Abstract
Peutz-Jeghers syndrome (PJS) is a familial cancer disorder due to inherited loss of function mutations in the LKB1/ STK11 serine/threonine kinase. PJS patients develop gastrointestinal hamartomas with 100% penetrance often in the second decade of life, and demonstrate an increased predisposition toward the development of a number of additional malignancies. Among mitogenic signaling pathways, the mammalian-target of rapamycin complex 1 (mTORC1) pathway is hyperactivated in tissues and tumors derived from LKB1-deficient mice. Consistent with a central role for mTORC1 in these tumors, rapamycin as a single agent results in a dramatic suppression of preexisting GI polyps in LKB1+/− mice. However, the key targets of mTORC1 in LKB1-deficient tumors remain unknown. We demonstrate here that these polyps, and LKB1- and AMPK-deficient mouse embryonic fibroblasts, show dramatic up-regulation of the HIF-1α transcription factor and its downstream transcriptional targets in an rapamycin-suppressible manner. The HIF-1α targets hexokinase II and Glut1 are up-regulated in these polyps, and using FDG-PET, we demonstrate that LKB1+/− mice show increased glucose utilization in focal regions of their GI tract corresponding to these gastrointestinal hamartomas. Importantly, we demonstrate that polyps from human Peutz-Jeghers patients similarly exhibit up-regulated mTORC1 signaling, HIF-1α, and GLUT1 levels. Furthermore, like HIF-1α and its target genes, the FDG-PET signal in the GI tract of these mice is abolished by rapamycin treatment. These findings suggest a number of therapeutic modalities for the treatment and detection of hamartomas in PJS patients, and potential for the screening and treatment of the 30% of sporadic human lung cancers bearing LKB1 mutations.
Keywords: AMPK, FDG-PET, glycolysis, hamartoma, polyposis
Peutz-Jeghers syndrome (PJS) is an inherited autosomal dominant disorder in that patients develop benign hamartomatous polyps in the gastrointestinal tract and are predisposed to developing cancer (1). Inactivating mutations in the LKB1/ STK11 tumor suppressor gene underlie PJS and have also been associated with sporadic lung adeno- and squamous carcinomas (2–8). Homozygous deletion of Lkb1 is embryonic lethal to mice while heterozygous deletion of Lkb1 results in late onset gastrointestinal polyposis between 6–13 months of age that closely models human PJS (9–13). Gastrointestinal hamartomas are benign tumors that consist of hyperplastic glandular epithelial cells, disorganized tissue architecture, and a characteristic arborizing smooth muscle stalk. Several hamartomatous syndromes involve inactivating mutations in genes that negatively regulate the mTORC1 pathway, which promotes cell growth and proliferation. In addition to PJS, these diseases include Cowden's disease, tuberous sclerosis complex, and neurofibromatosis type I, due to inactivating mutation in the PTEN, TSC1, TSC2, or NF1 genes, respectively (14).
The mammalian target of rapamycin (mTOR) is a central regulator of cell growth in all eukaryotes that is found in 2 functionally distinct multiprotein complexes (15). The mTOR complex 1 (mTORC1) is composed of mTOR and its scaffolding protein raptor. Signaling from mTORC1 is nutrient-sensitive, acutely inhibited by the bacterial macrolide rapamycin, and controls protein translation, cell growth, angiogenesis, and metabolism. Activation of mTORC1 results in phosphorylation of a number of downstream targets involved in promoting cell growth and proliferation. These substrates include proteins involved in the regulation of protein translation such as the p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) (16). Among the mRNAs known to be translationally up-regulated by mTORC1 are a number of key progrowth proteins including cyclin D1, cyclin D3, Mcl-1, c-myc, and the hypoxia inducible factor 1 alpha (HIF-1α) (17–21).
Accordingly, mTORC1 is activated by mitogenic stimuli acting through the PI3K/Akt and Erk signaling pathways (15). In contrast, under conditions of low intracellular ATP such as after nutrient deprivation or other stresses, the LKB1 tumor suppressor activates the AMP-activated protein kinase (AMPK), which then rapidly inhibits mTORC1 although phosphorylation of both raptor (22) and the TSC2 tumor suppressor (23, 24). Hence, treatment with AMPK activating drugs, or overexpression of LKB1 or AMPK, results in suppression of mTORC1, whereas targeted deletion of LKB1 in mice leads to increased mTORC1 activity in murine fibroblasts, liver, and in polyps of LKB1-heterozygous mice (24–26).
Despite the common feature of elevated mTORC1 signaling in these hamartoma syndromes, the important targets of mTORC1 in LKB1-deficient tumors remain to be defined. We show here that HIF-1α and its transcriptional targets in glucose metabolism are up-regulated in LKB1-deficient tumors in mice and human Peutz-Jeghers patients. Increased Glut1 and Hexokinase II expression in these polyps of Lkb1+/− mice allows them to be visualized by FDG-PET. Because rapamycin strongly suppresses polyposis in the Lkb1+/− mice, mTORC1 inhibitors and FDG-PET imaging may be useful clinically in the treatment of PJS patients.
Results
Rapamycin Reduces Tumor Burden and Proliferation in Lkb1+/− Mice.
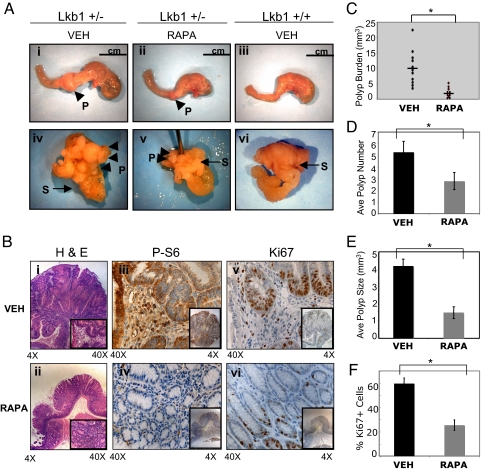
We investigated the effect of rapamycin on preexisting PJS-like polyps, by treating 9-month-old Lkb1+/− or Lkb1+/+ mice for a period of 2 months with rapamycin or vehicle (Fig. S1A). Our preliminary studies revealed that at 9 months of age, 100% of the Lkb1+/− mice have developed multiple gastrointestinal hamartomas, consistent with previous reports (10–13). Both Lkb1+/+ and Lkb1+/− mice tolerated rapamycin treatment with no obvious cytotoxicity or immunosuppression at the doses used. After 2 months of treatment, polyp size and number in each mouse were quantitated. The wild-type Lkb1+/+ mice were free of polyps, while all of the Lkb1+/− mice treated with vehicle presented severe polyp burden at or before 11–12 months of age, consistent with previous studies of these mice (10–13, 27). These mice had multiple large polyps in the stomach and pylorus and suffered from severe distention of the stomach and anemia (Fig. 1A i and iv). Histological analysis of H&E-stained polyps from untreated mice were classified as pedunculated, hyperplastic lesions consisting of differentiated glandular epithelium, stroma, and a smooth muscle stalk. In contrast, Lkb1+/− mice treated with rapamycin had a dramatic reduction in polyp burden. These mice uniformly had reduced polyp size (Fig. 1E) and lower frequency of polyps (Fig. 1D), no distention of the stomach (Fig. 1A ii and v) and appeared active and vigorous at 2 months of treatment. Comparison of the polyp burden between Lkb1+/− mice treated or untreated with rapamycin showed an 80% reduction in the overall mass of polyps (Fig. 1C). Polyps isolated from treated mice were greatly reduced in size, although still retained some disruption of the normal tissue architecture as shown by H&E staining (Fig. 1B ii).
Fig. 1.
Rapamycin reduces polyposis, mTORC1 signaling, and proliferation in Lkb1+/− polyps. (A) Top are images of whole stomach and duodenum and Bottom are images of the open stomachs (S) showing the exposed polyps (P) from Lkb1+/− mice treated with either vehicle (VEH) (i, iv) or rapamycin (RAPA) (ii, v) and Lkb1+/+ mice treated with vehicle (VEH) (iii, vi). (B) Immunohistochemical analysis of polyps from VEH- or RAPA-treated Lkb1+/− mice: H & E staining (i, ii), P-S6 staining (iii, iv), and Ki67 staining (v, vi). Results are representative of polyps from 5 mice of each treatment group. (C) A graph of the total polyp burden in Lkb1+/− mice treated with either VEH (○, n = 11) or RAPA (●, n = 10). The mean polyp burden for RAPA-treated mice (2.0 ± 1.2) was significantly reduced (∗, P = 0.00026; Student t test, 2 tail) compared with those mice treated with VEH (9.6 ± 5.5). (D) Average polyp number in VEH-treated mice (black bar, n = 11) or RAPA-treated mice (gray bar, n = 10). Only visible polyps between 1 and ≥5 mm were scored in both VEH- and RAPA-treated mice. The mean polyp number for RAPA-treated mice (2.8 ± 1.4) was significantly reduced (∗, P = 0.00022; Student t test, 2 tail) compared with VEH-treated mice (5.3 ± 1.8). (E) Average polyp size in VEH-treated mice (black bar, n = 11) or RAPA-treated mice (gray bar, n = 10). The mean polyp size in RAPA mice (1.2 ± 0.9) was significantly reduced (∗, P < 0.0001; Student t test, 2 tail) compared with VEH-treated mice (4.4 ± 0.8). (F) Average percentage of Ki67-positive epithelial cells in VEH-treated mice (black bar, n = 5) or RAP- treated mice (gray bar, n = 5). The mean percentage of Ki67-positive cells in RAPA mice (24.5 ± 6.1) was significantly reduced (∗, P < 0.0002; Student t test, 2 tail) compared with VEH-treated mice (59.7 ± 7.3).
We analyzed mTORC1 signaling in the polyps of Lkb1+/− mice to determine whether rapamycin was effectively inhibiting the pathway. Immunohistochemical staining of polyps for phospho-S6 (P-S6) revealed that untreated polyps displayed high levels of P-S6 staining indicative of hyperactive mTOR signaling, while the polyps from rapamycin treated mice were greatly reduced for P-S6 staining indicating successful inhibition of mTORC1 signaling (Fig. 1B iii and iv). These results were further corroborated by western blot analysis of polyp lysates (Fig. S1B).
Rapamycin has been shown to suppress tumor growth and induce apoptosis, resulting in cytostatic or cytotoxic responses in several genetically engineered mouse models with spontaneously arising tumors (15). We sought to examine the mechanism(s) by which rapamycin reduced polyp burden in the Lkb1+/− mice. We first analyzed expression of the proliferation marker Ki67 in rapamycin or vehicle-treated polyps. The highest expression of Ki67 was found in proliferating epithelial cells at the base of the crypts. While staining was extensive in the vehicle-treated polyps, rapamycin-treated polyps showed a clear reduction in Ki67 staining (Fig. 1 B v and vi and F). However, polyps from treated mice did not show evidence of apoptosis as detected by cleaved caspase-3 protein using immunohistochemistry or immunoblotting. These results suggest that in this tumor model rapamycin may be having a cytostatic effect rather than a cytotoxic effect, consistent with observations in other tumor types (15).
Rapamycin Down-Regulates Expression of HIF-1α and HIF-1α Targets.
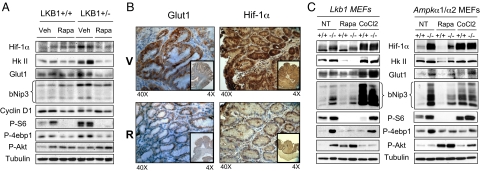
Activation of mTORC1 results in increased translation of a number of key downstream targets, including cyclin D1 and the hypoxia inducible factor 1 alpha gene (HIF-1α) (17–20). We examined the protein levels of cyclin D1 and HIF-1α in the stomachs and polyps of Lkb1+/+ and Lkb1+/− mice by western blot. HIF-1α but not cyclin D1 levels were elevated in polyps of vehicle-treated Lkb1+/− mice. In contrast, the polyps of mice treated with rapamycin showed reduced HIF-1α levels similar to the basal levels seen in Lkb1+/+ mice (Fig. 2A). Next, we examined protein expression of transcriptional targets of HIF-1α including Glut1, hexokinase II, and bNIP3, and observed that their expression was up in the polyps proportional to HIF-1α up-regulation and similarly, that the expression of these HIF-1α targets was suppressed by rapamycin (Fig. 2A). To confirm that HIF-1α and its targets were up-regulated within the epithelial cell population that are P-S6 and Ki67 positive, as opposed to originating from any stromal or infiltrating cells, we performed immunohistochemistry with anti- HIF-1α and Glut1 antibodies in vehicle- and rapamycin-treated LKB1+/− polyps. HIF-1α and Glut1 protein expression levels were much higher in epithelial cells in the untreated polyps and were diminished with rapamycin treatment (Fig. 2B).
Fig. 2.
Up-regulated HIF-1α and HIF-1α targets in LKB1-deficient polyps and fibroblasts are reduced by rapamycin. (A) Immunoblots of lysates made from GI tissue or polyps from Lkb1+/+ and Lkb1+/− mice treated VEH or RAPA. Immunoblots were probed against the indicated antibodies. (B) Immunohistochemical analysis of polyps from VEH- or RAPA-treated Lkb1+/− mice probed with antibodies against Glut1 or Hif-1α. Results are representative of polyps from 3 mice of each treatment group. (C) Immunoblots of lysates from Lkb1+/+ or Lkb1−/− MEFs (Left) or Ampk+/+ or Ampk−/− MEFs (Right) probed with antibodies against the indicated proteins. MEFs were either untreated (NT) or treated with RAPA or cobalt chloride (CoCl2).
To determine if increased HIF-1α levels were due to loss of the Lkb1 gene and not simply a consequence of hypoxia within the polyp microenvironment or a secondary mutation that arose during polyp formation, we first examined the functional levels of hypoxia present in the polyps and surrounding epithelium using hypoxyprobe-1 (28). No significant levels of hypoxia were observed in the polyps, in contrast to widespread HIF-1α elevation throughout the epithelial cells of the polyps (Fig. S2). To further extend this analysis in a controlled normoxic environment, and to rule out the potential impact of any secondary mutations that may have arisen in the polyps that might contribute to up-regulation of HIF-1α, we examined HIF-1α levels in primary non-immortalized wild-type and Lkb1-deficient MEFs grown in normoxic conditions. Expression of HIF-1α protein, and the HIF-1α targets hexokinase II and bNIP3, were dramatically increased in Lkb1−/− MEFs and all downregulated by rapamycin treatment (Fig. 2C). Because we have shown that AMPK is a key target of LKB1 that controls mTORC1 activity via its phosphorylation of TSC2 and raptor, we next examined whether HIF-1α and its targets were similarly up-regulated in immortalized MEFs lacking both catalytic isoforms of AMPK. Indeed, HIF-1α and its targets were up-regulated in Ampkα1/α2−/− fibroblasts compared with wild-type cells and treatment with rapamycin reduced their expression, paralleling suppression of 4ebp1 and S6K phosphorylation (Fig. 2C).
Lkb1+/− Polyps Exhibit Dramatic Increases in Glucose Metabolism.
One of the earliest defined biochemical hallmarks of tumor cells is the propensity to rely on glycolysis for ATP production, even when oxygen is not limiting, unlike their normal counterparts. This conversion from oxidative phosphorylation to glycolysis that accompanies tumorigenesis was termed the Warburg Effect after its discoverer Otto Warburg (for review, see ref. 29). In the past decade, interest in the Warburg effect has been renewed in part due to the increased use of 18F-fluoro-deoxyglucose (FDG)-positron emission tomography (PET) in human cancer patients to detect tumors due to their higher rates of glucose utilization (30). The molecular underpinning for increased FDG-PET has been hypothesized to involve increased levels of cell surface glucose transporters including GLUT1, as well the enzyme for the first committed step of glycolysis, hexokinase II (31). Because immunoblotting had revealed increased expression of both GLUT1 and hexokinase II in the polyps of LKB1+/− mice, we were interested in whether these tumors could be visualized by FDG-PET.
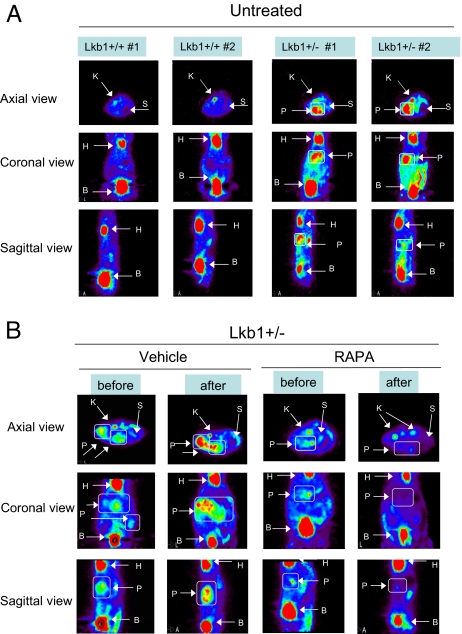
We analyzed 11-month-old Lkb1+/+ and Lkb1+/− mice by FDG PET to scan for the presence of GI polyps. In addition to the excretion to the bladder, we observed the expected uptake of FDG in the heart and kidney of all mice regardless of genotype. However, FDG PET images showed increased FDG in focal masses located in the Lkb1+/− mice in their midline below the heart where the stomach and pylorus are located, whereas the Lkb1+/+ were negative (P = 0.06) for FDG signal in this area (Fig. 3A). Several of the Lkb1+/− mice were killed after imaging, and it was confirmed that these animals had large polyps in the pylorus and stomach corresponding exactly to the regions of greatest FDG uptake. Treatment of animals with rapamycin for 4 weeks abolished the FDG-PET signal. Immediate autopsy of the animals imaged by FDG-PET revealed that the rapamycin-treated mice had minimal detectable GI polyps while the vehicle-treated mice all exhibited the presence of large GI polyps (Fig. 3B). These results demonstrate that FDG-PET analysis is a viable method by which to detect polyps in Lkb1+/− animals and confirms that rapamycin reverses polyp growth in Lkb1+/− mice. All experimental procedures in mice were approved by the Salk Institute and University of California at San Diego Institutional Animal Care and Use Committees.
Fig. 3.
Polyps from Lkb1+/− mice visualized by FDG PET analysis. (A) Left shows FDG PET images of axial, sagital and coronal views of untreated 12-month-old Lkb1+/+ mice. Right shows the same views of untreated Lkb1+/− mice. The FDG PET images of the mice are labeled accordingly: K, kidney; S, stomach; H, heart; B, bladder; and P, polyp. (B) Left shows FDG PET imaging of axial, sagittal, and coronal views of Lkb1+/+ and Lkb1+/− mice either treated with vehicle or rapamycin at 11 months of age. Right shows the same mice imaged after 1 month of receiving either vehicle or rapamycin. The images of the mice are labeled accordingly: K, kidney; S, stomach; H, heart; B, bladder; and P, polyp.
mTORC1 and HIF-1α Signaling Increased in Human PJS Polyps.
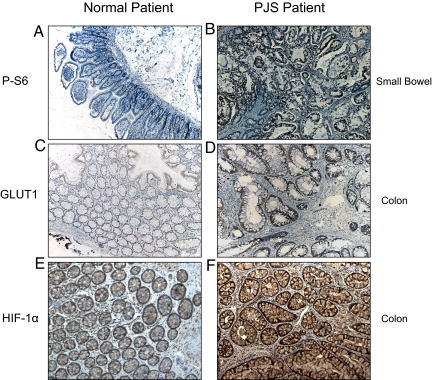
Finally, we examined whether the increased mTORC1 and HIF-1α dependent signaling we observed in the LKB1+/− murine model are relevant to human Peutz-Jeghers patients. mTORC1 signaling, HIF-1α protein, and GLUT1 protein expression were analyzed by immunohistochemistry in small bowel and colon samples from PJS patients and compared with samples of small bowel and colonic mucosa from normal patient controls. Expression of the mTOR target P-S6 was increased in the epithelium of small bowel of PJS patients compared with that of normal tissue (Fig. 4 A and B). Likewise, strong immunostaining of HIF-1α and GLUT1 was observed in glandular epithelial cells in 7 of 8 PJP colonic polyp specimens (Fig. 4 D and F). In the normal colonic mucosa specimens, weak immunohistochemical staining of HIF-1α and Glut1 was observed relative to the highly elevated levels observed in the PJS samples (Fig. 4 C and E). These results suggest that loss of the LKB1 gene leads to both mTORC1 hyperactivation and increased HIF-1α and GLUT1 expression in PJS patients in a manner that closely follows the murine model of PJS.
Fig. 4.
Polyps from human Peutz-Jeghers patients show increased P-S6, GLUT1, and HIF-1α expression. A and B represent immunohistochemistry performed on human small bowel samples from normal patients (Left) or Peutz Jeghers patients (Right) that were probed with antibodies against the mTORC1 marker P-S6. C–F represent immunohistochemistry performed on normal colonic mucosa (Left) and colonic Peutz-Jeghers polyps (Right) probed with antibodies against the GLUT1 protein (C and D) or the HIF-1α protein (E and F).
Discussion
Aberrant activation of the mTORC1 pathway has been observed in spontaneously arising tumors in mice genetically engineered for loss of the tumor suppressors Pten, Nf1, Tsc2, or Lkb1 (32–38). Mutations in these genes are responsible for the inherited cancer syndromes Cowden's disease, neurofibromatosis type I, tuberous sclerosis complex, and Peutz-Jeghers syndrome; collectively referred to as Phakomatoses, and all sharing overlapping clinical features including the development of hamartomas. Biochemical and cell biological studies from the past decade have revealed that these tumor suppressors all are direct components of the mTOR signaling pathway that serve to inhibit mTORC1 activity (15).
The underlying hypothesis is that mutational inactivation of these tumor suppressors in individual cells leads to cell-autonomous hyperactivation of mTORC1, promoting cell growth and ultimately resulting in tumors that are subsequently reliant on mTORC1 signaling for tumor maintenance. Consistent with this possibility, rapamycin analogs have been examined for their therapeutic efficacy in the suppression of tumors that arise in a number of the aforementioned mouse models. The Pten+/−, Nf1+/−, Tsc+/−, Lkb1+/−, and activated Akt transgenic mouse models have also proven to be responsive to the mTOR inhibitors rapamycin or rapamycin analogs RAD001 (Novartis), CCI0779 (Wyeth) and AP23573 (Ariad) (34, 39–43). These drugs have been proven to effectively inhibit mTORC1 in vivo and reduce tumor burden through mTORC1 dependent mechanisms, including suppression of cyclin D, Mcl-1, or HIF-1α and its targets (35, 39–41).
In recent clinical trials, rapamycin and its analog temsirolimus were shown to have palliative success in clinical trials on patients with PTEN-deficient glioblastomas and metastatic renal cell carcinoma (44, 45). Furthermore, in a pair of phase II clinical trials involving tuberous sclerosis (TSC) and lymphangioleiomyomatosis (LAM) patients, partial responses to the rapamycin analog Sirolimus were observed, including regression of angiomyoliomas with continuous therapy (46, 47), consistent with previous clinical observations in TSC patients given rapamycin (48, 49). Combined with data from mouse models, these clinical data suggest that hamartoma syndromes with hyperactivation of mTORC1 may be particularly responsive to rapamycin analogs as a single agent. To date there are no therapies to treat PJS and the only course of treatment is resection of arising gastrointestinal hamartomatous polyps. Consistent with a previous report (34), we found here that rapamycin greatly reduced the polyp burden in the Lkb1+/− mouse model of PJS. This suppression was correlated with inhibition of mTORC1 and downregulation of HIF-1α and its transcriptional targets. While these results are encouraging for the use of rapamycin analogs as therapeutics for PJS, like the recent phase II clinical trial findings with TSC patients, removal of the drug may result in rapid return of the initial tumor due to the largely cytostatic nature of the response (46). Perhaps new, targeted inhibitors directed at the kinase domain of mTOR will produce greater therapeutic response with targeted cytotoxicity, or perhaps kinase inhibitors that dually inactivate mTOR and PI3K, because PI3K provides a survival signal in most epithelial cell types. As observed in most cancers studied to date, combinations of targeted therapeutics, or of targeted and traditional chemo-therapeutics may find the ultimate utility in the treatment of this disease. Importantly, it is worth noting here that rapamycin treatment may not only be therapeutically useful for the hamartomas that arise in Peutz-Jeghers patients, but also in preventing and reducing any secondary malignancies that arise in these patients at additional sites (breast, pancreas, and ovary).
This study also finds the transcription factor HIF-1α as a relevant target of mTORC1 in LKB1-dependent hamartomas, and the up-regulation of HIF-1α targets Glut1 and hexokinase II may be responsible for the ability of these tumors to be visualized by FDG-PET, because both Glut1 and hexokinase II have been reported as rate-limiting steps for FDG uptake and imaging (31). HIF-1α has been shown to be an excellent correlate of rapamycin response in a transgenic model of prostate neoplasia dependent on activated Akt, and in VHL-deficient renal cell carcinoma xenografts (20, 39). Consistent with the findings here in spontaneously arising hamartomas in LKB1+/− mice, recent studies using human glioblastoma xenografts and transplanted murine breast carcinomas also found rapamycin-sensitivity of FDG-PET imaging of these tumors (50, 51). Data from LKB1 and AMPK-deficient MEFs demonstrate that HIF-1α and HIF-1α targets are dramatically up-regulated in these cells under normoxic conditions, indicating that HIF-1α up-regulation is not a secondary consequence of other tumor mutations or hypoxia present within the hamartomas. These findings also suggest that AMPK may be a key effector of LKB1 in the suppression of HIF-1α in the normal gastrointestinal epithelium that when disrupted gives rise to hamartomas. Interestingly, the increase in HIF-1α was observed in the deficient fibroblasts under conditions of increased cell density when basal AMPK activity is high in the wild-type cells, perhaps due to glucose depletion of the media. In cells that genetically lack the ability to activate AMPK, HIF-1α is up-regulated under these conditions.
Consistent with these findings, hypoxia-independent up-regulation of HIF-1α has been observed in murine embryonic fibroblasts deficient in TSC2 or TSC1 (52, 53). Furthermore, evidence from a number of laboratories in the past decade indicates that mTORC1 signaling stimulates increases in HIF-1α levels independent of VHL and hypoxia-driven stabilization, but instead dependent on increased translation of HIF-1α due to a functional 5′ TOP element in its 5′ UTR (20, 39, 52, 54–58). Indeed, fusion of the 5′ UTR of HIF-1α to multiple reporter constructs confers rapamycin-dependent accumulation of the fused cDNA (17, 20, 59).
It will be critical for future studies to define whether the distinct downstream targets of mTORC1 are key for tumorigenesis in different cell types. Preliminary evidence suggests that different effectors may be important, because in a mouse model of Neurofibromatosis type 1, rapamycin dramatically suppressed tumors, although no effects were observed on HIF-1α or on vascularization; instead, cyclin D appears to be the critical target of mTORC1 in this setting. Notably, no change in cyclin D1 was observed in LKB1+/− hamartomas, consistent with recent reports on tissue variability of the reliance of cyclin D on mTORC1 (18). Interestingly however, we did observe increases in cyclinD1 in LKB1- and AMPK-deficient fibroblasts paralleling increases in HIF-1α, suggesting that different cell types exhibit distinct effectors downstream of mTORC1.
Although most often used in the detection of malignant tumors, our findings suggest that FDG-PET may find clinical utility for the identification of polyps in PJS patients. Moreover, FDG-PET may be useful for monitoring the efficacy of treatment or surgical resection of these polyps. It will also be very interesting to determine whether secondary cancers that arise in PJS patients at other sites (breast, pancreas, and endometrium) can also be visualized by FDG-PET, and whether rapamycin analogs or mTOR kinase inhibitors will demonstrate clinical efficacy in the treatment of those tumors. Finally, given the prevalence of somatic LKB1 mutations in sporadic non-small cell lung carcinomas, it will be of great interest to examine if the presence of LKB1 mutations in NSCLC will correlate with the propensity of lung tumors to be imaged with FDG-PET, or their response to mTOR-targeted therapeutics.
Materials and Methods
For immunoblotting, anti-phospho-S6K1 (T389), phospho-ribosomal protein S6 (S235/236), elF4E, phospho-4ebp1 (Ser-65), hexokinase II (C64G5), phospho-Akt (Thr-308), and bNIP3 antibodies were obtained from Cell Signaling Technology. Antibodies against HIF-1α (C-term) polyclonal antibody (Cayman Chemicals), Glut1 (GT11-A Rabbit polyclonal antibody; Alpha Diagnostics Int.), Cyclin D1 (BD PharMingen) and tubulin (Sigma Chemicals) were also used. Rapamycin was obtained from LC Laboratories. A detailed description of all of the other methods appears in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Lauri Aaltonen and Sini Marttinen (Biomedicum Helsinki, Helsinki)for the Finnish Peutz-Jeghers patient samples, Keith Laderoute (SRI International, Menlo Park, CA) and Benoit Viollet (Institut Cochin, Paris) for their generous donation of the isogenic SV40-immortalized wild-type and AMPKa1/a2 double deficient MEFs, and Dr. Carl Ho (University of California San Diego, La Jolla, CA) for his assistance in analyzing the FDG-PET data. We thank Katja Lamia for critical reading of the manuscript. This work was supported in part from grants from the National Institutes of Health (P01 CA120964 to R.J.S. and C.-L.W.), National Cancer Institute (P50 CA128346 to D.R.V.), American Cancer Society (R.J.S.), and V Foundation for Cancer Research (R.J.S). D.B.S. was supported by training grant T32 CA009370 to the Salk Institute Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900465106/DCSupplemental.
References
- 1.Hemminki A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell Mol Life Sci. 1999;55:735–750. doi: 10.1007/s000180050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Cespedes M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 3.Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23:4037–4040. doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- 4.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 5.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 6.Jenne DE, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 7.Aretz S, et al. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat. 2005;26:513–519. doi: 10.1002/humu.20253. [DOI] [PubMed] [Google Scholar]
- 8.Hearle NC, et al. Exonic STK11 deletions are not a rare cause of Peutz-Jeghers syndrome. J Med Genet. 2006;43:e15. doi: 10.1136/jmg.2005.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ylikorkala A, et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001;293:1323–1326. doi: 10.1126/science.1062074. [DOI] [PubMed] [Google Scholar]
- 10.Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi H, et al. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 2002;62:2261–2266. [PubMed] [Google Scholar]
- 12.Jishage K, et al. Role of Lkb1, the causative gene of Peutz-Jegher's syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci USA. 2002;99:8903–8908. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi DJ, et al. Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis. Proc Natl Acad Sci USA. 2002;99:12327–12332. doi: 10.1073/pnas.192301399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Fingar DC, et al. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Averous J, Fonseca BD, Proud CG. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2008;27:1106–1113. doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- 19.Galmozzi E, et al. HER2 signaling enhances 5′UTR-mediated translation of c-Myc mRNA. J Cell Physiol. 2004;200:82–88. doi: 10.1002/jcp.20012. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GV, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 21.Mills JR, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci USA. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 24.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: Evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katajisto P, et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet. 2008;40:455–459. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- 28.Raleigh JA, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- 29.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Garber K. Energy deregulation: Licensing tumors to grow. Science. 2006;312:1158–1159. doi: 10.1126/science.312.5777.1158. [DOI] [PubMed] [Google Scholar]
- 31.Smith TA. The rate-limiting step for tumor [18F]fluoro-2-deoxy-D-glucose (FDG) incorporation. Nucl Med Biol. 2001;28:1–4. doi: 10.1016/s0969-8051(00)00177-3. [DOI] [PubMed] [Google Scholar]
- 32.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 33.Johannessen CM, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei C, et al. Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res. 2008;14:1167–1171. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- 35.Wendel HG, et al. Determinants of sensitivity and resistance to rapamycin-chemotherapy drug combinations in vivo. Cancer Res. 2006;66:7639–7646. doi: 10.1158/0008-5472.CAN-06-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onda H, et al. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci. 2002;21:561–574. doi: 10.1006/mcne.2002.1184. [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy S, et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumder PK, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 40.Wendel HG, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 41.Johannessen CM, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 42.Podsypanina K, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee L, Sudentas P, Dabora SL. Combination of a rapamycin analog (CCI-779) and interferon-gamma is more effective than single agents in treating a mouse model of tuberous sclerosis complex. Genes Chromosomes Cancer. 2006;45:933–944. doi: 10.1002/gcc.20357. [DOI] [PubMed] [Google Scholar]
- 44.Cloughesy TF, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 46.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies DM, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–203. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 48.Herry I, Neukirch C, Debray MP, Mignon F, Crestani B. Dramatic effect of sirolimus on renal angiomyolipomas in a patient with tuberous sclerosis complex. Eur J Intern Med. 2007;18:76–77. doi: 10.1016/j.ejim.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Franz DN, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 50.Wei LH, et al. Changes in tumor metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res. 2008;14:3416–3426. doi: 10.1158/1078-0432.CCR-07-1824. [DOI] [PubMed] [Google Scholar]
- 51.Namba R, et al. Rapamycin inhibits growth of premalignant and malignant mammary lesions in a mouse model of ductal carcinoma in situ. Clin Cancer Res. 2006;12:2613–2621. doi: 10.1158/1078-0432.CCR-05-2170. [DOI] [PubMed] [Google Scholar]
- 52.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 53.Laderoute KR, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zundel W, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 56.Elstrom RL, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 57.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dekanty A, Lavista-Llanos S, Irisarri M, Oldham S, Wappner P. The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J Cell Sci. 2005;118:5431–5441. doi: 10.1242/jcs.02648. [DOI] [PubMed] [Google Scholar]
- 59.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.