Abstract
L1 capsomeres purified from Escherichia coli represent an economic alternative to the recently launched virus-like particle (VLP)-based prophylactic vaccines against infection with human papillomavirus types 16 and 18 (HPV-16 and HPV-18), which are causative agents of cervical cancer. It was recently reported that capsomeres are much less immunogenic than VLPs. Numerous modifications of the L1 protein leading to the formation of capsomeres but preventing capsid assembly have been described, such as the replacement of the cysteine residues that form capsid-stabilizing disulfide bonds or the deletion of helix 4. So far, the influence of these modifications on immunogenicity has not been thoroughly investigated. Here, we describe the purification of eight different HPV-16 L1 proteins as capsomeres from Escherichia coli. We compared them for yield, structure, and immunogenicity in mice. All L1 proteins formed almost identical pentameric structures yet differed strongly in their immunogenicity, especially regarding the humoral immune responses. Immunization of TLR4−/− mice and DNA immunization by the same constructs confirmed that immunogenicity was independent of different degrees of contamination with copurifying immune-stimulatory molecules from E. coli. We hypothesize that immunogenicity correlates with the intrinsic ability of the capsomeres to assemble into larger particles, as only assembly-competent L1 proteins induced high antibody responses. One of the proteins (L1ΔN10) proved to be the most immunogenic, inducing antibody titers equivalent to those generated in response to VLPs. However, preassembly prior to injection did not increase immunogenicity. Our data suggest that certain L1 constructs can be used to produce highly immunogenic capsomeres in bacteria as economic alternatives to VLP-based formulations.
Certain types of human papillomavirus (HPV) are the cause of cervical cancer, most frequently HPV types 16 and 18 (HPV-16 and HPV-18), which are responsible for about 50% and 20% of cases, respectively (8, 15, 16). Recently, two vaccines that prevent infection with HPV-16 and HPV-18 have been introduced to the market. These vaccines are based on the viral major structural protein L1, which can spontaneously self-assemble in vitro into empty virus-like particles (VLPs) that resemble the native virions in size and shape. VLPs have been shown to be highly immunogenic, as they can induce high titers of neutralizing antibodies (29, 30). HPV virions and VLPs consist of 72 L1 pentamers, also called capsomeres, which are arranged in an icosahedral T=7 particle lattice with a diameter of 55 nm. Cryo-electron microscopic analysis has revealed the presence of 60 hexavalent and 12 pentavalent capsomeres (4).
Capsid assembly has been reported to be optimal at low pH (pH 5.4) and high ionic strength, whereas both high pH (pH 8.2) and the presence of reducing agents favor disassembly into capsomeres, the latter because the viral particles are stabilized by intercapsomeric disulfide bonds between two conserved cysteine residues at positions 175 and 428 (11, 35, 44). VLP formation is not affected by deletions of up to 9 amino acids (aa) from the N terminus and up to 34 aa from the C terminus of the L1 protein (11, 36). An N-terminally truncated L1 protein lacking 10 aa has been shown to assemble into particles consisting of 12 L1-pentamers with a T=1 lattice referred to as small VLPs (11, 12). Crystallographic analysis of the T=1 particles revealed that interpentameric contacts are established by hydrophobic interactions between the α-helices 2 and 3 of one capsomere and α-helix 4 of a neighboring capsomere (12). Consequently, a mutant L1 with helix 4 deleted formed homogenous capsomeres but failed in T=1 and T=7 particle assembly (7). Deletion of helices 2 and 3 impeded even pentamer formation, as a large fraction of the L1 protein was found to be insoluble, which suggests an essential role for these regions in L1 folding (7, 11).
VLP-based prophylactic vaccines have been shown to induce high titers of neutralizing antibodies, which protect against virus challenge and associated diseases in humans (24, 31). However, due to the relatively high production and distribution costs of the vaccines—they are expressed in and purified from eukaryotic cells and require a cold chain for storage—they will probably be largely unavailable to developing countries, where more than 80% of all cervical cancer cases occur (1, 38, 46).
L1 capsomeres represent a potentially lower cost alternative to VLPs, as they can be produced in large amounts from Escherichia coli and are considered more stable at room temperature (11, 34, 35). Capsomeres have been shown to induce high titers of neutralizing antibodies and T-cell responses upon oral, intranasal, and subcutaneous immunization and have also protected against viral challenge in the canine oral papillomavirus model (18, 19, 37, 42, 48, 53). Most of the immunization data for HPV capsomeres have been obtained from administration of full-length or N-terminally deleted (10 aa) wild-type L1 proteins (18, 37, 53). A recent report in which the L1 pentamers were derived from an L1 protein in which the conserved cysteines (aa 175 and 428) were replaced by alanines revealed that HPV-16 VLPs induce about 20- to 40-fold-higher humoral immune responses than capsomeres (47). The influence on immunogenicity of the other mutations and deletions of the L1 protein that prevent capsid assembly has so far not been studied in depth.
In a comparative analysis of eight differently modified HPV-16 L1 proteins purified as capsomeres from E. coli, we now report that their potential to induce humoral immune responses in mice correlates with their ability to assemble into particles larger than capsomeres. One of the constructs, L1ΔN10, encoded for capsomeres that exhibited immunogenicity similar to that of VLPs.
MATERIALS AND METHODS
Purification of the HPV-16 L1 capsomeres from E. coli.
All capsomere constructs were cloned into the pGex 4T-2 vector (Stratagene, La Jolla, CA) using the restriction enzymes BamHI/HindIII. The purification was performed by the method of Chen et al. (11) with minor modifications. Briefly, 2 liters of LB medium containing 1 mM ampicillin was inoculated 1:100 with an overnight culture of E. coli BL21 bacteria carrying the plasmid containing genes coding for L1 capsomeres and incubated at 37°C at 200 rpm until an optical density at 600 nm of 0.5 was reached. The cultures were cooled down to room temperature, and the expression of protein was induced by adding 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration). After incubation overnight at room temperature at 180 rpm, the bacteria were harvested, and the pellets were resuspended in 40 ml of buffer L (50 mM Tris-HCl [pH 8.2], 0.2 M NaCl, 1 mM EDTA, 2 mM dithiothreitol [DTT]), supplemented with a complete protease inhibitor cocktail tablet (Roche, Mannheim, Germany), and cells were lysed using a high-pressure homogenizer (Avestin, Ottawa, Canada). To prevent copurification of chaperones, lysates were incubated with 2 mM ATP and 5 mM MgCl2 (final concentrations) for 1 hour at room temperature, and urea was subsequently added slowly to a final concentration of 3.5 M. After 2 h of incubation at room temperature, the lysates were dialyzed for 16 to 18 h at 4°C against buffer L with three buffer exchanges. The lysates were cleared by centrifugation at 51,200 × g for 30 min, and supernatants were loaded on a 1-ml GSTrap column (GE Healthcare, Uppsala, Sweden) equilibrated in buffer L at a flow rate of 0.5 ml/min for 16 to 24 h at 4°C. Subsequently, columns were washed with 10 to 20 bed volumes of buffer L, and then the L1 protein was cleaved of the glutathione S-transferase (GST) tag by adding 40 units of thrombin protease (GE Healthcare) to the column and incubating overnight at room temperature. The eluates were dialyzed for 2 h at room temperature against modified buffer L, buffer LM (50 mM Tris-HCl [pH 8.2], 0.5 M NaCl, 1 mM EDTA, 5 mM DTT, 0.01% Tween 80), and then further purified by gel filtration chromatography using a Superdex 200 column (GE Healthcare). Protein concentrations were determined by Bradford assay using Rotiquant (Roth, Karlsruhe, Germany) and densitometrically on a Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel (SDS-PAG) by comparison to a bovine serum albumin (BSA) standard using ImageJ software (NIH, Bethesda, MD). Endotoxin concentrations were determined using the quantitative colorimetric QCL-1000 assay (Lonza, Walkersville, MD) following the manufacturer's instructions. Where indicated, lipopolysaccharide (LPS) was removed by treatment with Triton X-114 as reported previously (45).
SDS-PAGE and Western blot analysis.
Samples of purified proteins and sucrose gradient fractions were mixed with loading buffer (35 mM Tris-HCl [pH 8.6], 60 mM DTT, 3.6% glycerol, 1% SDS) and denatured for 10 min at 95°C. They were then separated by SDS-PAG electrophoresis (SDS-PAGE) using 12% polyacrylamide gels and stained with colloidal Coomassie blue dye (GelCode Blue stain reagent; Thermo Scientific, Rockford, IL) following the manufacturer's instructions.
For Western blot analysis, proteins were transferred from the SDS-PAG to a polyvinylidene difluoride membrane (Millipore, Eschborn, Germany). The L1 protein was detected using the HPV-16 L1-specific monoclonal antibody MD2H11. Bands were visualized by enhanced chemiluminescence for horseradish peroxidase detection (AppliChem, Darmstadt, Germany).
TEM.
For transmission electron microscopy (TEM), 3 μl of the sample at a concentration of 0.05 μg/μl was applied onto a carbon-coated grid and negatively stained with 2% uranyl acetate. Grids were analyzed using a transmission electron microscope CM200 FEG (FEI) operating at 200 kV. Pictures were taken at a magnification of 27,500-fold (VLPs) or 50,000-fold (capsomeres) using a slow-scan charge-coupled-device (2,000 × 2,000) camera.
Sedimentation analysis.
For this study, two kinds of sucrose gradient centrifugations were performed.
(i) Capsomere sucrose gradient.
The purified capsomeres (50 μg) were loaded onto 5 to 30% (wt/vol) linear sucrose gradients (in buffer LM) and centrifuged at 160,000 × g for 20 h at 4°C using a Beckman SW41Ti rotor. Fractions of 600 μl were collected from the bottom of the tube and analyzed by capture enzyme-linked immunosorbent assay (ELISA) and Western blotting. BSA (4S) (PAA Laboratories, Pasching, Austria), catalase (11S) (GE Healthcare), and thyroglobulin (19S) (GE Healthcare) were used to calibrate the gradient.
(ii) Assembly assay sucrose gradient.
L1 proteins were loaded before and after the assembly assay onto 5 to 50% (wt/vol) linear sucrose gradients (dissolved in buffer LM before and in phosphate-buffered saline [PBS], VLP buffer, or assembly buffer after the assembly assay) and centrifuged at 160,000 × g for 3 h at 4°C using the Beckman SW41Ti rotor. Fractions of 600 μl were collected from the bottom of the tube and analyzed by capture ELISA and Western blotting. Catalase (11S) (GE Healthcare) and HPV-16 VLPs purified from insect cells were used to calibrate the gradient.
Capture ELISA to analyze sucrose gradient fractions.
Each well in 96-well microtiter plates (Nunc, Roskilde, Denmark) was coated with 50 μl of the HPV-16 L1 conformation-specific monoclonal antibody Ritti01 (also referred to as 1.3.) (32, 36, 40) diluted 1:1,000 in PBS at 4°C overnight. After the plates were washed with PBS containing 0.05% Tween 20 [PBS-0.05% Tween 20], they were blocked with 5% skim milk in PBS-0.05% Tween 20 for 1 h at 37°C. Sucrose gradient fractions were diluted 1:10 or 1:50 in PBS-0.05% Tween 20 containing 5% skim milk, 50 μl was loaded onto each well on the plate, and the plates were incubated for 1 h at 37°C. The plates were washed with PBS-0.05% Tween 20 and subsequently incubated with 50 μl/well of a polyclonal serum generated against HPV-16 VLPs in a 1:5,000 dilution in 5% skim milk in PBS-0.05% Tween 20 for 1 h at 37°C. Afterwards, the plates were washed again, and 50 μl/well of a goat anti-rabbit peroxidase conjugate (1:5,000; Dianova, Hamburg, Germany) was added. After 1 h at 37°C, 50 μl of tetramethylbenzidine dihydrochloride (TMB) substrate (Sigma, Steinheim, Germany) was added to each well. After 5 to 30 min, the reaction was stopped with 50 μl of 1 M H2SO4 to each well. Absorbance was measured in an ELISA reader at 450 nm.
ELISA using different monoclonal HPV-16 L1-specific antibodies.
Each well in 96-well microtiter plates (Nunc) was coated with 0.5 μg/well (diluted in PBS) of purified L1 protein at 4°C overnight. The next day, the plates were washed four times with PBS-0.05% Tween 20 and blocked with 200 μl of 5% milk in PBS-0.05% Tween 20 per well for 1 h at 37°C. Afterwards, the wells of the plates were loaded with 50 μl/well of different HPV-16 L1-specific hybridoma supernatants kindly provided by N. D. Christensen (College of Medicine, Pennsylvania State University) (13, 14). Dilutions were chosen as previously determined by Rizk et al. (40). After 1 h of incubation at 37°C, the plates were washed, and 50 μl of a horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Dianova) diluted 1:5,000 was added to each well. Subsequently, 50 μl of TMB substrate (Sigma) was added to each well. The reaction was carried out for 5 to 30 min and then stopped by adding 50 μl of 1 M H2SO4 per well. Absorption was measured in an ELISA reader at 450 nm.
Assembly assay.
Purified HPV-16 L1 capsomeres (50 to 100 μg) were dialyzed in Slide-A-Lyzer dialysis chambers (Pierce) against PBS, VLP buffer (50 mM HEPES [pH 7.2], 0.5 M NaCl, 0.01% Tween 80) or assembly buffer (40 mM sodium acetate [pH 5.4], 1 M NaCl, 0.01% Tween 80) for 2 h at room temperature with one buffer exchange. Assemblies were analyzed by electron microscopy and sedimentation analysis.
Mice.
C57BL/6 mice (H2Db) were purchased from Charles River WIGA Laboratories (Sulzfeld, Germany). TLR4−/− (Sv129 × C57BL/6) mice (H2Db) (25) were originally obtained from C. Kirschning (TU Munich, Munich, Germany) and were bred and kept in the DKFZ (German Cancer Research Centre, Heidelberg, Germany) under specific-pathogen-free conditions. Alternatively, C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, ME) that carry a dominant-negative point mutation in the tlr4 gene were used. All mice were immunized at an age of 8 to 12 weeks.
Immunizations. (i) Protein immunizations.
Mice were immunized subcutaneously at biweekly intervals with 0.5 to 5 μg of purified L1 protein diluted in PBS, buffer LM, or assembly buffer. Control mice received PBS or buffer LM alone. To confirm the LPS insensitivity of the TLR4−/− mice (Sv129 × C57BL/6), 5,000 endotoxin units (EU) of LPS (O111:B4; Sigma) was added to the L1 proteins prior to injection. To study the effects of adjuvants on the immunogenicity of L1ΔN10 capsomeres and VLPs, C57BL/6 mice were vaccinated with 0.5 μg of the different particle forms adsorbed to 500 μg aluminum hydroxide (catalog no. A8222; Sigma) alone or in combination with an adjuvant system containing 50 μg of monophosphoryl lipid A (MPL) derived from Salmonella enterica serovar Minnesota R595 and 50 μg of synthetic trehalose dicorynomycolate in squalene and Tween 80 (catalog no. S6322; Sigma). Blood samples were taken with each immunization by puncture of the superficial temporal vein. Seven days after the last boost, the mice were sacrificed, and their spleens were used for enzyme-linked immunospot (ELISPOT) analysis. Final blood samples were taken by cardiac puncture, and antibody titers were determined by VLP capture ELISA.
(ii) DNA immunization.
For DNA immunizations, a humanized version of the HPV-16 L1 gene was used to generate the different L1 mutants, which were then subcloned into the pTHamp vector (23). Endotoxin-free plasmids were purified using the EndoFree plasmid maxikit (Qiagen, Hilden, Germany) and diluted in PBS to a final concentration of 3.3 μg/μl. Mice were anesthetized with isoflurane and shaved at the immunization site. The different DNA constructs (50 μg) were applied at biweekly intervals intradermally at the right flank using a tattoo device (6). Negative-control mice were immunized with the empty vector or a pTHamp vector coding for an irrelevant antigen (pTHamp HPV-16 E7). Seven days after the last boost, the mice were sacrificed, and their spleens were used for ELISPOT analysis. The final blood samples were taken by cardiac puncture, and antibody titers were determined by VLP capture ELISA.
VLP capture ELISA to determine L1-specific antibody titers.
The wells of 96-well microtiter plates (Nunc) were coated overnight with 50 μl/well of a purified polyclonal HPV-16 L1-specific serum in a 1:500 dilution in PBS at 4°C. The plates were washed with PBS-0.05% Tween 20 and blocked with PBS-0.05% Tween 20 containing 5% milk for 1 h at 37°C. Subsequently, HPV-16 VLPs (0.3 μg/well in 5% milk in PBS-0.05% Tween 20) purified from baculovirus-infected insect cells were added. After 1 h at 37°C, the plates were washed, and sera from immunized mice were applied in duplicate samples in a twofold dilution series starting from 1:50 and incubated for 1 h at 37°C. The plates were washed, and a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Dianova) was added (50 μl/well; 1:5,000 dilution). After 1 h at 37°C, 50 μl of TMB substrate (Sigma) was added to each well. The reaction was carried out for 5 to 30 min and then stopped with 50 μl of 1 M H2SO4 per well. Absorbance was measured in an ELISA reader at 450 nm. Antibody titers are given as the reciprocal of the highest dilution that resulted in a value above the cutoff (mean values of negative-control mice plus 3 standard deviations).
Neutralization assay.
HPV-16 pseudovirions incorporating a plasmid coding for the reporter protein secreted alkaline phosphatase (SEAP) were produced as described earlier (9, 39). For the neutralization assay, 293TT cells were seeded into 96-well cell culture plates in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal calf serum (GIBCO BRL, Germany) and 1% penicillin-streptomycin (Life Technologies, Germany) and incubated for 1 day at 37°C and 5% CO2. The sera of immunized mice were diluted 1:100 or 1:1,000 in medium and incubated with the HPV-16 pseudoviruses for 15 min at room temperature. Afterwards, the serum-pseudovirion mixtures were added to the 293TT cells in a volume of 200 μl per well and incubated for 5 days at 37°C and 5% CO2. For controls, 293TT cells were infected with pseudovirions alone (negative control) or with pseudovirions that were mixed with the HPV-16 L1-specific neutralizing monoclonal antibody Ritti01 (40) (positive control). Subsequently, the supernatant of the cells was collected and analyzed for the presence of SEAP using the chemiluminescence SEAP reporter gene assay following the manufacturer's instructions (Roche). Neutralizing activity was expressed as a percentage of neutralization compared to that of the positive control, which was set at 100%. All sera that showed neutralization above an arbitrary cutoff of 50% were regarded as neutralization positive. Neutralization titers were measured by determining the highest serum dilution that resulted in a neutralization value above the cutoff.
IFN-γ ELISPOT analysis.
Ex vivo ELISPOT analysis was performed 7 days after the last vaccination. MultiScreen-HTS ELISPOT plates (96-well plates) (Millipore, Germany) were incubated for 5 min with 70 μl of 70% ethanol per well, washed four times with PBS, and then coated with 100 μl/well of anti-mouse gamma interferon (IFN-γ) capture antibody (BD Pharmingen) (0.6 μg per well) in PBS at 4°C overnight. The plates were washed four times with PBS and then blocked with 150 μl per well of RPMI medium (Sigma) supplemented with 10% fetal calf serum (GIBCO BRL) and penicillin and streptomycin for 2 h at 37°C. Splenocytes of immunized mice were seeded into three wells in a twofold dilution series from 1 × 106 cells to 125,000 cells per well in a volume of 100 μl. For a negative control, 50 μl of medium per well was added to cells of one dilution series. For a positive control, one row of cells was stimulated nonspecifically with 1 μg of pokeweed mitogen (Sigma) per well. The third row received 10 μM of HPV-16 L1165-173 peptide (H2-Db) in a volume of 50 μl. After 16 to 18 h of incubation at 37°C, the cells were removed by washing four times with PBS-0.01% Tween 20 and once with PBS. Then, 0.1 μg of a biotinylated rat-anti-mouse IFN-γ antibody (BD Pharmingen) per well was added for 2 h at room temperature. Unbound detection antibody was removed by washing the wells six times with PBS-0.01% Tween 20 and once with PBS. Subsequently, 100 μl of streptavidin-alkaline phosphatase (BD Pharmingen) diluted 1:1,000 in PBS was added to each well, and the plates were incubated for 30 min at room temperature. The plates were washed three times with PBS-0.01% Tween 20 and three times with PBS and stained for up to 10 min with 100 μl of 5-bromo-4-chloro-3-indolylphosphate (BCIP)-Nitro Blue Tetrazolium liquid substrate system (Sigma) per well. The reaction was stopped by rinsing the plates extensively with cold water. Spots were quantified in an ELISPOT reader (Zeiss-Vison C; Zeiss, Oberkochen, Germany).
Statistical analysis.
Differences between data sets were tested using the Wilcoxon rank sum test. P values below 0.05 were considered statistically significant.
RESULTS
A previous report has shown that HPV-16 L1 capsomeres are less immunogenic than VLPs in terms of inducing antibody responses (47). This study used a modified L1 protein that forms capsomeres owing to the replacement of two cysteine residues (C175A and C428A). However, we wanted to extend this analysis to a comparison of differently modified L1 capsomere constructs—as several modifications of the L1 protein that inhibit capsid assembly have been described (7, 11, 12, 44)—and investigate whether there is any variation in their immunogenicity and whether any structural features can be used to predict this.
C-terminal deletions and the deletion of helix 4 lead to higher protein yields.
For our analysis, we designed eight differently modified HPV-16 L1 proteins (Fig. 1A). We expressed them as GST fusion proteins and purified these from E. coli using glutathione Sepharose chromatography and subsequent cleavage of the GST tag by the method of Chen et al. (11). All eight constructs had 10 N-terminal amino acids deleted; these amino acids conferred much higher solubility in bacteria compared to full-length L1 protein. This deletion has also been shown to prevent T=7 particle formation (11). In addition to the construct with only this 10-aa deletion, named L1ΔN10, we also included an L1 construct coding for two Cys→Ser exchanges at positions 175 and 428; these cysteines have been reported to be important for efficient VLP assembly (44) (double-cysteine mutant L1ΔN10ΔdCys). In two further constructs (L1ΔN10Δ414-431 and L1ΔN10Δ408-431), helix 4, which is also known to be essential for VLP formation, was deleted. The amino acids deleted from the L1ΔN10Δ408-431 protein included some adjacent to helix 4, and its structure has been characterized previously (11). We therefore also expressed an L1ΔN10Δ414-431 protein in which only helix 4 was deleted. For all four constructs, additional versions with C-terminal deletions of 29 aa were generated, as preliminary data suggested a positive effect of this deletion on solubility, and truncations of the C terminus of L1 are tolerated for up to 34 aa without affecting pentamer formation (36).
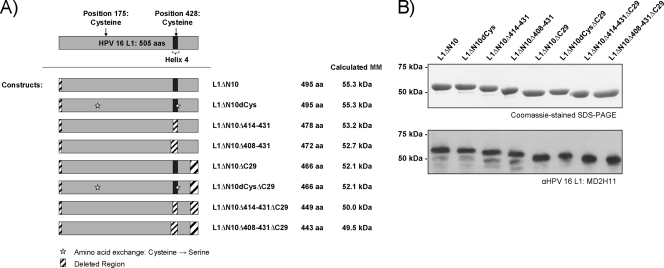
FIG. 1.
HPV-16 L1 capsomere constructs. (A) The different HPV-16 L1 capsomere constructs analyzed are depicted in cartoon format. For comparison, the wild-type L1 protein is displayed at the top. To the right of each construct map is its name, number of amino acids (aa), and the expected molecular mass (MM). (B) Equal amounts of the purified L1 proteins were analyzed by Coomassie blue-stained SDS-PAGE (top) and Western blotting using the L1-specific monoclonal antibody MD2H11 (bottom). αHPV 16 L1, anti-HPV-16 L1 antibody.
The purified proteins were analyzed by Coomassie blue staining following SDS-PAGE (Fig. 1B, top) and Western blotting (Fig. 1B, bottom), which revealed the expected molecular masses for each construct (Fig. 1A). All proteins were of high purity (95 to 99%). The average protein yields for each construct are shown in Table 1. The protein yields of the L1ΔN10 and L1ΔN10dCys capsomere constructs were similar to one another, as were the yields of the L1ΔN10ΔC29 and L1ΔN10dCysΔC29 proteins. Compared to L1ΔN10, the constructs with a full-length C terminus but lacking helix 4 gave up to 52% higher protein yields after purification. As no differences in expression levels across constructs were detected, the proteins lacking helix 4 are most likely more soluble. In comparison to their full-length analogues, constructs with C-terminal deletions resulted in an almost twofold increase in protein yields, and the highest protein yield was achieved for the constructs with both helix 4 and C-terminal deletions, resulting in 2.2- to 2.8-fold-higher yields than for L1ΔN10.
TABLE 1.
Average HPV-16 L1 protein yields for the different capsomere constructsa
| L1 capsomere construct | L1 protein yield (mg)/liter of bacterial culture | Amt (EU/mg) of LPS/mg of L1 |
|---|---|---|
| L1ΔN10 | 0.27 | 1,750 |
| L1ΔN10dCys | 0.23 | 2,400 |
| L1ΔN10Δ414-431 | 0.34 | 2,900 |
| L1ΔN10Δ408-431 | 0.41 | 850 |
| L1ΔN10ΔC29 | 0.50 | 860 |
| L1ΔN10dCysΔC29 | 0.45 | 780 |
| L1ΔN10Δ414-431ΔC29 | 0.59 | 1,450 |
| L1ΔN10Δ408-431ΔC29 | 0.75 | 410 |
The L1 proteins were expressed in and purified from E. coli as described previously by Chen et al. (11). Subsequently, gel filtration chromatography was performed using a Superdex 200 column. Protein amounts were determined by Bradford assay and by densitometry on a Coomassie blue-stained SDS-PAG using the ImageJ software (NIH). LPS contamination was measured using the colorimetric assay QCL-1000 (Lonza). The values in the table are the median values for at least three independent purifications.
The different L1 proteins form homogenous capsomeres.
Gel filtration of the L1 proteins under native conditions determined their molecular masses to be between 220 and 260 kDa, indicating the presence of pentamers (data not shown).
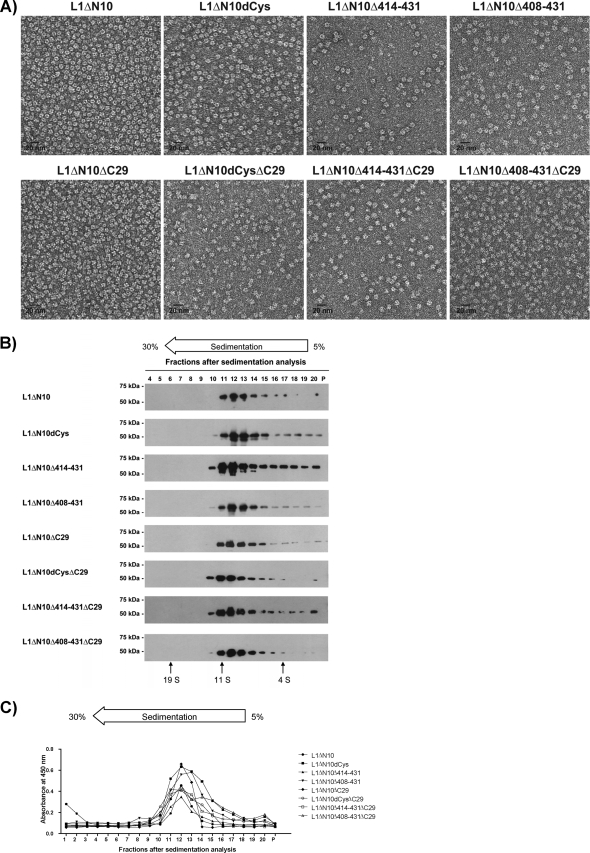
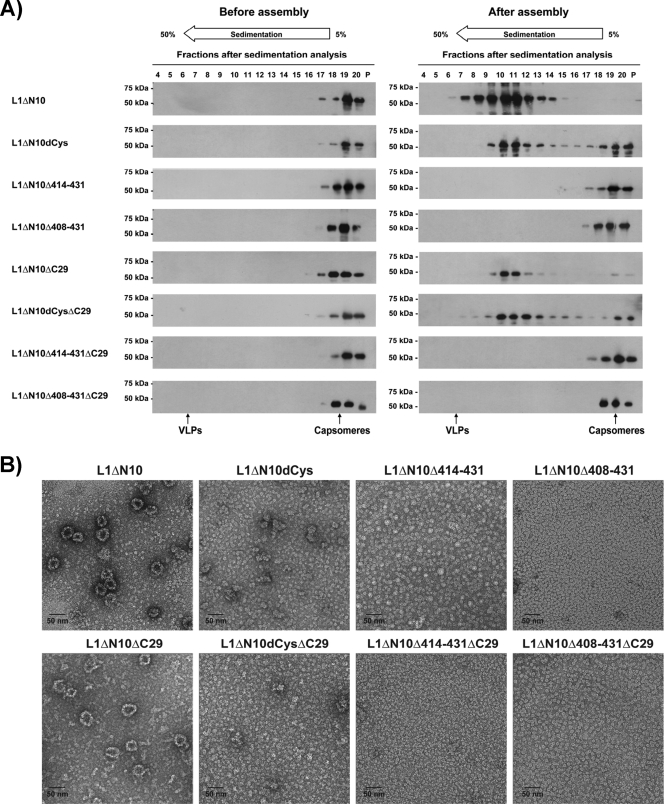
To further analyze their structure, electron microscopy (Fig. 2A) and sedimentation analyses (Fig. 2B and C) were performed. For all constructs, the electron microscopy analyses revealed homogenous 10-nm particles consistent in shape and size with previously reported L1 capsomeres (11, 22, 35). For the L1 proteins with the full-length C terminus (Fig. 2A, top row), a capsomere-characteristic donut-like structure was observed, whereas for the C-terminally truncated constructs, the black dot in the center of the capsomeres, originating from the staining of their cylindrical cavity (4), was only rarely visible.
FIG. 2.
Structural analysis of the HPV-16 L1 capsomeres. The structure of the purified HPV-16 L1 proteins in buffer LM was characterized by TEM employing negative staining (A) and sedimentation analysis. Fractions 4 to 20 and pellets (P) of the sucrose gradients were analyzed by Western blotting using the L1-specific monoclonal antibody MD2H11 (B), and fractions 1 to 20 and pellets were analyzed by capture ELISA (C). BSA (4S), catalase (11S), and thyroglobulin (19S) were used as calibration markers.
For the sedimentation analysis, fractions were analyzed by Western blotting (Fig. 2B) and capture ELISA (Fig. 2C). All L1 proteins were found mainly in fractions 11 to 13, corresponding to a sedimentation coefficient of approximately 11S. This result is consistent with the value reported for L1 pentamers (33, 37, 53). The capture ELISA was performed using a neutralizing HPV-16 L1-specific monoclonal antibody that recognizes a conformational epitope (Ritti01). This antibody bound to all the different L1 proteins recovered from fractions 11 to 13, suggesting that the pentamers were correctly folded.
These results show that all HPV-16 L1 constructs oligomerized into homogenous pentamers.
The different capsomeres are of variable immunogenicity.
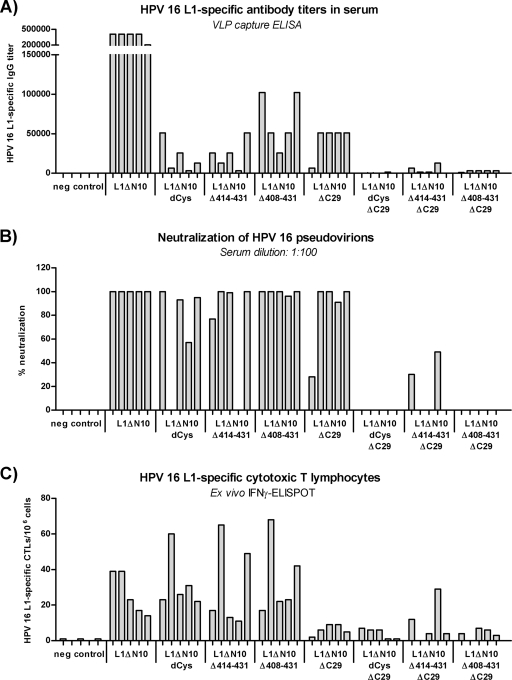
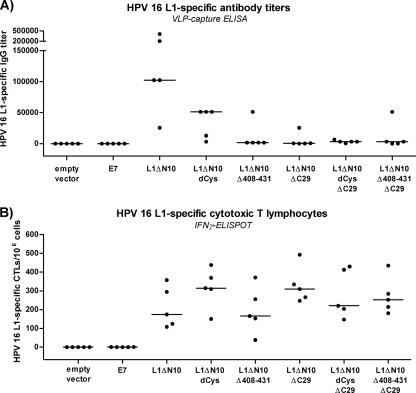
We investigated further the influence of the individual mutations and deletions on the immunogenicity of the capsomeres. Therefore, C57BL/6 mice (five mice per group) were immunized twice at a biweekly interval; the mice were injected subcutaneously with 5 μg of one of the different L1 proteins. Negative-control mice were immunized with PBS. Sera were analyzed for L1-specific IgG antibody responses by VLP capture ELISA (Fig. 3A) and for neutralization of HPV-16 pseudovirions (Fig. 3B). The induction of L1-specific cytotoxic T lymphocytes (CTLs) was determined by IFN-γ ELISPOT (Fig. 3C).
FIG. 3.
Comparison of the immunogenicity of the HPV-16 L1 capsomeres in C57BL/6 mice. C57BL/6 mice (five mice per group) were injected twice at a biweekly interval with 5 μg of one of the different L1 capsomere constructs. (A and B) One week after the last immunization, blood samples were collected, and sera were analyzed for L1-specific antibody titers by VLP capture ELISA (A) and for neutralization activity with HPV-16 pseudovirons (B). (C) Splenocytes were isolated and examined by IFN-γ ELISPOT analysis to determine the induction of L1-specific cytotoxic T lymphocytes. neg control, negative control.
In the groups treated with L1 capsomeres with the full-length C terminus, mice immunized with the L1ΔN10 protein (median antibody titer, 409,600) developed higher antibody titers than mice vaccinated with the cysteine-lacking capsomeres (median titer, 12,800; P = 0.01), or with either of the two Δh4 mutants (mutants in which helix 4 had been deleted) (median titer, 25,600 [P = 0.01] and 51,200 [P = 0.01]). The same result was observed for the C-terminally truncated L1 proteins L1ΔN10ΔC29 (median titer of 51,200), L1ΔN10dCysΔC29 (median titer of 100; P = 0.01), L1ΔN10Δ414-431ΔC29 (median titer of 1,600; P = 0.02), and L1ΔN10Δ408-431ΔC29 (median titer of 3,200; P = 0.01). For L1 capsomeres with either the full-length or truncated C terminus, helix 4 deletion led to slightly higher antibody responses than the corresponding cysteine-lacking L1 proteins, although these differences were not statistically significant. For all capsomeres, the C-terminal deletion resulted in antibody titers that were statistically significantly lower than the titers for their corresponding full-length C-terminus analogues (paired comparisons; for all cases, P < 0.05). Thus, the L1ΔN10 capsomeres induced the highest L1-specific humoral immune response of all L1 proteins tested.
The neutralizing activity of the sera (1:100 dilution) was tested in an HPV-16 pseudovirion assay (Fig. 3B). When an arbitrary cutoff of 50% neutralization was set, all sera with L1-specific antibody titers above 6,400 also displayed neutralizing capacity. Only the sera from mice vaccinated with L1ΔN10 were also neutralizing at a serum dilution of 1:1,000 (data not shown). These results suggest that all the L1 capsomeres have the potential to induce neutralizing antibodies once sufficiently high antibody titers are reached. Indeed, enhancing the IgG antibody response after immunization with the L1ΔN10Δ408-431ΔC29 protein using LPS as an adjuvant led to neutralization activity within these sera (data not shown).
In contrast to the humoral immune responses, the “dCys” modification and deletion of helix 4 had no influence on the cellular immune responses to the L1 proteins (Fig. 3C). Interestingly, the mice immunized with the L1 proteins containing the full-length C terminus showed higher L1-specific numbers of cytotoxic T lymphocytes compared to the mice injected with the respective analogues lacking the C terminus (L1ΔN10Δ408-431, P = 0.09; for all other cases, P < 0.05).
These results indicate that the L1 pentamers, although they showed similar structural characteristics, differ in their immunogenicity, especially regarding the L1-specific humoral immune responses.
The difference in immunogenicity is independent of LPS contaminations.
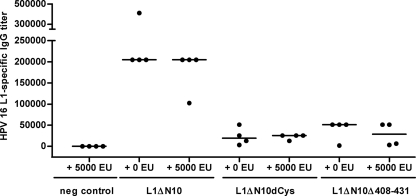
Protein preparations from E. coli are always at risk of contamination with endotoxins, which can enhance antigen-specific immune responses by activating Toll-like receptor 4 (TLR4) (50). A comparison of different proteins expressed in bacteria is challenging, as the amount of contaminating endotoxin can vary between purifications and for different proteins. Indeed, considerable amounts of LPS were detected in the L1 capsomere preparations (between 400 to 3,000 EU per mg of L1 protein [Table 1]). To investigate whether the differences in immunogenicity observed for the L1 proteins are due to various levels of LPS contamination, LPS-insensitive TLR4−/− knockout mice (major histocompatibility complex class I background of C57BL/6 mice) were immunized a total of three times at biweekly intervals with 5 μg of the L1ΔN10, L1ΔN10dCys, or L1ΔN10Δ408-431 capsomeres (four mice per group). To confirm the endotoxin resistance of these mice, each protein was also injected together with 5,000 EU of LPS. Control mice received PBS and 5,000 EU of LPS. After the third immunization, blood samples were collected, and sera were analyzed for L1-specific antibodies by VLP capture ELISA (Fig. 4).
FIG. 4.
Comparison of the immunogenicity of HPV-16 L1 capsomeres in TLR4−/− mice. Sv129 × C57BL/6 mice (four mice per group) were immunized three times at biweekly intervals with 5 μg of L1ΔN10, L1ΔN10dCys, or L1ΔN10Δ408-431 capsomeres either with or without the addition of 5,000 EU of LPS. Two weeks after the last injection, blood samples were collected, and L1-specific antibody titers in the sera were determined by VLP capture ELISA. The value for each individual mouse serum sample (•) and the median of each group (horizontal bar) are shown. neg control, negative control.
A comparison of the corresponding groups that had received the antigen either with or without additional LPS showed that even large amounts of LPS had no influence on the induction of L1-specific antibodies (P > 0.5), confirming the insensitivity of the mice to LPS. Similar to the immunization of wild-type mice (Fig. 3), strong differences were observed between the groups receiving the various L1 capsomeres. The mice immunized with the L1ΔN10 protein developed higher antibody titers (median titer, 204,800) than the mice that were vaccinated with L1ΔN10dCys (median titer, 25,600; P = 0.001) or L1ΔN10Δ408-431 (median titer, 51,200; P = 0.001). As with the wild-type mice (Fig. 3), there was no statistically significant difference for the L1-specific humoral immune responses between the groups that received the L1ΔN10dCys and L1ΔN10Δ408-431 capsomeres.
From these data, we conclude that the different capacity to induce antibody responses is not a consequence of varying amounts of contaminating endotoxin.
The difference in immunogenicity is also observed after DNA immunization.
Besides endotoxin, proteins from E. coli may also be contaminated with other molecules, such as unmethylated CpG DNA or bacterial chaperones, which are known to influence antigen-specific immune responses (5, 52). To study the immunogenicity of the different L1 proteins independent of all putative contaminations, we immunized C57BL/6 mice (five mice per group) three times at biweekly intervals, intradermally with 15 μg of endotoxin-free HPV-16 L1 plasmid DNA (codons modified for optimal usage in mammalian cells [see Materials and Methods]) encoding the L1ΔN10, L1ΔN10dCys, and L1ΔN10Δ408-431 proteins and their C-terminally truncated analogues. Control mice received either the empty pTHamp vector or pTHamp vector containing the HPV-16 E7 gene (pTHampE7). Beforehand, it was confirmed that the different constructs showed similar expression levels after transient transfection of 293TT cells (data not shown). Seven days after the third immunization, sera of the mice were analyzed for L1-specific antibodies by VLP capture ELISA (Fig. 5A), and splenocytes were analyzed for the presence of L1-specific CTLs by IFN-γ ELISPOT assay (Fig. 5B).
FIG. 5.
Comparison of the immunogenicity of the HPV-16 L1 capsomeres by DNA immunization. C57BL/6 mice (five mice per group) were immunized three times at biweekly intervals intradermally with 15 μg of endotoxin-free plasmid DNA coding for the different L1 capsomere constructs or for HPV-16 E7 (pTHampE7) and with empty vector. (A) One week after the last vaccination, blood samples were collected, and sera were analyzed for L1-specific antibodies by VLP capture ELISA. (B) Splenocytes were isolated and examined by IFN-γ-ELISPOT for L1-specific cytotoxic T lymphocytes. The value for each individual mouse (•) and the median of each group (horizontal bar) are shown.
In agreement with the protein immunization of the wild-type C57BL/6 mice (Fig. 3) and the TLR4−/− knockout mice (Fig. 4), DNA immunization revealed similar differences in immunogenicity between the individual constructs (Fig. 5). Mice vaccinated with the L1ΔN10 DNA construct (median titer, 102,400) developed higher antibody titers than the mice injected with L1ΔN10dCys DNA (median titer, 51,200; P = 0.056) or L1ΔN10Δ408-431 DNA (median titer, 1,600; P = 0.02). In contrast to the previous immunization experiments, the mice that were vaccinated with L1ΔN10dCys exhibited slightly higher antibody titers than the mice that received L1ΔN10Δ408-431. For the C-terminally truncated L1 constructs, only very low L1-specific antibody titers were measured, which is consistent with a previous report (32).
For all constructs, the L1-specific CTL response was similar and resulted in a very high number of cytotoxic T lymphocytes (median number of spots, 166 to 314 for 1 × 106 splenocytes; Fig. 5B). In line with previous reports (21, 49), we thus demonstrate that DNA immunization leads to efficient induction of cellular immune responses.
The results of the DNA immunization show that the construct coding for the L1ΔN10 protein is the most efficient at inducing L1-specific antibodies independent of any possible contamination that could be introduced during the purification from E. coli.
The L1 constructs display similar surface epitopes.
The results of protein immunizations comparing the different L1 capsomeres in wild-type C57BL/6 mice (Fig. 3) and TLR4−/− knockout mice (Fig. 4) as well as the DNA immunization (Fig. 5) showed that, despite the homogenous capsomere structures, the L1ΔN10 protein confers higher immunogenicity with regard to a humoral response than the constructs lacking the two cysteine residues, helix 4, or the 29 C-terminal amino acids. Aiming for a possible explanation, we compared the presence of a number of epitopes on the capsomeres by the binding of 30 different well-characterized HPV-16 L1-specific monoclonal antibodies (40) (Table 2). Of the 20 conformation-specific antibodies, 16 reacted similarly with the various capsomeres; antibodies H16.7E, H16.3A, H16.2F, and H16.E70 showed stronger binding to the L1ΔN10 and L1ΔN10ΔC29 proteins. The differences in binding were most noticeable for the H16.E70 antibody. It has been proposed that this antibody recognizes an epitope that is located on adjacent intra- or intercapsomeric L1 proteins (51), suggesting that the L1ΔN10 and L1ΔN10ΔC29 capsomeres, in contrast to the other L1 pentamers, at least partially assembled into higher-order structures in this assay. The H16.V5 epitope has been shown to be essential for the induction of a neutralizing antibody response (43, 51), and the different L1 capsomeres were bound to a very similar extent by this antibody. No differences in binding were observed for antibodies recognizing linear epitopes, which suggests that none of the proteins were folded incorrectly. Antibody H16.B20 did not bind the L1ΔN10Δ408-431 or L1ΔN10Δ408-431ΔC29 protein, most likely because most of the amino acids of the epitope (from positions 396 to 415) were deleted in these L1 proteins.
TABLE 2.
Comparison of the presence of important conformational and linear epitopes for the different L1 capsomere constructsa
| Monoclonal antibodyb | Epitope characteristics
|
Bindinge to:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsomeres purified from E. coli
|
VLPs (insect cells) (control) | ||||||||||
| Essential amino acidsc | Typed | L1ΔN10 | L1ΔN10dCys | L1ΔN10Δ414-431 | L1ΔN10Δ408-431 | L1ΔN10ΔC29 | L1ΔN10dCysΔC29 | L1ΔN10Δ414-431ΔC29 | L1ΔN10Δ408-431ΔC29 | ||
| H16.7E | 38-65 | C+ | 1.254 | 0.929 | 0.608 | 0.760 | 1.121 | 0.325 | 0.265 | 0.353 | 1.181 |
| H16.9A | 1-173 | C+f | 0.723 | 0.641 | 0.682 | 0.650 | 0.567 | 0.477 | 0.472 | 0.555 | 0.116 |
| H16.3A | 172-505 | C+ | 0.835 | 0.463 | 0.481 | 0.459 | 0.691 | 0.379 | 0.398 | 0.400 | 0.561 |
| H16.4A | 172-505 | C+ | 1.195 | 1.146 | 1.161 | 1.082 | 1.225 | 0.967 | 0.911 | 0.838 | 1.060 |
| H16.14J | 172-505 | C+ | 1.137 | 0.950 | 0.909 | 0.919 | 1.077 | 0.739 | 0.682 | 0.715 | 0.910 |
| H16.V5 | 260-290 | C+g,h | 1.303 | 1.274 | 1.338 | 1.285 | 1.327 | 1.237 | 1.202 | 1.177 | 1.257 |
| H16.5A | 266-297, 339-365 | C+ | 1.302 | 1.264 | 1.265 | 1.233 | 1.221 | 1.122 | 1.177 | 1.133 | 1.203 |
| H16.2F | 266-297, 339-365 | C+ | 1.158 | 0.786 | 0.803 | 0.820 | 0.964 | 0.666 | 0.616 | 0.745 | 1.005 |
| H16.6F | 266-297, 339-365 | C+ | 1.151 | 1.030 | 1.027 | 0.963 | 1.126 | 0.923 | 0.844 | 0.832 | 0.981 |
| H263.A2 | 266-297, 339-365 | C+ | 0.701 | 0.616 | 0.555 | 0.460 | 0.697 | 0.564 | 0.544 | 0.523 | 0.494 |
| H16.1A | 266-297, 339-365 | C+f | 1.088 | 0.973 | 0.963 | 0.916 | 1.101 | 0.804 | 0.823 | 0.857 | 0.849 |
| H16.E70 | 282 | C+g | 1.216 | 0.203 | 0.228 | 0.495 | 0.824 | 0.141 | 0.152 | 0.216 | 1.029 |
| H16.U4 | 427-445 | C+g,i | 0.992 | 0.929 | 0.982 | 0.963 | 0.766 | 0.768 | 0.782 | 0.816 | 0.632 |
| Ritti01 | ? | C+ | 0.749 | 0.712 | 0.648 | 0.537 | 0.769 | 0.714 | 0.630 | 0.598 | 0.483 |
| MM05 | ? | C+ | 0.699 | 0.672 | 0.750 | 0.693 | 0.668 | 0.656 | 0.630 | 0.657 | 0.105 |
| H16.8B | ? | C− | 0.597 | 0.702 | 0.666 | 0.605 | 0.687 | 0.630 | 0.682 | 0.623 | 0.097 |
| H16.11B | ? | C− | 0.743 | 0.779 | 0.830 | 0.775 | 0.642 | 0.623 | 0.714 | 0.745 | 0.113 |
| H16.13D | ? | C− | 0.802 | 0.611 | 0.826 | 0.880 | 0.722 | 0.345 | 0.649 | 0.753 | 0.069 |
| H263.C3 | ? | C− | 0.199 | 0.173 | 0.072 | 0.062 | 0.135 | 0.096 | 0.063 | 0.058 | 0.061 |
| V16H9 | ? | C− | 0.285 | 0.269 | 0.305 | 0.251 | 0.249 | 0.224 | 0.269 | 0.255 | 0.064 |
| MM07 | ? | L+ | 0.441 | 0.366 | 0.440 | 0.334 | 0.492 | 0.351 | 0.359 | 0.342 | 0.629 |
| H16.S1 | 111-130 | L−j | 0.088 | 0.091 | 0.147 | 0.091 | 0.103 | 0.100 | 0.100 | 0.093 | 0.184 |
| H16.C2 | 174-185 | L− | 0.699 | 0.490 | 0.897 | 0.765 | 0.766 | 0.359 | 0.761 | 0.746 | 0.718 |
| H16.H5 | 174-185 | L− | 0.835 | 0.760 | 1.038 | 0.831 | 0.882 | 0.619 | 0.829 | 0.849 | 0.999 |
| H16.15G | 172-505 | L− | 0.121 | 0.066 | 0.096 | 0.100 | 0.097 | 0.055 | 0.091 | 0.093 | 0.115 |
| CamVir1 | 204-210 | L−j | 1.011 | 1.069 | 1.104 | 1.022 | 1.051 | 1.030 | 1.079 | 1.058 | 0.525 |
| H16.J4 | 261-280 | L−j | 0.124 | 0.136 | 0.260 | 0.134 | 0.162 | 0.129 | 0.165 | 0.151 | 0.563 |
| MD2H11 | 299-315 | L− | 0.157 | 0.159 | 0.221 | 0.168 | 0.163 | 0.146 | 0.168 | 0.163 | 0.097 |
| H16.B20 | 396-415 | L− | 0.483 | 0.361 | 0.512 | 0.068 | 0.460 | 0.312 | 0.474 | 0.060 | 0.168 |
| H16.D9 | ? | L− | 0.256 | 0.443 | 0.880 | 0.458 | 0.529 | 0.548 | 0.630 | 0.514 | 0.173 |
The different L1 capsomeres purified from E. coli were directly coated onto the wells of a 96-well plate and compared in an ELISA by their binding of 30 different monoclonal L1-specific antibodies with well-defined epitope characteristics.
Information about the antibodies was mainly taken from Rizk et al. (40).
?, not known.
C, conformational; L, linear; +, neutralizing; −, nonneutralizing.
The median absorption values at 450 nm are shown. Boldface values indicate differences of at least 0.3 absorbance unit between certain capsomere constructs, with the higher value being underlined. For a control, the reactivity of these antibodies with HPV-16 VLPs purified from baculovirus-infected insect cells is shown.
Additional information in Christensen et al. (13).
Additional information in Day et al. (17).
Additional information in Ryding et al. (43).
Additional information in Carter et al. (10).
Additional information in Roden et al. (41).
These results demonstrate that the capsomeres show few differences in the presentation of a number of distinct linear and conformational epitopes. Therefore, the observed variations in immunogenicity cannot be explained by a lack of important epitopes or by misfolding.
The capsomere constructs show different assembly properties.
We have suggested that VLPs induce antibodies more strongly than capsomeres owing to more efficient B-cell receptor cross-linking as a result of the larger antigen arrays (47). To investigate the extent to which the various L1 capsomeres differed in their ability to assemble into higher organized structures and whether this correlated with their immunogenicity, the different L1 capsomeres were dialyzed against assembly buffer (see Materials and Methods) and subsequently analyzed by sedimentation analysis (Fig. 6A) and electron microscopy (Fig. 6B). All L1 proteins existed as capsomeres before assembly (Fig. 6A, left-hand panel). For L1ΔN10 and L1ΔN10dCys and their ΔC29 variants, a clear shift to a larger assembly form was observed after dialysis (Fig. 6A, right-hand panel). However, their sedimentation properties indicated particles smaller than T=7 VLPs. The constructs with helix 4 deleted did not show any assembly ability, which is consistent with previous reports (7, 11). Electron microscopy revealed VLP-like structures with a diameter of about 35 to 40 nm for L1ΔN10 and L1ΔN10ΔC29, but smaller and more heterogeneous formations together with a high number of capsomeres for the “dCys” proteins. This difference could be explained either by unspecific aggregation of the “dCys” proteins during the assembly process or by the formation of instable assemblies that disintegrated during the procedure of negative staining.
FIG. 6.
Assembly characteristics of the different HPV-16 L1 capsomeres. The L1 capsomeres were dialyzed against assembly buffer, and the resulting particles were characterized by sedimentation analysis before and after assembly (A) and TEM employing negative staining after assembly (B). The sucrose fractions were analyzed by Western blotting using the HPV-16 L1-specific monoclonal antibody MD2H11 (A). Catalase (11S) and HPV-16 VLPs purified from insect cells were used as calibration markers.
The assembly conditions described do not necessarily reflect the situation in an immunization experiment. Therefore, we also compared the assembly properties of the L1ΔN10 and L1ΔN10dCys capsomeres by dialysis against PBS and by dilution in PBS and analyzed the resulting particles by sedimentation analysis. The results are summarized in Table 3. Both proteins showed very similar assembly characteristics, as judged by sedimentation analysis. However, electron microscopy revealed that only the L1ΔN10 protein formed small VLPs, whereas L1ΔN10dCys again formed heterogeneous aggregates. These findings suggest that capsomeres formed by these two L1 proteins can oligomerize to a certain extent when diluted in PBS prior to injection, though the particles formed by the L1ΔN10dCys capsomeres were apparently less stable or less well-structured. As the sedimentation properties of these two proteins were similar after preparation for immunization, we conclude that there might be some differences upon injection that could explain the differences in immunogenicity. In an attempt to simulate this situation, we tested the stability of the assembled products of the L1ΔN10 and L1ΔN10dCys capsomeres obtained by dialysis against or dilution in PBS at body temperature (incubation overnight and subsequent sedimentation analysis at 37°C). Under these more physiological conditions, the L1ΔN10 protein formed larger particles with a few capsomeres remaining (Table 3). In contrast, the L1ΔN10dCys protein formed only capsomeres, suggesting that the larger particles broke apart after exposure to PBS at 37°C owing to lower stability.
TABLE 3.
Assembly characteristics of the HPV-16 L1ΔN10 and L1ΔN10dCys capsomeresa
| Capsomere construct | Assembly procedure
|
Resulting assembly structurec | ||
|---|---|---|---|---|
| Dialysis/dilution | Buffer | Temp (°C)b | ||
| L1ΔN10 | Dialysis | Assembly buffer | RT | Small VLPs (Ø, 35-40 nm) |
| Dialysis | PBS | RT | Small VLPs (Ø, 35-40 nm) | |
| Dialysis | VLP buffer | RT | Small VLPs (Ø, 35-40 nm) | |
| Dialysis | PBS | 37 | Small VLPs (Ø, 35-40 nm) and capsomeres | |
| Dilution | Assembly buffer | RT | Small VLPs (Ø, 35-40 nm) | |
| Dilution | PBS | RT | Small VLPs (Ø, 30 nm) and capsomeres | |
| Dilution | PBS | 37 | Small VLPs (Ø, 30 nm) and capsomeres | |
| L1ΔN10dCys | Dialysis | Assembly buffer | RT | Small VLPs, aggregates (?) |
| Dialysis | PBS | RT | Small VLPs, aggregates (?) | |
| Dialysis | PBS | 37 | Capsomeres | |
| Dilution | PBS | RT | Small VLPs, aggregates (?) | |
| Dilution | PBS | 37 | Capsomeres | |
The L1ΔN10 and L1ΔN10dCys proteins were dialyzed against or diluted in PBS, VLP buffer, or assembly buffer at room temperature or 37°C. The resulting assembly forms were characterized by electron microscopy and sedimentation analysis and subsequent ELISA and Western blot analysis of the sucrose gradient fractions. Results of the analyses are given in the Resulting assembly structure column.
RT, room temperature.
The diameter of the particles (Ø) is given in parentheses. Aggregates were not clearly definable by electron microscopic analysis, which is indicated by a question mark in parentheses.
These results demonstrate that the L1ΔN10 capsomeres (and the L1ΔN10ΔC29 capsomeres) assembled into larger particles after dilution in PBS and that, most likely, these assemblies were stable even after injection into the mice, whereas the “dCys” proteins seemed to oligomerize into similar structures that might have broken up after injection at body temperature. The L1 proteins with helix 4 deleted did not assemble into larger particles even under optimal conditions. Therefore, the difference in immunogenicity between the L1 capsomeres analyzed might be explained in large part by their assembly properties.
Preassembly of the L1ΔN10 capsomeres into larger particles does not increase immunogenicity.
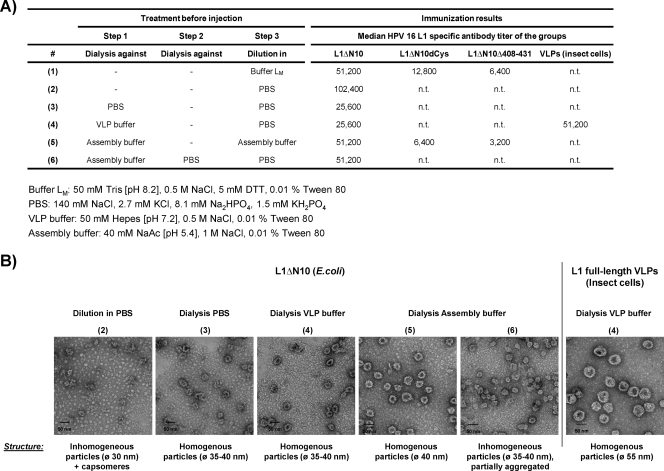
The ability of L1ΔN10 capsomeres to assemble into stable larger particles seems to be the reason for their higher immunogenicity. We therefore wanted to investigate whether immunogenicity can be increased further by a controlled assembly prior to immunization. In order to determine the best assembly protocol, different treatments were applied to the L1ΔN10 protein by dialysis against or dilution in different buffers (Fig. 7A, treatments 1 to 6). The dilution of the antigen before immunization (step 3) was necessary because each mouse received equal amounts of the antigen in a total volume of 100 μl. As shown above, dilution in a certain buffer can also influence the assembly status of the capsomeres (Table 3). Prior to immunization, the L1ΔN10 assembly forms were characterized by electron microscopy (Fig. 7B) after the different treatments (treatments 1 to 6). Each treatment resulted in the formation of particles with a diameter of about 30 to 40 nm, yet with different efficiencies (for detailed results, see the legend to Fig. 7B).
FIG. 7.
Immunogenicity of the HPV-16 L1 capsomeres after a preassembly. (A) The L1ΔN10, L1ΔN10dCys, and L1ΔN10Δ408-431 capsomeres were treated using different protocols (treatments 1 to 6 [#1 to #6]) prior to immunization as indicated. The median antibody titers of each group are summarized (n.t., not tested). The components of the buffers are shown at the bottom of the panel (NaAc, sodium acetate). (B) The resulting assembly structures of the L1ΔN10 protein were analyzed by TEM employing negative staining. A description of the structures visible is noted below each picture. C3H/HeJ mice (five mice per group) were injected three times at biweekly intervals with 1 μg of the pretreated capsomeres. For comparison, one group of mice received HPV-16 VLPs purified from insect cells. Negative-control mice were injected with PBS. Two weeks after the last vaccination, blood samples were collected, and L1-specific antibodies were detected by VLP capture ELISA of the sera. The diameter of the particles (Ø) is shown in parentheses.
To measure the immunogenicity of each assembly form and to compare it with HPV-16 VLPs purified from insect cells, we immunized C3H/HeJ mice (five mice per group). These mice carry a dominant-negative point mutation in the tlr4 gene and are therefore less sensitive to LPS contaminations. The C3H/HeJ mice were immunized with the pretreated L1ΔN10 capsomeres. Additionally, one group of mice received HPV-16 VLPs purified from insect cells and prepared according to treatment 4. Negative-control mice were treated with buffer LM. Two weeks after the last immunization, sera were analyzed for L1-specific antibodies by VLP capture ELISA (median titers in Fig. 7A). The results indicate that preassembly did not significantly affect the immunogenicity of the L1ΔN10 capsomeres. The mice that received L1ΔN10 particles after any of the treatments did not develop statistically significant higher L1-specific antibody responses than the mice injected with L1ΔN10 capsomeres; that is, the L1ΔN10 protein diluted in buffer LM. Most importantly, the mice immunized with VLPs derived from baculovirus-infected insect cells did not develop higher L1-specific antibody titers than any of the groups injected with the L1ΔN10 protein derived from E. coli. Therefore, we conclude that the ability of the L1ΔN10 protein to spontaneously assemble into larger particles is sufficient to induce high titers of antibodies.
This was confirmed by the results obtained from mice that were immunized with the L1ΔN10dCys and L1ΔN10Δ408-431 proteins after dilution in buffer LM (treatment 1) or after dialysis against assembly buffer (treatment 5). As shown in Fig. 6, these proteins do not assemble beyond the pentameric structure. Mice treated with these two proteins developed significantly lower antibody titers compared to all groups injected with the L1ΔN10 protein (in all cases, P < 0.05). Although we have shown that the L1ΔN10dCys capsomeres formed larger particles upon dialysis against assembly buffer (Fig. 6), no difference in antibody titer was seen in the mice that had received this protein after different pretreatments (treatments 1 and 5).
Adjuvants enhance the immunogenicity of L1ΔN10 capsomeres.
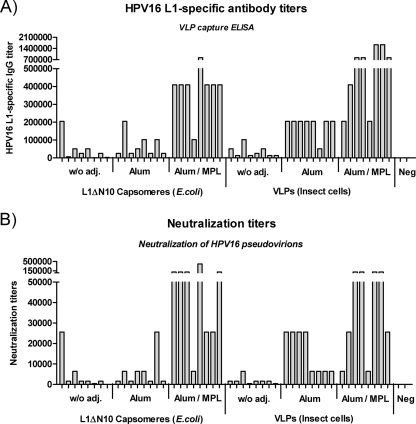
To verify our observations from the TLR4-deficient C3H/HeJ mice (Fig. 7) and to investigate the influence of adjuvants, the immunogenicity of the L1ΔN10 capsomeres purified from E. coli was compared to the immunogenicity of VLPs purified from insect cells in wild-type C57BL/6 mice with and without adjuvants. Endotoxin contamination was removed from the L1ΔN10 capsomeres by treatment with Triton X-114, a protocol that we recently found to be suitable for the removal of LPS from L1 particles (2, 45). C57BL/6 mice (eight mice per group) were injected subcutaneously three times at biweekly intervals with 0.5 μg of VLPs or LPS-free L1ΔN10 capsomeres. Additional mouse groups received the antigens adsorbed to aluminum hydroxide (alum) alone or to aluminum hydroxide in combination with the Sigma adjuvant system (Sigma), which contains monophosphoryl lipid A as active substance (alum/MPL). Negative-control mice (n = 2) received PBS. Seven days after the third immunization, we analyzed sera for L1-specific antibody titers by VLP capture ELISA (Fig. 8A) and by HPV-16 pseudovirion assay for neutralizing activity (Fig. 8B).
FIG. 8.
Immunogenicity of L1ΔN10 capsomeres and VLPs in combination with adjuvants. C57BL/6 mice (eight mice per group) were immunized subcutaneously three times at biweekly intervals with 0.5 μg of LPS-free L1ΔN10 capsomeres purified from E. coli or VLPs purified from baculovirus-infected insect cells. Additional groups received the antigens adsorbed to alum alone or alum in combination with an adjuvant system (Sigma) containing monophosphoryl lipid A (alum/MPL). Negative-control mice (n = 2) were injected with PBS. (A) Seven days after the third immunization, sera were analyzed for L1-specific antibody titers by VLP capture ELISA, and (B) neutralization titers were determined using HPV-16 pseudovirions. w/o adj., without adjuvant; Neg, negative control.
In the absence of adjuvants, the mice vaccinated three times with the L1ΔN10 capsomeres or VLPs developed similar antibody titers (median titers, 25,600 and 19,200; P = 0.78), but it should be noted that after only two immunizations, the VLP-immunized mice had around two- to threefold-higher L1-specific antibody titers than those receiving L1ΔN10 capsomeres (data not shown). The addition of alum led to an increase in antibody titer with both antigens, though the enhancing effect was much higher for VLPs (median titer, 204,800) than for L1ΔN10 capsomeres (median titer, 38,400). The adjuvant combination alum/MPL increased the L1-specific antibody response even further and without statistical differences in the median titers of the VLP-immunized and capsomere-immunized mice (VLPs, 819,200; L1ΔN10 capsomeres, 409,600; P = 0.24). In each case, the neutralization titers correlated with the L1-specific antibody titers (Fig. 8B). Without adjuvant, the neutralization titers were similar after immunization with VLPs or L1ΔN10 capsomeres (median titer in both groups, 1,600), whereas the addition of alum led to a stronger increase in titers for mice receiving VLPs (median titer, 16,000) than in mice receiving L1ΔN10 capsomeres (median titer, 4,000; P = 0.04). This difference could not be observed after immunization with alum/MPL, as similar titers were measured for VLPs (median titer, 64,000) and L1ΔN10 capsomeres (median titer, 102,400) (P = 0.61). These data demonstrate that L1ΔN10 capsomeres and VLPs induce similar humoral immune responses that can be increased strongly when using an appropriate adjuvant.
DISCUSSION
In the present study, we show by the comparison of HPV-16 L1 capsomeres encoded by eight different constructs that immunogenicity is partially a function of the modifications made to the L1 protein to prevent capsid assembly. Replacement of the cysteine residues at positions 175 and 428 with serine residues, as well as deletion of helix 4, reduced immunogenicity with respect to the induced humoral immune responses. These differences were independent of different endotoxin concentrations or other bacterial contaminations in the L1 preparations, both of which can influence the induced immune responses (50, 52).
The structural analysis of the different L1 proteins revealed that all constructs formed homogenous L1 pentamers with a diameter of 10 nm. We further characterized the capsomeres in detail by analytical ultracentrifugation and determined a sedimentation coefficient of about 10.2S to 10.4S for all constructs (data not shown; L. Schädlich, unpublished data), which is similar to the value previously reported for capsomeres (33, 53). The binding of a panel of well-characterized L1-specific antibodies indicated that all the capsomeres were correctly folded. The H16.V5 antibody recognizes an epitope essential for the generation of neutralizing L1-specific antibodies (43, 51) and bound all L1 pentamers to the same extent, suggesting that these L1 proteins are potentially capable of inducing neutralizing antibodies upon injection. Indeed, we found that after reaching a certain antibody titer for all constructs (e.g., by the use of an adjuvant), neutralizing antibodies could be detected (data not shown).
Given that the different L1 capsomeres were almost identical in both structure and epitope presentation, we propose that the variation in immunogenicity is likely owing to their different assembly properties. Antigens with a highly organized structure are known to lead to more efficient B-cell receptor cross-linking (3). The L1ΔN10 capsomeres, which led to the induction of the highest antibody titers, were indeed seen to assemble into larger particles under all conditions tested, and these were stable even at 37°C, a condition closer to the situation in vivo. The particles formed by the L1ΔN10 protein had a diameter of about 35 to 40 nm, which is larger than the T=1 assemblies that resulted from capsomeres expressed from the same construct reported by Chen et al. (11, 12), which had a diameter of 32 nm. This discrepancy might be explained by the different protein concentrations and dilution factors used for assembly.
As has been shown by others (7, 11), L1 pentamers lacking helix 4 did not assemble into particles larger than capsomeres, and we hypothesize that this is the reason they induced much lower antibody titers. The L1ΔN10dCys protein, which also induced very low antibody responses, formed particles with sedimentation characteristics similar, to an extent, to those of the L1ΔN10 protein. However, these particles were less stable, especially at more physiological conditions. Our observation of the assembly of L1ΔN10dCys capsomeres contradicts previous reports that used HPV-11 L1 Cys424Gly or HPV-16 L1 Cys428Ser, where the formation of larger particles was not observed (26, 27, 33). However, HPV-16 L1 Cys175Ser has been shown to assemble into heterogeneous rod-shaped structures (27). So far, an L1 protein with substitutions of both cysteines for serine residues has not been analyzed for its assembly properties. We speculate that the combination of both exchanges might facilitate the formation of different but much weaker interactions, as we were not able to detect these assemblies by electron microscopy and they were not stable at 37°C. The H16.E70 antibody has been suggested to bind to an epitope between adjacent capsomeres (51), and it is interesting to note that this antibody bound the L1ΔN10 assemblies, but not the L1ΔN10dCys assemblies.
We cannot exclude the possibility that helix 4 alone constitutes a highly immunogenic epitope necessary for the induction of strong antibody responses. Indeed, all of the constructs that showed lower immunogenicity had modifications in this region, as the cysteine residue at position 428 is part of this helix. On the other hand, none of the numerous monoclonal neutralizing L1-specific antibodies generated so far are directed against this epitope, arguing that the inability to assemble into particles larger than capsomeres is more likely to be the reason for the lower immunogenicity.
Whether assembly of the L1ΔN10 capsomeres leads to a more efficient B-cell receptor cross-linking (3) or to more efficient activation, uptake, and presentation via major histocompatibility complex class I and II molecules by dendritic cells, and therefore to a stronger T-helper cell response (3, 20), needs to be analyzed in detail. However, preliminary data obtained by loading bone marrow-derived dendritic cells with the L1 capsomeres with the dCys modification or with helix 4 deleted showed no differences in antigen presentation compared to the L1ΔN10 protein (L. Schädlich, unpublished observations). Additionally, these alterations had no effect on the induction of CD8+ T-cell responses, which are primed by peptide-loaded dendritic cells.
All C-terminally truncated L1 capsomeres induced both lower humoral and cellular immune responses after protein vaccination compared to the L1 proteins containing a full-length C terminus. This was also the case for the L1ΔN10ΔC29 capsomeres, which had assembly properties similar to those of the L1ΔN10 protein. This difference might be explained by a specific copurification of immune-stimulatory molecules from E. coli with the full-length C-terminus constructs. Another reason could be the potentially less efficient binding and uptake of the truncated proteins by dendritic cells, as the C terminus of the L1 protein has been reported to contain the heparin-binding region (28). However, the cytotoxic T-cell responses after DNA immunization were similar for the constructs both with and without the C terminus. Remarkably, for the plasmids encoding the C-terminally truncated L1 proteins, only very low titers of L1-specific antibodies could be detected. This result is in line with previously reported findings that suggested that the immunogenicity of L1 proteins expressed after DNA immunization might at least indirectly be dependent on their intracellular localization, as the C terminus contains two nuclear localization signals (32). L1 proteins that were transported into the nucleus elicited much higher antibody responses than C-terminally truncated L1 proteins, which remained in the cytoplasm.
Of all constructs tested, the L1ΔN10 capsomeres were the most potent inducers of strong neutralizing antibody responses. This is likely owing to their ability to assemble into larger particles after dilution in PBS, as this was done prior to immunization. However, a controlled assembly prior to vaccination did not lead to an increase in the antibody titers of C3H/HeJ mice. In all buffers analyzed, the L1ΔN10 pentamers elicited very similar humoral immune responses, even under buffer conditions that prevented capsid assembly (buffer L, pH 8.2). This suggests that the pentamers might oligomerize upon injection as a result of the physiological conditions in vivo. Interestingly, there was no detectable difference between the humoral immune responses induced by the E. coli-derived L1ΔN10 protein and those induced by VLPs purified from baculovirus-infected insect cells in both LPS-insensitive C3H/HeJ and wild-type C57BL/6 mice.
However, proteins purified from E. coli might, for example, also be contaminated with unmethylated CpG DNA, which is known to stimulate the immune response via activation of TLR9. A comparison of the immunogenicity of protein preparations from different expression systems is always challenging, as they can be contaminated with distinct immune-stimulatory molecules specific to the organism in which they are expressed.
The immunogenicity of the L1ΔN10 capsomeres could be enhanced by the addition of the adjuvants alum and alum/MPL, with the latter proving most effective. As the current VLP-based vaccines are coadministered with adjuvants, our observation that alum/MPL enhances the immunogenicity of VLPs and L1ΔN10 capsomeres to the same extent is an important demonstration of the suitability of capsomeres to compete with VLPs.
Reducing the production costs of prophylactic vaccines that protect against infection with HPV-16 is a crucial step toward their introduction in developing countries. The expression and purification of L1 capsomeres in and from bacteria offer economic advantages that allow rapid biomass production in inexpensive culture medium and high-cell density fermentors. Currently, one of the most efficient methods for the production of HPV-16 L1 from E. coli involves its expression as a GST fusion protein (11). However, for scaling-up purposes, the rather cost-intensive thrombin-mediated cleavage of the L1 protein from the GST tag might need to be replaced by a less costly procedure.
Our analysis has demonstrated the high immunogenicity of E. coli-derived L1 capsomeres. We have further shown that there is a strong correlation between immunogenicity and the intrinsic ability of the protein to assemble into stable larger particles. The assembly properties of any given L1 protein are therefore a guide to how immunogenic it is likely to be. We thus provide an important and encouraging perspective, which expands previous work that has shown that the pentameric structure alone is not highly immunogenic (47), and highlight once again the potential of L1 capsomeres as cost-effective alternatives to the licensed VLP-based vaccines.
Acknowledgments
We thank Neil D. Christensen for providing the monoclonal HPV-16 L1-specific antibodies and Ivonne Rubio for supplying the HPV-16 pseudovirions. We further thank Robert L. Garcea for the pGex L1ΔN10Δ408-431ΔC29 construct.
This study has been partially supported by a grant of the Bill & Melinda Gates Foundation (Grand Challenges in Global Health) awarded to L.G. and a grant by the Wilhelm Sander Stiftung awarded to M.M.
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Agosti, J. M., and S. J. Goldie. 2007. Introducing HPV vaccine in developing countries—key challenges and issues. N. Engl. J. Med. 3561908-1910. [DOI] [PubMed] [Google Scholar]
- 2.Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132191-195. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., and R. M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15235-270. [DOI] [PubMed] [Google Scholar]
- 4.Baker, T. S., W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown. 1991. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 601445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrios, C., C. Georgopoulos, P. H. Lambert, and G. Del Giudice. 1994. Heat shock proteins as carrier molecules: in vivo helper effect mediated by Escherichia coli GroEL and DnaK proteins requires cross-linking with antigen. Clin. Exp. Immunol. 98229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bins, A. D., A. Jorritsma, M. C. Wolkers, C. F. Hung, T. C. Wu, T. N. Schumacher, and J. B. Haanen. 2005. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 11899-904. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, B., J. Dasgupta, and X. S. Chen. 2007. Structure-based engineering of papillomavirus major capsid l1: controlling particle assembly. Virol. J. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, and International Biological Study on Cervical Cancer (IBSCC) Study Group. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87796-802. [DOI] [PubMed] [Google Scholar]
- 9.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119445-462. [DOI] [PubMed] [Google Scholar]
- 10.Carter, J. J., G. C. Wipf, S. F. Benki, N. D. Christensen, and D. A. Galloway. 2003. Identification of a human papillomavirus type 16-specific epitope on the C-terminal arm of the major capsid protein L1. J. Virol. 7711625-11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, X. S., G. Casini, S. C. Harrison, and R. L. Garcea. 2001. Papillomavirus capsid protein expression in Escherichia coli: purification and assembly of HPV11 and HPV16 L1. J. Mol. Biol. 307173-182. [DOI] [PubMed] [Google Scholar]
- 12.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5557-567. [DOI] [PubMed] [Google Scholar]
- 13.Christensen, N. D., N. M. Cladel, C. A. Reed, L. R. Budgeon, M. E. Embers, D. M. Skulsky, W. L. McClements, S. W. Ludmerer, and K. U. Jansen. 2001. Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types. Virology 291324-334. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223174-184. [DOI] [PubMed] [Google Scholar]
- 15.Clifford, G., S. Franceschi, M. Diaz, N. Munoz, and L. L. Villa. 2006. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24(Suppl. 3)S26-S34. [DOI] [PubMed] [Google Scholar]
- 16.Clifford, G. M., J. S. Smith, M. Plummer, N. Munoz, and S. Franceschi. 2003. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 8863-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day, P. M., C. D. Thompson, C. B. Buck, Y. Y. Pang, D. R. Lowy, and J. T. Schiller. 2007. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 818784-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell, K., R. Koesters, M. Linnebacher, C. Klein, and L. Gissmann. 2006. Intranasal immunization with human papillomavirus type 16 capsomeres in the presence of non-toxic cholera toxin-based adjuvants elicits increased vaginal immunoglobulin levels. Vaccine 242238-2247. [DOI] [PubMed] [Google Scholar]
- 19.Fligge, C., T. Giroglou, R. E. Streeck, and M. Sapp. 2001. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology 283353-357. [DOI] [PubMed] [Google Scholar]
- 20.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20621-667. [DOI] [PubMed] [Google Scholar]
- 21.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12442-447. [DOI] [PubMed] [Google Scholar]
- 22.Hagensee, M. E., N. H. Olson, T. S. Baker, and D. A. Galloway. 1994. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J. Virol. 684503-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanke, T., J. Schneider, S. C. Gilbert, A. V. Hill, and A. McMichael. 1998. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine 16426-435. [DOI] [PubMed] [Google Scholar]
- 24.Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet 3641757-1765. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 26.Ishii, Y., S. Ozaki, K. Tanaka, and T. Kanda. 2005. Human papillomavirus 16 minor capsid protein L2 helps capsomeres assemble independently of intercapsomeric disulfide bonding. Virus Genes 31321-328. [DOI] [PubMed] [Google Scholar]
- 27.Ishii, Y., K. Tanaka, and T. Kanda. 2003. Mutational analysis of human papillomavirus type 16 major capsid protein L1: the cysteines affecting the intermolecular bonding and structure of L1-capsids. Virology 308128-136. [DOI] [PubMed] [Google Scholar]
- 28.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 2745810-5822. [DOI] [PubMed] [Google Scholar]
- 29.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 8912180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 676929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 3471645-1651. [DOI] [PubMed] [Google Scholar]
- 32.Kuck, D., C. Leder, A. Kern, M. Müller, K. Piuko, L. Gissmann, and J. A. Kleinschmidt. 2006. Efficiency of HPV 16 L1/E7 DNA immunization: influence of cellular localization and capsid assembly. Vaccine 242952-2965. [DOI] [PubMed] [Google Scholar]
- 33.Li, M., P. Beard, P. A. Estes, M. K. Lyon, and R. L. Garcea. 1998. Intercapsomeric disulfide bonds in papillomavirus assembly and disassembly. J. Virol. 722160-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, M., T. P. Cripe, P. A. Estes, M. K. Lyon, R. C. Rose, and R. L. Garcea. 1997. Expression of the human papillomavirus type 11 L1 capsid protein in Escherichia coli: characterization of protein domains involved in DNA binding and capsid assembly. J. Virol. 712988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy, M. P., W. I. White, F. Palmer-Hill, S. Koenig, and J. A. Suzich. 1998. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J. Virol. 7232-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller, M., J. Zhou, T. D. Reed, C. Rittmuller, A. Burger, J. Gabelsberger, J. Braspenning, and L. Gissmann. 1997. Chimeric papillomavirus-like particles. Virology 23493-111. [DOI] [PubMed] [Google Scholar]
- 37.Öhlschläger, P., W. Osen, K. Dell, S. Faath, R. L. Garcea, I. Jochmus, M. Müller, M. Pawlita, K. Schäfer, P. Sehr, C. Staib, G. Sutter, and L. Gissmann. 2003. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J. Virol. 774635-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkin, D. M., and F. Bray. 2006. The burden of HPV-related cancers. Vaccine 24(Suppl. 3)S11-S25. [DOI] [PubMed] [Google Scholar]
- 39.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321205-216. [DOI] [PubMed] [Google Scholar]
- 40.Rizk, R. Z., N. D. Christensen, K. M. Michael, M. Müller, P. Sehr, T. Waterboer, and M. Pawlita. 2008. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J. Gen. Virol. 89117-129. [DOI] [PubMed] [Google Scholar]
- 41.Roden, R. B., A. Armstrong, P. Haderer, N. D. Christensen, N. L. Hubbert, D. R. Lowy, J. T. Schiller, and R. Kirnbauer. 1997. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J. Virol. 716247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose, R. C., W. I. White, M. Li, J. A. Suzich, C. Lane, and R. L. Garcea. 1998. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J. Virol. 726151-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryding, J., L. Dahlberg, M. Wallen-Ohman, and J. Dillner. 2007. Deletion of a major neutralizing epitope of human papillomavirus type 16 virus-like particles. J. Gen. Virol. 88792-802. [DOI] [PubMed] [Google Scholar]
- 44.Sapp, M., C. Fligge, I. Petzak, J. R. Harris, and R. E. Streeck. 1998. Papillomavirus assembly requires trimerization of the major capsid protein by disulfides between two highly conserved cysteines. J. Virol. 726186-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schädlich, L., T. Senger, C. J. Kirschning, M. Müller, and L. Gissmann. 2009. Refining HPV 16 L1 purification from E. coli: reducing endotoxin contaminations and their impact on immunogenicity. Vaccine 271511-1522. [DOI] [PubMed] [Google Scholar]
- 46.Schiller, J. T., and D. Nardelli-Haefliger. 2006. Second generation HPV vaccines to prevent cervical cancer. Vaccine 24(Suppl. 3)S147-S153. [DOI] [PubMed] [Google Scholar]
- 47.Thönes, N., A. Herreiner, L. Schädlich, K. Piuko, and M. Müller. 2008. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J. Virol. 825472-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thönes, N., and M. Müller. 2007. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology 369375-388. [DOI] [PubMed] [Google Scholar]
- 49.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 2591745-1749. [DOI] [PubMed] [Google Scholar]
- 50.Wakelin, S. J., I. Sabroe, C. D. Gregory, I. R. Poxton, J. L. Forsythe, O. J. Garden, and S. E. Howie. 2006. “Dirty little secrets”—endotoxin contamination of recombinant proteins. Immunol. Lett. 1061-7. [DOI] [PubMed] [Google Scholar]
- 51.White, W. I., S. D. Wilson, F. J. Palmer-Hill, R. M. Woods, S. J. Ghim, L. A. Hewitt, D. M. Goldman, S. J. Burke, A. B. Jenson, S. Koenig, and J. A. Suzich. 1999. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 734882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto, S., T. Yamamoto, S. Shimada, E. Kuramoto, O. Yano, T. Kataoka, and T. Tokunaga. 1992. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol. Immunol. 36983-997. [DOI] [PubMed] [Google Scholar]
- 53.Yuan, H., P. A. Estes, Y. Chen, J. Newsome, V. A. Olcese, R. L. Garcea, and R. Schlegel. 2001. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 757848-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]