Abstract
Primary HIV-1 infection (PHI) is marked by a flu-like syndrome and high levels of viremia that decrease to a viral set point with the first emergence of virus-specific CD8+ T-cell responses. Here, we investigated in a large cohort of 527 subjects the immunodominance pattern of the first virus-specific cytotoxic T-lymphocyte (CTL) responses developed during PHI in comparison to CTL responses in chronic infection and demonstrated a distinct relationship between the early virus-specific CTL responses and the viral set point, as well as the slope of CD4+ T-cell decline. CTL responses during PHI followed clear hierarchical immunodominance patterns that were lost during the transition to chronic infection. Importantly, the immunodominance patterns of human immunodeficiency virus type 1 (HIV-1)-specific CTL responses detected in primary, but not in chronic, HIV-1 infection were significantly associated with the subsequent set point of viral replication. Moreover, the preservation of the initial CD8+ T-cell immunodominance patterns from the acute into the chronic phase of infection was significantly associated with slower CD4+ T-cell decline. Taken together, these data show that the specificity of the initial CTL response to HIV is critical for the subsequent control of viremia and have important implications for the rational selection of antigens for future HIV-1 vaccines.
In the first weeks after human immunodeficiency virus type 1 (HIV-1) acquisition, viral loads peak at high levels, accompanied by a flu-like syndrome (15). A rapid depletion of the CD4+ T-cell population occurs during this acute infection, in particular, within the gastrointestinal tract-associated lymphoid tissue (6, 19, 20), marking a nonrecoverable scar on the immune system. With the resolution of the clinical syndromes, viral loads decrease to a set point, which persists at this level for months to years until progressive CD4+ T-cell decline results in the onset of AIDS. It has been shown that the initial viral set point following primary infection is a very strong predictor of the disease-free period until the onset of AIDS (18, 21, 22).
The initial decrease in the viral load during primary HIV-1 infection (PHI) is temporally associated with the first emergence of virus-specific CD8+ T-cell responses, and several studies have provided strong evidence that HIV-1-specific CD8+ T-cell responses are capable of controlling viral replication (5, 16, 24, 25, 27, 31, 33). However, significant numbers of virus-specific CD8+ T cells are detectable both in chronically infected individuals who progress rapidly to AIDS and in those who do not experience HIV-1 disease progression for decades (1, 11), and the characteristics that define a protective HIV-1-specific CD8+ T-cell response are not known. In particular, the level of control over viral replication is not predicted by the overall breadth, magnitude, or function of virus-specific CD8+ T-cell responses in chronic HIV-1 infection (1, 4, 11, 26, 28).
Here, we demonstrate in a large cohort of individuals identified during PHI that immunodominance patterns of virus-specific CD8+ T-cell responses detected in PHI, but not in chronic HIV-1 infection, are strongly associated with the subsequent set point of viral replication. These data show that the specificity of the initial CD8+ T-cell response to HIV is critical for the subsequent control of viremia and have important implications for the rational selection of antigens for future HIV-1 vaccines.
MATERIALS AND METHODS
A total of 527 HIV-1-infected subjects participated in this study, including 99 subjects with untreated chronic HIV-1 infection enrolled at Massachusetts General Hospital in Boston and 428 subjects with PHI recruited from primary-infection cohorts in North America, Germany, and Australia (Massachusetts General Hospital, Boston, MA; Fenway Community Health Center, Boston, MA; AIDS Research Institute, University of California, San Francisco; McGill University, Montreal, Canada; Infection Network of the National Centre in HIV Epidemiology and Clinical Research, Darlinghurst, Australia; Jessen-Praxis, Berlin, Germany; and the University of California, San Diego) (Table 1) . A total of 224 (52%) of the subjects with PHI were identified during acute HIV-1 infection (AHI) (defined by negative HIV p24 antibody testing by enzyme-linked immunosorbent assay in the presence of detectable HIV-1 RNA or positive HIV p24 antibody testing by enzyme-linked immunosorbent assay and an evolving [≤3 bands positive] HIV Western blot), and 204 subjects (48%) were identified with early HIV-1 infection (EHI) (defined by documented HIV-1 acquisition within the previous 6 months). The study was approved by the respective institutional review boards and was conducted in accordance with the human experimentation guidelines of Massachusetts General Hospital.
TABLE 1.
Origins of acute and chronic samples
| Origin | No. (n = 527) | % |
|---|---|---|
| Boston | 252 | 48 |
| San Francisco | 82 | 16 |
| Montreal, Canada | 75 | 14 |
| Sydney, Australia | 66 | 13 |
| Berlin, Germany | 33 | 6 |
| San Diego | 19 | 4 |
HLA typing.
High- and intermediate-resolution HLA class I typing was performed by sequence-specific PCR according to standard procedures.
IFN-γ ELISPOT assay.
HIV-1-specific CD8+ T-cell responses were assessed with frozen peripheral blood mononuclear cell samples collected during untreated infection for chronically infected individuals or 8 weeks (±10 days) following diagnosis with PHI. HIV-1-specific CD8+ T-cell responses were quantified by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay, using a panel of 222 HLA class I matched optimal epitope responses as previously described (11). A response was considered positive only if there were ≥55 spot-forming cells (SFCs)/106 PBMC and SFC/106 PBMC at least three times greater than mean background of the SFC/106 PBMC in the negative wells and three times greater than the standard deviation of the SFC/106 PBMC within the negative controls.
Assessment of the viral set points for individuals identified during PHI.
Longitudinal viral-load data were collected from all study subjects. Two infectious-disease physicians independently determined the average viral set points for all untreated subjects. Only subjects with untreated HIV-1 infection and multiple (four or more) available viral loads 6 months after first presentation were included in the analysis. Subjects were excluded (i) if they had ever received antiretroviral therapy during the first 6 months of infection, (ii) if results for less than four independent viral-load measurements were available during the viral set point period, and (iii) if one of the two infectious-disease physicians concluded that no stable viral set point was reached. Viral set points were defined following the algorithm previously described by Fellay et al. (10). Viral set points were obtained for 110 out of 428 study subjects. The main reasons for the absence of a viral set point in the remaining 318 study subjects were as follows: treatment during acute infection (n = 242), loss to follow-up (n = 38), treatment before the establishment of a viral set point (n = 26), or lack of a stable viral set point (n = 12). The 110 subjects, for whom a viral set point was obtained were representative of the cohort of 428 subjects in terms of gender, race, and HLA distribution.
Assessment of the slope of CD4 decline and viral set points in subjects from the MACS cohort.
The calculation of the slope of CD4 decline was performed with clinical data derived from the Multicenter AIDS Cohort Study (MACS) (n = 322). For each HLA allele, the rate of CD4+ T-cell decline in individuals with the given allele was estimated using a mixed-effects model as previously described (3). The correlation coefficient comparing the CD4+ T-cell decline with the frequency of epitope recognition and the test for association using Pearson's product-moment correlation are reported. The mean log10 viral set point of the MACS cohort was determined 1.5 to 3 years after infection.
Assessment of CD8+ T-cell immunodominance and contribution.
An immunodominant epitope-specific CD8+ T-cell response was defined as a response to the most frequently recognized epitope restricted by the respective HLA class I allele in the study population. Similarly, the contribution of epitope-specific CD8+ T-cell responses to the total response was defined as the epitope-specific CD8+ T-cell response giving on average the highest IFN-γ secretion on the population level compared to the other measured epitope-specific responses in a given individual and, therefore, taking the magnitude and breadth of the each measured HLA-restricted CD8+ T-cell response into account. Each HLA class I allele was treated independently for this analysis; therefore, one subject might appear in the average of multiple data points for different HLA class I alleles.
Statistical analysis.
Statistical analysis and graphical presentation were done using Graph Pad Prism 5.0, Microsoft Excel, SAS 9.1 (SAS Institute), and R 2.7.1 (R Foundation for Statistical Computing, Vienna, Austria). The results are given as means ± standard deviations or medians with ranges. Correlations were assessed by Spearman rank analysis. Statistical analysis of significance (P values) was based on two-tailed t tests and linear regression analysis.
RESULTS AND DISCUSSION
The first emerging virus-specific cytotoxic T-lymphocyte (CTL) responses during PHI have been associated with the decrease in the HIV-1 viral load and the establishment of a set point of viral replication (5, 16). Here, we systematically assessed in a cohort of 428 subjects with PHI and 99 subjects with chronic HIV-1 infection the impacts of the immunodominance patterns of these early virus-specific CD8+ T-cell responses on the plasma viral load and CD4+ T-cell count. The study cohort consisted primarily of male subjects (>97%) of Northern European descent (87% Caucasian), and the HLA class I distribution reflected the general distribution in a Caucasian population (Table 2) (http://www.allelefrequencies.net). A total of 224 subjects enrolled in this study were identified during AHI (AHI group), while 204 subjects were identified within 6 months of infection (EHI group). The average peak viral load in the AHI group was 3,034,510 copies/ml (range, 399 to 95,000,000 copies/ml) and was significantly higher than in the EHI group (238,698 copies/ml; range, 49 to 8,470,000; P = 0.0001) (see Fig. S1 in the supplemental material). Following antiretroviral treatment guidelines, therapy initiation has been widely recommended in the acute phase of HIV-1 infection in the past, and the majority of subjects therefore received treatment during PHI. However, for 110 of the 428 subjects who remained treatment-naïve, a viral replication set point was defined, and this average viral set point was with 42,752 copies/ml (range, 49 to 588,000), significantly lower than the peak viral load in the AHI group (P < 0.0001) or the EHI group (P = 0.001) at baseline but similar to the viral load in a control cohort of 99 individuals enrolled with untreated viremic chronic HIV-1 infection (chronic-infection cohort; average viral load at time of presentation, 42,664 copies/ml; range, 49 to 988,000) (see Fig. S1 in the supplemental material)). The 110 individuals for whom a viral set point was determined did not differ in their clinical characteristics and HLA class I genotypes from the overall cohort of 428 individuals with PHI.
TABLE 2.
Major histocompatibility complex class 1 allele frequencies
| Allele | Frequency (%)
|
||
|---|---|---|---|
| Acute (n = 428) | Set point group (n = 110) | Chronic (n = 99) | |
| HLA-A | |||
| A1 | 25 | 26 | 22 |
| A2 | 54 | 49 | 42 |
| A3 | 25 | 27 | 25 |
| A11 | 13 | 20 | 10 |
| A23 | 5 | 5 | 5 |
| A24 | 15 | 21 | 11 |
| A25 | 5 | 5 | 5 |
| A26 | 5 | 6 | 6 |
| A28 | 0 | 0 | 0 |
| A29 | 5 | 0 | 8 |
| A30 | 6 | 4 | 10 |
| A31 | 3 | 0 | 2 |
| A32 | 5 | 0 | 10 |
| A33 | 4 | 4 | 7 |
| A68 | 9 | 7 | 14 |
| A74 | 1 | 0 | 5 |
| HLA-B | |||
| B7 | 19 | 21 | 17 |
| B8 | 19 | 15 | 12 |
| B14 | 7 | 4 | 6 |
| B15 | 13 | 13 | 16 |
| B18 | 11 | 7 | 13 |
| B27 | 6 | 9 | 7 |
| B35 | 19 | 15 | 15 |
| B37 | 3 | 0 | 2 |
| B38 | 3 | 5 | 5 |
| B39 | 4 | 5 | 2 |
| B40 | 13 | 19 | 7 |
| B42 | 0 | 0 | 2 |
| B44 | 27 | 23 | 26 |
| B50 | 3 | 0 | 4 |
| B51 | 11 | 10 | 14 |
| B52 | 2 | 0 | 4 |
| B53 | 4 | 0 | 8 |
| B55 | 4 | 0 | 2 |
| B57 | 7 | 8 | 10 |
| B58 | 3 | 0 | 9 |
| B62 | 1 | 0 | 0 |
| B71 | 0 | 0 | 0 |
| HLA-Cw | |||
| Cw1 | 7 | 10 | 9 |
| Cw3 | 18 | 23 | 22 |
| Cw4 | 25 | 22 | 32 |
| Cw5 | 16 | 11 | 19 |
| Cw6 | 11 | 11 | 24 |
| Cw7 | 50 | 47 | 37 |
| Cw8 | 9 | 6 | 12 |
| Cw12 | 11 | 14 | 17 |
| Cw14 | 1 | 0 | 2 |
| Cw15 | 5 | 5 | 8 |
| Cw18 | 1 | 0 | 0 |
HIV-1-specific CTL responses were quantified by IFN-γ ELISPOT assay against a panel of 222 previously described optimal HIV-1 epitopes in all 527 subjects (30). The peptides used for the screening of each study subject were selected based on the subject's HLA class I genotype. Study participants were tested for CTL responses directed against a median of 37 (range, 5 to 66) optimal HIV-1-specific CTL epitopes in the primary-infection cohort and 35 (range, 7 to 69) optimal epitopes in the chronic-infection cohort (P = 0.64). An average of 5 peptides (range, 0 to 23) corresponding to optimal HIV-1-specific CTL epitopes induced peptide-specific IFN-γ production from CTLs in subjects from the primary-infection cohort. In line with previous studies (13), HIV-1-specific CTL responses were directed against a significantly larger number of HIV-1 epitopes in chronic infection, with an average of 8 peptides (range, 0 to 19) inducing IFN-γ production of CTLs in the chronic-infection cohort (P = 1.18 × 10−8). Within the primary-infection cohort, significantly fewer HIV-1-specific CTL epitopes were targeted in the AHI group (mean number of targeted peptides, 4 [range, 0 to 23]), compared to the EHI group (mean number of targeted peptides, 6 [range, 0 to 21]) (P = 6.3 × 10−7).
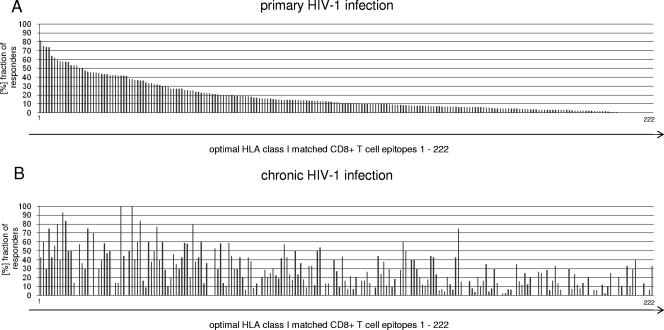
During PHI, some individual epitopes were targeted at a very high frequency in subjects expressing the respective HLA class I molecule, while other epitopes restricted by the same HLA class I molecule were rarely recognized. Figure 1A and Table S1 in the supplemental material summarize the frequencies of recognition for all tested HIV-1-specific CTL epitopes in 428 subjects with PHI. In line with previous data (2), the most frequently recognized HIV-1-specific CTL epitopes were predominantly restricted by HLA class I alleles associated with slower HIV-1 disease progression, such as HLA-B27, HLA-B57, or HLA-B51. In contrast, CTL responses restricted by HLA class I alleles associated with a more rapid course of HIV-1 disease (including HLA-B35, HLA-A1, and HLA-A68) targeted epitopes less frequently during PHI (Fig. 1A; see Table S1 in the supplemental material). The pattern of recognition frequencies of HIV-1-specific CTL epitopes observed during PHI was substantially different in chronically infected individuals (Fig. 1B). While frequently recognized epitopes during PHI were targeted in up to 80% of the 428 subjects with primary infection expressing the respective HLA class I alleles, the same epitopes were less frequently targeted in the chronic-infection cohort. For example, the frequency of CTL responses directed against HLA-B27 KK10 or HLA-B57 TW10 dropped from 81% to 42% (chi-square test; P = 0.008) and from 74% to 30% (P = 0.07), respectively, while subdominant responses or responses not present during PHI represented immunodominant HIV-1-specific CTL responses in chronic infection (Fig. 1; see Table S1 in the supplemental material). Taken together, these studies of the immunodominance patterns of HIV-1-specific CTL responses demonstrate significant differences in the frequencies of recognition of individual epitopes between PHI and chronic HIV-1 infection.
FIG. 1.
Immunodominance pattern of CD8+ T-cell responses targeted during PHI (A) and chronic HIV-1 infection (B). (A) The recognition of described HLA class I matched HIV-1-specific CD8+ T-cell epitopes is shown ranked based on the frequencies of recognition of these epitopes by CD8+ T cells in subjects (n = 428) expressing the respective HLA alleles ([%] fraction of responders) during PHI. The order of recognition, HLA class I restrictions, protein locations, and sequences of these optimal epitopes (1 to 222) are listed in Table S1 in the supplemental material. (B) Frequencies of recognition by CD8+ T cells during chronic infection for the same optimal epitopes (n = 99).
Previous studies suggested that the virus-specific CTL responses that develop in PHI are responsible for the initial control over viral replication (2, 5, 8, 9, 16, 23). In addition, several studies have demonstrated that the early viral set point developing 4 to 6 months after the acute phase of infection is a strong determinant for HIV-1 disease progression (21, 22). We hypothesized that this early viral set point, and the subsequent rate of HIV-1 disease progression, are determined by the immunodominance patterns of the first virus-specific CTL responses. For a subset of 110 treatment-naïve individuals with PHI, viral-load set points 6 months after infection were available. In line with previous observations, subjects expressing the strongly protective HLA class I alleles HLA-B57 and HLA-B27 (n = 19) showed a significantly lower average viral set point, with 20,749 copies/ml (range, 418 to 94,400 copies/ml) compared to the 91 subjects who did not express these protective HLA class I alleles (average viral set point, 55,140 copies/ml; range, 49 to 588,000 copies/ml; P = 0.009) (see Fig. S1 in the supplemental material).
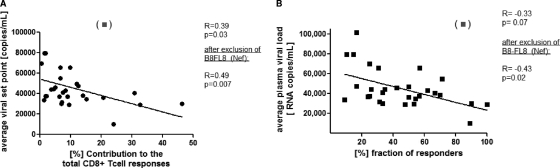
We next assessed whether the contributions of the most immunodominant HIV-1-specific CTL responses restricted by a given HLA class I allele to the total virus-specific CTL response during PHI impacted the average plasma viral set point in the individuals expressing the allele. Interestingly, the contributions of the immunodominant responses by each HLA class I allele to the total virus-specific CTL response were significantly correlated with the average viral set point for individuals expressing those alleles in the 110 individuals for whom viral set points were available, with the exception of HLA-B8 (R = −0.39; P = 0.03) (Fig. 2A). The most immunodominant epitope-specific CD8+ T-cell response restricted by HLA-B8 was directed against the epitope FLEKEGGL (B8-FL8) in Nef and contributed 21% to the total magnitude of all measured virus-specific responses. However, individuals expressing HLA-B8 developed an average plasma viral load of over 110,000 copies/ml. Previous studies have demonstrated that this epitope escapes fairly early (7) but is still recognized with lower functional avidity (29) by CD8+ T-cell responses. The mechanisms accounting for this different association for the immunodominant HLA-B8-restricted epitope in Nef and its impact on viral control warrant further investigation. In contrast, no correlation between CTL responses detected in chronic infection and the viral load was observed (R = 0.06; P = 0.74) (data not shown), as described previously (1, 11). Taken together, these data show that study subjects expressing HLA class I alleles restricting a dominant CTL response that contributed strongly to the initial total virus-specific CD8+ T-cell response in PHI had a significantly lower viral set point.
FIG. 2.
Correlation between immunodominance patterns of HIV-1-specific CD8+ T-cell responses and the viral set point. The early viral set points 6 months after infection were determined for 110 subjects identified during PHI who remained treatment naïve. (A) Correlation between the contributions of the immunodominant HIV-1-specific CD8+ T-cell responses restricted by each HLA class I allele to the total virus-specific CD8+ T-cell responses and the average viral set point for the respective HLA class I allele (n = 110). The average viral load showed a significant inverse correlation (R = −0.39; P = 0.03) with the percent contribution of immunodominant HIV-1-specific immune responses restricted by the respective HLA class I allele to the total response, and it became more prominent (P = 0.007) after removal of the outlier B8-FL8 (Nef) (▪) based on Cook's outlier analysis (R = −0.49). (B) Correlation between the frequencies of recognition ([%] fraction of responders expressing the respective HLA allele) of the most immunodominant HIV-1-specific CD8+ T-cell epitope restricted by each HLA class I allele and the average viral set point for subjects (n = 110) expressing the respective HLA class I allele. The average viral load showed an inverse correlation (P = 0.07) with the (%) recognition of the immunodominant CD8+ T-cell epitope restricted by the respective HLA class I allele (R = −0.33) and became statistically significant (R = −0.43; P = 0.03) after removal of the outlier B8-FL8 (Nef) (▪) based on Cook's outlier analysis.
We next assessed whether the frequency of recognition of immunodominant epitopes during PHI among the 110 treatment-naïve individuals (Fig. 1A; see Table S2 in the supplemental material) had an impact on the viral set point. The most immunodominant CTL epitope was determined for each HLA class I allele, and the frequencies of recognition of these immunodominant epitopes during PHI were then correlated with the average viral set point reached after 6 months in individuals expressing the respective HLA class I alleles. Similar to the contributions to the total virus-specific response, the frequencies of recognition of these immunodominant epitopes during PHI were closely correlated with the early viral set point (R = −0.33; P = 0.07), and this correlation reached significance after the exclusion of the previously mentioned outlier B8-FL8 in Nef (Cook D > 0.7 [in Cook's outlier analysis]; R = −0.43; P = 0.02) (Fig. 2B). Again, no correlation between the frequency of recognition of immunodominant epitopes during chronic HIV-1 infection and the viral load was observed (data not shown).
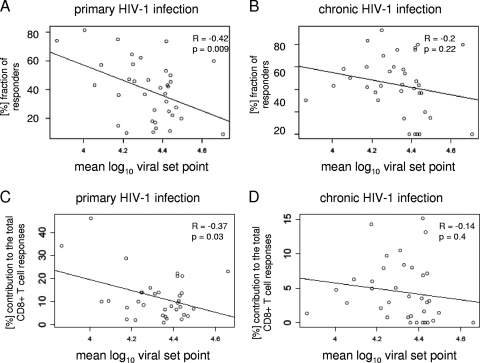
In order to avoid a bias introduced from subjects expressing the highly protective HLA alleles HLA-B57 and HLA-B27, we performed a further subanalysis excluding the subjects expressing these alleles. Even in the absence of both HLA-B27+ and HLA-B57+ individuals, the frequency of epitope recognition and the contributions of the immunodominant epitope-specific responses to the total virus-specific response remained significantly correlated with the early viral set point (R = −0.40, P = 0.04, and R = −0.41, P = 0.03, respectively [the outlier HLA-B8FL8 was excluded from the analysis in both cases]). To extend the viral set point analysis beyond the 110 subjects identified within the primary-infection cohort, we also correlated the frequencies of recognition of immunodominant epitopes determined in the cohort of all 428 individuals with PHI with the viral set point levels described in the MACS cohort for individuals expressing the respective HLA class I alleles. In line with the findings above, we again observed a significant inverse correlation between the frequency or contribution of epitope recognition in PHI and plasma viral load set points derived from the MACS cohort (R = −0.42, P = 0.009, and R = 0.37, P = 0.03, respectively [no outlier was excluded]) (Fig. 3A and C). In contrast, there was no correlation between the frequencies or contributions by which these early immunodominant epitopes were targeted in chronic HIV-1 infection and the viral set point (R = −0.2, P = 0.22, and R = −0.14, P = 0.4, respectively) (Fig. 3B and D). Taken together, these findings support the hypothesis that the early and strong recognition of epitopes during the initial phase of HIV-1 infection is an important contributor to the effective control of HIV-1 replication.
FIG. 3.
Correlation of the frequency and contribution of HIV-1-specific CD8+ T-cell responses during primary infection and the viral set point derived from the MACS cohort. The fraction of responders to the immunodominant epitope detected during PHI for each HLA class I allele was calculated for the 428 subjects during PHI (A) and the 99 subjects during chronic HIV-1 infection (B) and correlated with the mean log10 viral set point derived from the MACS cohort for each HLA class I allele. The numbers on the x axes represent the average mean log10 viral loads, ranging from 3.8 to 4.8. The individual immunodominant epitopes for each HLA class I allele and their frequencies of recognition in primary and chronic infection are shown in Table S2 in the supplemental material. We observed a significant inverse correlation between the fraction of responders and the plasma viral set point for the subjects expressing the respective HLA class I allele (determined 1.5 to 3 years after infection) (R = −0.42; P = 0.009), while no correlation was observed with the frequency of epitope recognition determined in the chronic phase of HIV-1 infection and the mean log10 viral set point derived from the MACS cohort (R = −0.2; P = 0.22). (C) Correlation between the contribution of the immunodominant HIV-1-specific CD8+ T-cell responses restricted by each HLA class I allele to the total virus-specific CD8+ T-cell responses and the average viral set point for the respective HLA class I allele derived from the MACS cohort. (D) The average viral load showed an inverse correlation (R = −0.37; P = 0.03) with the (%) contribution of HIV-1-specific immune responses restricted by the respective HLA class I allele to the total number of responses, while no correlation was observed with the frequency of epitope recognition of the early immunodominant CD8+ T-cell responses determined in the chronic phase of HIV-1 infection and the mean log10 viral set point derived from the MACS cohort (R = −0.14; P = 0.4).
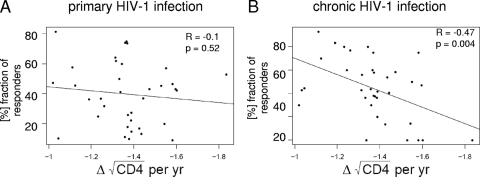
Opportunistic infection commonly occurs when CD4+ T-cell counts drop below a certain level (<200 cells/μl), and clinical guidelines acknowledge the importance of the rate of CD4+ decline for the initiation of antiretroviral therapy. To address whether the recognition of specific primary- or chronic-phase immunodominant epitopes had an impact on the rate of CD4+ T-cell decline, we calculated the average slope of CD4 decline over a year for each HLA class I allele and correlated this with the frequencies of recognition of the immunodominant epitopes restricted by the respective HLA allele detected in acute or chronic infection. The slopes of CD4+ T-cell decline for each HLA class I allele were calculated using data from subjects in the MACS cohort (3). No significant correlation between the frequencies at which immunodominant epitopes were recognized in primary infection and the slope of CD4+ decline in the MACS cohort was observed (Fig. 4A), suggesting that the immunodominance patterns of HIV-1-specific CD8+ T-cell responses in primary infection are not a good predictor of CD4+ T-cell decline. Interestingly, however, the frequencies at which epitopes that were immunodominant in primary infection were still recognized in the chronic phase of HIV-1 infection, based on the results for the 99 chronically infected individuals studied (Fig. 1B; see Table S1 in the supplemental material), were significantly inversely correlated with the slope of CD4+ T-cell decline derived from the MACS cohort (R = −0.47; P = 0.004) (Fig. 4B). In contrast, the immunodominance patterns of HIV-1-specific CD8+ T-cell responses observed in chronic infection alone (see Table S1 in the supplemental material) were not correlated with the slope of CD4+ decline (R = 0.22; P = 0.17) (data not shown). These data suggest that the preservation of frequently recognized immunodominant HIV-specific CTL responses from the acute phase into the chronic phase of infection is associated with lower rates of CD4+ T-cell loss on the population level.
FIG. 4.
Correlation between the frequency of HIV-1-specific CD8+ T-cell response during primary infection and the slope of CD4 decline derived from the MACS cohort. The fraction of responders to the immunodominant epitope detected during PHI for each HLA class I allele was calculated during PHI (n = 428) (A) and chronic HIV-1 infection (n = 99) (B) and correlated with the slope of CD4 decline derived from the MACS cohort for each respective HLA class I allele. The numbers on the x axes represent the average slopes of CD4 decline, ranging from −1.8 (representing a faster loss of CD4 cells) to −1 (representing a slower loss of CD4 cells). The individual immunodominant epitopes for each HLA class I allele and their frequencies of recognition in primary and chronic infection are shown in Table S2 in the supplemental material.
Very little is known about the consequences of the immunodominance patterns of initial HIV-1-specific CD8+ T-cell responses for the early viral set point and disease progression. Here, we used a large cohort of individuals with PHI that allowed, for the first time, correlation between individual epitope-specific CD8+ T-cell responses and markers of HIV-1 disease progression for a significant number of frequent HLA class I alleles. While the approach using peptides corresponding to described optimal CD8+ T-cell epitopes within HIV-1 did not allow us to determine the full breadth of HIV-1-specific T-cell responses and might have underestimated total virus-specific responses (30), we showed that study subjects expressing HLA class I alleles that restricted a dominant virus-specific CD8+ T-cell response in PHI had a significantly lower viral set point.
Correlating the immunodominance patterns of HIV-1-specific CD8+ T-cell responses with the slopes of CD4+ T-cell decline using data from the MACS cohort, for which CD4+ T-cell slopes are well characterized over years of infection, our data furthermore suggest that the preservation of the initial immunodominance patterns of HIV-1-specific CD8+ T-cell responses from primary to chronic infection is associated with protection from CD4+ T-cell loss. These data suggest that CD8+ T cells targeting immunodominant viral epitopes that are not rapidly lost due to early viral escape by sequence evolution (7, 9) or activation-induced deletion of epitope-specific T-cell clones (12, 14, 17, 32) might be able to mediate persistent immune control over the virus and protection from CD4+ T-cell loss. However, it was not possible to determine from this study whether the lower viral set points or slower losses of CD4+ T cells observed were directly due to the presence or absence of an immunodominant epitope restricted by the respective HLA class I allele, as the number of subjects not targeting individual immunodominant HLA-restricted CD8+ T-cell responses was low, despite the size of the cohort. In the case of HLA-B27 and HLA-B57, for example, only a single study subject with available viral set points did not target the corresponding immunodominant epitope. These data suggest that larger cohorts of individuals identified during primary infection with well-documented courses of HIV-1 disease will be required to link disease outcome directly to the presence or absence of an epitope-specific CD8+ T-cell response in primary infection.
In conclusion, these data from a large cohort of individuals identified during PHI demonstrate that immunodominant and persisting virus-specific CD8+ T-cell responses induced during PHI are significantly associated with the early plasma set point of HIV-1 replication and the subsequent slopes of CD4+ T-cell decline. These data show that the specificity and persistence of the initial HIV-1-specific CTL response might be critical predictors for the subsequent control of HIV-1 and have important implications for the rational selection of antigens for the design of future HIV-1 vaccines.
Supplementary Material
Acknowledgments
We thank all of the patients for participating in this study. We also thank all physicians, nurses, and clinical providers who helped to make this study possible. We thank in particular Robert Finlayson (Taylor Square Clinic, Darlinghurst, Sydney, Australia), Robert MacFarland (407 Doctors, Darlinghurst, Sydney, Australia), Cassy Workman (AIDS Treatment Initiative, Darlinghurst, Sydney, Australia), Mark Bloch (Holdsworth House General Practice, Darlinghurst, Sydney, Australia), and David Cooper (St. Vincent's Hospital, Darlinghurst, Sydney, Australia) for enrolling study participants from the respective Australian sites. In addition we thank Gerald Spott (UCSF, San Francisco); Suzanne Bazner, Jenna Rychert, Sarah Placek, Kristin Moss, Agnes Sarkozi, and Aisha Tiala Darrah (MGH, Boston), and the Fenway Community Health Center, Boston, MA, for their help with the samples, as well as Yaoyu Wang for his help with the analysis.
This study was supported by the National Institutes of Health (PO1 AI074415; RO1 AI50429; HHSN261200800001E Acute Infection Early Disease Research Network U01 AI052403; contract N01-CO-12400). The research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. In addition, A.D.K. is supported by a Program Grant and Practitioner Fellowship from the Australian National Health and Medical Research Council (NHMRC). The NCHECR is supported by the Commonwealth Department of Health and Ageing, Australia. The work of S.L. was supported by National Institutes of Health grant AI43638. J.-P.R. is a physician-scientist supported by Fonds de Recherche en Santé du Quebec (FRSQ). H.S. was supported by an MBRC Testeon Fellowship Award. M.A. is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation. We thank all the members of the Montreal HIV Infection Study receiving financial support from FRSQ. This study was supported in part by the Philip T. and Susan Ragon Foundation.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Published ahead of print on 20 May 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., E. T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M. N. Johnston, N. Burgett, M. E. Swartz, A. Yang, G. Alter, X. G. Yu, A. Meier, J. K. Rockstroh, T. M. Allen, H. Jessen, E. S. Rosenberg, M. Carrington, and B. D. Walker. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 3e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashirova, A. A., G. Bleiber, Y. Qi, H. Hutcheson, T. Yamashita, R. C. Johnson, J. Cheng, G. Alter, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, M. May, F. Maldarelli, L. Jacobson, J. O'Brien, S. A. Telenti, and M. Carrington. 2006. Consistent effects of TSG101 genetic variability on multiple outcomes of exposure to human immunodeficiency virus type 1. J. Virol. 806757-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumme, Z., C. Brumme, J. Carlson, H. Streeck, M. John, Q. Eichbaum, B. Block, B. Baker, C. Kadie, M. Markowitz, H. Jessen, A. Kelleher, E. Rosenberg, J. Kaldor, Y. Yuki, M. Carrington, T. Allen, S. Mallal, M. Altfeld, D. Heckerman, and B. Walker. 2008. Marked epitope and allele-specific differences in rates of mutation in HIV-1 Gag, Pol and Nef CTL epitopes in acute/early HIV-1 infection. J. Virol. 829216-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 776867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, J., J. McNevin, U. Malhotra, and M. J. McElrath. 2003. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 1713837-3846. [DOI] [PubMed] [Google Scholar]
- 10.Fellay, J., K. V. Shianna, D. Ge, S. Colombo, B. Ledergerber, M. Weale, K. Zhang, C. Gumbs, A. Castagna, A. Cossarizza, A. Cozzi-Lepri, A. De Luca, P. Easterbrook, P. Francioli, S. Mallal, J. Martinez-Picado, J. M. Miro, N. Obel, J. P. Smith, J. Wyniger, P. Descombes, S. E. Antonarakis, N. L. Letvin, A. J. McMichael, B. F. Haynes, A. Telenti, and D. B. Goldstein. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 782187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gougeon, M. L., H. Lecoeur, A. Dulioust, M. G. Enouf, M. Crouvoiser, C. Goujard, T. Debord, and L. Montagnier. 1996. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 1563509-3520. [PubMed] [Google Scholar]
- 13.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes, B. F., G. Pantaleo, and A. S. Fauci. 1996. Toward an understanding of the correlates of protective immunity to HIV infection. Science 271324-328. [DOI] [PubMed] [Google Scholar]
- 15.Kassutto, S., and E. S. Rosenberg. 2004. Primary HIV type 1 infection. Clin. Infect. Dis. 381447-1453. [DOI] [PubMed] [Google Scholar]
- 16.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichterfeld, M., X. G. Yu, S. K. Mui, K. L. Williams, A. Trocha, M. A. Brockman, R. L. Allgaier, M. T. Waring, T. Koibuchi, M. N. Johnston, D. Cohen, T. M. Allen, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2007. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J. Virol. 814199-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyles, R. H., A. Munoz, T. E. Yamashita, H. Bazmi, R. Detels, C. R. Rinaldo, J. B. Margolick, J. P. Phair, J. W. Mellors, et al. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. J. Infect. Dis. 181872-880. [DOI] [PubMed] [Google Scholar]
- 19.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 20.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellors, J. W., J. B. Margolick, J. P. Phair, C. R. Rinaldo, R. Detels, L. P. Jacobson, and A. Munoz. 2007. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA 2972349-2350. [DOI] [PubMed] [Google Scholar]
- 22.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 2721167-1170. [DOI] [PubMed] [Google Scholar]
- 23.Oxenius, A., D. A. Price, A. Trkola, C. Edwards, E. Gostick, H. T. Zhang, P. J. Easterbrook, T. Tun, A. Johnson, A. Waters, E. C. Holmes, and R. E. Phillips. 2004. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J. Infect. Dis. 190713-721. [DOI] [PubMed] [Google Scholar]
- 24.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 1782746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safrit, J. T., C. A. Andrews, T. Zhu, D. D. Ho, and R. A. Koup. 1994. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J. Exp. Med. 179463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekaly, R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shacklett, B. L., C. A. Cox, M. F. Quigley, C. Kreis, N. H. Stollman, M. A. Jacobson, J. Andersson, J. K. Sandberg, and D. F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173641-648. [DOI] [PubMed] [Google Scholar]
- 28.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J. P. Routy, E. S. Rosenberg, R. P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 817725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 2341563-1566. [DOI] [PubMed] [Google Scholar]
- 32.Xu, X. N., M. A. Purbhoo, N. Chen, J. Mongkolsapaya, J. H. Cox, U. C. Meier, S. Tafuro, P. R. Dunbar, A. K. Sewell, C. S. Hourigan, V. Appay, V. Cerundolo, S. R. Burrows, A. J. McMichael, and G. R. Screaton. 2001. A novel approach to antigen-specific deletion of CTL with minimal cellular activation using α3 domain mutants of MHC class I/peptide complex. Immunity 14591-602. [DOI] [PubMed] [Google Scholar]
- 33.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 713120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.