SYNOPSIS
Objectives
This study sought to determine if (1) using a hands-free technique (HFT)—whereby no two surgical team members touch the same sharp item simultaneously—≥75% of the time reduced the rate of percutaneous injury, glove tear, and contamination (incidents); and (2) if a video-based intervention increased HFT use to ≥75%, immediately and over time.
Methods
During three and four periods, in three intervention and three control hospitals, respectively, nurses recorded incidents, percentage of HFT use, and other information in 10,596 surgeries. The video was shown in intervention hospitals between Periods 1 and 2, and in control hospitals between Periods 3 and 4. HFT, considered used when ≥75% passes were done hands-free, was practiced in 35% of all surgeries. We applied logistic regression to (1) estimate the rate reduction for incidents in surgeries when the HFT was used and not used, while adjusting for potential risk factors, and (2) estimate HFT use of about 75% and 100%, in intervention compared with control hospitals, in Period 2 compared with Period 1, and Period 3 compared with Period 2.
Results
A total of 202 incidents (49 injuries, 125 glove tears, and 28 contaminations) were reported. Adjusted for differences in surgical type, length, emergency status, blood loss, time of day, and number of personnel present for ≥75% of the surgery, the HFT-associated reduction in rate was 35%. An increase in use of HFT of ≥75% was significantly greater in intervention hospitals, during the first post-intervention period, and was sustained five months later.
Conclusion
The use of HFT and the HFT video were both found to be effective.
Operating room (OR) personnel risk acquiring bloodborne diseases from percutaneous injuries with contaminated sharp items or bloody contaminations due to splashes or glove tears.1 To reduce risk, use of the hands-free technique (HFT), whereby no two people touch the same sharp item at the same time, has been recommended2–4 together with such procedures as double-gloving5 and using blunt suture needles for tissues under the skin.6 Implementation of HFT consists of laying down a sharp item in a “neutral zone” (e.g., a location on the surgical field or a container) for it to be retrieved.
Surgical personnel wear masks and protective apparel that increase the likelihood of miscommunication, and often do not work together regularly. They can become distracted, misunderstand requests, or lose control of instruments during surgery. HFT is recommended to increase the predictability of how items are passed.
One organization has recommended HFT for almost two decades. However, its effectiveness has been evaluated in only one comprehensive 2002 study.7 A total of 3,765 surgeries were followed in an inner-city U.S. hospital where there was a policy endorsing HFT. At the end of surgeries, circulating and scrub nurses estimated the extent of HFT use by the surgical team, and circulators recorded other surgery-related information including length, type and emergency status, blood loss, number of people present, and noise level. They also recorded any percutaneous injuries, glove tears, or other types of bloody exposures that occurred. After adjusting for potential confounders, it was found that when HFT was used 75% to 100% of the time, the risk of injuries, glove tears, and contaminations decreased by 59% (95% confidence interval [CI] 28, 77) in surgeries with 100 cubic centimeters (cc) or more blood loss.
To investigate further, we undertook a multihospital Canadian study using a different design and a larger sample of surgeries. This quasi-experimental intervention study aimed to determine (1) whether using HFT ≥75% of the time during surgery resulted in decreased frequency of percutaneous injuries, contaminations, and glove tears, and (2) whether a video-based intervention increased overall HFT use to ≥75% in surgeries immediately post-intervention and five months later.
METHODS
Sample and data collection
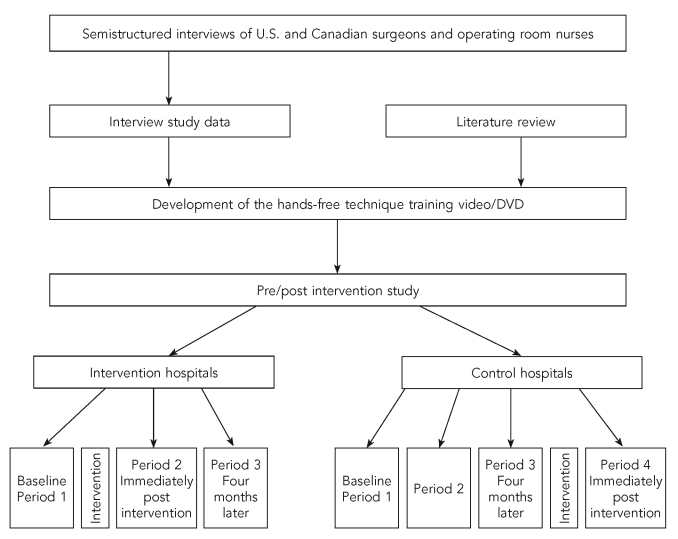
We collected data on eligible surgeries and eligible percutaneous injuries, glove tears, and contaminations from July 2004 to April 2006 at three control hospitals in Hamilton, Ontario (Hc1, Hc2, and Hc3), and three intervention hospitals—two located in Sudbury, Ontario (Si1 and Si2), and one located in Edmonton, Alberta (Ei1) (Figure). All participating hospitals were private, publicly funded, not-for-profit hospitals affiliated with universities.
Figure.
Pre/post hands-free technique intervention study in six hospitals in Canada
Data were to have been collected simultaneously in control and intervention hospitals, but due to the withdrawal in August 2004 of four intervention hospitals in London, Ontario, simultaneous data collection in intervention and control sites could not occur, as it took several months to identify the two hospitals in Sudbury and one hospital in Edmonton as substitute intervention sites.
In all hospitals, we collected data during baseline Period 1, after a one-week interval (Period 2), and five months later (Period 3). After Period 1, we implemented the intervention during a week in the Sudbury (Si1 and Si2) and Edmonton (Ei1) intervention hospitals. Due to low overall HFT use of ≥75% and low incident rates, we also implemented the intervention in the three Hamilton control hospitals after Period 3. Therefore, while data collection in the Sudbury and Edmonton hospitals ended after Period 3, we also collected data during Period 4 in the Hamilton hospitals.
Eligible surgeries included all elective and emergency surgeries that lasted at least 30 minutes, were carried out anytime of day on weekdays, and had a full-time circulating nurse present. All but anesthesia personnel were eligible to report percutaneous injuries, mucocutaneous contaminations, and glove tears.
To promote good response, we included a double-sided, one-page questionnaire based on one used previously7 in the packet of forms usually completed for each surgery. At the end of eligible surgeries, circulating nurses recorded the type and length of surgery and its emergency status, amount of blood loss, time of day, perceived loudness, number of people present for at least 75% of its duration, and, after consulting scrub personnel, the percentage of HFT used by the surgical team for 0%, 25%, 50%, 75%, or 100% of the time. When eligible personnel (i.e., surgeons, their assistants, residents, scrub and circulating nurses, and medical and nursing students) sustained a percutaneous injury, glove tear, or mucocutaneous contamination, circulators recorded details as soon as possible. Data were collected for a minimum of 350 surgeries/hospital in each period and included:
Sharp items: routinely identified sharps or anything that could perforate
Hand-free passing: laying down a sharp item onto a neutral zone by one person and retrieval by another, or the same person
Neutral zone: a section of the surgical field, mayo stand, overbed table, or rectangular basin, depending on the size and number of sharp items required during surgery
Percutaneous injury: pricking or stabbing sensation with or without blood8
Glove tear: visibly torn glove with or without blood on the skin
Mucocutaneous contamination: blood or diluted blood in contact with intact or non-intact skin or mucous membrane of the eyes, nose, mouth, or genitalia
Case surgery: surgery in which one eligible person had an injury, glove tear, or contamination; one incident/surgery used in analyses
Intervention
We developed the video in collaboration with a professional filmmaker, and informed by a review of the literature and results of semistructured telephone interviews with U.S. and Canadian surgeons, as well as OR nurses, about barriers to and facilitators of HFT use. In the video, a surgeon and OR nurse opinion leader provided knowledge about HFT and why it should be used. Voiceover and written text provided the most up-to-date information about rates of percutaneous injury and other exposures occurring during surgery, hepatitis B and C and human immunodeficiency virus seroconversion rates, and other related information. Additionally, in several scenarios, nurse actors demonstrated how HFT could be put into practice in a variety of surgical subspecialties, identifying neutral zones case by case, depending on the tools and sharp items being used.
Primarily, implementation of the intervention consisted of nurses attending interactive educational sessions provided at each hospital and viewing the 20-minute video, then viewing the video at anytime during the following one-week period (Figure). We also made efforts to have the surgeons and surgical residents in intervention hospitals participate in the same type of interactive sessions during the intervention week, but there was little uptake.
After the withdrawal of the intervention hospitals in August 2004, for feasibility reasons, it was also necessary to base the video production at one of the Hamilton control hospitals (Hc3) after the completion of baseline data collection.
Quality control
Independent observers, with at least a month of experience, covertly estimated HFT use in a subset of 30 operations in which the observation time was ≥75% of the surgery, while also measuring noise with a sound-level meter. When comparing nurses' and observers' HFT use estimates, the estimated kappa was 0.88 (95% CI 0.80, 0.96), which is considered “very good.”9
Data analyses
We based analyses on operations for which the amount of HFT use was recorded. As in the previous HFT study,7 we categorized the HFT variable as “used” in surgeries when it was used 75% to 100% of the time, and as “not used” when it was used about 0%, 25%, or 50% of the time. We considered all injuries, glove tears, and contaminations incidents.
Risk factors recognized to have the potential to confound the relationship between HFT use and incidents8,10–14 were structured. For surgical subspecialty, to achieve approximately equal numbers and homogeneous risk profiles in categories, we combined surgeries with similar procedural characteristics as: “general,” “orthopedic,” “cardiothoracic,” “urology/gynecology/vascular,” and “other” (neuro, plastics, otolaryngology, eye, and miscellaneous). We classified time of day as “day” when the surgery was conducted between 8 a.m. and 2:59 p.m., and otherwise as “other.” We dichotomized noise into “quiet” (surgeries in which quiet talking could easily be heard) and “not quiet” (those in which “normal” and “loud” talking could be heard).
We treated blood loss (in cc), surgery duration (in minutes), and number of personnel present for ≥75% of the surgery as continuous variables. We used multiple imputation procedures to impute values for blood loss data missing for 27% of surgeries, and duration data missing for 2% of surgeries. We based imputed values on their relationships with the rest of the risk factors in the rest of the dataset;15,16 we generated 10 imputations. The imputed value used for blood loss was 263 cc and the imputed value used for duration was 108 minutes. Imputation does not mean that the data estimated should be used as if they are real for an individual surgery; however, the inferences drawn from such imputed data give better inferences than using the data with missing observations.
Because of heightened HFT-related activity due to the video production at control hospital 3, including recruitment of OR personnel to demonstrate HFT use and live filming during surgeries, an unanticipated intervention took place at that site. Consequently, we considered Hc3 an intervention hospital and analyzed it as such.
Initially, we examined patterns in HFT use and incidents during each data collection period potentially suggestive of a Hawthorne effect. We grouped surgeries in each period into fifths by date, and conducted regressions for each period to determine if the fifths were related to HFT use or incident rates, controlling for type of surgery and hospital. Analyses revealed no indication that surgeries at the beginning of each period were associated with higher reported HFT use. And although they suggested higher incident rates in the first fifth of Period 1 only, cross-tabulation showed that they were higher in only two of the six study hospitals. This suggested no consistent Hawthorne effect on incident reporting in the study overall.
Because of the low frequency of incidents, we estimated relative risks of an incident—when the HFT was and was not used—using odds ratios (ORs). To account for the variation in risk according to other features of the surgery, we used unconditional logistic regression to estimate adjusted risk ratios and produce 95% CIs,17 using SPSS® software.18
To evaluate the HFT's effectiveness, we assessed first-effect modification using interaction terms between HFT and blood loss, surgery duration, and type of surgery—included in a model with the other potential risk factors—as well as hospital. We evaluated it by the likelihood ratio test at a criterion level of 0.05, chosen because we evaluated several interaction terms simultaneously.19 If we found no interaction, we conducted the analysis using a model with the HFT variable and potential confounder variables.
The model included the HFT use variable as the dependent variable, the interaction between hospital status (intervention or control) and period, as well as the potential confounders. We used evaluation of the interaction between hospital status and period to determine whether change in HFT use to ≥75% between Period 2 and Period 1, and Period 3 and Period 1, differed in intervention compared with control hospitals.
RESULTS
We based the main analyses on 10,596 out of 17,461 eligible surgeries done on weekdays for which the percentage of HFT use was recorded. Thus, we had a 60.7% response rate. We excluded surgeries on weekends and holidays, and from Period 3 at Si2, because of low response. The percentages of surgeries by study period were: 34.3% (Period 1), 25.2% (Period 2), 26.7% (Period 3), and 13.8% (Period 4).
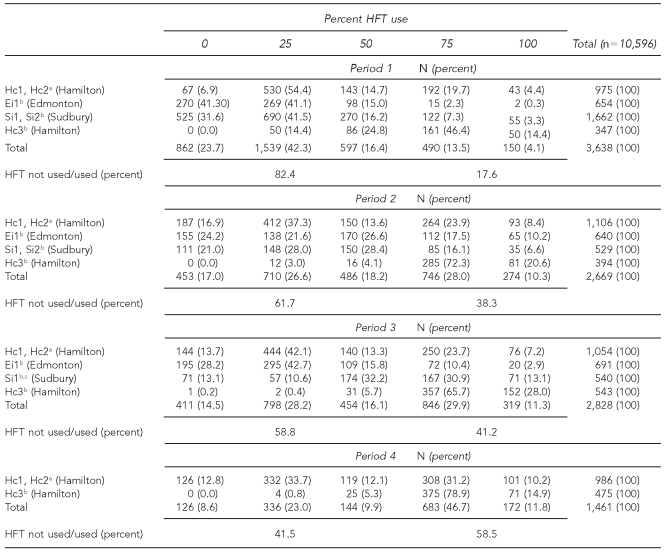
Overall, HFT use was 17.5% (0% category), 31.9% (25% category), 15.9% (50% category), 26.1% (75% category), and 8.6% (100% category). A total of 6,916 (65.3%) surgeries had HFT use of 0%, 25%, and 50%, and 3,680 surgeries (34.7%) had HFT use of 75% and 100% (Table 1).
Table 1.
HFT use at three control and three intervention hospitals in Canada by hospital intervention status by period, July 2004 to April 2006
aControl hospitals
bAt intervention hospitals, production of the video at Hc3 resulted in an unanticipated intervention. As such, it was treated as an intervention site in analyses.
cSi2 was excluded in Period 3 because of a low response rate.
HFT = hands-free technique
Table 1 shows that at the control hospitals, 75% to 100% HFT use rates increased from 24.1% to 32.2% between Period 1 and Period 2, but this was a smaller increase than at the intervention hospitals. At Ei1, rates increased from 2.6% to 27.7%; at Si1 and Si2, rates rose from 10.6% to 22.7%; and at Hc3, they increased from 60.8% to 92.9%. Overall, HFT use of 75% and 100% increased from 17.6% in Period 1 to 38.3% in Period 2. Between Period 2 and Period 3, HFT use of 75% and 100% declined at Ei1 by 14.4% and increased at Si1 (the Sudbury hospital remaining in the study) from 24.9% to 44.1%. Overall, HFT use of 75% and 100% increased from 38.3% in Period 2 to 41.2% in Period 3.
HFT use varied significantly (p<0.05) by emergency status, time of day, type of surgery, perceived loudness of surgery, duration of surgery, and blood loss. HFT use of 75% or 100% was 35.0% during non-emergencies vs. 27.0% during emergencies. In surgeries starting between 8 a.m. and 2:59 p.m., HFT use was 35.0%, compared with 31.0% otherwise. Among subspecialties, we found the highest HFT use in orthopedics (40.0%) and general surgery (39.0%), and the lowest use in cardiothoracic surgery (17.0%). Use of HFT was 37.0% in surgeries perceived to be quiet and 33.0% otherwise. In addition, mean duration of surgery when HFT was used was 90 minutes, compared with 117 minutes otherwise. Similarly, mean blood loss was 203 cc when used, compared with 297 cc when not used.
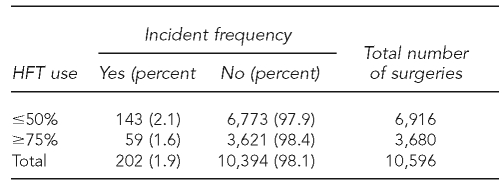
A total of 205 incidents occurred in 202 surgeries; in the three surgeries during which two incidents occurred, we used only the most risky incident in the analysis. Therefore, we used a total of 49 injuries, 125 glove tears, and 28 contaminations in the analyses. As seen in Table 2, in 6,916 surgeries in which HFT was used ≤50% of the time, 143 incidents occurred for a rate of 2.1%. In the 3,680 surgeries in which HFT was used ≥75% of the time, 59 incidents occurred for a rate of 1.6%. This resulted in a crude OR of 0.77 (95% CI 0.57, 1.05).
Table 2.
Frequency of incidents at three control and three intervention hospitals in Canada, by HFT use, July 2004 to April 2006a
aCrude odds ratio = 0.77 (95% confidence interval 0.57, 1.05)
HFT = hands-free technique
Incident rates varied (p<0.05) by hospital, period, surgical category, duration of surgery, blood loss, and number of non-anesthesiology personnel present. Among hospitals, Si1 had the highest incident rate overall at 3.7%, and Hc2 and Si2 (at 1.3%) had the lowest overall rate. The overall incident rate declined between baseline Period 1 (3.0%) and Period 3 (1.1%). The incident rate was 3.6% in cardiothoracic surgery, 2.2% in urology/gynecology/vascular, 1.8% in orthopedic, 1.4% in general, and 1.0% in other surgeries. We found the mean duration of surgery and blood loss to be higher in surgeries when an incident occurred: 174 minutes vs. 107 minutes, and 445 cc vs. 261 cc, respectively. The mean number of non-anesthesiology personnel present ≥75% of the time during surgery was also higher when an incident occurred: 5.8 vs. 5.2. We observed no association between incident rate and emergency status of surgeries, whether surgeries took place during the day, or nurses' perceptions of the loudness of surgeries.
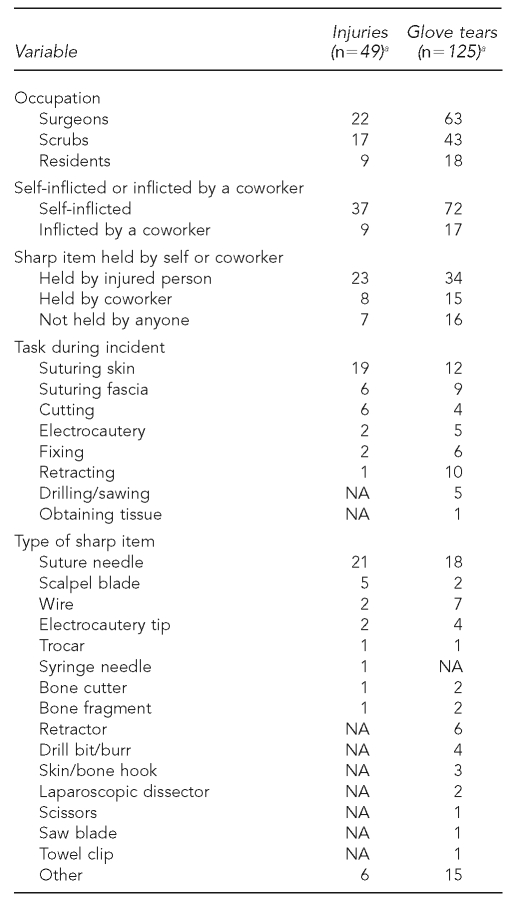
Table 3 shows information on injury and glove tear rates by occupational group, task, and type of sharp used. Surgeons were most frequently affected by injuries and glove tears, suturing skin was the most common task performed during incidents, and suture needles were the most common sharp item involved in incidents.
Table 3.
Number of injuries and tears by occupation, task, and type of sharp item at three intervention and three control hospitals in Canada, 2004–2006
aMissing data resulted in numbers not summing to the total number of surgeries with injuries and tears; because of excessive missing data, contaminations are not included in the table.
NA = not applicable
Logistic regression analyses
After determining that the prespecified interaction terms were not significant, we used logistic regression analysis to assess the effect of HFT use on incidents. We dropped noise from the model as it was not significant and not a previously established confounder.
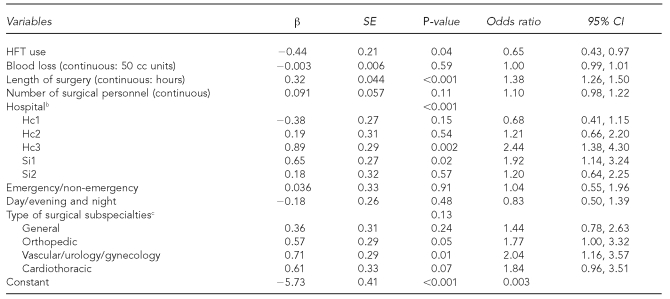
This model (Table 4) showed that when simultaneously controlling for other risk factors, HFT use ≥75% of the time in surgery was associated with a 35.0% reduction in the incident rate (OR=0.65, 95% CI 0.43, 0.97). We also found that the incident rate increased with duration of surgery and by hospital; specifically, compared with Ei1, incident rates were higher at Si1 and Hc3.
Table 4.
Logistic regression: effect of HFT use ≥75% of the time on incidents at three control and three intervention hospitals in Canada, July 2004 to April 2006a
aFull model analysis was carried out with 10,526 cases and 199 incidents; 70 surgeries were missing.
bEi1 was the reference category.
cThe reference category was “other” subspecialties consisting of neuro, plastics, eye, and ear/nose/throat.
HFT = hands-free technique
SE = standard error
CI = confidence interval
cc = cubic centimeter
It should be noted that adjusted ORs for each type of incident, although not statistically significant for injuries and glove tears, did show the same pattern: injuries: OR=0.87 (95% CI 0.38, 1.96); glove tears: OR=0.69 (95% CI 0.42, 1.14); and contaminations: OR=0.28 (95% CI 0.09, 0.88).
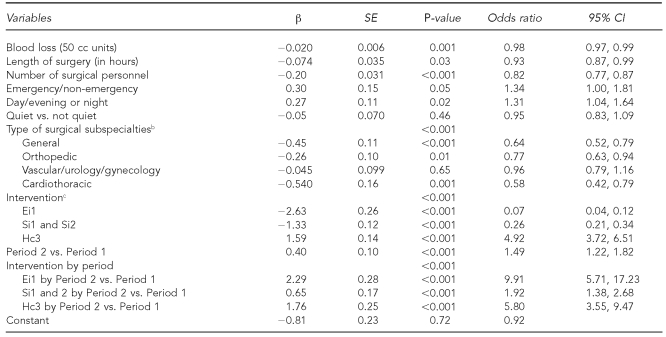
Logistic regression analysis carried out to assess the intervention's effect on HFT use ≥75% of the time demonstrated that increases in HFT use between Period 1 and 2 were greater (p<0.001) at Ei1, Si1, Si2, and Hc3 than at the control hospitals (Hc1 and Hc2) after adjusting for other potential influences on HFT use (Table 5). The significant interaction between the intervention and the period variable indicates that an increase in HFT use to ≥75% in Period 2 was greater in intervention hospitals.
Table 5.
Logistic regression: intervention's ability to increase HFT use to 75% and 100% in Period 2 compared with Period 1, at three control and three intervention hospitals in Canada, July 2004 to April 2006a
aFull-model analysis was conducted with 6,193 surgeries (Periods 1 and 2); 114 surgeries were missing.
bThe reference category was “other” subspecialties consisting of neuro, plastics, eye, and ear/nose/throat.
cHc1 and Hc2 were the reference categories.
HFT = hands-free technique
SE = standard error
CI = confidence interval
cc = cubic centimeter
Similarly, regression showed that HFT increases in intervention hospitals were sustained above baseline in Period 3, compared with HFT use in control hospitals (p<0.001) (results not shown). Other factors associated with HFT use in each of the models comparing Periods 2 and 3 to Period 1 included blood loss, length of surgery, and number of personnel; as they increased, the rate of HFT use decreased.
Regression also determined that HFT use ≥75% of the time increased (p<0.001) during Period 4 after implementing the intervention in the control hospitals (Hc1 and Hc2), but not in Hc3, the hospital that received the unanticipated intervention, where HFT use ≥75% of the time was already 94% in Period 3 (results not shown).
DISCUSSION
Many aspects of surgery do not lend themselves to standardization because of their complexity, but the routine transfer of sharp items between surgical -personnel does.20 HFT is an interdependent team practice mainly involving those “scrubbed into” an operation that should be seen as part of a system of regularizing OR work practices among a diverse group of skilled workers, who may not regularly work together. Predictability is increased by streamlining transfers. The need for enhanced predictability is highlighted by the stories of a U.S. scrub nurse who sustained an injury when a cardiac surgeon tried to hand back a contaminated scalpel while she was passing an instrument to another surgeon,21 and of a Canadian circulating nurse who sustained a needlestick when a surgeon handed her a syringe full of blood with an uncapped needle to send for testing; within weeks the nurse had developed acute hepatitis C and died.22
Increased hands-free passing decreases the level of vigilance required by surgical team members. Specifically, when most or all passes are hands-free, scrub nurses lay most or all sharp items onto a neutral zone for retrieval by surgeons and residents, and surgeons and residents lay most or all sharp items for retrieval by scrub nurses. This is unlike passing sharp items hand-to-hand, as that requires scrubbed personnel to ensure that items are correctly and securely placed in a recipient's hand, or to remember to announce that a sharp item will be passed.
In this multihospital, quasi-experimental intervention study, we demonstrated that use of HFT most of the time in surgery reduced work-related injuries, glove tears, and contaminations by 35%. The study confirms previous research results that found the rate of percutaneous injury, glove tear, and contamination was reduced by about 60% in surgeries in which HFT was used most of the time, and there was blood loss of ≥100 cc.7 Applying these new study results, 50 fewer incidents might be expected if HFT was used ≥75% of the time in the 6,916 surgeries in which it was used ≤50%; that is, incidents would have been reduced from 143 to 93 (143 × 0.35 = 50).
This study also found that the intervention (the newly developed HFT training video shown during interactive training sessions) increased HFT use to ≥75% of the time immediately after the intervention, and that increases were sustained for about five months. Although we found an overall tendency toward increased HFT use in all hospitals during the study, the intervention hospitals showed a greater increase. Specifically, in the control sites, use of HFT most of the time in surgery increased from about 24% to 32% between Period 1 and Period 2; in the Edmonton hospital, HFT use increased from about 3% to 28%; in the Sudbury hospitals, use of HFT increased from about 11% to 23%; and in the Hamilton intervention hospital, it increased from 61% to 93%.
We included both a video and interactive training in the intervention because both have been found to impact professional practice. For example, a Cochrane review found that interactive training resulted in moderate to large improvements in the professional practice of physicians and other health professionals,23 and videos are recognized as a good means of showing viewers, including nurses and physicians, how to do something properly.24,25
The intervention also appeared to sustain increased use of HFT in the longer term in some intervention hospitals. When comparing Period 3 with Period 1, there was increased use of the HFT in 13% vs. 3% of Edmonton surgeries; in 44% vs. 11% of Sudbury surgeries; and in 94% vs. 61% of surgeries in the Hamilton intervention hospital. In the Hamilton control sites, the percentage of surgeries in which HFT was used ≥75% of the time remained almost the same in Period 3 as in Period 2.
In addition to the intervention, it should be noted that sustained increased use of HFT may have occurred for another reason: whether or not a policy recommending HFT use was in place or was being considered. In the Sudbury hospital, in which data were collected during Period 3 and an HFT policy was implemented after Period 2, use of HFT most of the time in surgery increased by 19%. In the Edmonton hospital where there was no policy, HFT use of ≥75% decreased by 14%. Use of HFT most of the time in surgery occurred in 43% of baseline surgeries in the Hamilton hospitals, where an HFT policy had been in place for five years, and in 11% of baseline surgeries in the Sudbury hospitals, where an HFT policy was being formulated. In the Edmonton hospital, where a policy was not being considered, increased HFT occurred in 3% of baseline surgeries. While use of HFT ≥75% of the time occurred in 35% of all surgeries included in this study, increased HFT use occurred in 48% of surgeries that took place in hospitals with an HFT policy at the start of the study or implemented a policy during the study. We found higher use in this percentage of surgeries to be consistent with findings in a previous HFT study by Stringer et al.7 In that study, the participating hospital had an HFT policy in place and employed HFT ≥75% of the time in 42% of included surgeries carried out in 1995–1996.
Limitations
A limitation of this study with regard to its data collection methods was that it relied on circulating nurses to collect information on risk factors, consult with scrub personnel to quantify use of HFT for each case, and record details of incidents as they occurred. However, to optimize the inclusion of surgeries and reporting of incidents as much as possible, we used weekly raffles for prizes and various other incentives to maintain the interest of nurses and other surgical personnel and to promote the study. Nurses and independent observers demonstrated high reliability for HFT estimates. As explained previously, we did not identify evidence of a Hawthorne effect related to the collection of data on incidents or HFT use.
Nonetheless, substantial underreporting of incidents in this study is apparent when its incident rates are compared with rates reported in previous OR studies, in which circulating nurses7,8,11,14 or personnel without other tasks recorded incidents.10,12,13 In our study, percutaneous injuries were reported in 0.46%, glove tears in 1.18%, and contaminations in 0.26% of surgeries. In previous studies in which nurses recorded incidents, percutaneous injuries occurred in 1.1% to 3.1% of surgeries, and contaminations occurred in 1.4% to 25.0% of surgeries.7,8,11,14 In the study in which glove tears were also recorded, they occurred in 1.4% of surgeries.7 Despite low incident rates associated with underreporting, this study demonstrated the protective effect of HFT use most of the time in surgery.
Another potential limitation was that while we had low rates of missing data on other characteristics of the surgeries, blood loss data were missing in 27% of surgeries. To address this issue, we imputed blood loss data using multiple imputation, a technique recognized for generating valid inferences for missing data, while reducing the risk of biased results and increasing study power, which is especially valuable in large datasets such as ours.15
We also had an overall response rate of 61%, despite the implementation of rigorous efforts to maximize inclusion of eligible surgeries. However, in Period 1, the response rates in the control and intervention sites were 74% and 70%, respectively, indicating that there was little difference between them in the likelihood of inclusion of eligible surgeries. The data from the included surgeries from six hospitals were representative of the wide range of surgeries conducted in the study hospitals, as well as hospitals in the U.S. and Canada generally, containing expected percentages of surgeries by subspecialty and duration.
In this study, we could not measure characteristics such as the age and previous experience of individual surgical team members or of the surgical team as a whole. While this may have resulted in uncontrolled confounding, such characteristics have not been identified as related to risk. A previous OR study found that surgeons with at least 10 years of experience did not have a decreased risk of percutaneous injury when compared with surgeons who had less than 10 years of experience.8 Lack of individual-level data also made it difficult to assess for the potential effect of clustering at the level of the surgery. However, because surgeries with the same surgeon have increasingly varying configurations of surgical teams—as OR nurses are increasingly required to work in several subspecialties—this effect is unlikely to be large. Further, we adjusted the analyses for baseline differences in numerous measured potential confounders, such as hospital and duration of surgery, as described previously.
Risk-reduction methods such as HFT, which are found to be effective, should be instituted, especially considering the substantial risk during surgery of occupational exposure and patient recontact by sharps after occupational injury,13,14 yet implementation remains less than optimal.26 The semistructured interview study conducted to inform development of our intervention video identified dramatic resistance to using HFT among surgeons.27 They invoked a lack of evidence supporting HFT use, and reluctance to shift their gaze from a surgical wound or a microscope, as the main reasons. However, their rationale concerning a lack of evidence was undermined by the fact that most of the surgeons who stated this did not regularly double-glove—a measure repeatedly shown to reduce the risk of percutaneous injury and contamination.5,28 With regard to shifting gaze from a surgical wound, surgeons who adopt the technique have been able to acquire this skill in a short period of time and use it in all types of procedures, with rare exceptions.
Our experience in the intervention phase of this study was consistent with findings from that qualitative study; specifically, surgeons showed little willingness to participate in the study's video-based interactive sessions. Also, nurses who wanted to implement HFT told us that they frequently felt obstructed by surgeons' unwillingness to change, and that they needed administrative or regulatory support to use it most of the time in their practice.
U.S. OR personnel have been gaining experience implementing HFT as a result of the Occupational Safety and Health Administration's (OSHA's) Bloodborne Pathogens Standard introduced in 199129 and strengthened in 2001.30 OSHA highlights HFT on its hospital e-tool, Surgical Suite Module,31 and recommends it as a work practice to reduce bloodborne risk.30 An OSHA Interpretation states: “Where feasible, hospitals must implement the use of … proper work practices, such as designated neutral or safe zones, which allow hands-free passing of sharps.”32 As part of OSHA's standard, if surgeons refuse to pass sharp items using HFT, they must complete an Exception Form33 outlining how use of the technique could potentially harm their patients. No Canadian province has legislation comparable to the Bloodborne Pathogens Standard. Although our study has demonstrated the effectiveness of a video-based interactive intervention, additional support such as that provided by OR policies recommending use of the HFT and by OSHA interpretations and enforcement is likely also required for HFT use to be adopted more widely in Canada and elsewhere.
CONCLUSION
This study corroborated the results of the previous comprehensive study on the effectiveness of hands-free passing by again finding that HFT use most of the time reduces the risk of incidents. It also found that video-based, interactive discussions increased HFT use.
However, even though HFT is inexpensive to implement, requires little training, and does not appear to have adverse effects, major barriers to implementation existed in this study, mainly in the form of resistance from surgeons. A strong desire for change among motivated nurses is not sufficient; a system of incentives and penalties is required to ensure appropriate uptake of safety practices in the OR, including uptake by surgeons. OR administrators and enforcement agencies can help enact and sustain implementation.
Acknowledgments
The authors thank Ontario's Workplace Safety and Insurance Board for funding this research; Peter Karuna for producing the video; Kathy Radcliff for facilitating its development; Alaine Young and Pat Collington for help with problem solving throughout the study; the nurse assistants who championed the study in their workplaces; and the circulating and scrub nurses and technicians for completing hands-free technique questionnaires at the end of each surgery, and recording incidents and details when they occurred.
REFERENCES
- 1.Fry DE. Occupational risks of blood exposure in the operating room. Am Surg. 2007;73:637–46. [PubMed] [Google Scholar]
- 2.Occupational Safety and Health Administration (US) Standard interpretations: the use of safety-engineered devices and work practice controls in operating rooms; hospital responsibility to protect independent practitioners under BBP standard. [cited 2008 Aug 28];2007 Available from: URL: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=INTERPRETATIONS&p_id=25620.
- 3.Association of Operating Room Nurses. Recommended practices for standard and transmission-based precautions in the perioperative practice setting. AORN J. 1999;69:404–6. doi: 10.1016/s0001-2092(06)62499-x. [DOI] [PubMed] [Google Scholar]
- 4.American College of Surgeons. Statement on sharps safety. Bull Am Coll Surg. 2007;92:34–7. [PubMed] [Google Scholar]
- 5.St.Germaine RL, Hanson J, de Gara CJ. Double gloving and practice attitudes among surgeons. Am J Surg. 2003;185:141–5. doi: 10.1016/s0002-9610(02)01217-5. [DOI] [PubMed] [Google Scholar]
- 6.Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures—New York City, March 1993–June 1994. MMWR Morb Mortal Wkly Rep. 1997;46(2):25–9. [PubMed] [Google Scholar]
- 7.Stringer B, Infante-Rivard C, Hanley JA. Effectiveness of the hands-free technique in reducing operating theatre injuries. Occup Environ Med. 2002;59:703–7. doi: 10.1136/oem.59.10.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerberding JL, Littell C, Tarkington A, Brown A, Schecter WP. Risk of exposure of surgical personnel to patients' blood during surgery at San Francisco General Hospital. N Engl J Med. 1990;322:1788–93. doi: 10.1056/NEJM199006213222506. [DOI] [PubMed] [Google Scholar]
- 9.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 10.Panlilio AL, Foy DR, Edwards JR, Bell DM, Welch BA, Parrish CM, et al. Blood contacts during surgical procedures. JAMA. 1991;265:1533–7. [PubMed] [Google Scholar]
- 11.Popejoy SL, Fry DE. Blood contact and exposure in the operating room. Surg Gynecol Obstet. 1991;172:480–3. [PubMed] [Google Scholar]
- 12.Quebbeman EJ, Telford GL, Hubbard S, Wadsworth K, Hardman B, Goodman H, et al. Risk of blood contamination and injury to operating room personnel. Ann Surg. 1991;214:614–20. doi: 10.1097/00000658-199111000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokars JI, Bell DM, Culver DH, Marcus R, Mendelson MH, Sloan EP, et al. Percutaneous injuries during surgical procedures. JAMA. 1992;267:2899–904. [PubMed] [Google Scholar]
- 14.White MC, Lynch P. Blood contact and exposures among operating room personnel: a multicenter study. Am J Infect Control. 1993;21:243–8. doi: 10.1016/0196-6553(93)90416-2. [DOI] [PubMed] [Google Scholar]
- 15.Newgard CD, Haukoos JS. Advanced statistics: missing data in clinical research—part 2: multiple imputation. Acad Emerg Med. 2007;14:669–78. doi: 10.1197/j.aem.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Fox-Wasylyshyn SM, El-Masri MM. Handling missing data in self-report measures. Res Nurs Health. 2005;28:488–95. doi: 10.1002/nur.20100. [DOI] [PubMed] [Google Scholar]
- 17.Kleinbaum DG. Logistic regression: a self-learning text. New York: Springer-Verlag Telos; 1994. [Google Scholar]
- 18.SPSS Inc. SPSS: Version 13.0. Chicago: SPSS Inc; 2005. [Google Scholar]
- 19.Newby PK. Medford (MA): Tufts University Open Courseware; 2004. [cited 2008 Aug 28]. Lecture 12: regression II: the realities of model building. Available from: URL: http://ocw.tufts.edu/Content/1/lecturenotes/194258. [Google Scholar]
- 20.Grote G, Zala-Mezö E. GIHRE-Kolleg (group interaction in high risk environments) of the Daimler-Benz-Foundation. Zurich: Swiss Federal Institute of Technology; 2004. Mar, [cited 2008 Aug 28]. The effects of different forms of coordination in coping with work load: cockpit versus operating theatre. Report on the psychological part of the project. Also available from: URL: http://e-collection.ethbib.ethz.ch/ecol-pool/bericht/bericht_379.pdf. [Google Scholar]
- 21.Pueschel M. U.S. Medicine. 2004. Jul, [cited 2008 Aug 28]. VA invention reduces OR scalpel injuries. Available from: URL: http://www.usmedicine.com/article.cfm?articleID=896&issueID=64. [Google Scholar]
- 22.Haines T, Stringer B. Could the death of a BC OR nurse have been prevented by using the hands-free technique? Can Oper Room Nurs J. 2007;25(8):10–1. 19–20. [PubMed] [Google Scholar]
- 23.O'Brien MA, Freemantle N, Oxman A, Wolf F, Davis D, Herrin J. Poster presented at the 9th International Cochrane Colloquium. Lyon, France: 2001. Oct, Continuing education meetings and workshops: effects on professional practice and health care outcomes; pp. 9–13. [DOI] [PubMed] [Google Scholar]
- 24.Steinke EE. Use of videotaped interventions in research. West J Nurs Res. 2001;23:627–43. doi: 10.1177/01939450122045429. [DOI] [PubMed] [Google Scholar]
- 25.Meade CD. Producing videotapes for cancer education: methods and examples. Oncol Nurs Forum. 1996;23:837–46. [PubMed] [Google Scholar]
- 26.Dagi TF, Berguer R, Moore S, Reines HD. Preventable errors in the operating room—part 2: retained foreign objects, sharps injuries, and wrong site surgery. Curr Probl Surg. 2007;44:352–81. doi: 10.1067/j.cpsurg.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Stringer B, Haines T, Goldsmith CH, Blythe J, Harris KA. Perioperative use of the hands-free technique: a semistructured interview study. AORN J. 2006;84:233–5. 238–48. doi: 10.1016/s0001-2092(06)60491-2. [DOI] [PubMed] [Google Scholar]
- 28.Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;3:CD003087. doi: 10.1002/14651858.CD003087. [DOI] [PubMed] [Google Scholar]
- 29.Occupational Safety and Health Administration (US) Occupational exposure to bloodborne pathogens; final rule. 29 C.F.R. 1910.1030. Federal Register. 1991;56:64004–182. [PubMed] [Google Scholar]
- 30.Occupational Safety and Health Administration (US) Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries; final rule. 29 C.F.R. 1910.1030. Federal Register. 2001;66:5317–25. [PubMed] [Google Scholar]
- 31.Stringer B, Infante-Rivard C, Hanley JA. NIOSH's Best Practices in Workplace Surveillance. Cincinnati: 2001. Nov, [cited 2008 Aug 28]. The effectiveness of the hands-free technique in reducing operating room injuries; pp. 7–9. Also available from: URL: http://www.osha.gov/SLTC/etools/hospital/surgical/surgical.html#BloodbornePathogens. [Google Scholar]
- 32.Fairfax RE. San Antonio: 2007. Jan 18, [cited 2008 Aug 28]. Letter to Frederik E, director of safety, Baptist Medical Center. Available from: URL: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=INTERPRETATIONS&p_id=25620. directorate of enforcement programs. [Google Scholar]
- 33.Centers for Disease Control and Prevention (US) 2004. [cited 2008 Aug 28]. Workbook for designing, implementing, and evaluating a sharps injury prevention program. Available from: URL: http://www.cdc.gov/sharpssafety/index.html. [Google Scholar]