Abstract
As part of influenza pandemic preparedness, policy decisions need to be made about how best to utilize vaccines once they are manufactured. Since H5N1 avian influenza virus has the potential to initiate the next human pandemic, isolates of this subtype have been used for the production and testing of prepandemic vaccines. Clinical trials of such vaccines indicate that two injections of preparations containing adjuvant will be required to induce protective immunity. However, this is a working assumption based on classical serological measures only. Examined here are the dose of viral hemagglutinin (HA) and the number of inoculations required for two different H5N1 vaccines to achieve protection in ferrets after lethal H5N1 challenge. Ferrets inoculated twice with 30 μg of A/Vietnam/1194/2004 HA vaccine with AlPO4, or with doses as low as 3.8 μg of HA with Iscomatrix (ISCOMATRIX, referred to as Iscomatrix herein, is a registered trademark of CSL Limited) adjuvant, were completely protected against death and disease after H5N1 challenge, and the protection lasted at least 15 months. Cross-clade protection was also observed with both vaccines. Significantly, complete protection against death could be achieved with only a single inoculation of H5N1 vaccine containing as little as 15 μg of HA with AlPO4 or 3.8 μg of HA with Iscomatrix adjuvant. Ferrets vaccinated with the single-injection Iscomatrix vaccines showed fewer clinical manifestations of infection than those given AlPO4 vaccines and remained highly active. Our data provide the first indication that in the event of a future influenza pandemic, effective mass vaccination may be achievable with a low-dose “single-shot” vaccine and provide not only increased survival but also significant reduction in disease severity.
The emergence in 2004 and continued persistence of highly pathogenic H5N1 influenza A virus in bird populations is justifiably considered a potential pandemic threat (19). The virus has become endemic in many areas of the world and has demonstrated an ability to infect humans through transmission from poultry, thus far with limited human-to-human spread (26). Of great concern is that the case fatality rate for H5N1 infection of humans is reported to be >60%, compared to 0.1% for the 1957 and 1968 pandemics and 2 to 3% for the 1918 pandemic, which together resulted in at least 50 million deaths (14, 20). For these reasons, the development of strategies to minimize the impact if the virus mutates to acquire efficient human-to-human spread is essential.
Vaccination is considered the best method to ultimately control an influenza pandemic and should be implemented as soon as the pandemic strain is identified and vaccines produced (9, 23). To maximize coverage, pandemic vaccines will need to be available rapidly and will have to include the minimal dose of antigen to achieve solid immunity. This poses several major problems. One is that the human population is predominantly immunologically naive to the emerging subtype of virus, and so very large numbers of people will need to be protected as quickly as possible, which will place a huge demand on vaccine supply. The use of an adjuvant to lower the dose of antigen required (8) may ameliorate this problem to some degree, but there are few adjuvants that are suitable for human use, particularly those in ready supply in the event of a pandemic. In addition, we have little understanding of what levels and what type of immunity will provide protection from death or severe disease due to H5N1 infection (19).
Clinical trials with candidate H5N1 vaccines have been initiated with traditional virus preparations (egg-grown whole or detergent-disrupted “split” virions) and alternative vaccine strategies (recombinant protein, live-attenuated, and adjuvant-containing vaccines) (24). Using split virus alone, high amounts of antigen, containing 90 μg of hemagglutinin (HA), given twice, were required to elicit what is considered to be a protective antibody response in ca. 50% of subjects (25). Adjuvants, such as those based on aluminum salts (3) or the oil-in-water adjuvants MF59 (2, 17, 22) and ASO3 (13, 21), have provided considerable antigen dose reduction, but in all clinical trials and preclinical animal evaluation to date, two doses of vaccine have been required to achieve what is considered to be adequate anti-HA antibody levels or protection, respectively (8, 24).
One aim of the present study was to determine how suitable the ferret model is for making assumptions about human responsiveness to influenza vaccination. To do this, we evaluated in ferrets the same H5N1 pandemic vaccines, formulated with or without AlPO4 adjuvant, that had been examined in phase 1 and II randomized trials in healthy adults (18). We then sought to compare whether the responses to these vaccines were protective against lethal H5N1 challenge and whether the protective effects could be achieved with less antigen by using the more potent saponin-based Iscomatrix (ISCOMATRIX, referred to as Iscomatrix herein, is a registered trademark of CSL Limited) adjuvant. The Iscomatrix adjuvant has been shown to be safe and well tolerated in humans and to induce strong and long-lived antibody and cytotoxic T-cell responses in both humans and animal studies (7). Finally, the encouraging results with these adjuvants led us to examine whether protection from severe disease and death could be achieved after only a single injection of the H5N1 vaccines.
MATERIALS AND METHODS
Ferrets.
Healthy juvenile female or male ferrets, less than 12 months old and typically 4 to 6 months of age and 700 to 1,500 g in weight, were supplied by the Institute of Medical and Veterinary Science, Adelaide, South Australia, Australia. Ferrets were tested by hemagglutination inhibition (HI) tests and found to be seronegative to currently circulating influenza type A (H1N1 and H3N2) and B viruses prior to use. Approximately 10 days prior to virus challenge, temperature transponders were surgically implanted beneath the skin of the flank. All experiments were conducted with the approval of the CSIRO AAHL Animal Ethics Committee.
Viruses.
The wild-type H5N1 human influenza virus, A/Vietnam/1203/04 (which differs in the HA from A/Vietnam/1194/04 by only a single amino acid) and A/Indonesia/5/05 were obtained from the World Health Organization Collaborating Centre for Influenza Reference and Research, Parkville, Victoria, Australia. Stock viruses were propagated in the allantoic cavity of 10-day-embryonated chicken eggs at 35°C for 24 to 36 h and stored at −70°C. All experiments with these highly pathogenic viruses were conducted at the BSL3+ containment facility of the Commonwealth Scientific and Industrial Research Organization's Australian Animal Health Laboratory at Geelong, Victoria, Australia.
Vaccine formulations.
The vaccine used was based on the reverse-engineered strain (NIBRG-14) of the A/Vietnam/1194/04 human isolate of clade 1 H5N1 virus supplied by NIBCS, Mill Hill, United Kingdom. The purified inactivated detergent-disrupted vaccine was prepared from this virus by CSL Limited in an identical manner to that used for seasonal influenza vaccine. In brief, virus was propagated in embryonated eggs, mixed with β-propiolactone (ICN Pharmaceuticals, Inc., Costa Mesa, CA), subjected to rate zonal centrifugation on a sucrose gradient, and treated with sodium taurodeoxycholate (Sigma, St. Louis, MO) to yield a “split” virus preparation (5). The concentration of viral antigen was expressed in terms of HA protein, which was determined by standard single radial immunodiffusion and compared to a known standard of the relevant strain. The vaccine was used either in the absence of adjuvant or formulated with aluminum phosphate (AlPO4) or Iscomatrix adjuvant (60 μg/dose) in phosphate-buffered saline (PBS; pH 6.2) prior to inoculation. The AlPO4-containing vaccines were equivalent to those used in phase I (ClinicalTrials.gov identifier no. NCT00136331) and phase II (ClinicalTrials.gov identifier no. NCT00320346) clinical trials in healthy adults.
Vaccination and viral challenge of ferrets.
Two equivalent 0.5-ml doses of vaccine were administered to groups of four (unless otherwise indicated) ferrets, 21 days apart. Vaccines were delivered by the intramuscular route into the quadriceps muscle of the hind legs, using a 1-ml syringe with a 27-gauge needle. The route, antigen doses, and timing of immunization followed that of the phase I clinical trial, which examined vaccines containing 7.5 or 15 μg of HA, with or with AlPO4, and the phase II trial, which evaluated 30 (and 45) μg of HA of vaccine formulated with AlPO4 (18). In some experiments, ferrets received only a single inoculation of vaccine. Three to four weeks after the last inoculation, the ferrets were challenged intranasally with 106 50% egg infectious doses of challenge virus (A/Vietnam/1203/2004 or A/Indonesia/5/05) in 0.5 ml as described previously (10) under ketamine/medetomidine anesthesia (50:50, 0.1 ml/kg, reversed with atipemazole).
Monitoring and sample collection.
Animals were visually inspected daily throughout the study and twice daily after challenge. Ferrets were euthanized when reaching a previously determined endpoint or 14 days after challenge. The humane endpoint was defined as 10% body weight loss within 7 days of challenge or exhibition of signs consistent with involvement of other organ systems (e.g., tremor or abdominal discomfort). In control animals, this occurs typically within the first 7 days after viral challenge. In preliminary studies, these signs were found to correlate with the requirement to euthanize ferrets on subsequent days on humane grounds; thus, they have been utilized as surrogates for lethality.
Reaction site observations (i.e., erythema and edema) were assessed at 2, 24, and 48 h after each vaccination. General clinical observations were documented prior to challenge and, after challenge, a detailed clinical signs sheet and an evaluation of activity based on a five-level score were recorded daily. Animals were weighed while under sedation at the time of vaccination and challenge and at days 3, 5, 7, and 14 postchallenge. Rectal temperature was also determined at sedation by using digital thermometers to augment data derived remotely from the implanted temperature transponders (16).
Blood samples were collected immediately prior to each vaccination and prior to viral challenge from either the jugular or axillary veins. A further blood sample to monitor boosting of antibody responses by the challenge virus was collected at 14 days postchallenge or at the time of euthanasia. In addition, nasal washes and oral and rectal swabs, each into 1 ml of PBS, were taken on days 3, 5, and 7 postchallenge for virus isolation. A range of tissues were collected at autopsy and fixed in 10% neutral buffered formalin. Sections were prepared and stained with hematoxylin and eosin.
Immunological and virological evaluation.
Serum samples were assessed by micro-HI using chicken red blood cells (or horse red cells where indicated) and VN by standard methods. The supernatants from nasal washes and swab media were assessed for infectious virus by inoculation into the allantoic cavity of at least three 9- to 11-day-embryonated fowl eggs. The eggs were incubated at 35 to 37°C for up to 5 days. Allantoic fluids from eggs containing dead or dying embryos as they arose, and all eggs remaining at the end of the incubation period were tested for virus by hemagglutination of fowl red blood cells. All allantoic fluids with HA activity were considered positive for the virus. For some experiments, nasal washes were titrated for virus growth in Vero cells and reported as the 50% tissue culture infective dose.
RESULTS
Challenge experiments indicate not only protection from death but also from clinical disease in ferrets immunized with split influenza vaccines formulated with AlPO4.
Ferrets were immunized with the same formulations of 7.5- and 15-μg HA vaccines, with or without AlPO4, that had been evaluated in humans. Table 1 shows the serum HI and virus-neutralizing (VN) antibody responses of these animals after the first and second of two inoculations given 3 weeks apart. Ferrets did not mount any detectable responses to the vaccines without AlPO4. However, responses were observed 21 days after the first immunization with each of the vaccines containing AlPO4, and these increased after the second immunization. By 4 weeks after the second immunization, all ferrets in both the 7.5 and 15 μg of HA plus AlPO4 groups had HI and VN titers of 16 or greater, representing at least a fourfold rise for both assays. An additional group of 10 ferrets was immunized with 30 μg of HA plus AlPO4, and all of these had HI titers between 16 and 64 and VN titers between 32 and 256 at 4 weeks after secondary immunization (data not shown).
TABLE 1.
Serological responses to human H5N1 candidate vaccines in ferrets before and after viral challenge
| Vaccinea and ferret | Serum antibody titer
|
Fd | DPe | |||||
|---|---|---|---|---|---|---|---|---|
| Primaryb
|
Secondaryb
|
Terminal bleedc
|
||||||
| HI | VN | HI | VN | HI | VN | |||
| 7.5 μg of HA | ||||||||
| 37 | <4 | <4 | <4 | <4 | <4 | <4 | ||
| 38 | <4 | <4 | <4 | <4 | 64 | 128 | + | |
| 39 | <4 | <4 | <4 | <4 | <4 | <4 | + | 4 |
| 40 | <4 | <4 | <4 | <4 | 64 | 256 | + | |
| 15 μg of HA | ||||||||
| 41 | <4 | <4 | <4 | <4 | <4 | <4 | + | 7 |
| 42 | <4 | <4 | <4 | <4 | <4 | <4 | + | 6 |
| 43 | <4 | <4 | <4 | <4 | <4 | <4 | + | 3 |
| 44 | <4 | <4 | <4 | <4 | 128 | 512 | + | |
| 7.5 μg of HA + AlPO4 | ||||||||
| 45 | <4 | 4 | 16 | 16 | 16 | 32 | + | 7 |
| 46 | 32 | 16 | 32 | 64 | 128 | 512 | 12 | |
| 47 | 16 | 8 | 32 | 32 | 64 | 128 | ||
| 48 | <4 | 4 | 32 | 64 | 32 | 128 | ||
| 15 μg of HA + AlPO4 | ||||||||
| 49 | <4 | 4 | 32 | 16 | 64 | 128 | ||
| 50 | 64 | 32 | 64 | 128 | 32 | 128 | ||
| 51 | <4 | 4 | 64 | 32 | 64 | 256 | ||
| 52 | 32 | 16 | 32 | 64 | 32 | 128 | ||
| PBS | ||||||||
| 53 | <4 | <4 | <4 | <4 | <4 | <4 | + | 7 |
| 54 | <4 | <4 | <4 | <4 | <4 | <4 | + | 7 |
| 55 | <4 | <4 | <4 | <4 | <4 | <4 | + | 8 |
| 56 | <4 | <4 | <4 | <4 | 512 | >4,096 | + | |
Vaccines were administered on days 0 and 21.
HI and VN tests were performed against A/Vietnam/1203/04 virus on sera obtained 3 weeks after the primary immunization and 4 weeks after the secondary immunization immediately prior to homologous viral challenge.
Sera were tested again on day 14 postchallenge or earlier at the time of culling.
F, fever; +, Rectal temperature >40°C on at least one sampling day (day 3, 5, or 7) postchallenge.
DP, day postchallenge of euthanasia at the humane endpoint; blanks indicate ferrets surviving to day 14 when the experiment was terminated.
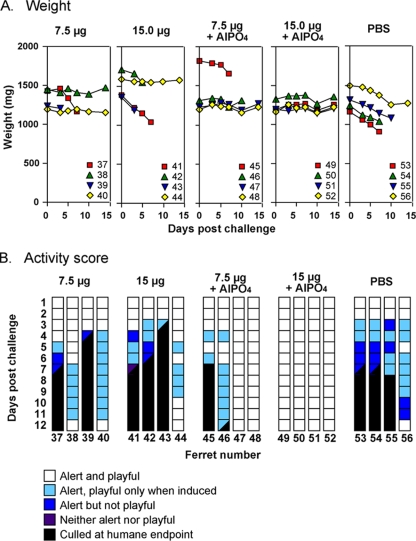
To address whether these responses were protective, the animals were challenged with A/Vietnam/1203/2004 virus 4 weeks after the second immunization, and the weight (Fig. 1A) and activity (Fig. 1B) of each animal was monitored for 14 days. All four ferrets in the PBS control group showed a dramatic drop in weight after viral challenge and a decrease in activity accompanied by fever and nasal discharge from day 3 onward. Virus was present in the nasal washings and oral swabs at all sampling times (data not shown). Two control ferrets were culled at a predetermined humane endpoint on day 7 and a third on day 8. The remaining control ferret survived, its weight stabilized and, despite the development of a cough, transient diarrhea, and conjunctival discharge, was alert throughout most of the observation period. This ferret developed very high HI (512) and VN (>4,000) antibody titers postchallenge (Table 1). Histopathological evaluation of organs from the PBS control ferrets culled prior to day 14 revealed multiorgan involvement, including nonsuppurative meningoencephalitis, necrotizing bronchioloalveolitis, hepatitis, pancreatitis, and nephritis. No involvement of the heart, intestine, spleen or bladder was observed.
FIG. 1.
Changes in weight and activity score of ferrets immunized with vaccines formulated with or without AlPO4. Ferrets were immunized with vaccines based on the reverse-engineered A/Vietnam/1194/04 NIBRG-14 (H5N1) virus containing 7.5 μg of HA (ferrets 37 to 56), 15 μg of HA (ferrets 41 to 44), 7.5 μg of HA formulated in AlPO4 (ferrets 45 to 48), or 15 μg of HA formulated in AlPO4 (ferrets 49 to 52) or PBS (ferrets 53 to 56). Ferrets received two inoculations of vaccine on days 1 and 21 and were challenged with wild-type A/Vietnam/1203/04 virus 4 weeks after the last immunization. (A) The weights of individual ferrets on days 0, 3, 5, 7, 10, and 14 after challenge. (B) Activity scores for each day after challenge out to day 12. Scores on days 13 and 14 were the same as day 12. Scores are depicted for each ferret by a strip of colored squares corresponding to the activity of the animal on the different days as indicated in the legend. Squares that are half black indicate the activity score on the day of culling at the humane end point.
In ferrets immunized with vaccines that did not contain AlPO4 adjuvant, there were two of four and one of four survivors in the 7.5- and 15-μg HA groups, respectively (Table 1). Despite fever, nasal discharge, and a slight decrease in activity, these ferrets maintained their weight and had largely recovered by day 12 (Fig. 1). The other ferrets in these two groups were culled on or before day 7. In the group of animals immunized with the vaccine containing 7.5 μg of HA and AlPO4 adjuvant two ferrets needed to be culled (Fig. 1) despite their having substantial prechallenge responses (VN titers 16 to 32) to the vaccine (Table 1). Clinical signs and postmortem histology were indicative of predominantly brain rather than respiratory tract involvement in both culled animals (data not shown). The remaining two ferrets in this group and all animals in the group that was immunized with the 15 μg of HA plus AlPO4 vaccine were largely free of clinical signs, showed no temperature rise and no drop in activity, and maintained their weight throughout the 14-day observation period (Fig. 1). Similarly, all 10 ferrets immunized with the vaccine containing 30 μg of HA plus AlPO4 showed no clinical signs of infection, decrease in activity, or a substantial temperature rise and either maintained or gained weight over the 14-day period.
Protection against morbidity and mortality can also be obtained with split influenza virus formulated with Iscomatrix adjuvant but with at least eightfold lower antigen doses.
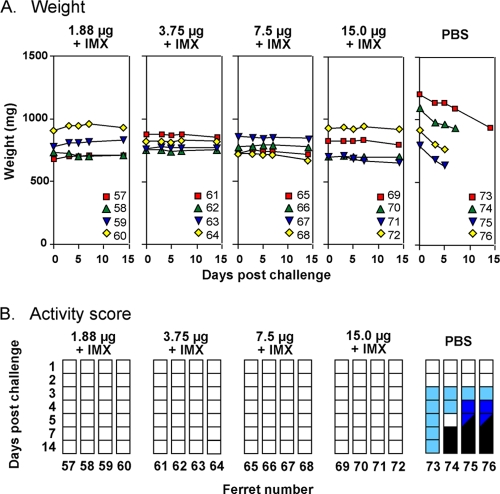
In an effort to further reduce the amount of antigen required for protection, the same viral challenge model was used to evaluate vaccines containing the more potent Iscomatrix adjuvant. Ferrets were immunized twice with a vaccine containing 15, 7.5, 3.8, or 1.9 μg of HA formulated with Iscomatrix adjuvant and subsequently challenged with A/Vietnam/1203/2004 virus. Irrespective of the antigen dose, all ferrets seroconverted after the second immunization by the VN test (summarized in Table 2), and all but one animal in the 1.9-μg group by the HI test, and survived the lethal challenge (Fig. 2). In addition, none of these ferrets showed a rapid drop in weight or any other any clinical sign of infection, except for one that had a transient temperature rise. This was in marked contrast to the PBS control ferrets, all of which lost weight (9.5 to 19.8%) and showed a decrease in activity score (Fig. 2), with three culled within 1 week postchallenge due to the development of pneumonia and/or neurological disease. The disease-free state induced by as little as 1.9 μg of HA in the presence of Iscomatrix adjuvant was similar to that achieved with 15 μg of HA formulated with AlPO4, indicating a dose reduction of at least eightfold.
TABLE 2.
Serological responses to vaccines formulated with Iscomatrix adjuvant before and after viral challenge
| Vaccinea | HI or VN testb
|
Fd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary
|
Secondary
|
Terminal bleedc
|
|||||||||||
| HI
|
VN
|
HI
|
VN
|
HI
|
VN
|
||||||||
| GMT | Resp | GMT | Resp | GMT | Resp | GMT | Resp | GMT | Resp | GMT | Resp | ||
| 1.9 μg of HA + IMX | 3 | 0/4 | 7 | 1/4 | 23 | 3/4 | 91 | 4/4 | 40 | 3/3 | 416 | 3/3 | 0/4 |
| 3.8 μg of HA + IMX | 7 | 2/4 | 8 | 1/4 | 45 | 4/4 | 128 | 4/4 | 64 | 4/4 | 512 | 4/4 | 0/4 |
| 7.5 μg of HA + IMX | 11 | 3/4 | 32 | 3/4 | 76 | 4/4 | 362 | 4/4 | 64 | 4/4 | 512 | 4/4 | 0/4 |
| 15 μg of HA + IMX | 8 | 2/4 | 16 | 2/4 | 108 | 4/4 | 256 | 4/4 | 152 | 4/4 | 431 | 4/4 | 1/4 |
| PBS | <4 | 0/4 | <4 | 0/4 | <4 | 0/4 | <4 | 0/4 | 5 | 1/4 | 6 | 1/4 | 4/4 |
Vaccines were inoculated on days 0 and 21. IMX, Iscomatrix adjuvant.
HI and VN tests were performed against A/Vietnam/1203/04 virus on sera taken 3 weeks after the primary immunization and 3 weeks after the secondary immunization immediately prior to homologous viral challenge. The GMT was calculated using a value of 2 when the titer was below detection limits (<4). The fraction of responder animals (Resp) was determined by a >4-fold increase in titer (16 or more).
Sera were tested again on day 14 postchallenge or earlier at the time of culling for the PBS group.
F, fever; the fraction of ferrets with rectal temperatures >40°C at on at least one sampling point (day 3, 5, or 7) postchallenge.
FIG. 2.
Changes in weight and activity score of ferrets immunized with vaccines formulated with Iscomatrix adjuvant. Ferrets were immunized with vaccines based on the reverse engineered A/Vietnam/1194/04 NIBRG-14 (H5N1) virus containing 1.9, 3.8, 7.5, or 15 μg of HA plus 60 μg of Iscomatrix adjuvant (IMX) or with PBS. Ferrets received two inoculations of vaccine on days 1 and 21 and were challenged with wild-type A/Vietnam/1203/04 virus 3 weeks after the last immunization. (A) Weights of individual ferrets on days 0, 3, 5, 7, and 14 after challenge. (B) Activity scores for each of the indicated days after challenge. Scores are depicted as in Fig. 1.
Two additional groups of five ferrets were inoculated twice with 3.8 μg of HA plus Iscomatrix adjuvant or 30 μg of HA plus AlPO4 as described above but were not challenged until 15 months after the second inoculation. Serum samples taken at 2, 3, 6, 9, and 12 months after immunization showed very low levels of HI antibody but, when the more sensitive horse red blood cells rather than chicken red blood cells were used in the assay, responses could be detected at all time points and ranged from a maximum of 160 at 2 months to 40 at 12 months; the mean responses to the two vaccines never varied more than twofold throughout (data not shown). After challenge, all ferrets survived and had subclinical infections only.
Protection of the upper respiratory tract is greatly enhanced in ferrets immunized with split influenza virus formulated with Iscomatrix adjuvant.
Despite being free of clinical disease, many of the ferrets throughout the study continued to have virus present in nasal washings and oral swabs for up to 10 days after viral challenge. Figure 3 shows the fraction of ferrets that had virus isolated from nasal washings or throat swabs taken on days 3, 5, and 7 postchallenge. Unexpectedly, all of the vaccines appeared to have the ability to reduce the duration of viral shedding. Only one of nine (11%) control ferrets that remained alive on day 7 postchallenge had stopped shedding virus, whereas the proportion of all immunized ferrets that had cleared virus from their nose and throat by day 7 was between 50 and 100% depending on the vaccine. At day 5 postchallenge, none of the animals immunized with vaccines containing 7.5 or 15 μg of HA with or without AlPO4 adjuvant had cleared virus from the upper respiratory tract but 70% of ferrets immunized with 30 μg of HA plus AlPO4 had cleared the virus. Significant differences in virus shedding compared to the control group were observed on all sampling days in this highest-dose AlPO4-containing vaccine group.
FIG. 3.
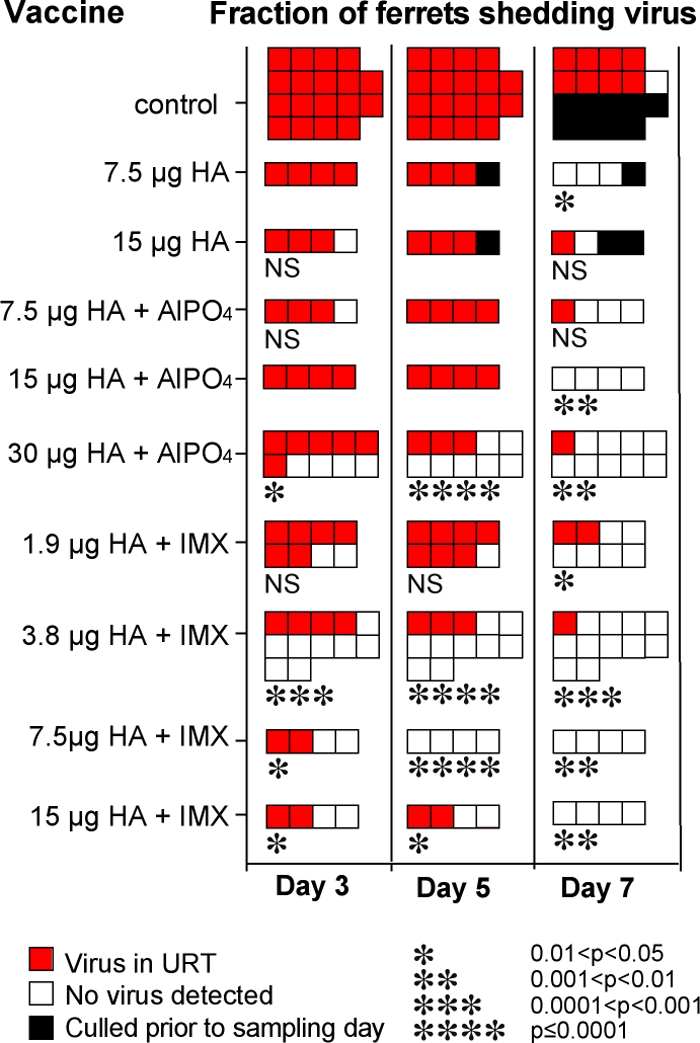
Influence of vaccine type on the presence or absence of virus in nose and throat samples taken on days 3, 5, and 7 postchallenge with homologous clade A/Vietnam/1203/04 virus. The data are combined from the different experiments described in the present study that included ferrets immunized with the indicated vaccines or PBS control animals. Nasal washings and also oral swabs taken into PBS were sampled on the indicated days postchallenge and tested for the presence of infectious virus by amplification in eggs. The data indicate the fraction of ferrets: red square, virus present in nasal and/or throat samples; white square, no virus detected in either nasal or throat samples; black square, animal previously culled. Statistical analyses of the vaccine groups compared to the control group for the relevant day postinfection were performed using the Fisher exact test with 95% confidence levels. The P values for those groups that were significantly different from the control group are shown as asterisks below the group, the number of stars indicates the P value as defined at the bottom of the figure. NS, not significantly different. No indicator, statistics unable to be performed as all shedding virus (therefore not different).
Overall, the rate of viral clearance in ferrets immunized with vaccines containing Iscomatrix adjuvant was much greater than for vaccines containing the equivalent antigen dose formulated in AlPO4. Compared to the control animals, ferrets immunized with vaccines containing 3.8, 7.5, or 15 μg of HA and Iscomatrix adjuvant showed a significant decrease in the proportion that shed virus even at day 3 after challenge, and this virus-positive proportion decreased from as early as day 5 in the 3.8- and 7.5-μg HA dose groups. Only 3 of the 28 ferrets given vaccines containing Iscomatrix adjuvant were still shedding virus on day 7 postchallenge. The 3.8-μg HA vaccine formulated with Iscomatrix adjuvant induced a particularly potent virus-clearing response, with 8 of 12 (66%) ferrets having no detectable virus in their noses and throats on day 3 and highly significant viral clearance at each time point. In terms of viral shedding, this vaccine was not significantly different (Fisher exact test) from the vaccine containing AlPO4 and eightfold more (30 μg) HA.
Protective cross-clade responses can be achieved with vaccines formulated with AlPO4 and Iscomatrix adjuvant.
To test whether the vaccines induced cross-reactive immunity capable of protecting against related H5N1 isolates, ferrets were immunized twice with vaccines prepared from the A/Vietnam/1194/04 clade 1 virus, containing either 15 μg of HA plus AlPO4 or 15 or 3.8 μg of HA plus Iscomatrix adjuvant and then subsequently challenged with the clade 2.1.3 virus A/Indonesia/5/05. In general, HI and VN titers were slightly lower against the clade 2 virus than the homologous clade 1 virus prior to challenge (Table 3). Nevertheless, all immunized ferrets survived the clade 2 viral challenge, which proved to be lethal to all four of the nonimmunized control animals. The immunized ferrets had subclinical infections (data not shown), reduced viral shedding compared to the controls, and managed to maintain and, in most cases, increase their weight over the 7 days postchallenge (Table 3). Consistent with the data from homologous challenge experiments (Fig. 3), there were fewer cases of viral shedding in the Iscomatrix vaccine groups compared to the AlPO4 group (Table 3).
TABLE 3.
Responses to vaccines formulated with AlPO4 or Iscomatrix adjuvant after infection with the heterologous clade 2 virus
| Vaccinea | Prechallenge GMT (fraction of vaccine responders)b
|
GMT (terminal bleed)c
|
Fractions of animals with virus in the nose and throatd (day 3, day 5, day 7) | Median % wt change (range)e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HI clade 1 | HI clade 2 | VN clade 1 | VN clade 2 | HI clade 1 | HI clade 2 | VN clade 1 | VN clade 2 | |||
| 15 μg of HA + AlPO4 | 45 (4/4) | 27 (4/4) | 108 (4/4) | 23 (3/4) | 1,024 | 256 | 1,218 | 724 | 4/4, 2/4, 1/4 | +4 (+3 to +10) |
| 15 μg of HA + IMXf | 51 (3/3) | 64 (3/3) | 161 (3/3) | 81 (3/3) | 815 | 323 | 512 | 1,024 | 3/3, 1/3, 0/3 | +5 (+5 to +14) |
| 3.8 μg of HA + IMX | 32 (4/4) | 19 (4/4) | 76 (4/4) | 27 (3/4) | 1,218 | 609 | 2,435 | 2,896 | 3/4, 1/4, 0/4 | +7 (0 to +9) |
| PBS | <4 | 4 | <4 | <4 | 4 | 3 | <4 | <4 | 4/4, 4/4, 4/4 | -15 (-13 to-17) |
Vaccines were administered on days 0 and 21. IMX, Iscomatrix adjuvant.
HI and VN tests were performed against the homologous clade virus A/Vietnam/1203/04 (clade 1) and the A/Indonesia/5/05 (clade 2.1.3) virus on sera taken 4 weeks after the secondary immunization immediately prior to challenge with the heterologous A/Indonesia/5/05 clade 2 virus. Shown is the GMT of the group and the fraction of vaccine responders (titers of 16 or above) in parentheses.
Sera were tested again on day 14 postchallenge or earlier at the time of culling, and the GMT is shown.
Nasal washings and oral swabs were taken on days 3, 5, and 7 postchallenge and inoculated into eggs. The respective fractions of animals with virus detected are indicated.
Median and range of percent change in weight from the day of viral challenge to 7 days postchallenge.
Only three animals completed the study in this group for reasons unrelated to influenza infection.
Protection against lethal H5N1 challenge can be achieved with only a single inoculation of vaccine.
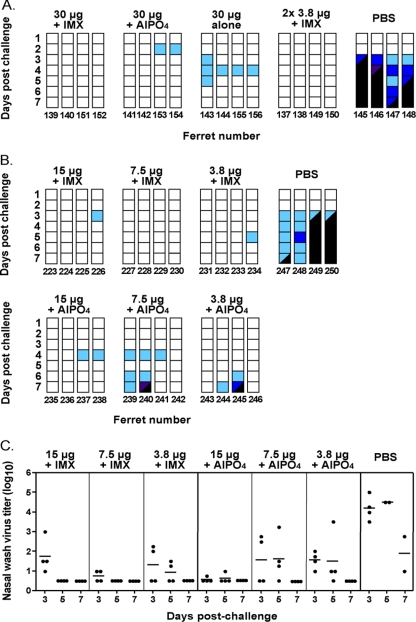
Another means of significantly reducing the amount of vaccine antigen required and/or the time to achieve protective immunity within a population is by use of a vaccine that is sufficiently potent to induce the required responses with only a single inoculation. Govorkova et al. (11) had previously observed protection against death due to A/Vietnam/1203/04 infection with a single dose of 7 and 15 μg of HA in the form of whole inactivated virus vaccine and AlOH adjuvant. However, these experiments were performed in ferrets that had previously been infected with influenza virus of a heterologous subtype, and so protection may have been due to recently induced cytotoxic T-cell responses. In the present study we tested vaccines in the naive ferrets that contained 30 μg of HA either alone or formulated with AlPO4 or Iscomatrix adjuvant given as a single inoculation and challenged the animals with A/Vietnam/1203/04 4 weeks later. As a positive control, a group of ferrets was immunized twice with the vaccine containing 3.8 μg of HA plus Iscomatrix adjuvant, a schedule that we knew to be totally protective against clinical disease and death. Table 4 shows the serology data, weight change, temperature status, and virus isolation from nose and throat, and Fig. 4A shows the activity scores postchallenge. All vaccinated ferrets survived the challenge, which was fatal for all four of the control animals. However, the course of infection differed between vaccine groups. Ferrets immunized with two inoculations of 3.8 μg of HA plus Iscomatrix adjuvant had very mild and largely subclinical infections, as expected, with additional clinical signs restricted to mucus in the nasal wash of ferrets 149 and 150 on day 5 only and a transient temperature rise in ferret 137 on day 7. This, plus the fact that half the animals showed no virus in the nose or throat samples from day 3 onward, was in accordance with the observations for similarly immunized animals elsewhere in the present study. Importantly, ferrets immunized with just a single inoculation of 30 μg of HA plus Iscomatrix adjuvant showed no clinical signs of infection whatsoever, and three of the four animals had no virus in their nose or throat samples from day 3 onward. Ferrets immunized with a single inoculation of 30 μg of HA plus AlPO4 remained healthy except for the presence of mucus in the nasal washes from days 3 to 5 postchallenge in all animals and conjunctive discharge in ferrets 153 and 154, which was preceded by a drop in their activity scores. In contrast, some ferrets immunized with 30 μg of HA in the absence of adjuvant had a spike in temperature on day 3 postchallenge and a transient drop in activity score and had virus in their noses and throats on days 3 and 5 postchallenge. Individual animals showed signs of depression, transient conjunctivitis, and mucus in the nasal wash but, nevertheless, showed better activity scores (Fig. 4A) and reduced weight loss (Table 4, experiment A) compared to animals vaccinated with two doses of 15 μg of HA (Fig. 1). The ferrets vaccinated once with 30 μg of HA alone fully recovered despite the fact that they had not seroconverted to the vaccine.
TABLE 4.
Responses to single inoculation vaccines
| Expt and vaccinea | Prechallenge GMT (fraction of vaccine responders)b
|
GMT (terminal bleed)c
|
Fractions of animals with virus in the nose and throatd (day 3, day 5, day 7) | Median % wt change (range)e | Fever >40°Cf | Survivalg | ||
|---|---|---|---|---|---|---|---|---|
| HI | VN | HI | VN | |||||
| Expt A | ||||||||
| 2 × 3.8 μg + IMX | 16 (2/4) | 128 (4/4) | 76 | 1,448 | 2/4, 1/4, 0/4 | +1.5 (−4 to +3) | 1/4 | 4/4 |
| 30 μg of HA + IMX | 8 (1/4) | 45 (3/4) | 54 | 1,024 | 1/4, 1/4, 0/4 | +2.5 (0 to +4) | 0/4 | 4/4 |
| 30 μg of HA + AlPO4 | 19 (3/4) | 108 (4/4) | 45 | 609 | 3/4, 2/4, 0/4 | +2 (−2 to +5) | 1/4 | 4/4 |
| 30 μg of HA | <4 (0/4) | <4 (0/4) | 91 | 861 | 4/4, 4/4, 0/4 | 0 (−1 to +2) | 2/4 | 4/4 |
| PBS | <4 (0/4) | <4 (0/4) | <4 | <4 | 4/4, 2/2, 1/1 | -9.5 (−7 to −11) | 3/4 | 0/4 |
| Expt B | ||||||||
| 15 μg of HA + IMX | 19 (3/4) | 27 (3/4) | 152 | 512 | 4/4, 3/4, 0/4 | -0.1 (−8 to 1) | 0/4 | 4/4 |
| 7.5 μg of HA + IMX | 14 (2/4) | 54 (4/4) | 91 | 215 | 2/4, 2/4, 0/4 | -0.8 (−2 to 0) | 1/4 | 4/4 |
| 3.8 μg of HA + IMX | 10 (1/4) | 8 (1/4) | 215 | 304 | 2/4, 2/4, 1/4 | -1.1 (−3 to +3) | 2/4 | 4/4 |
| 15 μg of HA + AlPO4 | 16 (3/4) | 23 (3/4) | 152 | 362 | 0/4, 2/4, 0/4 | -1.3 (−2 to −0.2) | 0/4 | 4/4 |
| 7.5 μg of HA + AlPO4 | 7 (1/4) | 7 (2/4) | 152 | 512 | 3/4, 3/4, 0/4 | -5.1 (−12 to −2) | 3/4 | 3/4 |
| 3.8 μg of HA + AlPO4 | 11 (2/4) | 10 (2/4) | 256 | 512 | 4/4, 4/4, 0/4 | -0.9 (−13 to 1) | 1/4 | 3/4 |
| PBS | <4 (0/4) | <4 (0/4) | 10 | 4 | 4/4, 2/2, 2/2 | -11 (−17 to −8) | 4/4 | 1/4 |
Vaccines were administered as a single inoculation, except 3.8 μg of HA plus IMX, given as two inoculations on days 0 and 21.
HI and VN tests were performed against A/Vietnam/1203/04 virus on sera taken 4 weeks after the single or last immunization, immediately prior to challenge with the homologous virus. Shown is the GMT of the group and fraction of vaccine responders (titers of 16 or above) in parentheses.
Sera were tested again on day 14 postchallenge or earlier at the time of culling, and the GMT is shown.
Nasal washings and oral swabs were taken on days 3, 5, and 7 postchallenge and inoculated into eggs.
That is, the percent change in weight from the day of viral challenge to 7 days postchallenge or earlier in culled animals.
The fraction of ferrets with rectal temperatures >40°C at on at least one sampling point (days 3, 5, and 7) after challenge is indicated.
The fraction of ferrets surviving to day 14 when the experiment was terminated is indicated.
FIG. 4.
Changes in activity scores of ferrets immunized with a single inoculation of vaccine. (A) Ferrets were immunized with vaccines containing 30 μg of HA and either Iscomatrix adjuvant (IMX), AlPO4, or no adjuvant. Ferrets were challenged with the homologous clade virus 4 weeks later, and activity scores up to day 7 are shown. As a positive control, a group of ferrets was immunized with two inoculations of vaccine containing 3.8 μg of HA plus IMX given 3 weeks apart and challenged 4 weeks later. (B) Ferrets were inoculated once with 3.8-, 7.5-, or 15-μg doses of vaccine with either Iscomatrix or AlPO4 adjuvant. The activity was recorded and is presented as in panel A. (C) Nasal washes from the ferrets described in panel B, sampled on days 3, 5, and 7 postchallenge, were titrated in Vero cells. The limit of detection for this assay was 100.5.
Antigen dose titration of the vaccines containing adjuvant revealed that complete protection against death could be achieved with a single dose as low as 3.8 μg of HA when formulated with Iscomatrix adjuvant or 15 μg of HA with AlPO4 (Table 4, experiment B, and Fig. 4B). Overall, the clinical signs of infection were fewer and limited to transient conjunctival and nasal discharge (data not shown), weight loss was somewhat less severe (Table 4B), and activity was less compromised (Fig. 4B) with vaccines containing Iscomatrix adjuvant compared to AlPO4 at these lower doses of antigen. Virus titers in nasal washes (Fig. 4C) were significantly reduced in all vaccinated groups compared to the control group (analysis of variance with Tukey's multiple comparison test performed for each sampling day), and no virus was detected in the nasal wash of any of the vaccinated ferrets on day 7 postinfection. A single inoculation of 15 or 7.5 μg of HA plus Iscomatrix adjuvant also yielded virus-free samples on day 5 postinfection, indicating a faster rate of viral clearance compared to AlPO4 vaccines. However, at the lowest dose of 3.8 μg of HA, there was no significant difference in viral clearance between the two adjuvant groups.
DISCUSSION
Successful pandemic vaccines that can be registered and implemented relatively quickly build on existing technology that has proven to be safe and effective for seasonal influenza vaccines and for which the manufacturing process is already in place and well validated (6, 24). For this reason, we have used inactivated, detergent-split, purified egg-grown influenza virus as a starting point for these pandemic vaccines and added components as adjuvants that have already been registered, in the case of AlPO4, or that are in development and clinically proven, as is the case for Iscomatrix adjuvant (7). Our studies have provided data in the ferret model that support the conclusion that the recently licensed H5N1 vaccine consisting of split virus in the form of 30 μg of HA plus AlPO4 adjuvant delivered as two inoculations given 21 days apart is highly immunogenic and further show that the responses induced are completely protective against death and disease after lethal H5N1 challenge. In the ferret model we also show, for the first time, that protection against death from lethal challenge can be achieved with only a single inoculation of this vaccine and, although some clinical signs of disease were apparent, these were relatively minor. This is very encouraging data that may inform process during mass vaccination and imply that provision of a single inoculation to a larger number of people may have a more beneficial outcome than trying to achieve better seroconversion with two inoculations in a smaller number of people.
That said, the precision with which these findings translate from the ferret to humans remains unknown. The phase I and II clinical trial results reported by Nolan et al. (18) showed some degree of responsiveness to 7 and 15 μg of HA vaccines in humans, whereas ferrets showed no response in the absence of adjuvant. The addition of AlPO4 adjuvant to these vaccines enabled a very modest rise in the frequency of significant responses in humans, most notably for VN antibodies, to give ca. 40% responders to the 15 μg of HA plus AlPO4 vaccine, but allowed HI and VN responses to become detectable in ferrets. Increasing the antigen dose to 30 μg in the vaccine formulated with AlPO4 resulted in a further increase in the frequency of human responders (>50%) and robust immunity in ferrets. It therefore appears that the ability to mount a seemingly successful response in both species requires roughly similar antigen doses. Although protection data must be extrapolated with caution, these data would give hope to the idea that similar fully protective responses can be generated in humans using this type of vaccine. This notion is further strengthened by the fact that the disease pathogenesis appears similar in ferrets to that observed in humans (4, 12), as previously noted in other studies (1, 10, 11).
In making assumptions about the success of vaccines in humans solely on the basis of seroconversion, some caution should be exercised. Here, as in other studies (11, 15), there are several instances where the classical serological measures of immunity normally associated with protection did not provide a good correlation with disease outcome. One such example is the group of ferrets that received a single inoculation of 30 μg of HA alone (Table 4, experiment A), where all survived viral challenge without prior seroconversion to the vaccine. Additional studies of the mechanisms underpinning vaccine-induced protection are warranted.
Vaccines formulated with Iscomatrix adjuvant were even more potent in terms of their ability to achieve protection of ferrets against clinical disease with very low antigen doses. Those containing as little as 1.9 μg of HA were effective in preventing disease in all animals tested, although the vaccine containing 3.8 μg of HA and Iscomatrix adjuvant as two inoculations gave more complete seroconversion. This latter vaccine also demonstrated cross-clade protection and was still completely protective against death and disease when animals were challenged 15 months after the last immunization. Most importantly, a single immunization with any of 3.8, 7.5, 15, or 30 μg of HA plus Iscomatrix adjuvant provided complete protection against death, an outcome only achieved at the higher antigen levels with AlPO4. This implies the possibility of an effective single immunization approach to H5N1 pandemic vaccination that has the advantage of reducing both the time to achieve protective immune status, as well as the amount of antigen required.
Although all vaccines reduced the period of viral shedding from the upper respiratory tract, vaccines containing Iscomatrix adjuvant were most effective in this regard when given in a two injection regime. The majority of animals vaccinated twice with >3.8 μg of HA and Iscomatrix adjuvant had cleared detectable virus from the site of inoculation by day 3, whereas those inoculated with comparable antigen doses of the other vaccines were still shedding virus 2 days later. Single-dose vaccines also showed significant benefit in reducing the viral load in the upper respiratory tract compared to PBS control animals. Titration of nasal washings in Vero cells from these animals confirmed this; the geometric mean titer (GMT) of infectious virus for each vaccinated group was at least 2 logs lower than for the control ferrets at the equivalent time point. These data indicate that the vaccines might have a role in decreasing the rate of person-to-person spread of the virus within a community, as well as having benefits for the vaccinee.
These studies confirm that the recently licensed human pandemic vaccine containing 30 μg of HA plus AlPO4 adjuvant has the potential to be completely protective against lethal H5N1 challenge and may even prevent death and significantly reduce disease after a single administration. The data also highlight the further dose reduction effect of Iscomatrix adjuvant, even after a single inoculation, to provide disease-free protective immunity. The present study provides the incentive for further evaluation of vaccines containing Iscomatrix adjuvant in humans.
Acknowledgments
We thank Tim Hancock for participation in the ferret experiments, Eleanor Cummins for formulation of vaccines, and Alan Hampson for advice in the initial stages.
This study was supported by the National Health and Medical Research Council of Australia's Urgent Research into a Potential Avian Influenza-Induced Pandemic granting scheme. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Baras, B., K. J. Stittelaar, J. H. Simon, R. J. Thoolen, S. P. Mossman, F. H. Pistoor, G. van Amerongen, M. A. Wettendorff, E. Hanon, and A. D. Osterhaus. 2008. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS ONE 3e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein, D. I., K. M. Edwards, C. L. Dekker, R. Belshe, H. K. Talbot, I. L. Graham, D. L. Noah, F. He, and H. Hill. 2008. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 197667-675. [DOI] [PubMed] [Google Scholar]
- 3.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 3671657-1664. [DOI] [PubMed] [Google Scholar]
- 4.Cardona, C. J., Z. Xing, C. E. Sandrock, and C. E. Davis. 2008. Avian influenza in birds and mammals. Comp. Immunol. Microbiol. Infect. Dis. [DOI] [PubMed]
- 5.Coulter, A., T. Y. Wong, D. Drane, J. Bates, R. Macfarlan, and J. Cox. 1998. Studies on experimental adjuvanted influenza vaccines: comparison of immune stimulating complexes (Iscoms) and oil-in-water vaccines. Vaccine 161243-1253. [DOI] [PubMed] [Google Scholar]
- 6.Daems, R., G. Del Giudice, and R. Rappuoli. 2005. Anticipating crisis: toward a pandemic flu vaccination strategy through alignment of public health and industrial policy. Vaccine 235732-5742. [DOI] [PubMed] [Google Scholar]
- 7.Drane, D., C. Gittleson, J. Boyle, and E. Maraskovsky. 2007. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev. Vaccines 6761-772. [DOI] [PubMed] [Google Scholar]
- 8.El Sahly, H. M., and W. A. Keitel. 2008. Pandemic H5N1 influenza vaccine development: an update. Expert Rev. Vaccines 7241-247. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, N. M., D. A. Cummings, C. Fraser, J. C. Cajka, P. C. Cooley, and D. S. Burke. 2006. Strategies for mitigating an influenza pandemic. Nature 442448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 792191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govorkova, E. A., R. J. Webby, J. Humberd, J. P. Seiler, and R. G. Webster. 2006. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 194159-167. [DOI] [PubMed] [Google Scholar]
- 12.Korteweg, C., and J. Gu. 2008. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am. J. Pathol. 1721155-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroux-Roels, I., A. Borkowski, T. Vanwolleghem, M. Drame, F. Clement, E. Hons, J. M. Devaster, and G. Leroux-Roels. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370580-589. [DOI] [PubMed] [Google Scholar]
- 14.Li, F. C., B. C. Choi, T. Sly, and A. W. Pak. 2008. Finding the real case-fatality rate of H5N1 avian influenza. J. Epidemiol. Community Health 62555-559. [DOI] [PubMed] [Google Scholar]
- 15.Lipatov, A. S., E. Hoffmann, R. Salomon, H. L. Yen, and R. G. Webster. 2006. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J. Infect. Dis. 1941040-1043. [DOI] [PubMed] [Google Scholar]
- 16.Mungall, B. A., D. Middleton, G. Crameri, J. Bingham, K. Halpin, G. Russell, D. Green, J. McEachern, L. I. Pritchard, B. T. Eaton, L. F. Wang, K. N. Bossart, and C. C. Broder. 2006. Feline model of acute nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 8012293-12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 3571937-1943. [DOI] [PubMed] [Google Scholar]
- 18.Nolan, T. M., P. C. Richmond, M. V. Skeljo, G. Pearce, G. Hartel, N. T. Formica, K. Hoschler, J. Bennet, D. Ryan, K. Papanaoum, R. L. Basser, and M. C. Zambon. 2008. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine 264160-4167. [DOI] [PubMed] [Google Scholar]
- 19.Poland, G. A., and S. Sambhara. 2008. Vaccines against influenza A (H5N1): evidence of progress. J. Infect. Dis. 198629-631. [DOI] [PubMed] [Google Scholar]
- 20.Potter, C. 1998. Chronicle of influenza pandemics, p. 3-18. In K. Nicholson, R. G. Webster, and A. J. Hay (ed.), Textbook of influenza. Blackwell Science, Oxford, England.
- 21.Rumke, H. C., J. M. Bayas, J. R. de Juanes, C. Caso, J. H. Richardus, M. Campins, L. Rombo, X. Duval, V. Romanenko, T. F. Schwarz, R. Fassakhov, F. Abad-Santos, F. von Sonnenburg, M. Drame, R. Sanger, and W. R. Ballou. 2008. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 262378-2388. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson, I., R. Bugarini, K. G. Nicholson, A. Podda, J. M. Wood, M. C. Zambon, and J. M. Katz. 2005. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 1911210-1215. [DOI] [PubMed] [Google Scholar]
- 23.Subbarao, K., and T. Joseph. 2007. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 7267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbarao, K., and C. Luke. 2007. H5N1 viruses and vaccines. PLoS Pathog. 3e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 3541343-1351. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. 2008. H5N1 avian influenza timeline of major events. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/ai_timeline/en/index.html.