Abstract
Mature, fully active human immunodeficiency virus protease (PR) is liberated from the Gag-Pol precursor via regulated autoprocessing. A chimeric protease precursor, glutathione S-transferase-transframe region (TFR)-PR-FLAG, also undergoes N-terminal autocatalytic maturation when it is expressed in Escherichia coli. Mutation of the surface residue H69 to glutamic acid, but not to several neutral or basic amino acids, impedes protease autoprocessing in bacteria and mammalian cells. Only a fraction of mature PR with an H69E mutation (PRH69E) folds into active enzymes, and it does so with an apparent Kd (dissociation constant) significantly higher than that of the wild-type protease, corroborating the marked retardation of the in vitro N-terminal autocatalytic processing of TFR-PRH69E and suggesting a folding defect in the precursor.
In the infected cell, human immunodeficiency virus (HIV) protease is initially translated as part of the Gag-Pol precursor (1, 16), in which it is flanked N terminally by the transframe region (TFR, transframe peptide [TFP] plus p6pol) and C terminally by the reverse transcriptase (14, 16) (Fig. 1). The N-terminal cleavage is critical for protease (PR) maturation (14, 15, 19), while the C-terminal cleavage has negligible influence on protease activity (5, 14). The mature protease exists as stable dimers (dimer dissociation constant [Kd], <10 nM) with the catalytic site formed at the dimer interface by two aspartic acids, each contributed by one monomer (13). In contrast, a protease precursor, TFR-PR, has very low catalytic activity due to a much higher Kd (13, 14). Therefore, precursor dimerization coupled with intramolecular N-terminal cleavage appears critical for protease maturation (14, 18). However, the underlying in vivo macromolecular mechanism and essential viral determinants remain largely undefined.
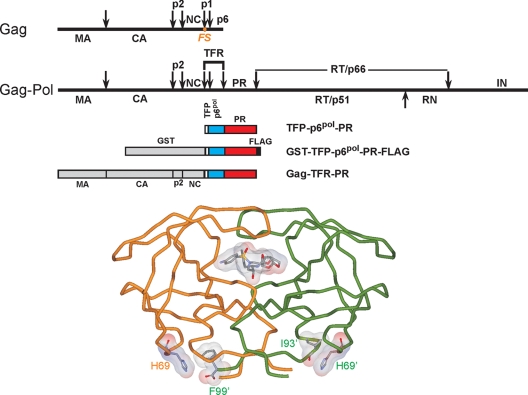
FIG. 1.
Structural organization of Gag and Gag-Pol polyproteins in HIV-1 and tube representation of the three-dimensional structure of the mature protease dimer in complex with the inhibitor DRV (Protein Data Bank accession number 2IEN [20]). Straight arrows indicate the specific sites of cleavage by the viral protease. The TFR, N-terminal to the protease, consists of the transframe octapeptide TFP and 48 amino acids of p6pol. The nomenclature of HIV-1 proteins is according to Leis at al. (9), as follows: CA, capsid; NC, nucleocapsid; RT, reverse transcriptase; RN, RNase H; and IN, integrase. Residues H69, F99, and I93 (proximal to H69) and DRV are shown as stick and surface representations. The protease precursor constructs used for monitoring the autocatalytic maturation reaction in vitro (TFR-PR) and in E. coli (GST-TFR-PR-FLAG) as well as the proviral construct expressed in 293T cells in vivo (Gag-TFR-PR) are all drawn to scale. The position of the frameshift (FS) that produces Gag-Pol is indicated as an orange dot. Calculated molecular weights of the domains indicated for the precursors are 26,583, 1,010, 5,357, 10,727, and 1,112 for GST, TFP, p6pol, PR, and FLAG, respectively. The proviral DNA construct contains the region for the expression of Gag and Gag-TFR-PR.
The GST-fused protease precursor undergoes autoprocessing in Escherichia coli.
When expressed in E. coli, either alone or as a larger precursor, the HIV protease is associated mainly with inclusion bodies (11, 12, 14). In an attempt to study protease maturation in vivo, we engineered a pGEX-3X-derived vector expressing a glutathione S-transferase (GST)-TFR-PR-FLAG fusion precursor by overlapping PCR mutagenesis (2, 3) (Fig. 1). In this construct, the TFR sequence is derived from pNL4-3 proviral DNA, the PR is the previously described pseudo-wild-type protease (12), and the C terminus of mature protease is fused in-frame to a FLAG (DYKDDDDK) peptide via a single valine residue. The resulting plasmid was transformed into E. coli BL21(DE3) (Novagen, San Diego, CA). Upon protein expression, cells were lysed in a 110-liter microfluidizer (Microfluidics, Newton, MA) in 1/10 of the culture volume of ice-cold 1× phosphate-buffered saline buffer containing 1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride and centrifuged (17,000 × g for 20 min at 4°C). PR-FLAG was found in the soluble fraction (Fig. 2A, lane 1), which was catalytically active (assayed using substrate IV, Lys-Ala-Arg-Val-Nle-[p-NO2-Phe]-Glu-Ala-Nle-NH2 [California Peptide Research, Napa, CA]) (12, 14) and demonstrated the expected molecular weight (calculated, 11,821; found, 11,824). The cleavage products containing the N-terminal GST were also recovered from the soluble lysates (Fig. 2A, lane 2). This approach is very similar to that of earlier studies (14) using a maltose binding protein (MBP) domain in the place of GST except that the MBP fusion precursor is not processed in E. coli.
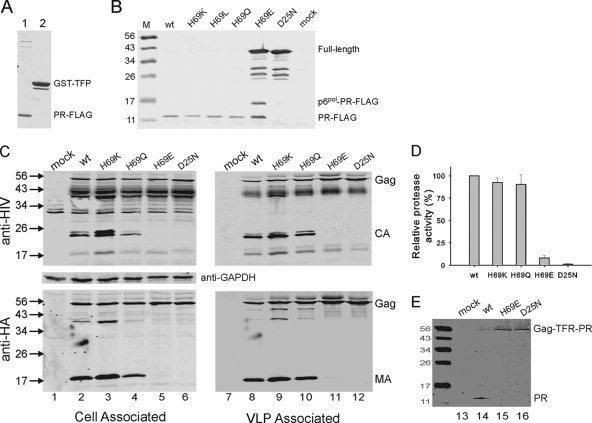
FIG. 2.
Protease autocatalytic maturation in E. coli and transfected 293T cells. (A) E. coli BL21(DE3) cells expressing GST-TFR-PR-FLAG were collected and divided into soluble and insoluble fractions. PR-FLAG (lane 1) and GST-containing proteins (lane 2) associated with the soluble fraction were affinity purified with anti-FLAG and anti-GST matrices (Sigma, St. Louis, MO) by following the manufacturer's instructions. They were then resolved by 13.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie brilliant blue R-250 staining. (B) Lysates derived from equal volumes of cultured bacteria expressing the indicated mutations were resolved by 15% SDS-PAGE and immunoblotted with mouse anti-FLAG M2 (Sigma) as the primary antibody and IR800 goat anti-mouse as the secondary antibody. M denotes the molecular mass standards in kDa. (All secondary antibodies used in Fig. 2 were obtained from Rockland Immunochemicals, Inc., Gilbertsville, PA). wt, wild type. (C) pNL4-3-derived proviral constructs expressing pseudo-wild-type or mutant PRs were transfected into 293T cells (4). At 48 h posttransfection, VLPs and postnuclear cell lysates were subjected to SDS-PAGE and Western blot analysis as described previously (2, 10). About 10% of cell lysates and 25% of VLPs derived from one well of a six-well plate were analyzed. Virus-specific Gag proteins were detected with human anti-HIV immunoglobulins and mouse anti-HA (Sigma) as primary antibodies and IR800 goat anti-human and IR700 goat anti-mouse as secondary antibodies. The blot was stripped and reprobed for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (clone 6C5; Fisher Scientific, Pittsburgh, PA) as a loading control. (D) Relative protease activity in VLPs was expressed as the ratio of MA to the sum of the band intensities in each lane and normalized relative to the ratio observed for the wild type (set to 100%). The graph presents averages of results from two independent experiments, with standard deviations. (E) The mature and precursor proteases in the VLPs produced by the indicated constructs were detected by polyclonal rabbit anti-PR antibodies and IR800 goat anti-rabbit antibodies.
The H69E mutation abolishes precursor autoprocessing in E. coli.
By examining charge distribution on the surface of HIV type 1 (HIV-1) mature protease, we noticed that residue 69 is part of a positively charged cluster. A similarly located basic patch is also present in HIV-2 protease (8). A previous study using bacterially expressed Gag-Pol precursor demonstrated that changing H69 to R, L, Y, N, and Q, individually, did not impair protease maturation; however, the H69D mutation abolished autoprocessing (11). To evaluate further the charge effect of H69 on protease maturation, we altered the parental H to K, L, Q, and E, individually. GST-TFR-PRD25N-FLAG, in which the Asp residue critical for catalytic activity was mutated to Asn (7, 11, 17), was also constructed to produce the full-length GST fusion precursor serving as a negative control. Mature protease and precursors were detected with anti-FLAG antibody by Western blotting (Fig. 2B). Three constructs (bearing the H69K, H69L, and H69Q mutations) produced mature protease as effectively as the wild-type control. In marked contrast, the H69E mutation resulted in accumulation of the precursor, similar to that observed with the D25N negative control (Fig. 2B). A likely intermediate, p6pol-PRH69E-FLAG, and mature PRH69E-FLAG were detected as well, an indication of some residual autocatalytic activity, although it apparently is insufficient to complete protease autoprocessing. Thus, substitution of either acidic amino acid for H69 prevents normal protease autoprocessing.
The H69E mutation also diminishes protease maturation in the context of proviral constructs.
We further subcloned these sequences into a pNL4-3-derived proviral construct in which a stop codon was placed at the end of the protease coding sequence, leading to the production of Gag-TFR-PR polyprotein as a result of frameshifting (Fig. 1). These constructs also carry the hemagglutinin (HA) peptide (underlined) flanked by linker amino acids (ASYPYDVPDYID) in the C-terminal region (between residues 127 and 128) of the matrix (MA) domain of Gag to permit quantitative detection of all of the MA-containing proteins by means of a monoclonal anti-HA antibody. When transfected into 293T cells (ATCC, Manassas, VA), the plasmids mediated comparable levels of Gag expression, indicated by approximately equal amounts of total Gag proteins detected in cell lysates (Fig. 2C, left panels). Detection of capsid protein (p24), the final product of Gag processing, in virus-like particles (VLPs) suggested adequate protease activity as a result of effective protease maturation. VLPs produced by H69K and H69Q mutants displayed a pattern similar to that of the pseudo-wild-type control (Fig. 2C, right panels). In contrast, only the full-length Gag precursor was detected in H69E VLPs (Fig. 2C), which resembled the D25N negative control (Fig. 2C, lane 12).
Intensities of bands containing the MA domain (detected with monoclonal anti-HA antibody) in VLPs were quantified using an Odyssey infrared dual-laser scanning unit (LI-COR Biotechnology, Lincoln, NE) and Totallab software (Nonlinear Dynamics Inc., Newcastle upon Tyne, United Kingdom). Protease activity was calculated from the ratio of MA to the sum of the band intensities in each lane relative to the wild-type ratio normalized to 100%. The H69E mutation reduced protease activity by >90% (Fig. 2D). Additionally, only the full-length unprocessed Gag-TFR-PR precursor was detected by a polyclonal rabbit anti-PR serum in VLPs produced by the H69E and D25N precursors (Fig. 2E). The H69D mutation in the context of pNL4-3 also impaired protease maturation, whereas H69N had no major impact (our unpublished observation). In summary, we suggest that protease maturation is modulated by charge properties of the residue 69, being impaired by acidic amino acids while not being affected by neutral or positively charged amino acids.
The H69E mutation abrogates precursor autoprocessing in vitro.
We next tested the ability of TFR-PRH69E to undergo autocatalytic maturation in vitro (12, 14). Site-directed mutagenesis, protein purification, and kinetic analysis were carried out as previously described (6, 12, 14). At comparable concentrations, maturation of the wild-type precursor was virtually complete in 60 min (Fig. 3A), whereas no processing was detected after 30 min for TFR-PRH69E. At a 2.2-fold-higher protein concentration of TFR-PRH69E, processing occurred over a 2- to 24-h time period to give the expected products (12, 14), p6pol and mature PRH69E (Fig. 3B). In contrast to our observation with the wild type, TFR-PRH69E processing appeared to stop when only 20 to 25% of the precursor was converted. The precursor in the reaction mixture is not further processed by the released mature PRH69E over a 24-h period (Fig. 3B), possibly due to folding defects of the mutant precursor that render the N-terminal cleavage site inaccessible to the mature protease.
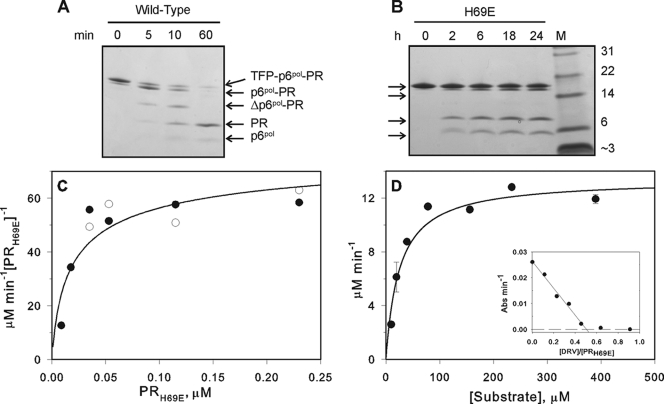
FIG. 3.
Time course of autocatalytic maturation of protease precursors in vitro and kinetic characterization of mature PRH69E. Processing reactions for the wild-type and H69E precursor proteins were conducted at final concentrations of 3.1 and 8.8 μM (A and B, respectively) in 50 mM sodium acetate, pH 5, at room temperature. Reactions were terminated as indicated (in min [A] or h [B]) and subjected to SDS-PAGE on 10 to 20% Tris-Tricine premade gels. M and Δ denote the molecular mass standards in kDa and “truncated,” respectively. The four arrows in panel B indicate TFP-p6pol-PR, p6pol-PR, PR, and p6pol, from top to bottom. The intermediate cleavage that occurs within p6pol to generate Δp6pol-PR in the wild-type precursor (A) was not observed in the H69E mutant precursor (B). (C) Initial rates of cleavage of substrate IV (0.4 mM) were measured at 28°C as a function of protein concentration (expressed as monomers) in 50 mM sodium acetate buffer, pH 5, containing 250 mM NaCl, and an apparent Kd of 30 nM was obtained by curve fitting an equation for the fraction of dimeric protease as a function of protein concentration (21) to the data. Filled and open symbols represent data from two separate experiments. (D) Kinetic parameters (Km and kcat) for hydrolysis of the chromogenic peptide, substrate IV, by 0.11 μM PRH69E (expressed as dimers) were determined under the same conditions. The inset shows a titration with the active-site-directed inhibitor DRV indicating that only ∼50% of the refolded PRH69E population possessed a functional active site.
Mature PRH69E is comparable to PR in its catalytic activity but not in its folding efficiency.
The H69E effect on autoprocessing could be attributed to at least (i) reduced enzymatic activity or (ii) impaired precursor dimerization coupled with N-terminal cleavage. To investigate these possible factors, we also examined the kinetic properties of mature PRH69E. Proteases were purified and stored under denaturing conditions, and a previously described quench protocol was used to refold the proteins (13, 17). Refolded PRH69E had an apparent dimer Kd of ∼30 nM (Fig. 3C and Table 1) compared with a Kd of <10 nM for wild-type PR. The kinetic constants Km and kcat for hydrolysis of substrate IV were measured at a concentration at which the protein is expected to be almost entirely dimeric (Fig. 3D; Table 1). The apparent kcat determined for PRH69E is lower than that previously measured for PR. However, titration of refolded PRH69E (Fig. 3D, inset) showed that only ∼50% of the nominal protein concentration possessed an active site capable of binding the inhibitor darunavir (DRV) (20). Thus, the actual Kd for active PRH69E is expected to be smaller, and the kcat larger, than the present apparent values by a factor of up to 2. Since the H69E mutation does not greatly affect the catalytic properties of active, mature PRH69E relative to those of PR, its effect on protease maturation is substantially larger than expected from a comparison of the mature enzymes. The apparent misfolding of ∼50% of the mature PRH69E resembles what we observed previously, namely, that in vitro autoprocessing of the H69E precursor does not proceed to completion even after an extended time. This suggests that a negative charge at residue 69 may adversely affect folding, such that only a fraction forms the correct geometric arrangement to undergo maturation.
TABLE 1.
Comparison of kinetic and equilibrium constants for mature PRH69E with those for wild-type PR
Measurements with PRH69E were made in 50 mM sodium acetate buffer, pH 5, containing 250 mM NaCl at 28°C (this study). wt, wild type.
This is an apparent value based on total protein concentration; active-site titration indicated that only ∼50% of the protein was catalytically competent.
See reference 12.
At 25°C (6).
Acknowledgments
We thank Annie Aniana for technical assistance.
This work was supported in part by the Intramural Research Program of the NIDDK, NIH. We acknowledge the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, for providing human anti-HIV antibodies (catalog no. 3957), rabbit HIV-1 protease antiserum (catalog no. 4105), and DRV (reagent 11447 from Tibotec Pharmaceuticals).
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220868-871. [DOI] [PubMed] [Google Scholar]
- 2.Chen, C., F. Li, and R. C. Montelaro. 2001. Functional roles of equine infectious anemia virus Gag p9 in viral budding and infection. J. Virol. 759762-9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, C., and R. C. Montelaro. 2003. Characterization of RNA elements that regulate Gag-Pol ribosomal frameshifting in equine infectious anemia virus. J. Virol. 7710280-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 72745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry, E., C. Liang, L. Rong, Y. Quan, P. Inouye, X. Li, N. Morin, M. Kotler, and M. A. Wainberg. 1998. Characterization of human immunodeficiency virus type-1 (HIV-1) particles that express protease-reverse transcriptase fusion proteins. J. Mol. Biol. 28443-56. [DOI] [PubMed] [Google Scholar]
- 6.Ishima, R., D. A. Torchia, S. M. Lynch, A. M. Gronenborn, and J. M. Louis. 2003. Solution structure of the mature HIV-1 protease monomer: insight into the tertiary fold and stability of a precursor. J. Biol. Chem. 27843311-43319. [DOI] [PubMed] [Google Scholar]
- 7.Kohl, N. E., E. A. Emini, W. A. Schleif, L. J. Davis, J. C. Heimbach, R. A. Dixon, E. M. Scolnick, and I. S. Sigal. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA 854686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalevsky, A. Y., J. M. Louis, A. Aniana, A. K. Ghosh, and I. T. Weber. 2008. Structural evidence for effectiveness of darunavir and two related antiviral inhibitors against HIV-2 protease. J. Mol. Biol. 384178-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leis, J., D. Baltimore, J. M. Bishop, J. Coffin, E. Fleissner, S. P. Goff, S. Oroszlan, H. Robinson, A. M. Skalka, H. M. Temin, et al. 1988. Standardized and simplified nomenclature for proteins common to all retroviruses. J. Virol. 621808-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, F., C. Chen, B. A. Puffer, and R. C. Montelaro. 2002. Functional replacement and positional dependence of homologous and heterologous L domains in equine infectious anemia virus replication. J. Virol. 761569-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb, D. D., R. Swanstrom, L. Everitt, M. Manchester, S. E. Stamper, and C. A. Hutchison III. 1989. Complete mutagenesis of the HIV-1 protease. Nature 340397-400. [DOI] [PubMed] [Google Scholar]
- 12.Louis, J. M., G. M. Clore, and A. M. Gronenborn. 1999. Autoprocessing of HIV-1 protease is tightly coupled to protein folding. Nat. Struct. Biol. 6868-875. [DOI] [PubMed] [Google Scholar]
- 13.Louis, J. M., R. Ishima, D. A. Torchia, and I. T. Weber. 2007. HIV-1 protease: structure, dynamics, and inhibition. Adv. Pharmacol. 55261-298. [DOI] [PubMed] [Google Scholar]
- 14.Louis, J. M., I. T. Weber, J. Tozser, G. M. Clore, and A. M. Gronenborn. 2000. HIV-1 protease: maturation, enzyme specificity, and drug resistance. Adv. Pharmacol. 49111-146. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig, C., A. Leiherer, and R. Wagner. 2008. Importance of protease cleavage sites within and flanking human immunodeficiency virus type 1 transframe protein p6* for spatiotemporal regulation of protease activation. J. Virol. 824573-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oroszlan, S., and R. B. Luftig. 1990. Retroviral proteinases. Curr. Top. Microbiol. Immunol. 157153-185. [DOI] [PubMed] [Google Scholar]
- 17.Sayer, J. M., F. Liu, R. Ishima, I. T. Weber, and J. M. Louis. 2008. Effect of the active site D25N mutation on the structure, stability, and ligand binding of the mature HIV-1 protease. J. Biol. Chem. 28313459-13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang, C., J. M. Louis, A. Aniana, J. Y. Suh, and G. M. Clore. 2008. Visualizing transient events in amino-terminal autoprocessing of HIV-1 protease. Nature 455693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tessmer, U., and H. G. Krausslich. 1998. Cleavage of human immunodeficiency virus type 1 proteinase from the N-terminally adjacent p6* protein is essential for efficient Gag polyprotein processing and viral infectivity. J. Virol. 723459-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tie, Y., P. I. Boross, Y. F. Wang, L. Gaddis, A. K. Hussain, S. Leshchenko, A. K. Ghosh, J. M. Louis, R. W. Harrison, and I. T. Weber. 2004. High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor (UIC-94017) active against multidrug-resistant clinical strains. J. Mol. Biol. 338341-352. [DOI] [PubMed] [Google Scholar]
- 21.Todd, M. J., N. Semo, and E. Freire. 1998. The structural stability of the HIV-1 protease. J. Mol. Biol. 283475-488. [DOI] [PubMed] [Google Scholar]