Abstract
There is an urgent need for human immunodeficiency virus (HIV) vaccines that induce robust mucosal immunity. Influenza A viruses (both H1N1 and H3N2) were engineered to express simian immunodeficiency virus (SIV) CD8 T-cell epitopes and evaluated following administration to the respiratory tracts of 11 pigtail macaques. Influenza virus was readily detected from respiratory tract secretions, although the infections were asymptomatic. Animals seroconverted to influenza virus and generated CD8 and CD4 T-cell responses to influenza virus proteins. SIV-specific CD8 T-cell responses bearing the mucosal homing marker β7 integrin were induced by vaccination of naïve animals. Further, SIV-specific CD8 T-cell responses could be boosted by recombinant influenza virus-SIV vaccination of animals with already-established SIV infection. Sequential vaccination with influenza virus-SIV recombinants of different subtypes (H1N1 followed by H3N2 or vice versa) produced only a limited boost in immunity, probably reflecting T-cell immunity to conserved internal proteins of influenza A virus. SIV challenge of macaques vaccinated with an influenza virus expressing a single SIV CD8 T cell resulted in a large anamnestic recall CD8 T-cell response, but immune escape rapidly ensued and there was no impact on chronic SIV viremia. Although our results suggest that influenza virus-HIV vaccines hold promise for the induction of mucosal immunity to HIV, broader antigen cover will be needed to limit cytotoxic T-lymphocyte escape.
Developing a safe and effective human immunodeficiency virus (HIV) vaccine is one of the defining scientific challenges of our time. Induction of peripheral CD8 T-cell immunity to HIV did not protect against sexual exposure to HIV type 1 (HIV-1) in humans in a recent efficacy trial (11, 43). In simian immunodeficiency virus (SIV)-macaque studies, peripheral CD8 T-cell immunity can effectively control viremia (40) but is often observed to have a transient or limited role in delaying SIV disease in macaques (32). The gradual accumulation of immune escape at CD8 T-cell epitopes undermines the effectiveness of CD8 T-cell immunity to SIV (6, 22, 46). It is likely that inducing mucosal CD8 T-cell immunity to HIV will be more effective at limiting viral replication during the very early phases of acute infection, prior to massive viral dissemination and destruction of large numbers of CD4 T cells (50). The induction of multifunctional mucosal CD8 T cells by live attenuated SIV vaccination of macaques is thought to play a significant role in the success of this strategy (25, 26); however, it is unfortunately too dangerous for clinical trials at present.
A series of mucosal viral and bacterial HIV vaccine vectors have been studied in recent years; however, none have yet proceeded to advanced clinical trials. Live attenuated poliovirus vectors have shown promise in SIV studies, but these viruses can in rare cases revert to virulence (14). Salmonella-based SIV vaccine vectors are able to induce CD8 T-cell responses which express the α4β7 integrin mucosal homing marker when administered orally (20, 24). However, there may be a much stronger link between concomitant genital tract immunity and immunity induced at respiratory mucosal sites compared to that induced at enteric sites (33, 38, 42). Vesicular stomatitis virus vectors that replicate in the nasal mucosa show promise in SIV-macaque trials but are potentially neurotoxic (55). Replication-competent adenovirus vectors have looked promising in some SHIV-macaque studies (49) but failed to provide significant protection in a recent SIV-macaque study (17) and could have similar issues of enhanced infection rates as seen in the recent efficacy trials of replication-incompetent adenovirus type 5 vectors.
A mucosal vector system that has several advantages over existing models but that is relatively unexplored is recombinant attenuated influenza viruses. Such viruses (i) have an existing reverse genetics system to readily generate and manipulate recombinant viruses (31, 34), (ii) are effective as anti-influenza vaccines and licensed for human use (e.g., “Flumist” vaccine [9]) with ready production capability, (iii) have robust respiratory mucosal replication that should facilitate genital mucosal immunity, and (iv) can be generated with a variety of hemagglutinin (H) and neuraminidase (N) glycoproteins, potentially enabling these viruses to be administered sequentially in prime-boost combinations to limit the effect of antivector humoral immunity (34). Mouse-adapted recombinant influenza virus-HIV vectors have been studied in mice and demonstrated significant induction of cellular immunity at mucosal sites (8, 27, 28, 44, 48). However, although several native influenza viruses replicate efficiently in the respiratory tracts of Asian macaque species (10, 12, 52), no studies to date have examined the immunogenicity or efficacy of recombinant attenuated influenza virus-SIV vectors in macaques.
MATERIALS AND METHODS
Recombinant influenza A viruses.
Recombinant influenza A viruses were generated using an eight-plasmid reverse genetics system as previously described (4, 31, 39, 59). Briefly, DNA constructs containing all eight influenza virus genome segments, (including the genetically manipulated NA segments) were transfected into a coculture of 293T and Madin-Darby canine kidney (MDCK) cells. All viral RNAs and mRNAs required for assembly and rescue of recombinant virus were generated through bidirectional transcription and translation from a single template. The two mouse-adapted strains of influenza A virus used in this study, i.e., X31 (H3N2, A/HKx31) and PR8 (H1N1, A/Puerto Rico/8/1934), share the same six internal gene segments but differ in their expression of HA and NA genes. The use of both strains in vaccination enables the analysis of secondary CD8+ T-cell responses in the absence of neutralizing antibody. The Mane-A*10-restricted CD8+ T-cell epitopes KP9 (SIV Gag164-172) (23), KSA10 (SIV Tat87-96), and KVA10 (SIV Tat114-123) (53) were inserted separately into the NA stalks of the respective viruses using recombinant PCR techniques as previously described (31) (primers and conditions are available on request). Transfection supernatants were used to amplify virus in 10-day-old embryonated eggs, and the presence of recombinant virus determined using a hemagglutination assay. The retention of the correctly inserted peptide epitope within the expanded virions was confirmed by sequencing.
Macaques and SIV infections.
Eleven pigtail macaques (Macaca nemestrina) were sourced from the Australian national macaque breeding facility and were free from simian retrovirus D and tuberculosis. All studies were approved by the institutional animal ethics committees. All 11 macaques expressed the common major histocompatibility complex (MHC) I allele Mane-A*10 as assessed by sequence-specific primer PCR as previously described (51). The macaques studied are described in Table 1. Two macaques were naïve and nine macaques were chronically infected with SIVmac251. The infected animals had been infected for 1 to 2 years, having participated in prior vaccination/challenge experiments (19, 36, 54), and were all primed with low-level SIV-specific CD8 T-cell responses. SIV infections were performed on naïve animals by intravenous challenge with 40 50% tissue culture infective doses of SIVmac251 as previously described (7, 19, 36). There were no detectable levels of escape mutants for the Mane-A*10-restricted KP9 Gag epitope within the SIVmac251 stock by either cloning and sequencing or quantitative real-time reverse transcription-PCR (qRT-PCR) as described below, consistent with the virus being derived originally from non-pigtail macaques.
TABLE 1.
Animals studied and influenza virus-specific T-cell epitopes identified
| SIV infection status | Influenza virus-SIV vaccination regimen | Animal no. | SIV Gag KP9 response (%) postvaccinationa | Influenza virus epitope(s) mappedb
|
|
|---|---|---|---|---|---|
| CD4 | CD8 | ||||
| Uninfected, naïve | Day 0, H1N1-KP9; day 28, H1N1-KP9; day 300, H3N2-KP9; day 328, H3N2-KP9 | 1335 | 0.06 | M191-107 | NP401-423c |
| 2374 | 0.04 | NP401-423 | |||
| SIV infected | Day 0, H1N1-KP9; day 28, H1N1-KP9; day 98, H3N2-KP9; day 126, H3N2-KP9 | 5821 | 8.78 | NP466-482, NP300-362d, M136-57, M151-112d | NP401-423 |
| 5827 | 10.22 | NP186-202, M136-57, NP300-362d, NP401-462d | NP311-332, NP456-472, NP482-498 M141-62, M186-107 | ||
| 086B | 3.79 | NP151-212d, NP300-362d | NP401-423, NP151-212d | ||
| 8305 | 0.48 | NP401-423 | |||
| Day 0, H3N2-KP9; day 28, H3N2-KP9; day 77, H1N1-KP9; day 105, H1N1-KP9 | 8454e | 0.74 | NP300-362d, NP186-202 | NP451-498d | |
| 9017e | 0.57 | ||||
| 9020e | 0.11 | ||||
| 1.3731e | 0.47 | NP451-498d, M186-107 | |||
| Day 0, H1N1-KP9, H1N1-KSA10, H1N1-KVA10 | 5612 | 8.74 | Not done | Not done | |
Peak CD8 T-cell response determined by KP9/Mane-A*10 tetramer, expressed as percentage of all CD8 T cells.
Common epitopes identified are in bold.
The NP401-423 response was mapped to minimal epitope NP402-410 (SAGQISIQP, SP9) as shown in Fig. 4B.
Pool of 10 overlapping peptides, not finely mapped.
Studied only for responses to epitopes already mapped in animals 5821, 5827, 086B, and 8305.
Infection of pigtail macaques with recombinant influenza virus-SIV.
All recombinant influenza virus-SIV vaccines were administered by both intratracheal and intranasal administration of 108 PFU influenza virus prepared in phosphate-buffered saline to each site. Virus was atraumatically dosed in the trachea and then in the nose with a pipette. The animals were sedated with Ketamine for all procedures. Each virus (H1N1 or H3N2) was administered twice (day 0 and 28), and animals were revaccinated with the alternate subtype virus (H3N2 or H1N1) 2 to 10 months later. Successful infection with the recombinant influenza virus-SIV vaccine was monitored by analyzing influenza virus RNA in serial swabs of the upper respiratory tract during the course of infection (60). Briefly, samples were analyzed with an SSIII qRT-PCR one-step kit (Invitrogen) with primers and probe targeting the matrix gene, using an ABI 7500 real-time PCR system (Applied Biosciences). Seroconversion to influenza A virus was assessed by a hemagglutination inhibition assay performed on serial serum samples as previously described (47). Briefly, 25 μl of H1N1 virus was incubated at room temperature with twofold dilutions of sera treated with RDE (Deka Seiken, Tokyo, Japan) for 1 h. Turkey red blood cells (25 μl, vol/vol) were added, left for 30 min, and observed for hemagglutination.
Immunogenicity studies.
SIV-specific CD8 T-cell responses were monitored on fresh whole blood by tetramer staining of CD3+ CD8+ lymphocytes using fluorescent Mane-A*10 tetrameric protein folded around either the KP9, KSA10, or KVA10 SIV peptide epitope as previously described (18, 53). Expression of the mucosal homing marker α4β7 integrin (13) was assessed by counterstaining tetramer-positive cells with fluorescent rat anti-human integrin β7 (BD Pharmingen, catalog no. 551082, clone FIB504). Influenza virus-specific T cells were assessed using an intracellular cytokine staining (ICS) assay on fresh blood samples by stimulation with overlapping influenza virus peptides and assessment of the specific expression of intracellular gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) as previously described (18). Briefly, 200 μl of fresh whole blood was stimulated with a single pool of 17-mer peptides, overlapping by 15 amino acids, spanning either the entire nucleoprotein (NP) (98 peptides) or matrix protein (M1) (48 peptides) (both purchased from Mimotopes, Clayton, Australia; purity, >65%) of A/Puerto Rico/8/1934 virus for 6 h at 37°C along with costimulatory antibodies to human CD28/CD49d (BD Biosciences/Pharmingen, San Diego CA) and 10 μg/ml brefeldin A (Sigma, St. Louis, MO). Cells were labeled with anti-CD3-AF700 (clone SP34-2), anti-CD4-fluorescein isothiocyanate (clone M-T477), and anti-CD8-peridinin chlorophyll protein (clone SK1); red blood cells were lysed with BD fluorescence-activated cell sorter lysing solution; and remaining leukocytes were permeabilized with BD permeabilizing solution 2 and then incubated with anti-human IFN-γ-allophycocyanin (clone B27) and anti-human TNF-α-PeCy7 (clone mAb11) (all from BD Biosciences) and fixed with 1% formaldehyde (Polysciences, Inc., Warrington, PA). Flow cytometric data were analyzed with FloJo software version 7.2.2 and influenza virus-specific T-cell frequencies calculated as the proportion of CD4 or CD8 T cells expressing the particular cytokine molecule. Background expression of cytokines following dimethyl sulfoxide stimulation alone was generally <0.2% and was subtracted from antigen-specific levels. To map individual influenza virus peptide-specific T-cell responses, pools of 10 peptides were mapped and then individual peptides tested with fresh blood samples. A particular influenza virus NP-specific CD8 T-cell response was finely mapped by purchasing 9- to 10-mer peptides spanning an overlapping pair of 17-mer peptides and assessing cytokine expression to fresh blood incubated with peptide dilutions.
qRT-PCR assay to detect virus escape mutant K165R.
As the vaccinations stimulated only SIV-specific CD8 T-cell responses that are known to be susceptible to immune escape (23), we studied escape from KP9-specific CD8 cells in the vaccinated/challenged animals 1335 and 2374. We utilized a qRT-PCR assay that specifically amplifies SIV that either is wild type (WT) at KP9 or carries the K165R mutation, which is the dominant escape mutation at this epitope as previously described and validated (41). The assays use a forward primer specific for either the WT sequence or the K165R KP9 escape mutant sequence. Briefly, for each time point after SIV infection 10 μl of RNA, extracted from EDTA-anticoagulated plasma, was subjected to reverse transcription, and then cDNA was amplified by qRT-PCR using either WT or escape mutant primers specific for the SIV Gag KP9 epitope. A reverse primer and 5′ 6-carboxyfluorescein-labeled minor groove binding DNA probe were also added for quantification against the SIV Gag epitope RNA standards using an Eppendorf Realplex4 cycler. Baselines were set two cycles earlier than real reported fluorescence, and the threshold value was determined by setting the threshold bar within the linear data phase. Samples amplifying after 40 cycles were regarded as negative, corresponding to ≤1.5 log10 SHIV/SIV RNA copies/ml of plasma.
RESULTS
Influenza virus-SIV vaccination studies of macaques.
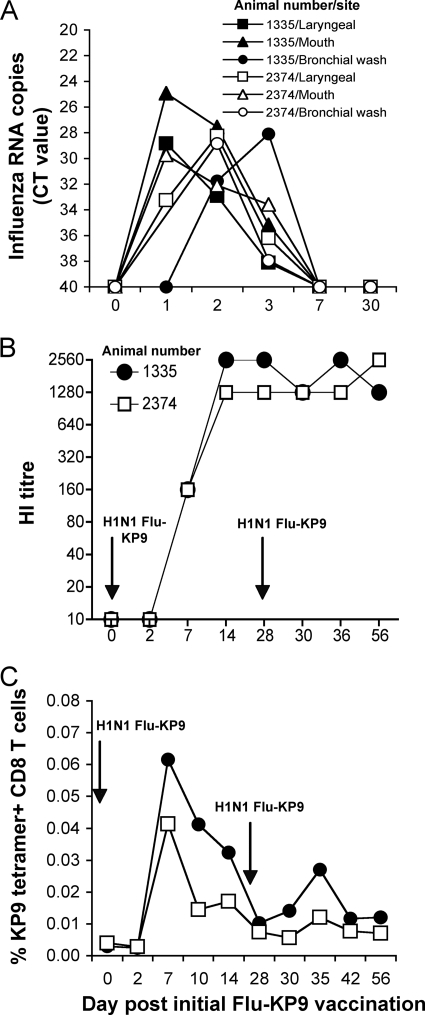
To assess the infectivity and immunogenicity of recombinant influenza virus-SIV expressing the SIV Gag KP9 epitope, we inoculated two naïve pigtail macaques, which expressed the Mane-A*10 allele (animals 1335 and 2374), with 108 PFU of recombinant H1N1 influenza virus (PR8)-SIV via the respiratory tract twice at a 4-week interval. Swabs of the respiratory tract were analyzed for influenza virus RNA daily. Influenza virus RNA was recovered from both animals at multiple sites after the first inoculation (Fig. 1A). Virus levels peaked at 1 to 2 days after inoculation and were cleared by day 7. Two days after a second inoculation with the same virus on day 28, no RNA was detected. This suggests both (i) an adaptive immune response generated to the initial infection and (ii) that the detection of influenza virus RNA after the first inoculation was due to replicating virus and not to sampling of the inoculum. To evaluate humoral recognition of influenza virus, we analyzed serial serum hemagglutination inhibition titers against the homologous parent H1N1 virus and observed a uniform seroconversion within 7 days of inoculation (Fig. 1B). The second inoculation did not further boost antibody levels.
FIG. 1.
Vaccination of pigtail macaques with recombinant influenza virus-SIV. Two naïve pigtail macaques expressing the Mane-A*10 allele were vaccinated twice with recombinant 108 PFU influenza A virus (H1N1 influenza virus KP9, PR8 strain) 4 weeks apart. (A) Recovery of influenza virus RNA from respiratory swabs by qRT-PCR. (B) Seroconversion to influenza A virus by hemagglutination inhibition (HI). (C) Detection of SIV Gag KP9-specific CD8 T cells in blood by KP9/Mane-A*10 tetramer staining. The proportion of all CD8 T cells staining for the KP9/Mane-A*10 tetramer is shown.
To determine immunogenicity against the inserted SIV epitope, we analyzed serial fresh blood samples for the presence of KP9-specific T cells using the Mane-A*10/KP9 tetramer. We observed a small but clearly detectable KP9-specific CD8 T-cell response after the initial inoculation (Fig. 1C). This response was marginally boosted after the second inoculation. The minimal boosting is consistent with humoral influenza virus immunity limiting replication of the vector.
Influenza virus-SIV vaccination of SIV-infected macaques.
To further characterize the immunogenicity of influenza virus-SIV vaccination of pigtail macaques, we conducted additional influenza virus-SIV vaccination studies of a series of eight Mane-A*10+ SIV-infected macaques used in prior therapeutic vaccine studies (Table 1). The macaques were infected with SIVmac251 1 to 2 years previously and had primed KP9-specific CD8 T-cell responses, and so they could be used to assess the immunostimulatory capacity of recombinant influenza virus-SIV prime-boost regimens. We studied two prime-boost regimens, each with four animals, with two doses of H1N1 influenza virus-SIV vaccination followed by two doses of H3N2 influenza virus-SIV vaccination or vice versa (Table 1; Fig. 2). As with the infection of the naïve animals, influenza virus RNA was recovered from the respiratory tracts of infected animals, and all animals seroconverted to influenza virus (data not shown). Both regimens were effective in reboosting KP9-specific CD8 T cells (Fig. 2). Following the H1N1-SIV prime/H3N2-SIV boost regimen, three of the four animals (animals 5821, 5827, and 086B) had substantial expansion of KP9-specific CD8 T cells, i.e., up to 10% of all CD8 T cells after the initial H1N1 influenza virus-SIV vaccination (Fig. 2A). These very large responses were not substantially reboosted by subsequent immunization with the H3N2-SIV virus. The four animals receiving first the H3N2 influenza virus-SIV recombinant virus had a more modest expansion of KP9-specific CD8 T cells after the initial vaccination (to ∼0.5%) (Fig. 2B), although these cells reexpanded to similar levels after the H1N1 boost. These studies suggest that SIV-specific CD8 T cells can be boosted by influenza virus-SIV vaccination of SIV-infected animals and that prime-boost immunization with heterologous influenza virus strains can maintain high levels of SIV-specific T cells.
FIG. 2.
Prime-boost vaccination of SIV-infected macaques with heterologous influenza virus-SIV vaccines. Groups of four Mane-A*10-expressing pigtail macaques with chronic SIV infection were administered either two doses of H1N1 influenza virus-KP9 SIV vaccine followed by two doses of H3N2 influenza virus-KP9 SIV vaccine (A) or two doses of H3N2 influenza virus-KP9 SIV vaccine followed by two doses of H1N1 influenza virus-KP9 SIV vaccine (B). SIV Gag KP9-specific CD8 T cells in blood were assessed by KP9/Mane-A*10 tetramer staining, shown as a proportion of total CD8 T cells.
Characterization of influenza virus-specific CD8 T cells.
The limited expansion of SIV-specific CD8 T cells following boosting with a heterologous influenza virus-SIV vector suggested that adaptive immune responses to influenza virus other than antibody responses could play a role in reducing the effectiveness of the boost. We therefore assessed serial fresh blood samples for influenza virus-specific T-cell responses by ICS throughout the course of the influenza virus-SIV vaccination study. To our knowledge, influenza virus-specific T-cell immunity has not previously been analyzed in pigtail macaque models or mapped in any macaque model. We studied responses to influenza virus NP and M1. These proteins are conserved for both the H1N1 and H3N2 strains used for vaccination, and NP is a common target for CD8 T-cell immunity in both mice and humans (57, 58). The majority of animals vaccinated with the recombinant influenza virus-SIV generated antiviral cytokine-producing CD8 and CD4 T cells in response to restimulation with overlapping peptides of NP (Fig. 3). T-cell responses to M1 protein were much smaller and less common (not shown). NP-specific CD8 T-cell responses begin to appear at day 35 following two doses of the initial recombinant influenza virus strain and were further boosted following one dose of the alternate heterologous recombinant influenza virus strain (Fig. 3A and C). A similar trend was observed for the generation of NP-specific CD4 T cells (Fig. 3B and D).
FIG. 3.
Influenza virus-specific T-cell immunity following influenza virus-SIV vaccination. Serial macaque fresh blood samples were assessed for the proportion of CD4 or CD8 T cells specifically expressing both IFN-γ and TNF-α following stimulation with a pool of overlapping peptides spanning the influenza virus NP protein. NP-specific CD8 and CD4 T-cell responses in four animals receiving H1N1 influenza virus-KP9 SIV followed by H3N2 influenza virus-KP9 SIV are shown in the left panels (A and B), and responses in animals receiving H3N2 influenza virus-KP9 SIV followed by H1N1 influenza virus-KP9 SIV shown in the right panels (C and D).
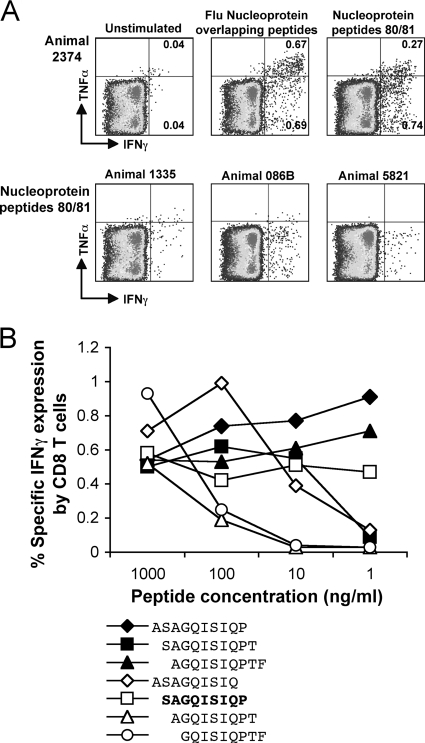
The reasonably robust T-cell responses to the whole influenza virus NP peptide pool suggested that the immune response in these pigtail macaques could target common NP epitopes. We therefore used smaller sets of 10 overlapping peptides to map CD4 and CD8 T-cell epitopes of NP and M1. The influenza virus-specific CD4 and CD8 T-cell epitopes mapped to individual peptides are shown in Table 1. During epitope mapping analysis of one animal (2374), peptides 80 and 81 of NP (NP401-423) stimulated a robust response CD8 T-cell response encompassing the majority of the IFN-γ and TNF-α-producing CD8 T cells to NP (Fig. 4A).
FIG. 4.
Identification of a common influenza virus NP CD8 T-cell epitope in pigtail macaques. Macaques responding to the pool of NP peptides had T-cell responses to individual or overlapping influenza virus NP peptides assessed on serial fresh blood samples. (A) Macaque 2374 had a robust CD8 T-cell response to NP, as evidenced by IFN-γ and TNF-α expression. The majority of NP-responding CD8 T cells were directed to two overlapping peptides, peptides 80 and 81 (NP401-423) of the pool of 98 overlapping NP 17-mer peptides. Three other macaques responding to the whole NP pool and also responding to these two NP peptides are also shown (lower fluorescence-activated cell sorter plots). (B) Fine mapping of the common NP CD8 T-cell response was performed by ICS on a series of 9- to 10-mer peptides spanning the overlap between NP peptides 80 and 81.
To finely map the NP-specific CD9 T-cell epitope, we purchased seven smaller peptides across this epitope and performed an ICS experiment on fresh blood from animal 2374 using reducing titrations of each peptide. The minimal epitope, being the smallest peptide that did not titrate out, was a 9-mer (SP9, NP402-410) (Fig. 4B). Defining this macaque influenza virus-specific CD8 T-cell epitope allowed us to screen blood samples from other influenza virus-SIV-infected macaques. A response to this NP epitope was common to four other vaccinated animals (Fig. 4A). These animals all share the MHC I allele Mane-A*10 but not any other MHC I alleles typed for, suggesting that the observed shared response to this influenza virus CD8 T-cell epitope is restricted by Mane-A*10.
SIV challenge of influenza virus-SIV vaccinated macaques.
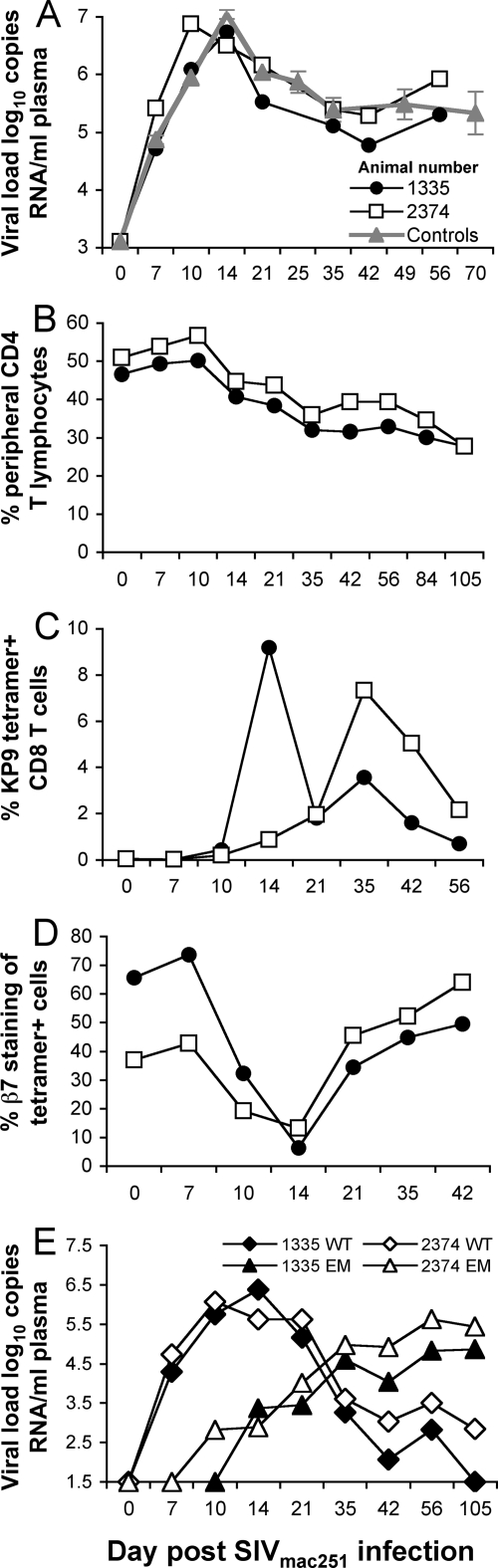
To assess the efficacy of the influenza virus-SIV vaccination, the naïve pigtail macaques vaccinated with the influenza virus-SIV recombinants were challenged with SIVmac251 6 weeks after the final vaccination. Both animals became infected, and acute SIV viral loads at 10 to 21 days after infection ranged from 5.52 to 6.88 log10 copies/ml (mean of 6.62 at day 14) (Fig. 5A). This is not significantly lower than the mean (± standard deviation) day 14 SIV viral load of 7.07 ± 0.54 log10 copies/ml for 36 unimmunized historical control animals infected with the same SIVmac251 stock (19). Levels of SIV viremia beyond acute infection remained high at 5 to 6 log10 copies/ml in influenza virus-SIV-immunized animals (mean, 5.60 log10 copies) and similar to that in historical control animals (mean ± standard deviation, 5.40 ± 1.08 log10 copies/ml [36]). Consistent with the high levels of continuing viremia, a gradual peripheral CD4 T-cell depletion ensued (Fig. 5B).
FIG. 5.
Outcome of SIVmac251 challenge of influenza virus-SIV-vaccinated macaques. Two naïve macaques vaccinated with recombinant influenza virus-SIV expressing the SIV Gag KP9 epitope (H1N1-KP9 and H3N2-KP9) were challenged intravenously with SIVmac251 and serial blood samples analyzed. (A) SIV plasma RNA. Viral load analyses (mean ± standard error) from 48 untreated historical pigtail macaques controls infected with the same SIVmac251 stock from 2 previously studies are shown in gray for comparison. (B) Total peripheral CD4 T cells. (C) SIV Gag KP9-specific CD8 T cells. (D) Expression of the α4β7 integrin on KP9/Mane-A*10 tetramer-positive CD8 T cells. (E) SIV KP9 WT and SIV KP9-K165R escape mutant (EM) virus levels determined by qRT-PCR.
Recall responses to the SIV KP9 epitope were studied with serial blood samples. After challenge with SIVmac251, a massive boosting of KP9-specific T cells to 8 to 10% of all CD8 T cells was observed in both macaques, demonstrating effective priming of this response (Fig. 5C).
Immune escape from SIV-specific CD8 T cells induced by influenza virus-SIV vaccines.
The high levels of viremia in the context of a very large recall CD8 T-cell response to SIV KP9 suggest that rapid early immune escape may have undermined the narrow SIV-specific immunity induced by vaccination. As the vaccination stimulated only SIV-specific CD8 T-cell responses that are known to be susceptible to immune escape (23), we studied escape from KP9-specific CD8 cells in the vaccinated/challenged animals, 1335 and 2374. Serial plasma samples were analyzed using a novel qRT-PCR that quantifies both WT viremia and the dominant K165R escape mutant viremia (41). We observed rapid early escape at the KP9 epitope in both animals (Fig. 5E). The escape mutant strain was first detected 10 to 14 days after challenge, and the level of the escape mutant virus exceed that of WT virus 5 weeks after infection and dominated the viral population thereafter.
Induction of β7 integrin-expressing SIV-specific CD8 T cells by influenza virus-SIV vaccination.
The α4β7 integrin is a specific mucosal homing marker on CD8 T cells. Other groups have demonstrated its role in mucosal homing of CD8 T cells in macaque models of SIV and other virus infections (13, 35). As the animals were immunized with the influenza virus-SIV recombinants via the respiratory tract, we analyzed the expression of the β7 subunit on peripheral KP9-specific CD8 T cells. Both after vaccination and immediately after SIV challenge, 40 to 60% of KP9-specific T cells expressed the β7 surface integrin molecule (Fig. 5D). Interestingly, a sharp transient decline in β7 expression on circulating KP9-specific T cells was observed at day 14, temporally associated with a dramatic increase in the CD8 T-cell response after challenge.
Expanded SIV antigen coverage with influenza virus-SIV vaccines.
Vaccines that stimulate a single CD8 T-cell epitope can provide valuable information for that population of CD8 T cells; however, they (i) are unlikely to adequately model vaccines that express multiple antigens and (ii) leave the host susceptible to immune escape (as we observed above). We therefore constructed influenza virus-SIV recombinants expressing two additional recently described Mane-A*10-restricted SIV CD8 T-cell epitopes located in the Tat protein (KSA10 and KVA10) (53), H1N1-KSA10 and H1N1-KVA10. We immunized a Mane-A*10+ SIV-infected macaque with 108 PFU of each virus simultaneously via the respiratory tract. There was a significant boost in CD8 T cells specific for all three SIV epitopes, particularly KP9 and KSA10 and to a lesser extent KVA10 (Fig. 6). This provides a model for the induction of broader SIV-specific CD8 T cells by mucosally administered vaccination.
FIG. 6.
Simultaneous induction of three SIV-specific CD8 T-cell responses using influenza virus-SIV vaccines. A Mane-A*10-expressing pigtail macaque with chronic SIV infection was simultaneously administered three H1N1 (PR8) influenza virus-SIV recombinants expressing either the SIV Gag KP9, SIV Tat KVA10, or SIV Tat KSA10 epitope. CD8 T-cell responses to each SIV epitope were followed by MHC I tetramer staining of fresh blood samples. (A) SIV-specific CD8 T-cell responses determined by tetramer staining after immunization. (B) Graph of responses over time.
DISCUSSION
Improved vaccines to prevent or ameliorate HIV infection are needed. We constructed a series of H1N1 and H3N2 recombinant influenza virus-SIVs and showed that these viruses can infect pigtail macaques via the respiratory mucosa and induce or boost SIV-specific CD8 T-cell responses. The SIV-specific CD8 T-cell responses expressed the α4β7 integrin mucosal homing marker and were boosted substantially after SIV challenge. Unfortunately, the SIV-specific CD8 T cells induced by the vaccines to a single SIV epitope rapidly selected for immune escape viral mutants, and no protection against chronic SIV infection was observed, illustrating that broad immunity will be required to control such viruses. Although we also show that it is possible to boost responses to at least three SIV-specific CD8 T-cell responses using panels of recombinant influenza virus-SIV recombinants, the breadth of immunity required to adequately limit virus replication is not yet clear. Our work with macaques on this novel mucosa-based vaccine platform provides opportunities to probe the requirements for effective mucosal T-cell immunity to viruses such as HIV.
Our study significantly expands on previous mouse work on recombinant influenza virus-HIV vaccine concepts (27, 28, 44, 45, 48). Murine studies showed the potential for recombinant influenza virus vaccines to be used in combination with other vaccine vectors (27, 28, 44). In our macaque trials we investigated sequential administration of heterologous influenza A virus strains and showed some boosting of CD8 T cells to the inserted SIV epitope. A potential limitation of this approach is the induction of cross-reactive influenza virus-specific T-cell immunity induced by the initial immunization that limits the effectiveness of the boosting vector. Indeed, we show that influenza virus-specific CD4 and CD8 T-cell immunity was readily induced in macaques with this immunization strategy. Further, we identified and finely mapped a common influenza virus NP CD8 T-cell epitope presented by multiple pigtail macaques. This facilitates the identification and characterization of influenza virus-specific CD8 T cells and allows future comparative studies of the effectiveness of CD8 T cells to acute (influenza virus) and chronic (SIV) viruses in pigtail macaques.
We studied mouse-adapted PR8 and X31 influenza A virus strains; these attenuated strains did not cause any symptoms and were cleared within 1 week of inoculation. Rapid clearance of the vector may have resulted in more transient SIV-specific immunity, although most vector-based vaccines, other than persistent viruses such as retroviruses and herpesviruses (15, 30), will be eliminated relatively quickly. The use of non-mouse-adapted influenza A virus strains with higher replicative capacity in primates should provide more robust and durable immunity that may not require frequent reboosting but could come at the cost of some symptoms or disease (12). The effectiveness of recombinant vaccines based on cold-adapted strains such as those used in the licensed live attenuated influenza virus vaccines requires further study. A key practical advantage of recombinant influenza virus-HIV vaccines is the ready availability of technologies, manufacturing capacity, and marketing of existing cold-adapted live attenuated influenza virus vaccines. Such technologies are rarely present for other live vectors and should, when suitable vaccine candidates are generated, substantially speed the development of this technology into clinical trials.
The ability to induce mucosal immunity is widely viewed as essential for improved HIV vaccine efforts. Influenza virus vectors delivered to the upper respiratory tract in mice robustly induce genital mucosal T-cell immunity (33). Our studies of the induction of SIV-specific CD8 T cells expressing the mucosal homing integrin α4β7 suggest that similar levels of genital mucosal immunity could be induced by influenza virus-HIV vector vaccines, although future biopsy- or autopsy-based studies are needed to confirm the presence and function of elevated numbers of mucosal SIV-specific T cells. The levels of α4β7 expression on circulating SIV-specific CD8 T cells are similar to those observed following live attenuated SIV infection (where virus also replicates at mucosal sites) and were comparable, if not better, than those in previous studies using DNA or viral vector vaccines administered intramuscularly (13, 35). Interestingly, we observed a sharp decline in peripheral β7+ SIV-specific CD8 T cells during acute SIV infection. One interpretation of this observation is that the β7+/tetramer-positive cells have left the circulation and headed toward SIV-infected mucosal tissues. If we can subsequently show that circulating β7+/tetramer-positive CD8 T cells do indeed accumulate rapidly in mucosal sites by sampling tissues in expanded studies, this could be indicative of an effective mucosal vaccination strategy. An alternative and more worrisome explanation, however, could be the specific targeting of α4β7-expressing CD8 T cells by SIV. Recent observations show that this integrin can bind HIV-1 and act as a facilitator of HIV-1 dissemination (5). CD4, rather than CD8, T cells are usually targeted by HIV/SIV infection; however, future studies should examine the infectivity of these T-cell populations.
An “Achilles heel” of CD8 T cell-based vaccine strategies is mutational escape (6, 29). Vaccinating with influenza virus recombinants expressing only a single SIV CD8 T-cell epitope (KP9, SIV Gag164-172) resulted in early, rapid, and complete immune escape of target virus using our novel qRT-PCR assay for the canonical K165R escape variant. Although escape may come at a “fitness cost” to the virus, this must have less impact on viral replication than effective CD8 T-cell immunity. The consequence of our strategy of vaccinating only against the KP9 epitope was a very high level of SIV-specific T cells (∼10% of all CD8 T cells) after challenge directed against a now-mutated viral CD8 T-cell epitope. Previous studies of rhesus macaques have elegantly shown the limitations of narrowly directed SIV-specific CD8 T-cell immunity when escape results (1-3, 6). Responses to other useful subdominant CD8 T-cell epitopes may be subverted by immunodominant “mono-epitope” strategies (16, 22).
A broader representation of SIV-specific CD8 T-cell epitopes, preferably supported by SIV-specific CD4 T-helper epitopes, is clearly required to adequately control virus replication. Although we show proof of principle that at least three SIV-specific CD8 T-cell responses (one Gag response and two Tat responses, all presented by Mane-A*10) can be boosted simultaneously by this recombinant influenza virus strategy, it could be cumbersome to construct sufficient numbers of influenza virus vectors to broadly cover relevant HIV/SIV T-cell epitopes in outbred populations. Expression of whole heterologous proteins from influenza virus proteins is technically possible and has been achieved with expression of the enhanced green fluorescence protein, interleukin-2, and tuberculosis proteins (21, 37, 56); however, the stability of such vectors will require careful study. We are currently constructing influenza virus vectors expressing whole SIV proteins.
In summary, we illustrate some of the promise and potential pitfalls of recombinant influenza virus-HIV vaccines expressing single SIV CD8 T-cell epitopes in macaques. Administration of these vectors to the respiratory tract reliably stimulates SIV-specific CD8 T cells that express mucosal homing integrin molecules. The vectors can be used in prime-boost regimens with heterologous influenza virus strains, although the usefulness of this approach is limited to some degree by conserved anti-influenza virus T-cell immunity. However, the ultimate utility of our current influenza virus-HIV vectors as effective vaccines requires further study, as immune escape from HIV-1 or SIV at CD8 T-cell epitopes is common. This strategy allows us to test the breadth of induced T-cell immune responses that will eventually be needed in future HIV vaccine strategies.
Acknowledgments
This work was supported by Australian National Health and Medical Research Council awards 299907 and 508902. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
We thank Caroline Fernandez, Thakshila Amarasena, Robert Goli, Leanne Smith, and Tania Cukalac for excellent assistance.
Footnotes
Published ahead of print on 13 May 2009.
REFERENCES
- 1.Allen, T. M., P. Jing, B. Calore, H. Horton, D. H. O'Connor, T. Hanke, M. Piekarczyk, R. Ruddersdorf, B. R. Mothe, C. Emerson, N. Wilson, J. D. Lifson, I. M. Belyakov, J. A. Berzofsky, C. Wang, D. B. Allison, D. C. Montefiori, R. C. Desrosiers, S. Wolinsky, K. J. Kunstman, J. D. Altman, A. Sette, A. J. McMichael, and D. I. Watkins. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J. Virol. 7610507-10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 764108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407386-390. [DOI] [PubMed] [Google Scholar]
- 4.Andreansky, S. S., J. Stambas, P. G. Thomas, W. Xie, R. J. Webby, and P. C. Doherty. 2005. Consequences of immunodominant epitope deletion for minor influenza virus-specific CD8+-T-cell responses. J. Virol. 794329-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9301-309. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. [DOI] [PubMed] [Google Scholar]
- 7.Batten, C. J., R. D. Rose, K. M. Wilson, M. B. Agy, S. Chea, I. Stratov, D. C. Montefiori, and S. J. Kent. 2006. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res. Hum. Retroviruses 22580-588. [DOI] [PubMed] [Google Scholar]
- 8.Bello, G., C. Casado, V. Sandonis, M. Alonso-Nieto, J. L. Vicario, S. Garcia, V. Hernando, C. Rodriguez, J. del Romero, and C. Lopez-Galindez. 2005. A subset of human immunodeficiency virus type 1 long-term non-progressors is characterized by the unique presence of ancestral sequences in the viral population. J. Gen. Virol. 86355-364. [DOI] [PubMed] [Google Scholar]
- 9.Belshe, R. B., K. M. Edwards, T. Vesikari, S. V. Black, R. E. Walker, M. Hultquist, G. Kemble, and E. M. Connor. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356685-696. [DOI] [PubMed] [Google Scholar]
- 10.Berendt, R. F. 1974. Simian model for the evaluation of immunity to influenza. Infect. Immun. 9101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 3721881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll, T. D., S. R. Matzinger, M. Genesca, L. Fritts, R. Colon, M. B. McChesney, and C. J. Miller. 2008. Interferon-induced expression of MxA in the respiratory tract of rhesus macaques is suppressed by influenza virus replication. J. Immunol. 1802385-2395. [DOI] [PubMed] [Google Scholar]
- 13.Cromwell, M. A., R. S. Veazey, J. D. Altman, K. G. Mansfield, R. Glickman, T. M. Allen, D. I. Watkins, A. A. Lackner, and R. P. Johnson. 2000. Induction of mucosal homing virus-specific CD8+ T lymphocytes by attenuated simian immunodeficiency virus. J. Virol. 748762-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crotty, S., B. L. Lohman, F. X. Lu, S. Tang, C. J. Miller, and R. Andino. 1999. Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: stimulation of humoral, mucosal, and cellular immunity. J. Virol. 739485-9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 2581938-1941. [DOI] [PubMed] [Google Scholar]
- 16.Davenport, M. P., L. Loh, J. Petravic, and S. J. Kent. 2008. Rates of HIV immune escape and reversion: implications for vaccination. Trends Microbiol. 16561-566. [DOI] [PubMed] [Google Scholar]
- 17.Demberg, T., J. D. Boyer, N. Malkevich, L. J. Patterson, D. Venzon, E. L. Summers, I. Kalisz, V. S. Kalyanaraman, E. M. Lee, D. B. Weiner, and M. Robert-Guroff. 2008. Sequential priming with simian immunodeficiency virus (SIV) DNA vaccines, with or without encoded cytokines, and a replicating adenovirus-SIV recombinant followed by protein boosting does not control a pathogenic SIVmac251 mucosal challenge. J. Virol. 8210911-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rose, R., C. J. Batten, M. Z. Smith, C. S. Fernandez, V. Peut, S. Thomson, I. A. Ramshaw, B. E. Coupar, D. B. Boyle, V. Venturi, M. P. Davenport, and S. J. Kent. 2007. Comparative efficacy of subtype AE simian-human immunodeficiency virus priming and boosting vaccines in pigtail macaques. J. Virol. 81292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Rose, R., C. S. Fernandez, M. Z. Smith, C. J. Batten, S. Alcantara, V. Peut, E. Rollman, L. Loh, R. D. Mason, C. M. Wilson, M. G. Law, A. J. Handley, and S. J. Kent. 2008. Control of viremia following immunotherapy of SIV-infected macaques with peptide pulsed blood. PLoS Pathog. 4e1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans, D. T., L. M. Chen, J. Gillis, K. C. Lin, B. Harty, G. P. Mazzara, R. O. Donis, K. G. Mansfield, J. D. Lifson, R. C. Desrosiers, J. E. Galan, and R. P. Johnson. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 772400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferko, B., C. Kittel, J. Romanova, S. Sereinig, H. Katinger, and A. Egorov. 2006. Live attenuated influenza virus expressing human interleukin-2 reveals increased immunogenic potential in young and aged hosts. J. Virol. 8011621-11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez, C. S., M. Z. Smith, C. J. Batten, R. De Rose, J. C. Reece, E. Rollman, V. Venturi, M. P. Davenport, and S. J. Kent. 2007. Vaccine-induced T cells control reversion of AIDS virus immune escape mutants. J. Virol. 814137-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez, C. S., I. Stratov, R. De Rose, K. Walsh, C. J. Dale, M. Z. Smith, M. B. Agy, S. L. Hu, K. Krebs, D. I. Watkins, H. O'Connor, D. M. P. Davenport, and S. J. Kent. 2005. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J. Virol. 795721-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchini, G., M. Robert-Guroff, J. Tartaglia, A. Aggarwal, A. Abimiku, J. Benson, P. Markham, K. Limbach, G. Hurteau, J. Fullen, et al. 1995. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res. Hum. Retroviruses 11909-920. [DOI] [PubMed] [Google Scholar]
- 25.Genesca, M., T. Rourke, J. Li, K. Bost, B. Chohan, M. B. McChesney, and C. J. Miller. 2007. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J. Immunol. 1794732-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genesca, M., P. J. Skinner, K. M. Bost, D. Lu, Y. Wang, T. L. Rourke, A. T. Haase, M. B. McChesney, and C. J. Miller. 2008. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gherardi, M. M., J. L. Najera, E. Perez-Jimenez, S. Guerra, A. Garcia-Sastre, and M. Esteban. 2003. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 777048-7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalo, R. M., D. Rodriguez, A. Garcia-Sastre, J. R. Rodriguez, P. Palese, and M. Esteban. 1999. Enhanced CD8+ T cell response to HIV-1 env by combined immunization with influenza and vaccinia virus recombinants. Vaccine 17887-892. [DOI] [PubMed] [Google Scholar]
- 29.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4630-640. [DOI] [PubMed] [Google Scholar]
- 30.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. Piatak, Jr., J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann, E., G. Neumann, Y. Kawaoka, G. Hobom, and R. G. Webster. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 976108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 767187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibraghimov, A. R., and R. G. Lynch. 1994. T cell specialization at environmental interfaces: T cells from the lung and the female genital tract of lpr and gld mice differ from their splenic and lymph node counterparts. Eur. J. Immunol. 241848-1852. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins, M. R., R. Webby, P. C. Doherty, and S. J. Turner. 2006. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J. Immunol. 1772917-2925. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman, D. R., J. Liu, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 1814188-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kent, S. J., R. De Rose, V. V. Mokhonov, E. A. Mohkonova, C. S. Fernandez, S. Alcantara, E. Rollman, R. D. Mason, L. Loh, V. Peut, J. Reece, X. J. Wang, K. M. Wilson, A. Suhrbier, and A. A. Khromykh. 2008. Evaluation of recombinant Kunjin replicon SIV vaccines for protective efficacy in macaques. Virology 374528-534. [DOI] [PubMed] [Google Scholar]
- 37.Kittel, C., S. Sereinig, B. Ferko, J. Stasakova, J. Romanova, A. Wolkerstorfer, H. Katinger, and A. Egorov. 2004. Rescue of influenza virus expressing GFP from the NS1 reading frame. Virology 32467-73. [DOI] [PubMed] [Google Scholar]
- 38.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162254-262. [PubMed] [Google Scholar]
- 39.La Gruta, N. L., K. Kedzierska, K. Pang, R. Webby, M. Davenport, W. Chen, S. J. Turner, and P. C. Doherty. 2006. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc. Natl. Acad. Sci. USA 103994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 45787-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh, L., J. Petravic, C. J. Batten, M. P. Davenport, and S. J. Kent. 2008. Vaccination and timing influence SIV immune escape viral dynamics in vivo. PLoS Pathog. 4e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohmeyer, J., J. Friedrich, F. Grimminger, U. Maus, R. Tenter, H. Morr, H. G. Velcovsky, W. Seeger, and S. Rosseau. 1999. Expression of mucosa-related integrin alphaEbeta7 on alveolar T cells in interstitial lung diseases. Clin. Exp. Immunol. 116340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 3721894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakaya, Y., T. Nakaya, M. S. Park, J. Cros, J. Imanishi, P. Palese, and A. Garcia-Sastre. 2004. Induction of cellular immune responses to simian immunodeficiency virus Gag by two recombinant negative-strand RNA virus vectors. J. Virol. 789366-9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakaya, Y., H. Zheng, and A. Garcia-Sastre. 2003. Enhanced cellular immune responses to SIV Gag by immunization with influenza and vaccinia virus recombinants. Vaccine 212097-2106. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8493-499. [DOI] [PubMed] [Google Scholar]
- 47.Oh, D. Y., I. G. Barr, J. A. Mosse, and K. L. Laurie. 2008. MDCK-SIAT1 cells show improved isolation rates for recent human influenza viruses compared to conventional MDCK cells. J. Clin. Microbiol. 462189-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palese, P., F. Zavala, T. Muster, R. S. Nussenzweig, and A. Garcia-Sastre. 1997. Development of novel influenza virus vaccines and vectors. J. Infect. Dis. 176(Suppl. 1)S45-S49. [DOI] [PubMed] [Google Scholar]
- 49.Patterson, L. J., J. Beal, T. Demberg, R. H. Florese, N. Malkevich, D. Venzon, K. Aldrich, E. Richardson, V. S. Kalyanaraman, I. Kalisz, E. M. Lee, D. C. Montefiori, F. A. Robey, and M. Robert-Guroff. 2008. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV89.6P challenge in Mamu-A*01 negative rhesus macaques. Virology 374322-337. [DOI] [PubMed] [Google Scholar]
- 50.Picker, L. J., and D. I. Watkins. 2005. HIV pathogenesis: the first cut is the deepest. Nat. Immunol. 6430-432. [DOI] [PubMed] [Google Scholar]
- 51.Pratt, B. F., D. H. O'Connor, B. A. Lafont, J. L. Mankowski, C. S. Fernandez, R. Triastuti, A. G. Brooks, S. J. Kent, and M. Z. Smith. 2006. MHC class I allele frequencies in pigtail macaques of diverse origin. Immunogenetics 58995-1001. [DOI] [PubMed] [Google Scholar]
- 52.Rimmelzwaan, G. F., T. Kuiken, G. van Amerongen, T. M. Bestebroer, R. A. Fouchier, and A. D. Osterhaus. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 756687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rollman, E., R. D. Mason, J. Lin, A. Brooks, and S. J. Kent. 2008. Protection afforded by live attenuated SIV is associated with rapid killing kinetics of CTLs. J. Med. Primatol. 3724-32. [DOI] [PubMed] [Google Scholar]
- 54.Rollman, E., M. Z. Smith, A. G. Brooks, D. F. Purcell, B. Zuber, I. A. Ramshaw, and S. J. Kent. 2007. Killing kinetics of simian immunodeficiency virus-specific CD8+ T cells: implications for HIV vaccine strategies. J. Immunol. 1794571-4579. [DOI] [PubMed] [Google Scholar]
- 55.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106539-549. [DOI] [PubMed] [Google Scholar]
- 56.Sereinig, S., M. Stukova, N. Zabolotnyh, B. Ferko, C. Kittel, J. Romanova, T. Vinogradova, H. Katinger, O. Kiselev, and A. Egorov. 2006. Influenza virus NS vectors expressing the Mycobacterium tuberculosis ESAT-6 protein induce CD4+ Th1 immune response and protect animals against tuberculosis challenge. Clin. Vaccine Immunol. 13898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsend, A. R., F. M. Gotch, and J. Davey. 1985. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell 42457-467. [DOI] [PubMed] [Google Scholar]
- 58.Wahl, A., F. Schafer, W. Bardet, R. Buchli, G. M. Air, and W. H. Hildebrand. 2009. HLA class I molecules consistently present internal influenza epitopes. Proc. Natl. Acad. Sci. USA 106540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webby, R. J., S. Andreansky, J. Stambas, J. E. Rehg, R. G. Webster, P. C. Doherty, and S. J. Turner. 2003. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc. Natl. Acad. Sci. USA 1007235-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yun, N. E., N. S. Linde, M. A. Zacks, I. G. Barr, A. C. Hurt, J. N. Smith, N. Dziuba, M. R. Holbrook, L. Zhang, J. M. Kilpatrick, C. S. Arnold, and S. Paessler. 2008. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1). Virology 374198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]