Abstract
Humans reason about the mental states of others; this capacity is called Theory of Mind (ToM). In typically developing adults, ToM is supported by a consistent group of brain regions: the bilateral temporoparietal junction (TPJ), medial prefrontal cortex (MPFC), precuneus (PC), and anterior temporal sulci (aSTS). How experience and intrinsic biological factors interact to produce this adult functional profile is not known. In the current study we investigate the role of visual experience in the development of the ToM network by studying congenitally blind adults. In experiment 1, participants listened to stories and answered true/false questions about them. The stories were either about mental or physical representations of reality (e.g., photographs). In experiment 2, participants listened to stories about people's beliefs based on seeing or hearing; people's bodily sensations (e.g., hunger); and control stories without people. Participants judged whether each story had positive or negative valance. We find that ToM brain regions of sighted and congenitally blind adults are similarly localized and functionally specific. In congenitally blind adults, reasoning about mental states leads to activity in bilateral TPJ, MPFC, PC, and aSTS. These brain regions responded more to passages about beliefs than passages about nonbelief representations or passages about bodily sensations. Reasoning about mental states that are based on seeing is furthermore similar in congenitally blind and sighted individuals. Despite their different developmental experience, congenitally blind adults have a typical ToM network. We conclude that the development of neural mechanisms for ToM depends on innate factors and on experiences represented at an abstract level, amodally.
Keywords: blindness, development, plasticity, temporoparietal junction, experience
Consider the following scenario: “While cleaning out her dorm room, Abigail sees an old love-letter lying under the bed. The handwriting looks like her beloved boyfriend's.” Based on this short description we can make inferences about Abigail's mental states, emotions, and actions. We infer that Abigail knows the letter is from her boyfriend; we recognize that she may feel happy to be reminded of him; and we might predict that she will put the letter away for safekeeping. This ability to reason about the mental states of others is called Theory of Mind (ToM).
A strikingly consistent group of brain regions supports ToM reasoning in a variety of tasks (1–3). These regions are located in right and left temporoparietal junction (TPJ), medial prefrontal cortex (MPFC), precuneus (PC), and anterior temporal sulci (aSTS). The distinct contribution of each of these regions is still controversial (2, 4–6) but this pattern of activation is highly reliable across a range of tasks involving ToM, presented both verbally (7, 8) and nonverbally (1, 9). Consequently, these regions are sometimes collectively called the ToM (or mentalizing) network.
Two key open questions remain: How do these functional regions develop in the life of an individual, and what processes lead to the typical location and functional profile of regions involved in ToM? One possibility is that the emergence of these regions is controlled by intrinsic biological factors, such as genes. However, a large body of research has shown that the function of cortical regions is at least partly determined by patterns of input from the environment received during development. For example, temporary visual deprivation of one eye in kittens leads to permanent under-representation of that eye in their visual cortices (10). Striking effects of visual deprivation are also observed in humans. The occipital cortices of congenitally blind adults support performance of language and tactile tasks (for review, see refs. 11 and 12).
One possibility is, therefore, that the functional organization of ToM regions is in part determined by experience during development. Which aspects of experience might play a role? Developmental psychologists have identified at least 3 kinds of experience that affect cognitive ToM development: visual, first-person, and linguistic. Children use these types of experience to learn about the invisible contents of other minds and to formulate a ToM. First, through vision children perceive the external consequences of other people's internal states: facial expressions as well as head, eye, and body movements. To explain these behaviors, children may formulate a causal model of the internal states that drive human actions (13–16). Second, children have their own first-person experiences of mental states. Upon observing the similarity between their own and other people's external actions and circumstances, children may infer that other people also experience internal states similar to their own (17–19). Third, children hear how other people talk about the mind, including descriptions of mental states like beliefs and emotions.
Would changes in these aspects of experience lead to changes in the neural mechanisms that support ToM? We begin to address this question by investigating the brain regions recruited for ToM in a group of adults who have had different developmental experiences: congenitally blind individuals.
Congenital blindness alters 2 sources of information about other minds. First, congenitally blind children cannot learn about other people's minds via visual observation of other people's facial expressions, head and eye movements, or hand and body movements. Second, congenital blindness alters first-person experiences of mental life. For sighted people, like Abigail in our example above, many mental states begin with visual experience: seeing the letter, recognizing the handwriting, etc. Another sighted person could imagine having the same experiences. By contrast, congenitally blind adults can understand Abigail's experience and could share its more abstract features (e.g., feeling happy to reminded of her boyfriend) but could not literally have the same experience.
Despite not having access to some information about the mind during development, congenitally blind adults eventually develop a functional and effective ToM, including an understanding of other people's experience of sight (20). Visual deprivation does, however, change the trajectory of ToM development. Blind children appear to be delayed in passing ToM tasks (21, 22). It is therefore possible that, in congenitally blind adults, similar ToM performance is supported by different neural structures than in sighted adults. Indeed, striking changes in the neural substrate of certain cognitive tasks, for example verbal memory or verb generation, have been identified in congenitally blind individuals (23–25). These data raise the question of whether the functional organization of ToM regions may also be altered in blind adults. Alternatively, absence of visual experience may not alter the neural development of ToM. It is possible, for instance, that the emergence of the ToM network is controlled by intrinsic biological factors, independent of experience during development. If so, ToM reasoning in blind adults may be accomplished by the very same neural structures as in sighted adults. In either case, the pattern of neural regions supporting ToM in congenitally blind adults would provide clues about the developmental processes that produce the typical ToM network.
How might congenital blindness alter the ToM network? One hypothesis is that blindness would affect lateral regions involved in ToM [left (LTPJ) and right (RTPJ) TPJ], which are anatomically nearby brain regions involved in the visual perception of human bodies and actions. In sighted adults, visual perception of human eye, head, hand, and body movements leads to activation in the right posterior superior temporal sulcus (pSTS), which is located just anterior to the RTPJ (26, 27). If visual information, processed in the pSTS, is normally a crucial input to the RTPJ during development, then the location or selectivity of the RTPJ might be different in the brains of congenitally blind people.
A second hypothesis is that blindness would specifically affect medial regions implicated in ToM (the PC and MPFC). The MPFC, in particular, is sensitive to the similarity of another person to the self. Some authors (28, 29) have suggested that the MPFC represents other people's mental states in terms of the subject's own similar first-person experience. Blindness eliminates the first-person experience of seeing. We might therefore observe a different functional response in the MPFC when congenitally blind individuals think about someone else's experiences of seeing, compared with thinking about someone's experience of hearing.
The goal of the present study was to test these predictions. More generally our goal was to examine the anatomical position (experiment 1) and functional selectivity (experiment 2) of brain regions involved in ToM in congenitally blind and sighted adults.
In experiment 1, participants listened to stories about mental (e.g., beliefs) and physical (e.g., photographs) representations and answered true/false questions about them. We identified brain regions recruited more during stories about mental representations than about physical representations. We then tested the anatomical position of these ToM brain regions, across groups, in 2 ways: (i) using whole brain analyses to look for any region where the neural activation in this task differed in the sighted versus the blind group, and (ii) by specifically comparing across groups the anatomical position of brain regions within the ToM network. Thus, experiment 1 allowed us to test, for example, whether the absence of a visual experience of human actions, typically processed by the pSTS, leads to the displacement or even nonexistence of the lateral (TPJ) components of the ToM network.
Experiment 2 probed the response profile of ToM regions of congenitally blind adults in greater detail. Participants listened to stories describing mental experiences based on seeing, hearing, or bodily sensations (e.g., hunger, aches) or news events not involving people. After each story, participants made a valence judgment (how bad does she feel; how bad is this news). By comparing the response to these 4 conditions, we tested the functional selectivity of ToM regions in two ways: (i) by testing whether each region distinguished between mental and bodily sensations (a measure of the regions' selectivity for mental state information) and (ii) by testing whether the response to hearing versus seeing experiences was different in any region for the blind versus the sighted. Thus, experiment 2 allowed us to test, for example, whether, in the absence of first-person experiences of sight, the MPFC would show less recruitment during the attribution of sight to other people.
Results
Behavioral Results.
Experiment 1.
Early blind (EB) and sighted participants did not differ in the their accuracy of response to belief and photo stories. There was no effect of group, condition, or group by condition interaction (P > 0.20) on accuracy. Participants were faster to respond to questions in the photo than in the belief condition [F(1,29) = 9.09, P < 0.01]. EB participants were marginally slower overall than sighted participants [F(1,28) = 3.64, P < 0.10]. The group by condition interaction did not approach significance (P > 0.30). EB and sighted participants did not differ in their accuracy or reaction time in the noise condition [see supporting information (SI) Table S1 for accuracy and reaction time data].
Experiment 2.
There was no difference in reaction times for seeing and hearing stories in EB or sighted participants. We performed a 2 × 2 ANOVA using condition (seeing/hearing) as a within-subjects factor and group (EB/sighted) as a between-subjects factor. No main effects or interactions reached significance (P > 0.25).
Due to the absence of a reaction time differences between the hearing and seeing conditions, these were collapsed into a single belief condition for the remainder of the behavioral analyses. We then looked for reaction time differences among belief, feeling, and control conditions among sighted and EB participants, as well as group-by-condition interactions. EB and sighted participants did not differ in reaction time (P > 0.30). There was a main effect of condition (belief/feeling/control) [F(2,65) = 4.03, P < 0.05] but no group-by-condition interaction (P > 0.15). In post hoc comparisons, participants were reliably faster to respond in the control condition than in the belief condition (Tukey's honestly significant differences test, P < 0.05). No other differences were reliable (see Table S1 for reaction time data).
Neuroimaging Results.
Experiment 1: Neural bases of reasoning about beliefs.
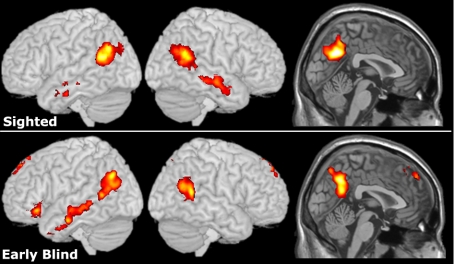
In sighted participants, reasoning about beliefs activated the bilateral PC (BA7), TPJ (BA39/40), and the middle and inferior temporal gyri (BA21/20). Similarly, in EB subjects reasoning about beliefs activated the bilateral PC (BA7), bilateral TPJ (BA22/39), left middle temporal gyrus (BA22), left inferior frontal gyrus (LIFG; BA47) and left medial frontal gyrus (LMFG; BA8) (Fig. 1). A comparison between groups did not reveal any regions that were differentially activated. The LIFG and LMFG reached significance in EB but not in sighted participants. However, the differences between groups in these regions were not reliable either in whole-brain or region of interest (ROI) analyses. A closer inspection of the data revealed that the LIFG effect was highly variable across participants and was driven by two subjects in the EB group (see Fig. S1). The LMFG showed a subthreshold effect in sighted participants, so activity in this region did not differ significantly between the two groups.
Fig. 1.
Belief stories and physical representations stories (experiment 1). There was a greater response to stories about beliefs than stories about physical representations in sighted and EB groups (whole-brain random effects analyses, P < 0.05 corrected). Areas of activation are overplayed onto a standardized MNI template.
We then compared the loci of ToM regions in sighted and EB participants. For each subject, we identified the peak active voxel for belief–reality in anatomically defined TPJ, STS, PC, vMPFC, and dMPFC ROI. We then compared the x, y, and z coordinates of these peak voxels across groups. Sighted and EB participants did not differ in the anatomical loci of any active regions (P > 0.1).
No differences in ToM regions were observed between the EB and sighted groups. However, during language comprehension, EB participants had greater activation than sighted participants in the visual cortices (physical story–backwards speech; group-by-condition interaction, P < 0.05, corrected) (Fig. S2). There was more language-related activity for EB adults than for sighted adults in the right inferior occipital gyrus, right fusiform gyrus, left middle occipital gyrus, and left fusiform gyrus.
To compare the amount of neural reorganization in the ToM and language networks, we performed a multivoxel pattern analysis to compare activation by group in each task over the entire cerebral cortex. We computed a correlation between voxel-wise t values in the EB and sighted groups' ToM networks (belief–physical; r = 0.55) and between the EB and sighted groups' language networks (physical–backward speech; r = 0.45). The spatial layouts of the ToM networks were more similar for EB and sighted groups than the spatial layouts of their language networks (z = 2.07, P < 0.05). To rule out the possibility that the language network was in general more variable across subjects, we compared the language and ToM networks across two halves of our sighted participants. Across the two halves of sighted participants, the language network (r = 0.61) was numerically, but not significantly, less variable than the ToM network (r = 0.58). Said differently, the similarity between EB and sighted adults on the ToM task is the same as the similarity within the sighted adults. The similarity on the language task, on the other hand, was lower between EB and sighted adults than within sighted adults (Fig. S3).
Experiment 2.
Regional specificity across groups.
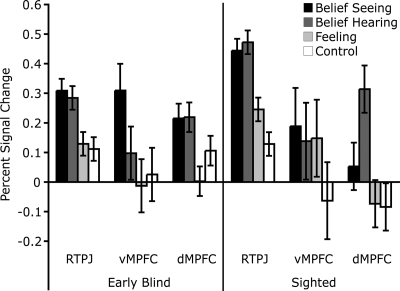
The RTPJ of EB and sighted adults was equally selective for belief content. For EB and sighted participants, stories about people's beliefs activated the RTPJ more than control stories (which were not about people) [EB: t(9) = 5.17, P < 0.001; sighted: t(10) = 3.44, P < 0.01] and more than stories about people's bodily feelings [EB: t(9) = 2.75, P < 0.05; sighted: t(10) = 2.64; P < 0.05]. Bodily feeling stories and control stories did not differ from each other (P > 0.2). There was no difference between groups in the size of the belief–bodily feeling difference (interaction not reliable, P > 0.3). Similarly, none of the other ROIs examined were differentially selective across the two groups of participants (LTPJ, PC, vMPFC, and dMPFC) (see Fig. 2). Whole-brain analysis did not reveal any regions that responded differentially across groups in the belief vs. bodily feeling or belief vs. control contrasts.
Fig. 2.
PSC in RTPJ, vMPFC, and dMPFC ROIs in sighted and EB groups (experiment 2). The graph shows PSC in BOLD signal while participants listened to stories about beliefs based on seeing, stories about beliefs based on hearing, stories about bodily feelings, and stories about events without human protagonists. Error bars represent standard errors of the difference between conditions calculated separately for each group.
Reasoning about beliefs based on hearing and seeing in EB and sighted adults.
The RTPJ, LTPJ, PC and MPFC responded similarly to the hearing and seeing belief conditions in both EB and sighted participants (all group-by-condition interaction effects and main effects, P > 0.2). The dMPFC showed a trend toward a group-by-condition interaction [F(1,20) = 3.74, P = 0.06]. However, this interaction was primarily driven by a greater response to Hearing Belief trials in sighted participants [t(11) = 2.78, P < 0.05]. The hearing and seeing conditions were not different in EB subjects (P > 0.3). Whole-brain analysis failed to uncover any regions that responded differentially across group in the seeing vs. hearing contrast.
Follow-up memory experiment.
Experiment 2 did not reveal any differences between the seeing and hearing conditions across groups. To check that participants did encode the visual or aural source of the protagonists' beliefs, we performed a surprise memory test after the fMRI experiment. Seven of the control subjects performed a forced-choice memory task. They read part of the original story (e.g., Abigail was reminded of her boyfriend) and recalled whether the source of the belief was visual or aural.
Participants were significantly above chance in remembering whether the protagonist experienced the event through seeing or hearing (mean ± SD: 70 ± 14%; t(6) = 3.65, P < 0.01; signed-rank test S = 10.5, P < 0.05). These data demonstrate that participants did encode the modality of the character's experience. (The signed-rank test was used because of the relatively small N.)
Discussion
ToM in the Brains of Blind Individuals.
The first key result of the current study is that EB and sighted individuals recruit the same neural network for ToM processing, including the RTPJ, LTPJ, PC, vMPFC, and dMPFC. The components of this network are similarly localized and equally selective for belief content across groups. As in sighted individuals, the ToM network of EB participants responds more to passages about beliefs than passages about nonbelief representations [e.g., photographs (30)] or passages about bodily sensations [e.g., hunger, fatigue (8)].
In contrast to the preservation of the ToM network, we found that visual deprivation leads to reorganization in the neural systems engaged in language comprehension: The visual cortices of EB, but not sighted, individuals are engaged during speech comprehension.
These data illustrate that the ToM network is robust to complete visual deprivation from birth. More generally, our findings suggest that the visual experience of other people plays little role ontogenetically in structuring the neural mechanisms for ToM reasoning. Although visual brain regions become engaged in language comprehension in the absence of visual input, these changes do not affect the neural mechanisms of ToM.
The Role of Vision and Language in ToM Development.
What do the current results tell us about the role of vision in developing ToM? According to one interpretation of our data, visually observing other humans plays no role in ToM development. This interpretation is suspect, however, in light of developmental evidence. As noted in the introduction, young sighted children use visual information (observation of eye gaze, facial expression, and action) to make inferences about mental states (31). Visual information is also a common basis for joint and coordinated attention in typical development; young children follow adults' gazes and pointing and produce demonstrative points to guide adults' attention. Such coordinated attention is an important precursor of later ToM development in sighted children (32).
Potentially even stronger evidence for the role of vision in ToM development comes from studies of blind children. Congenitally blind children are delayed in passing standard ToM tasks (21, 22, 33–37). A major challenge to interpreting these data is disentangling whether blind children are delayed in acquiring ToM or whether blind children are confused by the experimental tasks. For example, blind children may reasonably expect that sighted people have access to more information then they do about the location or contents of objects. If a ball is moved from a box to a basket, it could be unclear whether the ball is visible inside the basket. Studies of blind children have attempted to deal with these challenges in different ways. Green et al. (37) asked blind children to make predictions about the false beliefs of another blind child. Taken together, the previous literature does suggest that visual deprivation can delay ToM development. Observing other humans, therefore, appears to facilitate ToM development. Our data indicate that despite this role of visual experience in typical development, absence of visual learning has no residual effect on neural mechanisms for ToM in adulthood.
Blind people may acquire a typical ToM in part by learning from what people say about mental states, including both epistemic states, like believing and knowing, and perceptual states, like seeing and hearing. In this respect, blind children's learning experience may not be different from sighted children's. There is a lot of evidence that linguistic experience is necessary for ToM development. During typical development, linguistic ability is correlated with false-belief task performance (38). Conversely, deaf children of hearing parents (that is, whose parents are nonnative signers) are selectively delayed in passing false-belief tasks (e.g., refs. 39 and 40). Deaf children of deaf parents (native signers), by contrast, are not delayed (41). Moreover, the child's performance on the false-belief task is predicted by the mother's use of mental state signs (42). Hearing people talk about mental states may thus be a critical source of evidence about other minds, for both sighted and blind children.
Reasoning About Seeing in Blind Adults.
The second key result of the current study is that having seen is not necessary for the development of normal neural representations of another person's experiences of seeing. The ToM network of EB individuals is recruited similarly for reasoning about beliefs formed based on seeing and based on hearing.
One concern for this claim might be that our EB participants were not truly representing another person's experience of seeing. This concern could take one of two forms: The blind participants might have attributed to the character a specific sensory mental state in another modality like touch or audition with which they do have first-person experience, or they might have attributed to the character a nonsensory (amodal) mental state with no sensory component at all. We believe, however, that both prior studies of blind individuals and our own evidence mitigate these concerns.
Prior behavioral evidence has demonstrated that congenitally blind adults and children interpret sight verbs applied to sighted people as describing visual experiences. By four years of age, blind children understand the word look, when applied to themselves, to mean manual exploration. By contrast, for sighted people, blind children understand that seeing does not require touching and can happen at a distance (20). This knowledge about what it means to see is illustrated in a dialogue between Kelli, who is a blind child, and an experimenter:
Experimenter: Could we see something with our eyes up in the sky?
Kelli: I can't because my eyes don't work.
Experimenter: Could you touch something up in the sky?
Kelli: No because it is way up here.
Experimenter: How about me? Could I see something up in the sky?
Kelli: Yeah.
(Although her knowledge of seeing wasn't perfect; at one point in the exchange Kelli suggests that people might be able to see with their mouth open.)
A congenitally blind adult interviewed by Landau and Gleitman (20) has an even stronger command of seeing, as illustrated in her definition of sight verbs including to examine:
To look at, scrutinize, look at in very fine detail.[Experimenter asks: How do people do that? Would you hold it close to you?] It depends on the size of the object you're looking at. What kind of perspective you want to get on it. If you wanted to get the full detail you would close [sic]. If you wanted to see a lot of detail you would look at it from far away. If you wanted to see just part of it you would look at it up close.
These data illustrate that blind individuals interpret sight verbs as describing vision when they are applied to sighted people.
To further decrease the probability of an amodal interpretation of our stories, our stimuli described mental experiences that were strongly linked to a specific modality. For example, in the story about Abigail, she was reminded of her boyfriend either by seeing handwriting (which has no natural analog outside of vision) or by hearing his footsteps (which are similarly restricted to audition). Our pilot data suggest that this approach was effective; the modality of the experience described in each story was salient and memorable. Thus we believe that in our study both blind and sighted participants represented the characters' modality-specific experiences.
A different concern may be that, although blind participants can reason about seeing in verbal stories, such reasoning is not typical of “real-life” mentalizing. By contrast, we think that in real life, mentalizing often occurs in response to verbal stimuli, including both single utterances and extended narratives (43). Moreover, previous studies suggest that mentalizing based on verbal and nonverbal stimuli engages the same ToM neural network (1, 9).
Our results therefore suggest that reasoning about beliefs does not involve simulating the sensory experiences that gave rise to those beliefs and argue against a strong version of Simulation Theory (ST). According to strong ST, we reason about others' beliefs by imagining ourselves having the experiences that gave rise to the beliefs. Understanding the beliefs of others would then critically depend on sharing their sensory and action experiences. In contrast, we find that never having seen does not alter how people represent mental states that arise from seeing.
We conclude that thinking about other people's thoughts, in both sighted and blind people, depends on abstract and propositional representations that can be acquired without first-person experience. Of course, our data do not rule out recapitulation of first-person experiences during mentalizing, in some circumstances. Memories of their own specific experiences may help people infer and empathize with another person's current situation (44). Nevertheless, our results suggest that detailed and sophisticated thoughts about thoughts can occur in the absence of such shared experiences.
The Role of Experience in the Development of the ToM Network.
We demonstrate that the absence of visual experience during development does not affect the location and functional profile of ToM brain regions. Our results do not resolve, of course, how the brain regions for ToM in the blind (or in the sighted) develop; that is, what mechanisms do lead to brain regions with these functions in these locations.
On the one hand, the brain regions for ToM might be predetermined biologically and not open to the influence of experience in the course of development. The alternative, which we favor, is that the development of neural mechanisms for ToM does depend on experience but that these experiences are represented at an abstract level, linguistically and/or amodally. Thus, blind people's observations of humans through hearing may provide sufficient replacement for sighted people's observations through vision. For example, hearing footsteps or the tone of someone's voice may provide equivalent information to observing actions and seeing faces, as far as development of the ToM mechanism is concerned.
An outstanding question is: How do other kinds of experiential change affect the ToM network? Of particular interest are the effects of changes in linguistic and social environment. As described above, linguistic input may provide a critical source of information for the development of a ToM. Another critical source of relevant experience may be social interactions with caregivers early in life. For example, performance on basic false-belief tasks is delayed in children who were raised in orphanages, even after controlling for IQ and language ability (45). In particular, performance is correlated with the institutional adult–child ratio; children who have fewer opportunities for interaction with adults are more likely to fail false-belief tasks. A number of recent studies have reported the neural effects of institutional rearing (46, 47), but none have directly investigated the ToM network.
In summary, the present data illustrate that the ToM network is remarkably resilient to complete visual deprivation. An important goal of further research should be to establish which aspects of experience are necessary and sufficient for typical development of the ToM network.
Materials and Methods
Participants.
Twenty-two sighted (16 females; mean age ± SD, 40 ± 19) and 10 EB subjects (5 females; mean age ± SD, 50 ± 7) participated in experiment 1. All of the blind participants and 13 of the sighted participants (mean age ± SD, 52 ± 16) also took part in experiment 2. All participants were native English speakers. One EB participant was ambidextrous and one was left-handed. One sighted participant was left-handed. The remainder of the blind and all of the sighted participants were right-handed according to self-report. Nine EB participants were congenitally blind and one lost sight between the ages of 2 and 3. All blind participants reported having at most faint light perception and no pattern discrimination (Table S2). None were able to perceive shapes, colors, or motion. None of the participants suffered from psychiatric or neurological disorders, had ever sustained head injury, or were on any psychoactive medications. The study was approved by the institutional review board. All subjects gave informed consent and were paid $30 an hour for taking part in the experiment.
Behavioral Procedure.
Experiment 1.
Participants listened to stories (12 sec) about mental and physical (e.g., photographic) representations of reality. In each story there was an inconsistency between the representation (belief or physical) and reality. Immediately after the story, participants were asked a true/false question (6 sec) that referred to either the situation in reality or to the representation. Prior studies showed no difference between the reality/representation conditions (48). Therefore all analyses collapse across the reality/representation dimension. Story and question pairs were separated by a12-sec intertrial interval (ITI). On half of the trials the correct response to the question was “true.” The belief and physical stories were thus matched on the logical structure as well as the syntactic construction of the statement but differed as to whether they required participants to reason about beliefs. The belief and physical conditions were also matched on average number of words per story and question (all condition differences P > 0.1). Additionally, we included a noise control condition that was similar in structure to the story trials. In the noise condition, participants heard a long string of reversed speech followed by a short string of reversed speech. They were asked to determine whether the short string of backward speech was a piece of the long string or a new string. The long strings of reverse speech were created by playing the stories backwards. The short strings were made by playing the questions backwards or by splicing out a piece of a backwards story. The resulting auditory stimuli sounded like unintelligible noise. The task was performed in 6 runs with 12 items per run (4 belief, 4 physical, and 4 backward–speech). Each run was 6 min and 12 sec long.
Experiment 2.
Participants heard stories (13 sec) in 4 conditions: seeing belief, hearing belief, bodily feeling, and control. The seeing belief stories described a character coming to believe something as a result of a seeing experience. The hearing belief stories described a character coming to believe something as a result of a hearing experience. The bodily feeling stories described a character experiencing bodily feelings (e.g., aches, exhaustion, relaxation). Seeing, hearing, and bodily feeling conditions were matched on the semantic and syntactic aspects of stories by having each item occur in every condition, across participants. Participants decided whether the main character in the story felt “very bad,” “a little bad,” “a little good,” or “very good” (8 sec). Half of the stories in each condition described an event that might make the character feel bad, whereas half described positive events. The control stories described events without human protagonists that were either positive or negative. Participants were told that if the story they heard did not have a main character (control condition), it was a news clip and they were to rate the story as to whether the news was “very bad,” “a little bad,” “a little good,” or “very good.” The control condition was similar in syntactic construction and number of words per story to the belief and bodily feeling conditions (P > 0.3). Stories and questions were separated by an 11-sec ITI. The experiment consisted of 4 runs, each 4 min and 34 sec long. There were 8 trials per run, with 2 in each condition. The stimuli for experiments 1 and 2 were digitally recorded by a female speaker at a sampling rate of 44,100 to produce 32-bit digital sound files. See Fig. S4 for examples of stories from experiments 1 and 2.
fMRI Data Acquisition and Analysis.
Structural and functional data were collected on a 3-tesla Siemens scanner at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research (Massachusetts Institute of Technology). For details of data collection and analysis see SI Materials and Methods. Briefly, data analysis was performed with SPM2 (www.fil.ion.ucl.ac.uk) and in-house software. Data were motion-corrected, smoothed, and normalized. BOLD signal differences between conditions were evaluated through random effects analysis both in the whole-brain and in functionally defined ROIs. All reported results are significant at P < 0.05 and were corrected for multiple comparisons. Voxel-by-voxel pattern correlation analysis was used to compare the similarity of activations between the sighted and EB groups.
Supplementary Material
Acknowledgments.
We thank the research participants and the Boston blind community for making this research possible; Lucy Chen, Jonathan K. Scholz, and the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research for help with fMRI analyses and data collection; and Michael Frank, Laura Shultz, and the reviewers for comments on earlier drafts of the manuscript. This research study was supported in part by National Institutes of Health Grants R01 MH067008 and R01 DC006842 (to M.B. and A.P.L.) and K24 RR01887, R01 EY12091, and R21 EY0116168 (to A.P.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900010106/DCSupplemental.
References
- 1.Gallagher HL, et al. Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 2.Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- 3.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 4.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 5.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cognit Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- 7.Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G. Thinking of mental and other representations: The roles of left and right temporo-parietal junction. Soc Neurosci. 2006;1:245–258. doi: 10.1080/17470910600989896. [DOI] [PubMed] [Google Scholar]
- 8.Saxe R, Powell LJ. It's the thought that counts: Specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- 9.Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: Dissociating theory of mind and executive control in the brain. Soc Neurosci. 2006;1:284–298. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- 10.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Ann Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 12.Amedi A, Merabet L, Bermophl F, Pascual-Leone A. The occipital cortex in the blind: Lessons about plasticity and vision. Curr Directions Psychol Sci. 2005;16:306–311. [Google Scholar]
- 13.Baron-Cohen S. Mindblindness: An essay on Autism and Theory of Mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 14.Baron-Cohen S. Mindreading: Evidence for both innate and acquired factors. Anthropol Psychol. 2006;17:26–27. [Google Scholar]
- 15.Brooks R, Meltzoff AN. The importance of eyes: How infants interpret adult looking behavior. Dev Psychol. 2002;38:958–966. doi: 10.1037//0012-1649.38.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langton SR, Watt RJ, Bruce II. Do the eyes have it? Cues to the direction of social attention. Trends Cognit Sci. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- 17.Meltzoff AN. ‘Like me’: A foundation for social cognition. Dev Sci. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meltzoff AN. The ‘like me’ framework for recognizing and becoming an intentional agent. Acta Psychol (Amsterdam) 2007;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants' perception of others' actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau B, Gleitman LR. Language and Experience: Evidence from the Blind Child. Cambridge, MA: Harvard Univ Press; 1985. [Google Scholar]
- 21.Brown R, Hobson RP, Lee A, Stevenson J. Are there “autistic-like” features in congenitally blind children? J Child Psychol Psychiatry. 1997;38:693–703. doi: 10.1111/j.1469-7610.1997.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 22.Peterson CC, Peterson JL, Webb J. Factors influencing the development of a theory of mind in blind children. Br J Dev Psychol. 2000;18:431–447. [Google Scholar]
- 23.Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat Neurosci. 2004;7:1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- 24.Merabet L, et al. Feeling by sight or seeing by touch? Neuron. 2004;42:173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- 25.Sadato N, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 26.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: A comparison of theory of mind tasks. J Cognit Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 27.Saxe R, Whitfield-Gabrieli S, Scholz J, Pelphrey KA. Brain Regions for Perceiving and Reasoning about Other People in School-aged Children. Child Dev. 2009 doi: 10.1111/j.1467-8624.2009.01325.x. in press. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins AC, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc Natl Acad Sci USA. 2008;105:4507–4512. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perner J, Aichhorn M. Theory of mind, language and the temporoparietal junction mystery. Trends Cognit Sci. 2008;12:123–126. doi: 10.1016/j.tics.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Baron-Cohen S, Cross P. Reading the eyes: Evidence for the role of perception in the development of a Theory of Mind. Mind and Language. 1992;7:172–186. [Google Scholar]
- 32.Tomasello . In: Joint Attention: Its Origins and Role in Development. Moore C, Dunham PJ, editors. Hillsdale, NJ: Erlbaum; 1995. pp. 103–120. [Google Scholar]
- 33.Minter M, Hobson RP, Martin B. Congenital visual impairment and ‘theory of mind’. Br J Dev Psychol. 1998;16:183–196. [Google Scholar]
- 34.Hobson RP, Bishop M. The pathogenesis of autism: Insights from congenital blindness. Philos Trans R Soc London Ser B. 2003;358:335–344. doi: 10.1098/rstb.2002.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobson RP, Lee A, Brown R. Autism and congenital blindness. J Autism Dev Disord. 1999;29:45–56. doi: 10.1023/a:1025918616111. [DOI] [PubMed] [Google Scholar]
- 36.Roch-Levecq A. Production of basic emotions by children with congenital blindness: Evidence for the embodiment of theory of mind. Br J Dev Psychol. 2006;24:507–528. [Google Scholar]
- 37.Green S, Pring L, Swettenham J. An investigation of first-order false belief understanding of children with congenital profound visual impairment. Br J Dev Psychol. 2004;22:1–17. [Google Scholar]
- 38.Hughes C, et al. Origins of individual differences in theory of mind: From nature to nurture? Child Dev. 2005;76:356–370. doi: 10.1111/j.1467-8624.2005.00850.x. [DOI] [PubMed] [Google Scholar]
- 39.Peterson CC, Siegal M. Representing inner worlds: Theory of Mind in autistic, deaf, and normal hearing children. Psychol Sci. 1999;10:126–129. [Google Scholar]
- 40.Figueras-Costa B, Harris P. Theory of Mind development in deaf children: A nonverbal test of false-belief understanding. J Deaf Stud Deaf Educat. 2001;6:92–102. doi: 10.1093/deafed/6.2.92. [DOI] [PubMed] [Google Scholar]
- 41.de Villiers PA. In: Why Language Matters for Theory of Mind. Astington JW, Baird JA, editors. Oxford: Oxford Univ Press; 2005. pp. 266–297. [Google Scholar]
- 42.Moeller MP, Schick B. Relations between maternal input and theory of mind understanding in deaf children. Child Dev. 2006;77:751–766. doi: 10.1111/j.1467-8624.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 43.Saxe R. Why and how to study Theory of Mind with fMRI. Brain Res. 2006;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Batson CD. “I've been there, too”: Effect on empathy of prior experience with a need. Personality Soc Psychol Bull. 1996;22:474–482. [Google Scholar]
- 45.Yagmurlu B, Berument SK, Celimli S. The role of institution and home contexts in theory of mind development. Appl Dev Psychol. 2005;26:521–537. [Google Scholar]
- 46.Chugani HT, et al. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 47.Marshall PJ, Fox NA. A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cognit Neurosci. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- 48.Aichhorn M, et al. Temporo-parietal junction activity in Theory-of-Mind tasks: Falseness, beliefs, or attention. J Cognit Neurosci. 2009;21:1179–1192. doi: 10.1162/jocn.2009.21082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.