium and the incubation continued for an additional 24 h, after which time the cultures were maintained in 10 ml of blasticidin-containing selection medium for 10 days. FcγR-expressing cells were sorted a minimum of two times for enrichment. For sorting, cells were removed from flasks with 2.5 ml of a nonenzymatic cell dissociation solution (Sigma-Aldrich, St. Louis, MO), resuspended in PBS containing 0.1% bovine serum albumin (BSA), and counted. Approximately 5 × 106 to 10 × 106 cells were stained with FITC-conjugated anti-FcγR for 1 h at 4°C. Stained cells were washed three times with cold PBS containing 0.1% BSA and suspended in the same solution at a density of 107 cells/ml. Parental TZM-bl cells were stained with the same FITC-conjugated monoclonal Ab as a negative control. Between 2 × 105 and 5 × 105 fluorescent-positive cells were sorted at 4°C in a BD FACSAria instrument (BD Biosciences) contained in a Bioprotect II hood. Collected cells were suspended in blasticidin-containing culture medium and maintained at 37°C for a minimum of 7 days. Expression of the FcγRs, CD4, CCR5, and CXCR4 in the sorted cell populations was verified by flow cytometry by using a BD FACSCalibur analyzer (BD Biosciences). Between 12,000 and 100,000 live events were acquired per sample. Compensation and analysis were performed by using FlowJo software (Tree Star).
Determination of IgG subclass binding specificity.
The IgG subclass specificities of FcγRs expressed on TZM-bl cells were assessed by flow cytometry. Briefly, 106 cells were incubated for 1 h at 4°C in PBS-0.1% BSA containing myeloma proteins (50 μg/ml). Cells were washed three times and stained with FITC-conjugated goat anti-human IgG (Fab specific). Flow cytometry was performed with a BD FACSCalibur analyzer as described above.
Neutralization assay.
Neutralization was measured as a reduction in Luc reporter gene expression after a single round of infection with Env-pseudotyped viruses as described previously (60). Briefly, 200 50% tissue culture infective doses of virus was incubated with serial threefold dilutions of test sample in duplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg/ml DEAE-dextran) were added to each well. One set of control wells received cells plus virus (virus control), and another set received cells only (background control). After a 48-h incubation, 100 μl of cells was transferred to a 96-well black solid plate (Costar) for measurements of luminescence using the Britelite luminescence reporter gene assay system (Perkin-Elmer Life Sciences). Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to those in virus control wells after subtraction of background RLUs. Assay stocks of molecularly cloned Env-pseudotyped viruses were prepared by transfection in 293T cells and titrated in TZM-bl cells as described previously (60). The HIV-1 subtype A, B, and C Env clones used for pseudoviruses have been described previously (8, 53, 60, 61) and may be obtained from the NIH ARRRP. All Env clones were from acute/early sexually acquired infections and are considered to possess a tier 2 neutralization phenotype. In addition, clones SC422661.8, SC05.8C11.2344, and 700010040.C9.4520 are considered to be true transmitted/early founder viruses (53).
In some experiments, freshly trypsinized cells were added to diluted test samples in 96-well flat-bottom culture plates and incubated for 2 h to allow the cells to attach. The medium was replaced completely with fresh medium (without test sample) prior to addition of virus. In other experiments, cells (5,000 cells in 100 μl of growth medium containing 75 μg/ml DEAE-dextran) were seeded in 96-well culture plates and incubated for 1 day prior to the start of the assay. In the latter case, serial dilutions of Ab with and without virus were prepared in separate 96-well plates and transferred to plates containing preseeded cells.
RESULTS
Expression of FcγRs on TZM-bl cells.
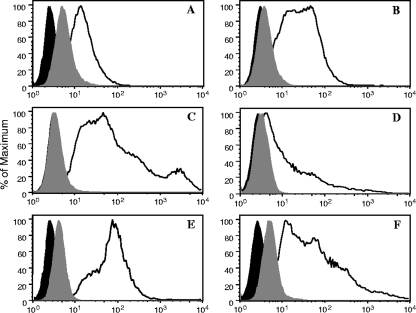
cDNAs for human FcγRs were amplified by PCR using primers complementary to regions flanking the receptor open reading frame. PCR products were cloned in the lentiviral vector pLenti6/V5 under the control of a cytomegalovirus promoter. Recombinant retroviral vectors were packed into virions in 293FT cells and used to transduce TZM-bl cells. Transduced cells were selected in blasticidin-containing medium and sorted by flow cytometry. Cells were sorted multiple times until no further enrichment of the FcγR-expressing population was achieved. Parental TZM-bl cells stained negative for all four FcγRs (data not shown). Figure 1 shows the relative FcγR surface expression on each cell line after at least two rounds of live sorting. As expected, cells transduced with FcγRIIa (HH form) and FcγRIIb stained positive with anti-CD32 (Fig. 1B and C); 52% and 91% of the cells were positive, respectively. Also as expected, cells transduced with FcγRI and FcγRIIIa (FF form) stained positive with anti-CD64 (26% positive) and anti-CD16 (51% positive), respectively (Fig. 1A and D).
FIG. 1.
Expression of the FcγRs on TZM-bl cells. Cell surface expression of the Fcγ receptors was analyzed by flow cytometry using the four established cell lines. Cell suspensions were stained with FITC-labeled mouse monoclonal Abs against the human FcγRs at 4°C. After washing, cells were fixed and analyzed using a BD FACSCalibur analyzer (BD Biosciences). The acquired data were analyzed using a FlowJo software (Tree Star). (A) Cells transduced with FcγRI cDNA stained with anti-human CD64. (B) Cells transduced with FcγRIIa cDNA stained with anti-human CD32. (C) Cells transduced with FcγRIIb stained with anti-human CD32. (D) Cells transduced with FcγRIIIa cDNA stained with anti-human CD16. (E) Cells transduced with FcγRI cDNA stained with anti-human FcɛR-γ chain. (F) Cells transduced with FcγRIIIa cDNA stained with anti-γ-chain. Black-filled curves correspond to nonstained cells; gray-filled curves correspond to cells stained with isotype control Abs.
As described previously (25, 69), we found that surface expression of FcγRI and FcγRIIIa required coexpression of the FcɛRγ subunit. A lentiviral vector encoding the cDNA for this subunit was therefore cotransduced at a 2:1 transducing unit ratio with the FcγRI and FcγRIIIa vectors into TZM-bl cells. Relative surface expression of the γ subunit in cells expressing FcγRI (99% positive) and FcγRIIIa (77% positive) is shown in Fig. 1E and F. Flow cytometry also was used to monitor the levels of CD4, CCR5, and CXCR4 expression, which were approximately equal on parental TZM-bl cells and the four FcγR-expressing cell lines for at least 40 passages (data not shown). FcγR expression was equally stable over 40 passages on the different FcγR cell lines.
TZM-bl cells expressing FcγRs exhibit differential binding of IgG subclasses.
The IgG binding capability of FcγRs expressed on TZM-bl cells was evaluated with human myeloma proteins. Pure IgG1, IgG2, IgG3, and IgG4 human myeloma proteins were incubated with a suspension of each FcγR-expressing TZM-bl cell line. Heat-aggregated myeloma proteins were used for cells expressing the low- to medium-affinity FcγRIIa, FcγRIIb, and FcγRIIIa, because these Fcγ receptors have low affinity for mIgG. Binding was quantified by flow cytometry by using FITC-conjugated goat anti-human IgG (Fab specific; Sigma-Aldrich, St. Louis, MO). The results are shown in Fig. 2. No significant binding to parental TZM-bl cells compared to the isotype control Ab was detected for any of the myeloma proteins. Cells expressing the high-affinity FcγRI bound relatively high levels of mIgG1, mIgG3, and mIgG4 and somewhat lower levels of mIgG2. Cells expressing the low- to medium-affinity FcγRIIa, FcγRIIb, and FcγRIIIa bound relatively high levels of heat-aggregated IgG3. In addition, FcγRIIb and FcγRIIIa bound relatively high levels of heat-aggregated IgG1, whereas FcγRIIa bound much lower levels of this subclass. Very weak binding of heat-aggregated IgG2 and IgG4 was detected with FcγRIIa, FcγRIIb, and FcγRIIIa. Our results mostly agree with the IgG subclass specificities of human FcγRs determined in a recent systematic study (10). Two exceptions were that (i) we detected IgG2 binding to FcγRI and (ii) we found that IgG1 bound more strongly than IgG4 to FcγRIIb. These two discrepancies could be related to the different expression and detection methods used in the two studies.
FIG. 2.
Binding of human IgG subclasses to TZM-bl cells expressing different FcγRs. Pure myeloma proteins of the four IgG subclasses were incubated with a suspension of each of the FcγR-expressing cells. After washing to remove unbound myeloma proteins, bound proteins were quantified by flow cytometry. Because the medium- to low-affinity receptors FcγRIIa, FcγRIIb, and FcγRIIIa bind mIgG poorly, the myeloma proteins were heat aggregated before use in these specific cases. Nonaggregated myeloma proteins were used for FcγRI-expressing cells.
HIV-1 infection and Ab-mediated neutralization in FcγR-expressing TZM-bl cells.
The susceptibility of each FcγR-bearing cell line to infection by HIV-1 was compared to that of parental TZM-bl cells by inoculating each cell line with an equal amount of three different R5 (CCR5-utilizing) strains of HIV-1, each belonging to a different genetic subtype (subtypes A, B, and C). Consistent with the observation that FcγR had no obvious effect on CD4 and HIV-1 coreceptor expression, infectivity in each cell line was approximately equal to that in parental TZM-bl cells (Table 1).
TABLE 1.
HIV-1 infectivity in FcγR-bearing TZM-bl cells
| FcγR | Mean RLU (SD, % of mean)a
|
||
|---|---|---|---|
| 6535.3 (subtype B) | Du156.12 (subtype C) | Q23.17 (subtype A) | |
| None | 124,737 (7.1) | 158,819 (12.0) | 106,343 (11.5) |
| FcγRI | 128,362 (8.3) | 88,694 (9.2) | 189,913 (6.4) |
| FcγRIIa | 146,455 (3.0) | 97,816 (12.1) | 140,250 (7.2) |
| FcγRIIb | 155,573 (3.3) | 102,269 (15.8) | 144,429 (6.2) |
| FcγRIIIa | 119,902 (9.3) | 113,960 (15.5) | 134,029 (6.9) |
Cells were inoculated with an equal amount of virus in 96-well culture plates. RLU were measured after 48 h. Values are the means for eight wells.
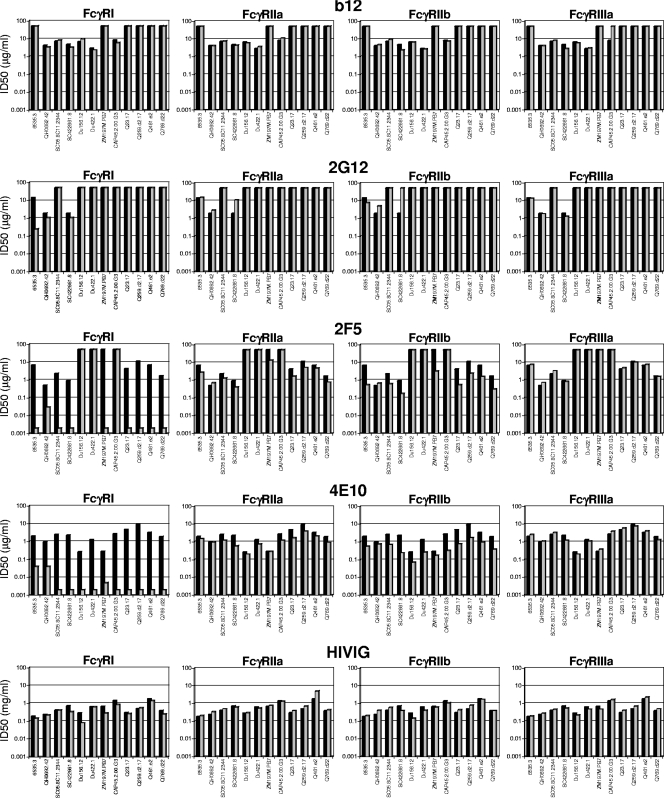
To determine whether the FcγRs can affect Ab-mediated neutralization of HIV-1, the neutralizing activities of b12, 2G12, 2F5, 4E10, HIVIG, and HIV-1-positive plasma samples were assessed against a multisubtype panel of 12 HIV-1 Env-pseudotyped viruses in parental TZM-bl cells and in each of the four FcγR-expressing TZM-bl cell lines (Fig. 3 and 4). Differences in neutralization potencies of ≥3-fold between parental TZM-bl cells and FcγR cells were considered significant (differences of <3-fold approach our normal range of interassay variability).
FIG. 3.
Effect of FcγR expression on HIV-1 neutralization by broadly neutralizing monoclonal Abs and HIVIG. The neutralizing activities of monoclonal Abs b12, 2G12, 2F5, and 4E10 (all IgG1) and HIVIG (purified IgG fraction prepared from pooled HIV-1-positive plasma samples) were assessed against four strains each of HIV-1 subtype B (6535.3, QH0692.42, SC05.8C11.2344, and SC422661.87), subtype C (Du156.12, Du422.1, ZM197M.PB7, and CAP45.2.00.G3), and subtype A (Q23.17, Q259.d2.17, Q461.a2, and Q769.d22) in parental TZM-bl cells (black bars) and in TZM-bl cells expressing FcγR (gray bars). ID50, 50% inhibitory dose.
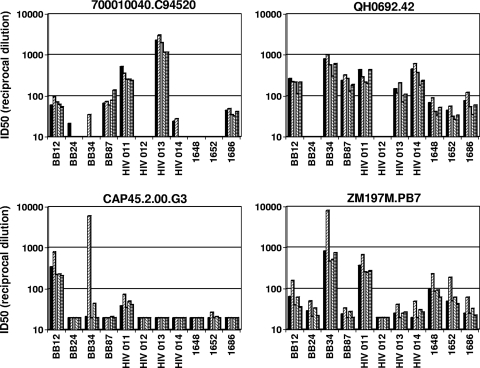
FIG. 4.
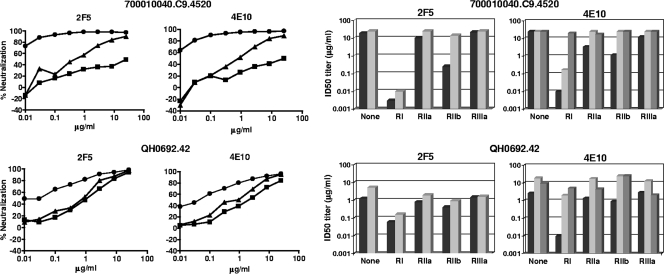
Effect of FcγR expression on HIV-1 neutralization by plasma samples from infected individuals. The neutralizing activities of plasma samples from individuals who were chronically infected with either HIV-1 subtype C (BB12, BB24, BB34, and BB87) or subtype B (HIV 011, HIV 012, HIV 013, HIV 014, 1648, 1652, and 1686) were assessed against two subtype B viruses (700010040.C94520 and QH0692.42) and two subtype C viruses (CAP45.2.00.G3 and ZM197M.PB7) in parental TZM-bl cells (black bars) and in TZM-bl cells expressing FcγRI (left-hatched bars), FcγRIIa (gray bars), FcγRIIb (right-hatched bars), and FcγRIIIa (horizontally hatched bars). Fifty percent inhibitory dose (ID50) neutralization titers that were below the level of detection (dilution of <1:20) were assigned a value of 10 and are not visible.
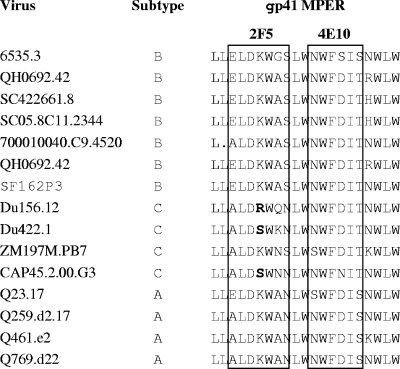
Substantial increases in the neutralization potencies of 2F5 and 4E10 against most viruses were observed in FcγRI-expressing cells, and moderate increases against most viruses were observed in FcγRIIb-expressing cells (Fig. 3). In the case of FcγRI-expressing cells, the neutralization potencies of 2F5 and 4E10 was increased as much as 5,000-fold, depending on the virus. Three subtype C viruses that resisted 2F5 (Du156.12, Du422.1, and CAP45.2.00.G3) contained an amino acid change that is known to affect the core epitope of this monoclonal Ab, whereas all viruses contained the core WFXI motif needed for 4E10 neutralization (Fig. 5) (64, 110). The effect of FcγRIIb was less pronounced (3-fold to 20-fold increases in neutralization potency, depending on the virus) but nonetheless was seen across multiple viruses.
FIG. 5.
Amino acid sequence alignment of the MPER region of the HIV-1 Env-pseudotyped viruses used in this study. The minimal epitopes for 2F5 and 4E10 are boxed. Key amino acid changes in the 2F5 epitope that are referred to in the text are shown in bold.
No FcγR effect was observed with b12, and only one significant effect was observed with HIVIG, that being a 3.6-fold increase in the potency of HIVIG when assayed in FcγRI-expressing cells against subtype C virus Du156.12 (Fig. 3). Mixed results were obtained with 2G12, where only three viruses were neutralized in parental TZM-bl cells. No FcγR effect was seen with one of these viruses (QH0692.42), whereas FcγRI increased 2G12 potency 58-fold against another virus (6535.3). Results with the third virus (SC422661.8) were unique in that FcγRIIa and FcγRIIb impaired the neutralizing activity of 2G12, suggesting a possible case of infection enhancement that countered neutralization. This observation was reproducible and was the only possible case of infection enhancement in our studies.
FcγR effects were rare with HIV-1-positive human plasma samples. However, one notable exception was a subtype C plasma sample (BB34); FcγRI increased the potency of this plasma sample by 280- and 10-fold, respectively, against the two subtype C viruses CAP45.2.00.G3 and ZM197M.PB7 (Fig. 4). A recent study identified gp41 MPER-specific neutralizing Abs as a major component of the neutralizing activity of this plasma sample (7).
Given the strong FcγRI effect on gp41 MPER-specific monoclonal Abs 2F5 and 4E10, we tested whether a similar effect would be seen with other gp41-specific human monoclonal Abs. Ten additional monoclonal Abs, in addition to 2F5 and 4E10, were assayed against a human-simian immunodeficiency virus strain containing a subtype B virus env, SF162P3, in TZM-bl and TZM-bl/FcγRI cells. As shown in Table 2, 2F5 and 4E10 exhibited ≥625- and ≥ 312-fold greater potencies, respectively, in FcγRI-expressing cells. All other results were negative except for those with monoclonal Ab 98.6 (cluster II region of gp41), which exhibited ≥16-fold-greater potency in FcγRI-expressing cells. A similar FcγRI effect on monoclonal Ab 98.6 was observed with three additional subtype B viruses (SF162.LS, 6535.3, and WITO4160.33) but not with three others (6101.1, PVO.4, and TRO.11) (data not shown). Interestingly, the epitope for this monoclonal Ab has been mapped to a region that overlaps the 2F5 epitope (34, 83, 99, 107).
TABLE 2.
Effect of FcγRI expression on other gp41-specific monoclonal Abs
| gp41 Aba | Epitope | 50% inhibitory dose (μg/ml)b in:
|
|
|---|---|---|---|
| TZM-bl cells | TZM-bl/FcγRI cells | ||
| 2F5 | MPER | >25 | 0.04 |
| 4E10 | MPER | >25 | 0.08 |
| M43 | Conformational | >25 | >25 |
| M47 | Conformational | >25 | >25 |
| 2.2B | Cluster II | >25 | >25 |
| 7B12 | Cluster I | >25 | >25 |
| 240-D | Cluster I | >3.8 | >3.8 |
| 246-D | Cluster I | >16.3 | >16.3 |
| F240 | Cluster I | >25 | >25 |
| 5F3 | Cluster I | >25 | >25 |
| 126-7 | Cluster I | >11.5 | >11.5 |
| 98.6 | Cluster II | >12.5 | 0.78 |
All Abs were subclass IgG1.
Neutralization of SHIV SF162P3 was assessed in parental TZM-bl cells and in TZM-bl cells that expressed FcγRI. Values are the concentration at which RLU were reduced 50% compared to those in virus control wells after subtraction of background RLU in cell control wells.
Additional requirements for an FcγR effect on HIV-1 neutralization.
In order to more firmly establish a biologic role for FcγR, it was necessary to demonstrate a requirement for the Fc region of Ab. In addition, because FcγRI bound IgG1 and IgG3 subclasses nearly equally whereas FcγRIIb showed a preference for IgG3 over IgG1 (Fig. 2), we also assessed a role for IgG subclass. Modified versions of 2F5 and 4E10 were assayed against two HIV-1 subtype B Env-pseudotyped viruses (700010040.C9.4520 and QH0692.42) in parental TZM-bl cells and in TZM-bl cells expressing either FcγRI or FcγRIIb. Neutralization curves for the IgG1 versions of 2F5 and 4E10 in the different cells lines are shown in Fig. 6 (left panel). As expected, FcγRI expression exerted a strong effect on the neutralization potencies of both monoclonal Abs against both viruses. FcγRIIb exerted a smaller effect that was more pronounced with virus 700010040.C9.4520 than with virus QH0692.42. All combinations exhibited true positive effects (based on ≥3-fold-increased potency compared to with parental TZM-bl cells), except for the combination of 2F5 assayed with QH0692.42 in cells expressing FcγRIIb, where no clear effect on neutralization was observed. Notably, the positive effects seen with 70010040.C9.4520 were among the most potent in this study.
FIG. 6.
Importance of IgG subclass and the Fc region of Ab. Left panel, neutralization curves for 2F5 (IgG1) and 4E10 (IgG1) assayed against two HIV-1 subtype B Env-pseudotyped viruses in parental TZM-bl cells (squares) and in TZM-bl cells expressing either FcγRI (circles) or FcγRIIb (triangles). Right panel, neutralization potencies of 2F5 IgG1 and 4E10 IgG1 (black bars), 2F5 IgG3 and 4E10 IgG3 (light gray bars), and 4E10 Fab (dark gray bars) in parental TZM-bl cells and TZM-bl cells expressing either FcγRI, FcγRIIa, FcγRIIb, or FcγRIIIa. ID50, 50% inhibitory dose.
Based on these results, the same two viruses were used to test a requirement for the Fc region of Ab and to compare the IgG3 and IgG1 versions of the two monoclonal Abs. A Fab version of 4E10, together with separate IgG1 and IgG3 versions of 2F5 and 4E10, was assayed in parental TZM-bl cells and in TZM-bl cells expressing each of the four FcγRs. As shown in Fig. 6 (right panel), none of the FcγRs increased the neutralization potency of 4E10-Fab, thus supporting a requirement for the Fc region in augmenting the potency of the complete IgG molecule. Comparisons of IgG subclass took into account the fact that the IgG3 versions of 2F5 and 4E10 were sometimes 2 to 7 times less potent than their IgG1 counterparts in parental TZM-bl cells. In this regard, the magnitude of an effect in an FcγR-expressing cell line was measured relative to the baseline potency of the corresponding IgG subclass in parental TZM-bl cells. After making this adjustment, FcγRI had a stronger effect on the IgG1 version than on the IgG3 version of 4E10 (Fig. 6, right panel). Thus, the potency of 4E10-IgG1 against viruses 700010040.C9.4520 and QH0692.42 in FcγRI-expressing cells was improved 2,500- and 268-fold, respectively, whereas the potency of 4E10-IgG3 was improved 147- and 9-fold, respectively. FcγRI affected both IgG subclasses of 2F5 nearly equally (<3-fold differences). FcγRIIb had an effect on the IgG1 versions of both monoclonal Abs assayed against virus 700010040.C9.4520 and on the IgG1 version of 4E10 assayed against virus QH0692.42. FcγRIIb had no effect on the IgG3 subclass of either monoclonal Ab.
Sequence of early events in the FcγR effect on neutralization.
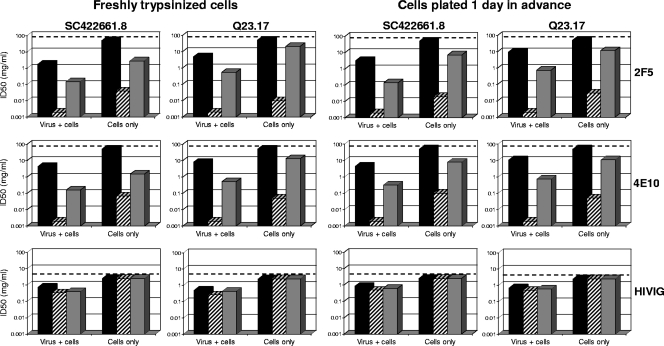
Most biologic effects of FcγR are mediated by preformed Ab-antigen IC. We sought to determine whether IC formation was necessary prior to FcγR engagement in order for FcγR to augment the neutralizing activities of 2F5 and 4E10. We also determined whether our use of freshly trypsinized cells in all other experiments might have altered the outcome of the assays. Parental TZM-bl cells and TZM-bl cells expressing individual FcγRs were preincubated for 2 hours with either 2F5, 4E10, or HIVIG, after which time the Abs were removed by one complete change of the medium before addition of virus. Excessive washing was avoided in order to minimize the removal of weakly bound Ab, especially in the case of the low-affinity FcγRIIb receptor. Assays were performed with two viruses (SC422661.8 and Q23.17) that were highly sensitivity to the FcγRI and FcγRIIb effect seen with 2F5 and 4E10 in Fig. 3. One set of assays used freshly trypsinized cells, whereas another used cells seeded in 96-well culture plates and incubated for 1 day prior to assay to allow recovery from trypsinization. The results in both cases were compared to those obtained at the same time under standard assay conditions (Ab incubated with both the virus and cells) using either freshly trypsinized cells or cells that were preseeded and incubated for 1 day prior to assay.
As seen in Fig. 7, nearly identical results were obtained using freshly trypsinized cells and cells that were preseeded and incubated for 1 day prior to assay. This outcome indicates that the FcγR effect is reproducible and independent of trypsinization. As additional evidence for the reproducibility of the FcγR effect, the results obtained under standard assay conditions shown in Fig. 7 were very similar to those shown in Fig. 3 that were obtained under the same standard assay conditions. Notably, no neutralization was detected in parental TZM-bl cells when only the cells were exposed to Ab. Thus, a single complete change of the Ab-containing medium was sufficient to remove all detectable neutralizing activity that was not dependent on FcγR expression. In contrast, preincubation of FcγRI-expressing cells with either 2F5 or 4E10, followed by removal of Abs prior to virus addition, led to potent neutralization of both viruses. In addition, moderate neutralization of both viruses was observed under these conditions when FcγRIIb-expressing cells were used. Notably, the magnitudes of these effects, though substantial, were diminished relative to the magnitudes seen under standard assay conditions. A diminished effect when only the cells were exposed to Ab suggests either that the Abs need to be present throughout the assay for maximum FcγR occupancy or that more than one mechanism of virus neutralization was involved. As a control, HIVIG neutralized only under standard assay conditions, where it was not affected by FcγR expression. No neutralization was detected with 2F5, 4E10, and HIVIG when cells expressing either FcγRIIa or FcγRIIIa were used under conditions where only the cells were exposed to Ab (data not shown).
FIG. 7.
Enhanced neutralizing activities of gp41 MPER-specific Abs captured by FcγRI and FcγRIIb on the cell surface prior to virus exposure. Neutralizing activities of 2F5, 4E10, and HIVIG were assessed against two strains of HIV-1 (subtype B strain SC422661.87 and subtype A strain Q23.17) in parental TZM-bl cells (black bars) and in TZM-bl cells expressing either FcγRI (hatched bars) or FcγRIIb (gray bars). The assays were performed either with freshly trypsinized cells or with preseeded cells that had been incubated for 1 day before the start of the assay. Two assay formats were used in both cases: (i) standard conditions in which virus was incubated with Ab for 1 h prior to addition of the combined mixture to cells (virus + cells) and (ii) a modified format in which cells were preincubated with Ab for 2 h, followed by one complete change of the medium to remove Ab prior to addition of virus (cells only). The highest Ab concentrations tested are indicated by a dashed line; thus, values below this dashed line signify positive neutralization. ID50, 50% inhibitory dose.
Monoclonal Ab binding to FcγR-expressing TZM-bl cells.
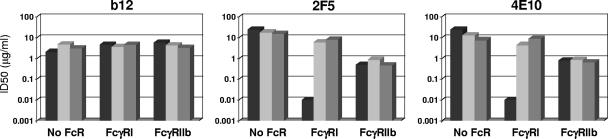
We sought to determine whether the differential effects of FcγR on b12, 2G12, 2F5, 4E10, and two IgG subclasses of 2F5 and 4E10 could be explained by FcγR binding capacity. Relative binding was assessed with mIgG because of technical difficulties in producing aggregates of the monoclonal Abs. As shown in Fig. 8, mIgG1 versions of b12, 2G12, 2F5, and 4E10 all demonstrated efficient binding to FcγRI-expressing TZM-bl cells (42 to 73% of cells staining positive with mean fluorescence intensity values of 349 to 928). IgG3 versions of 2F5 and 4E10 bound somewhat better than their IgG1 counterparts. Interestingly, the levels of IgG1-b12 and IgG1-2G12 binding were roughly equivalent to that of IgG1-4E10 binding and were only marginally below the level of 2F5 binding. These results are consistent with the myeloma protein results in Fig. 2 showing that FcγRI-expressing TZM-bl cells bind both IgG subclasses. Overall, differences in IgG subclass binding did not explain why an FcγRI effect on neutralization was seen with 2F5 and 4E10 but not with b12 and 2G12.
FIG. 8.
Relative binding of HIV-1 broadly neutralizing monoclonal Abs to FcγRI on TZM-bl cells. TZM-bl cells expressing FcγRI were incubated with either IgG1b12, 2G12, 2F5, or 4E10 for 1 h at 4°C. Cells were washed three times, stained with FITC-conjugated goat anti-human IgG (Fab specific), and analyzed by flow cytometry. Top, percentage of cells staining positive; bottom, mean fluorescence intensity (MFI) of positive cells. Background staining with an isotype control Ab was subtracted in both cases.
Little or no mIgG binding to cells expressing the low-affinity FcγRIIa, FcγRIIb, and FcγRIIIa was detected (data not shown). Low-affinity binding that was not detected by flow cytometry but nonetheless was capable of mediating the FcγRIIb effect described above when only cells were exposed to 2F5 and 4E10 (Fig. 7) might have occurred.
Effect of normal human serum.
The high-affinity FcγRI is the only FcγR that binds mIgG, making it possible that mIgG in normal human serum would compete with 2F5 and 4E10 for FcγRI binding. To test this possibility, the neutralizing activities of 2F5 and 4E10 were assessed in FcγRI-expressing TZM-bl cells in the presence and absence of either fresh or heat-inactivated normal human serum. Monoclonal Ab b12 was tested as a putative negative control. As shown in Fig. 9, 5% normal human serum, whether fresh or heat inactivated, had no effect on IgG1-b12 but completely abolished the FcγRI effect on the IgG1 versions of 2F5 and 4E10. Consistent with the known requirement for polymeric Ab, 5% fresh and heat-inactivated normal human serum had no measurable effect on neutralization in FcγRIIb-expressing cells.
FIG. 9.
Normal human serum abrogates the FcγRI effect on 2F5 and 4E10. Neutralization assays were performed with HIV-1 700010040.C9.4520 in parental TZM-bl cells and in TZM-bl cells expressing either FcγRI or FcγRIIb in the absence of serum (black bars) or in the presence of either 5% fresh normal human serum (light gray bars) or 5% heat-inactivated normal human serum (dark gray bars). The monoclonal Abs were of the IgG1 subclass. ID50, 50% inhibitory dose.
DISCUSSION
The role of FcγRs in determining the fate of HIV-1 IC was investigated by using lentiviral transduction to individually express each of four major FcγRs on the surface of a cell line that is highly permissive to HIV-1 infection. The TZM-bl cell line used for this purpose is one of the most popular cell lines used to measure neutralizing Abs. Expression of each FcγR on TZM-bl cells had no obvious effect on CD4, CCR5, and CXCR4 expression and HIV-1 susceptibility. Moreover, the physical integrity of each expressed FcγR was maintained as indicated by an ability to bind IgG.
These studies were initiated in part with the expectation that the new TZM-bl cell lines would be helpful in delineating the contributions of individual FcγRs to ADE of HIV-1 infection in vitro. However, only one possible case of FcR-mediated ADE was detected; this case involved the gp120 glycan-specific monoclonal Ab 2G12 assayed against a subtype B virus in FcγRI-expressing cells. The fact that ADE was so rarely detected might be an indication that FcγRs are relatively inefficient at facilitating HIV-1 infection, at least in TZM-bl cells.
Our major observation was that FcγRI and FcγRIIb potentiated the neutralizing activities of gp41 MPER-specific Abs while having little if any effect on other HIV-1-specific neutralizing Abs. Thus, both FcγRs augmented the potencies of broadly neutralizing monoclonal Abs 2F5 and 4E10, the nonneutralizing monoclonal Ab 98.6, and an HIV-1-positive plasma sample (BB34) that is known to contain gp41 MPER-specific neutralizing Abs. The FcγRI effect was quite substantial, potentiating the neutralizing activity of gp41 MPER-specific monoclonal Abs by as much as 5,000-fold depending on the virus. The FcγRIIb effect was not as great but was detected with several viruses. Engagement of FcγRI and FcγRIIb was shown to require the Fc segment of Ab, because no effect on neutralization was seen when a Fab version of 4E10 was tested.
Positive effects on HIV-1 neutralization were limited to FcγRI and FcγRIIb in that FcγRIIa and FcγRIIIa exerted little if any effect regardless of the monoclonal Ab or HIV-1-positive plasma tested. An element of epitope specificity was clearly involved, since no effect was seen with two gp120-specific monoclonal Abs (b12 and 2G12). A paucity of gp41 MPER-specific Abs might explain why HIV-1-positive plasma samples were minimally affected by the two FcγRs. In fact, only a minor fraction of the overall neutralizing activity in plasma samples from most HIV-1-infected individuals has been attributed to gp41 MPER-specific Abs (7, 88, 89, 108). Restriction of the FcγR effect to gp41 MPER-specific Abs could not be explained by IgG subclass, because IgG1 versions of b12 and 2G12 were not affected whereas IgG1 versions of 2F5 and 4E10 were. The restriction of this effect to MPER-specific Abs also could not be explained by differences in relative binding efficiencies, because b12 and 2G12 bound FcγRI about as well as did 4E10.
A critical question to ask is whether the FcγRI and FcγRIIb effects on HIV-1 neutralization seen here are predictive of biological consequences of IC engagement of these receptors in vivo. First, the TZM-bl cell line is not part of the immune system. Second, some cellular functions of FcγR might not be predicted by an effect on neutralization in TZM-bl cells. For example, FcγRIIIa was able to bind aggregated IgG1 and IgG3 subclasses quite well, but this receptor had no obvious effect on the neutralizing activity of either subclass of 2F5 and 4E10 in TZM-bl cells. Third, FcγRIIIa is often the only FcγR on natural killer cells, where it can play a major role in mediating ADCC when peripheral blood mononuclear cells are used as effectors. Such assays have been used to demonstrate ADCC activity with IgG1 versions of monoclonal Abs b12 (42, 43), 2G12 (97), and 98.6 (99), but yet these Abs showed no evidence of an FcγRIIIa effect on HIV-1 neutralization in our study. Finally, the high-affinity FcγRI binds mIgG and IC with similar affinities, which might account for our observation that 5% normal human serum abolished the FcγRI effect on 2F5 and 4E10. Competition from circulating mIgG may be expected in vivo, but it is not clear to what extent this will diminish the FcγRI effect on HIV-1 neutralization. The concentration of gp41 MPER-specific Abs relative to total IgG could be a determining factor, which might explain why FcγRI augmented the neutralizing activity of HIV-1-positive plasma sample BB34 in the context of total IgG. Similarly, we detected a positive FcγRI effect on 2F5 and 4E10 spiked at 0.2 mg/ml in normal human serum prior to assay (data not shown). Indeed, evidence in mice suggests that competition from circulating mIgG does not necessarily disable the specific functions of FcγRI in vivo (4, 6, 41, 50).
The mechanism by which FcγRI and FcγRIIb potentiated the neutralizing activities of gp41 MPER-specific Abs is unclear at this time; however, at least two mechanisms seem possible. One is that by prepositioning gp41 MPER-specific Abs at the cell surface, FcγRs might give Abs a kinetic advantage for virus inhibition. This kinetic advantage could be unique to gp41 MPER-specific Abs, whose epitopes are thought to be exposed for only a short time on a prehairpin intermediate conformation of gp41 during an early stage of fusion (30, 36). Support for this mechanism comes from our evidence that HIV-1 IC formation was not necessary prior to Ab-FcγR engagement in order for FcγRI and FcγRIIb to augment the neutralizing activities of 2F5 and 4E10 (Fig. 7). It has been suggested that the physical space surrounding the gp41 prehairpin intermediate is sterically constrained (54), where favorable access to Ab is achieved when the Fab is oriented parallel to the virus and cell membranes (14, 93). In this regard, the Fab portions of 4E10 and 2F5 appear to possess nonspecific lipid binding properties that have been suggested to provide a kinetic advantage for epitope binding (1, 30). The lipophilic character of these two monoclonal Abs has been attributed to the apex of the long hydrophobic CDR H3 loop, distal from the antigen contact residues at the base of CDR H3 (14, 70).
Given these unusual features of gp41 MPER Ab-epitope interactions, it is possible that FcγR engagement stabilizes a favorable orientation of Abs at the sterically constrained virus-cell interface to provide an additional kinetic advantage for binding membrane lipid and/or the prehairpin intermediate conformation of gp41. The lower hinge region of Ab-Fc has been shown to dock with the membrane-proximal D2 domain of FcR (105), which, in principle, could approximate the Fab close to the cell membrane. Further positioning of Fab could be influenced by differences in the flexibility of the hinge regions of various IgG subclasses (20, 81, 87), possibly explaining why FcγRI and FcγRIIb exerted a somewhat stronger effect on the IgG1 versions than on the IgG3 versions of 2F5 and 4E10 despite stronger binding of IgG3 by both receptors. This mechanism also offers a possible explanation for why FcγRIIb, with its much lower affinity for mIgG, exerted a weaker effect on the neutralizing activities of 2F5 and 4E10 than the high-affinity FcγRI receptor. Perhaps other neutralizing Abs were not affected by FcγR because their epitopes are less cryptic and thus allow more favorable energetics of Ab binding than do epitopes in the MPER of gp41.
Another mechanism by which FcγRs could potentially facilitate HIV-1 neutralization is phagocytosis. HeLa cells, from which the TZM-bl cell line was constructed, are known to exhibit properties of nonprofessional phagocytes (12, 32, 37). Thus, it is possible that TZM-bl cells were converted to professional phagocytic cells by introducing FcγR on their surface. Phagocytic destruction of preformed HIV-1 IC might explain why a full FcγR effect on neutralization was not seen when cells were preincubated with either 2F5 or 4E10 and nonbound Abs were removed prior to adding virus (Fig. 7).
Much like our findings, Holl et al. reported dramatic increases in the neutralizing activities of IgG1 versions of 2F5 and 4E10 and a much smaller effect on IgG1-b12 in primary cultures of human monocyte-derived macrophages and dendritic cells (DC) (44-46). Their studies also demonstrated a requirement for the Fc region of Ab and provided evidence for the involvement of FcγRI and FcγRII. Thus, by individual expression of human FcγRs in an epithelial cell line, our results reproduced their findings with broadly neutralizing monoclonal Abs against the MPER of gp41. Nonetheless, not all results were concordant, and in fact, our results were discordant with theirs for several monoclonal Abs, including 240-D, 246-D, F240, and 98.6. Discordant results with these highly strain-specific neutralizing Abs might be explained by different epitopes on the separate viruses used in the two studies or by the unusually high levels of CCR5 on TZM-bl cells (18).
Any FcγR-mediated antiviral effects on gp41 MPER-specific neutralizing Abs, whether by entry inhibition or phagocytosis, might be beneficial to several cell types in a setting of preexposure vaccination. For example, though FcγRs are rarely expressed on CD4+ lymphocytes, several additional HIV-1-susceptible cell types express multiple FcγRs and are involved in sexual transmission and the early establishment of long-lived viral reservoirs. In particular, macrophages may be among the first infection-susceptible cells that the virus encounters after mucosal exposure (31, 95), and they are thought to serve as a long-lived virus reservoir in chronic infection (9, 15). Macrophages are well known to express multiple FcγRs, including the recent demonstration of FcγRI, FcγRII, and FcγRIII expression on HIV-1-susceptible vaginal macrophages (90). Other HIV-1-susceptible cells that might benefit from the FcγR effect on gp41 MPER-specific neutralizing Abs include certain subsets of monocytes (57, 109) and DC (24, 73, 91).
In addition to these antiviral effects, an intriguing aspect of FcγRI and FcγRII is the role that they play in regulating adaptive immunity and peripheral tolerance (23, 68, 69). Indeed, it has been suggested that these receptors on DC play a role in regulating antigen uptake, antigen presentation, and cell activation (68, 82). Moreover, with the exception of T cells and natural killer cells, FcγRIIb is widely expressed on cells of the immune system, and in particular, it is the only FcγR on B cells (77). Inhibitory FcγRIIb plays an important role in regulating B-cell tolerance either by ITIM-mediated inhibition of B-cell receptor signaling or by inducing apoptosis independently of ITIM (79). In mice, both mechanisms appear to be capable of eliminating autoreactive class-switched B cells that escaped earlier checkpoints (68). The importance of this late checkpoint is further emphasized by a high susceptibility to autoimmune disease in FcγRIIb-deficient mice and humans (68).
FcγRIIb-mediated B-cell tolerance could be particularly relevant to 2F5 and 4E10, both of which have been suggested to be rare products of autoreactive B cells that escaped tolerance (1, 39). Under normal conditions, these autoreactive B cells might be eliminated from the body, which offers a partial explanation for why gp41 MPER-specific neutralizing Abs are uncommon in HIV-1-infected individuals (40). Our results showing that gp41 MPER-specific Abs interact with the inhibitory FcγRIIb are suggestive of a possible peripheral tolerance mechanism in which FcγRIIb-bearing B cells serve as a checkpoint for some Ab specificities without affecting other HIV-1-specific neutralizing Abs. We note that this mechanism is suggested based on neutralizing activity and that we have not determined whether this interaction leads to B-cell signaling. Interestingly, one HIV-1-positive plasma sample that is known to contain rare gp41 MPER-specific neutralizing Abs (BB34) was affected by FcγRI but was not affected by the potentially tolerizing FcγRIIb, possibly explaining why the Abs were able to be made in this individual. Likewise, the original 2F5 and 4E10 monoclonal Abs were isolated as IgG3 and, unlike their IgG1 counterparts, were not affected by FcγRIIb. It follows that a gp41 MPER-containing immunogen designed in some manner to circumvent FcγRIIb might evade tolerance and elicit broadly neutralizing Abs. Under these conditions, the introduction of additional modifications to increase engagement of activating FcγR on DC might lead to further improvement in immunogenicity, including the induction of virus-specific T-cell responses. Examples of such vaccine approaches are under consideration for the treatment of cancer (22, 52, 66, 76) and might prove useful if applied to HIV-1 vaccines. Safe application would depend in part on finding creative ways to circumvent FcγRIIb without unleashing pathogenic autoreactive cells that are normally held in check by this inhibitory receptor.
Finally, the medium- to low-affinity FcγRIIa and FcγRIIIa, which had no effect on HIV-1 neutralization, are encoded by genes with functional polymorphisms that affect ligand binding. The FcγRIIa gene can encode either a histidine (H) or an arginine (R) at position 131 (102). FcγRIIa containing H at this position binds human IgG2 and IgG3 with considerably higher affinity than receptors from RR individuals (23, 72). In the case of HIV-1, it has been shown that HIV-1 IC are more efficiently internalized by monocytes from HH subjects than by subjects who carry the RR alleles (28). The FcγRIIIa gene can encode either a phenylalanine (F) or valine (V) at position 158 (80). IgG1 and IgG3bind with higher affinity to the V form of this receptor (23, 100). In the present study, the separate cell lines expressed the H form of FcγRIIa and the F form of FcγRIIIa as being more prevalent in humans (56, 59, 92, 101).
In summary, FcγRI and FcγRIIb augmented the neutralizing activities of HIV-1 gp41 MPER-specific Abs by a mechanism that was highly dependent on the epitope and moderately dependent on the IgG subclass of the Ab. Though the mechanism of this FcγR effect is not clear, it could involve either enhanced inhibition of virus entry, phagocytosis, or both. In addition, the interaction of HIV-1 IC with FcγRIIb suggests a mechanism that could contribute to B-cell tolerance. These findings indicate that gp41 MPER-specific Abs are of greater importance than previously recognized. The findings also suggest new avenues to pursue to induce such Abs with vaccines.
Acknowledgments
We thank Jeffrey Ravetch for FcγR cDNA clones; Lynn Morris for the South African HIV-1-positive plasma samples; Suzan Zolla-Pazner, James Robinson, Dimiter Dimitrov, and the NIH AIDS Research & Reference Reagent Program for monoclonal Abs; the Duke Center for AIDS Research (CFAR) Flow Cytometry Facility for cell analyses; and Stephen Harrison and Ellis Reinherz for valuable advice when writing the manuscript. We also thank Anthony Geonnotti for critical reading of the manuscript.
This work was support by the National Institutes of Health, the Center for HIV/AIDS Vaccine Immunology (CHAVI), and the Duke CFAR.
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Alam, S. M., M. McAdams, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 1784424-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baravalle, G., M. Brabec, L. Snyers, D. Blaas, and R. Fuchs. 2004. Human rhinovirus type 2-antibody complexes enter and infect cells via Fcγ receptor IIB1. J. Virol. 782729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbato, G., E. Bianchi, P. Ingallinella, W. H. Hurni, M. D. Miller, G. Ciliberto, R. Cortese, R. Bazzo, J. W. Shiver, and A. Pessi. 2003. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J. Mol. Biol. 3301101-1115. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, N., A. L. Gavin, P. S. Tan, P. Mottram, F. Koentgen, and P. M. Hogarth. 2002. FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 16379-389. [DOI] [PubMed] [Google Scholar]
- 5.Baum, L. L., K. J. Cassutt, K. Knigge, R. Khattri, J. Margolick, C. Rinaldo, C. A. Kleeberger, P. Nishanian, D. R. Henrard, and J. Phair. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 1572168-2173. [PubMed] [Google Scholar]
- 6.Bevaart, L., M. J. H. Jansen, M. J. van Vugt, J. S. Verbeek, J. G. J. van de Winkel, and J. H. W. Leusen. 2006. The high-affinity IgG receptor, FcγRI, plays a central role in antibody therapy of experimental melanoma. Cancer Res. 661261-1264. [DOI] [PubMed] [Google Scholar]
- 7.Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of human immunodeficiency virus type 1 plasmas from subtype B and C chronic infections. J. Virol. 8211651-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blish, C. A., R. Nedellec, K. Mandaliya, D. E. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21693-702. [DOI] [PubMed] [Google Scholar]
- 9.Brown, A., H. Zhang, P. Lopez, C. A. Pardo, and S. Gartner. 2006. In vitro modeling of the HIV-macrophage reservoir. J. Leukoc. Biol. 801127-1135. [DOI] [PubMed] [Google Scholar]
- 10.Bruhns, P., B. Iannascoli, P. England, D. A. Mancardi, N. Fernandez, S. Jorieux, and M. Daëron. 2009. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 1133716-3725. [DOI] [PubMed] [Google Scholar]
- 11.Brunel, F. M., M. B. Zwick, R. M. F. Cardoso, J. D. Nelson, I. A. Wilson, D. R. Burton, and P. E. Dawson. 2006. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J. Virol. 801680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne, G. I., and J. W. Moulder. 1978. Parasite-specific phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect. Immun. 19598-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 3002065-2071. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso, R. M. F., M. B. Zwick, R. L. Stanfield, R. Kunert, J. M. Binley, H. Kaninger, D. R. Burton, and I. A. Wilson. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22163-173. [DOI] [PubMed] [Google Scholar]
- 15.Cassol, E., M. Alfano, P. Biswas, and G. Poli. 2006. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J. Leukoc. Biol. 801018-1030. [DOI] [PubMed] [Google Scholar]
- 16.Cavacini, L., C. Emes, A. Wisnewski, J. Power, G. Lewis, D. Montefiori, and M. Posner. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res. Hum. Retroviruses. 141271-1280. [DOI] [PubMed] [Google Scholar]
- 17.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93681-684. [DOI] [PubMed] [Google Scholar]
- 18.Choudhry, V., M.-Y. Zhang, I. Harris, I. A. Sidorov, B. Vu, A. S. Dimitrov, T. Fouts, and D. S. Dimitrov. 2006. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem. Biophys. Res. Commun. 3481107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor, R. I., N. B. Dinces, A. L. Howell, J. L. Romet-Lemonne, J. L. Pasquali, and M. W. Fanger. 1991. Fc receptors for IgG (FcγRs) on human monocytes and macrophages are not infectivity receptors for human immunodeficiency virus type 1 (HIV-1): studies using bispecific antibodies to target HIV-1 to various myeloid cell surface molecules, including the FcγR. Proc. Natl. Acad. Sci. USA 889593-9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dall'Acqua, W. F., K. E. Cook, M. M. Damschroder, R. M. Woods, and H. Wu. 2006. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J. Immunol. 1771129-1138. [DOI] [PubMed] [Google Scholar]
- 21.David, A., A. Saez-Cirion, P. Versmisse, O. Malbec, B. Iannascoli, F. Herschke, M. Lucas, F. Barre-Sinoussi, J.-F. Mouscadet, M. Daeron, and G. Pancino. 2006. The engagement of activating FcγRs inhibits primate lentivirus replication in human macrophages. J. Immunol. 1776291-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desjarlais, J. R., G. A. Lazar, E. A. Zhukovsky, and S. Y. Chu. 2007. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov. Today 12898-910. [DOI] [PubMed] [Google Scholar]
- 23.Dijstelbloem, H. M., C. G. M. Kallenberg, and J. G. J. van de Winkel. 2001. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 22510-516. [DOI] [PubMed] [Google Scholar]
- 24.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 982574-2576. [DOI] [PubMed] [Google Scholar]
- 25.Ernst, L. K., A. M. Duchemin, and C. L. Anderson. 1993. Association of the high-affinity receptor for IgG (FcγRI) with the gamma subunit of the IgE receptor. Proc. Natl. Acad. Sci. USA 906023-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez, N., M. Renedo, C. Garcia-Rodriguez, and M. Sanchez Crespo. 2002. Activation of monocytic cells through Fcγ receptors induces the expression of macrophage-inflammatory proteins MIP-1α, MIP-1β and RANTES. J. Immunol. 1693321-3328. [DOI] [PubMed] [Google Scholar]
- 27.Forthal, D. N., P. B. Gilbert, G. Landucci, and T. Phan. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J. Immunol. 1786596-6603. [DOI] [PubMed] [Google Scholar]
- 28.Forthal, D. N., G. Landucci, J. Bream, L. P. Jacobson, T. B. Phan, and B. Montoya. 2007. FcγRIIa genotype predicts progression of HIV infection. J. Immunol. 1797916-7923. [DOI] [PubMed] [Google Scholar]
- 29.Forthall, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural killer effector cells. J. Virol. 756953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey, G., H. Peng, S. Rits-Volloch, M. Morelli, Y. Cheng, and B. Chen. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA 1053739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvin, S. R., and M. S. Cohen. 2006. Genital tract reservoirs. Curr. Opin. HIV AIDS 1162-166. [DOI] [PubMed] [Google Scholar]
- 32.Ghigo, E., C. Capo, M. Aurouze, C. H. Tung, J. P. Gorvel, D. Raoulty, and J. L. Mege. 2002. Survival of Tropheryma whipplei, the agent of Whipple's disease, requires phagosome acidification. Infect. Immun. 701501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goncalvez, A. P., R. E. Engle, M. St. Claire, R. H. Purcell, and C.-J. Lai. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 1049422-9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorny, M. K., V. Gianakakos, S. Sharpe, and S. Zolla-Pazner. 1989. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 861624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselin, E. J., K. Wardwell, D. R. Gosselin, N. Alter, J. L. Fisher, and P. M. Guyre. 1992. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J. Immunol. 1493477-3481. [PubMed] [Google Scholar]
- 36.Gustchina, E., C. A. Bewley, and G. M. Clore. 2008. Sequestering of the prehairpin intermediate of gp41 by peptide N36Mut(e,g) potentiates the human immunodeficiency virus type 1 neutralizing activity of monoclonal antibodies directed against the N-terminal helical repeat of gp41. J. Virol. 8210032-10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzmán-Verri, C., E. Chaves-Olarte, C. von Eichel-Streiber, I. López-Goňi, M. Thelestam, S. Arvidson, J-P Gorvel, and E. Moreno. 2001. GTPases of the Rho subfamily are required for Brucella abortus internalization of nonprofessional phagocytes. J. Biol. Chem. 27644435-44443. [DOI] [PubMed] [Google Scholar]
- 38.Hager-Braun, C., H. Katinger, and K. B. Tomer. 2006. The HIV-neutralizing monoclonal antibody 4E10 recognizes N-terminal sequences on the native antigen. J. Immunol. 1767471-7481. [DOI] [PubMed] [Google Scholar]
- 39.Haynes, B. F., J. Fleming, E. W. St Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 3081906-1908. [DOI] [PubMed] [Google Scholar]
- 40.Haynes, B. F., M. A. Moody, L. Verkoczy, G. Kelsoe, and S. M. Alam. 2005. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum. Antibodies 1459-67. [PMC free article] [PubMed] [Google Scholar]
- 41.Heijnen, I. A., M. J. van Vugt, N. A. Fanger, R. F. Graziano, T. P. de Wit, F. M. Hofhuis, P. M. Guyre, P. J. Capel, J. S. Verbeek, and J. G. van de Winkel. 1996. Antigen targeting to myeloid-specific human Fcγ RI/CD64 triggers enhanced antibody responses in transgenic mice. J. Clin. Investig. 97331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hessell, A. J., L. Hangartner, M. Hunter, C. E. G. Havenith, F. J. Beurskens, J. M. Bakker, C. M. S. Lanigan, G. Landucci, D. N. Forthal, P. W. H. I. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449101-104. [DOI] [PubMed] [Google Scholar]
- 43.Hezareh, M., A. J. Hessell, R. C. Jensen, J. G. J. van de Winkel, and P. W. H. I. Parren. 2001. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 7512161-12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holl, V., S. Hemmerter, R. Burrer, S. Schmidt, A. Bohbot, A.-M. Aubertin, and C. Moog. 2004. Involvement of FcγR I (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 1736274-6283. [DOI] [PubMed] [Google Scholar]
- 45.Holl, V., M. Peressin, T. Decoville, S. Schmidt, S. Zolla-Pazner, A.-M. Aubertin, and C. Moog. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 806177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holl, V., M. Peressin, S. Schmidt, T. Decoville, S. Zolla-Pazner, A.-M. Aubertin, and C. Moog. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 1074466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homsy, J., M. Meyer, M. Tateno, S. Clarkson, and J. A. Levy. 1989. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 2441357-1360. [DOI] [PubMed] [Google Scholar]
- 48.Huber, V. C., J. M. Lynch, D. J. Bucher, J. Le, and D. W. Metzger. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 1667381-7388. [DOI] [PubMed] [Google Scholar]
- 49.Hulett, M. D., and P. M. Hogarth. 1994. Molecular basis of Fc receptor function. Adv. Immunol. 571-127. [DOI] [PubMed] [Google Scholar]
- 50.Ioan-Facsinay, A., S. J. de Kimpe, S. M. M. Hellwig, P. L. van Lent, F. M. A. Hofhuis, H. H. van Ojik, C. Sedlik, S. A. da Silveira, J. Gerber, Y. F. de Jong, R. Roozendaal, L. A. Aarden, W. B. van den Berg, T. Saito, D. Mosser, S. Amigorena, S. Izui, G. J. B. van Ommen, M. van Vugt, J. G. J. van de Winkel, and J. S. Verbeek. 2002. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 16391-402. [DOI] [PubMed] [Google Scholar]
- 51.Jouault, T., F. Chapuis, R. Olivier, C. Parravicini, E. Bahraoui, and J.-C. Gluckman. 1989. HIV infection of monocytic cells: role of antibody-mediated virus binding to Fcγ receptors. AIDS 3125-133. [PubMed] [Google Scholar]
- 52.Kalergis, A. M., and J. V. Ravetch. 2002. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J. Exp. Med. 1951653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L.-H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 1057552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein, J. S., P. N. P. Gnanapragasam, R. P. Galimidi, C. P. Foglesong, A. P. West, Jr., and P. J. Bjorkman. 2009. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc. Natl. Acad. Sci. USA 1067385-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuster, H., H. Thompson, and J. P. Kinet. 1990. Characterization and expression of the gene for the human Fc receptor γ subunit. Definition of a new gene family. J. Biol. Chem. 2656448-6452. [PubMed] [Google Scholar]
- 56.Kyogoku, C., N. Tsuchiya, K. Matsuta, and K. Tokunaga. 2002. Studies on the association of Fcγ receptor IIA, IIB, IIIA and IIIB polymorphisms with rheumatoid arthritis in the Japanese: evidence for a genetic interaction between HLA-DRB1 and FCGR3A. Genes Immun. 3488-493. [DOI] [PubMed] [Google Scholar]
- 57.Lambotte, O., Y. Taoufik, M. G. de Goer, C. Wallon, C. Goujard, and J. F. Delfraissy. 2000. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 23114-119. [DOI] [PubMed] [Google Scholar]
- 58.Laurence, J., A. Saunders, E. Early, and J. E. Salmon. 1990. Human immunodeficiency virus infection of monocytes: relationship to Fcγ receptors and antibody-dependent viral enhancement. Immunology 70338-343. [PMC free article] [PubMed] [Google Scholar]
- 59.Lehrnbecher, T., C. B. Foster, S. Zhu, S. F. Leitman, L. R. Goldin, K. Huppi, and S. J. Chanock. 1999. Variant genotypes of the low-affinity Fcγ receptors in two control populations and a review of low-affinity Fcγ receptor polymorphisms in control and disease populations. Blood 944220-4232. [PubMed] [Google Scholar]
- 60.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li, M., J. F. Salazar-Gonzalez, C. A. Derdeyn, L. Morris, C. Williamson, J. E. Robinson, J. M. Decker, Y. Li, M. G. Salazar, V. R. Polonis, K. Mlisana, S. A. Karim, K. Hong, K. M. Greene, M. Bilska, J. Zhou, S. Allen, E. Chomba, J. Mulenga, C. Vwalika, F. Gao, M. Zhang, B. T. M. Korber, E. Hunter, B. H. Hahn, and D. C. Montefiori. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in southern Africa. J. Virol. 8011776-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLain, L., and N. J. Dimmock. 1997. A human CD4+ T-cell line expresses functional CD64 (FcγRI), CD32 (FcγRII), and CD16 (FcγRIII) receptors but these do not enhance the infectivity of HIV-1-IgG complexes. Immunology 90109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montefiori, D. C. 1997. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin. Immunopathol. 18371-390. [DOI] [PubMed] [Google Scholar]
- 64.Montero, M., N. E. van Houten, X. Wang, and J. K. Scott. 2008. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 7254-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272263-267. [DOI] [PubMed] [Google Scholar]
- 66.Nimmerjahn, F., and J. V. Ravetch. 2007. Antibodies, Fc receptors and cancer. Curr. Opin. Immunol. 19239-245. [DOI] [PubMed] [Google Scholar]
- 67.Nimmerjahn, F., and J. V. Ravetch. 2005. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 3101510-1512. [DOI] [PubMed] [Google Scholar]
- 68.Nimmerjahn, F., and J. V. Ravetch. 2008. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 834-47. [DOI] [PubMed] [Google Scholar]
- 69.Nimmerjahn, F., and J. V. Ravetch. 2006. Fcγ receptors: old friends and new family members. Immunity 2419-28. [DOI] [PubMed] [Google Scholar]
- 70.Ofek, G., M. Tang, A. Sambor, H. Katinger, J. R. Mascola, R. Wyatt, and P. D. Kwong. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 7810724-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pantophlet, R., E. Ollmann Saphire, P. Poignard, P. W. H. I. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parren, P. W. H. I., P. A. M. Warmerdam, L. C. M. Boeije, J. Arts, N. A. C. Westerdaal, A. Vlug, P. J. A. Capel, L. A. Aarden, and J. G. J. van de Winkel. 1992. On the interaction of IgG subclasses with the low affinity FcγRIIa (CD32) on human monocytes, neutrophils, and platelets. J. Clin. Investig. 901537-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patterson, S., A. Rae, N. Hockey, J. Gilmore, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 756710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 722855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Porges, A. J., P. B. Redecha, R. Doebele, L. C. Pan, J. E. Salmon, and R. P. Kimberly. 1992. Novel Fcγ receptor I family gene products in human mononuclear cells. J. Clin. Investig. 902102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pricop, L., P. Redecha, J. L. Teilaud, J. Frey, W. H. Fridman, C. Sautes-Fridman, and J. E. Salmon. 2001. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 166531-537. [DOI] [PubMed] [Google Scholar]
- 77.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19275-290. [DOI] [PubMed] [Google Scholar]
- 78.Ravetch, J. V., and J. P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9457-492. [DOI] [PubMed] [Google Scholar]
- 79.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 29084-89. [DOI] [PubMed] [Google Scholar]
- 80.Ravetch, J. V., and B. Perussia. 1989. Alternative membrane forms of FcγRIII(CD16) on human natural killer cells and neutrophils: cell type-specific expression of two genes that differ in single nucleotide substitutions. J. Exp. Med. 170481-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redpath, S., T. E. Michaelson, I. Sandlie, and M. R. Clark. 1998. The influence of the hinge region length in binding of human IgG to human Fcγ receptors. Hum. Immunol. 59720-727. [DOI] [PubMed] [Google Scholar]
- 82.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatability complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson, W. E., Jr., M. K. Gorny, J. Y. Xu, W. M. Mitchell, and S. Zolla-Pazner. 1991. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J. Virol. 654169-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodrigo, W. W. S. I., X. Jin, S. D. Blackley, R. C. Rose, and J. J. Schlesinger. 2006. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human FcγRIA (CD64) or FcγRIIA (CD32). J. Virol. 8010128-10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 767293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saphire, E. O., P. W. H. I. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2001. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science 2931155-1159. [DOI] [PubMed] [Google Scholar]
- 87.Saphire, E. O., R. L. Stanfield, M. D. M. Crispin, P. W. H. I. Parren, P. M. Rudd, R. A. Dwek, D. R. Burton, and I. A. Wilson. 2002. Contrasting IgG structures reveal extreme asymmetry and flexibility. J. Mol. Biol. 3199-18. [DOI] [PubMed] [Google Scholar]
- 88.Sather, D. N., J. Armann, L. K. Ching, A. Mavrantoni, G. Sellhorn, Z. Caldwell, X. Yu, B. Wood, S. Self, S. Kalams, and L. Stamatatos. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scheid, J. F., H. Mouquet, N. Feldhahn, M. S. Seaman, K. Velinzon, J. Pietzsch, R. G. Ott, R. M. Anthony, H. Zebroski, A. Hurley, A. Phogat, B. Chakrabarti, Y. Li, M. Connors, F. Pereyra, B. D. Walker, H. Wardemann, D. Ho, R. T. Wyatt, J. R. Mascola, J. V. Ravetch, and M. C. Nussenzweig. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 4586360640. [DOI] [PubMed] [Google Scholar]
- 90.Shen, R., H. E. Richter, R. H. Clements, L. Novak, K. Huff, D. Bimczok, S. Sankara-Walters, S. Dandekar, P. R. Clapham, L. E. Smythies, and P. D. Smith. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 833258-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sullivan, K. E., A. F. Jawad, L. M. Piliero, N. Kim, X. Luan, D. Goldman, and M. Petri. 2003. Analysis of polymorphisms affecting immune complex handling in systemic lupus erythematosus. Rheumatology 42446-452. [DOI] [PubMed] [Google Scholar]
- 93.Sun, Z. Y. J., K. J. Oh, M. Kim, J. Yu, V. Brusic, L. Song, Z. Qiao, J. H. Wang, G. Wagner, and E. L. Reinherz. 2008. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 2852-63. [DOI] [PubMed] [Google Scholar]
- 94.Takeda, A., C. U. Tuazon, and F. A. Ennis. 1988. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science 242580-583. [DOI] [PubMed] [Google Scholar]
- 95.Trapp, S., S. G. Turville, and M. Robbian. 2006. Slamming the door on unwanted guests: why preemptive strikes at the mucosa may be the best strategy against HIV. J. Leukoc. Biol. 801076-1083. [DOI] [PubMed] [Google Scholar]
- 96.Trischmann, H., D. Davis, and P. J. Lachmann. 1995. Lymphocytic strains of HIV-1 when complexed with enhancing antibodies can infect macrophages via Fcγ RIII, independently of CD4. AIDS Res. Hum. Retroviruses 11343-352. [DOI] [PubMed] [Google Scholar]
- 97.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 701100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tyler, D. S., C. L. Nastala, S. D. Stanley, T. J. Matthews, H. K. Lyerly, D. P. Bolognesi, and K. J. Weinhold. 1989. GP120 specific cellular cytotoxicity in HIV-1 seropositive individuals. Evidence for circulating CD16+ effector cells armed in vivo with cytophilic antibody. J. Immunol. 1421177-1182. [PubMed] [Google Scholar]
- 99.Tyler, D. S., S. D. Stanley, S. Zolla-Pazner, M. K. Gorny, P. P. Shadduck, A. J. Langlois, T. J. Matthews, D. P. Bolognesi, T. J. Palker, and K. J. Weinhold. 1990. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J. Immunol. 1453276-3282. [PubMed] [Google Scholar]
- 100.Vance, B. A., T. W. Huizinga, K. Wardwell, and P. M. Guyre. 1993. Binding of monomeric human IgG defines an expression polymorphism of Fcγ RIII on large granular lymphocyte/natural killer cells. J. Immunol. 1516429-6439. [PubMed] [Google Scholar]
- 101.Van der Pol, W. L., M. D. Jansen, W. J. Sluiter, B. van de Sluis, F. G. Leppers-van de Straat, T. Kobayashi, R. G. Westendorp, T. W. Huizinga, and J. G. van de Winkel. 2003. Evidence for non-random distribution of Fcγ receptor genotype combinations. Immunogenetics 55240-246. [DOI] [PubMed] [Google Scholar]
- 102.Warmerdam, P. A., J. G. van de Winkel, A. Vlug, N. A. Westerdaal, and P. J. Capel. 1991. A single amino acid in the second Ig-like domain of the human Fcγ receptor II is critical for human IgG2 binding. J. Immunol. 1471338-1343. [PubMed] [Google Scholar]
- 103.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 105.Woof, J. M., and D. R. Burton. 2004. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. 41-11. [DOI] [PubMed] [Google Scholar]
- 106.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 2801884-1888. [DOI] [PubMed] [Google Scholar]
- 107.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 654832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuste, E., H. B. Sanford, J. Carmody, J. Bixby, S. Little, M. B. Zwick, T. Greenough, D. R. Burton, D. D. Richman, R. C. Desrosiers, and W. E. Johnson. 2006. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV-1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 803030-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu, T., D. Muthui, S. Holte, D. Nickle, F. Feng, S. Brodie, Y. Hwangbo, J. I. Mullins, and L. Corey. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 7510892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]