Abstract
Alternative RNA splicing may provide unique opportunities to identify drug targets and therapeutics. We identified an alternative spliced transcript for B-type natriuretic peptide (BNP) resulting from intronic retention. This transcript is present in failing human hearts and is reduced following mechanical unloading. The intron-retained transcript would generate a unique 34 amino acid (aa) carboxyl terminus while maintaining the remaining structure of native BNP. We generated antisera to this carboxyl terminus and identified immunoreactivity in failing human heart tissue. The alternatively spliced peptide (ASBNP) was synthesized and unlike BNP, failed to stimulate cGMP in vascular cells or vasorelax preconstricted arterial rings. This suggests that ASBNP may lack the dose-limiting effects of recombinant BNP. Given structural considerations, a carboxyl-terminal truncated form of ASBNP was generated (ASBNP.1) and was determined to retain the ability of BNP to stimulate cGMP in canine glomerular isolates and cultured human mesangial cells but lacked similar effects in vascular cells. In a canine-pacing model of heart failure, systemic infusion of ASBNP.1 did not alter mean arterial pressure but increased the glomerular filtration rate (GFR), suppressed plasma renin and angiotensin, while inducing natriuresis and diuresis. Consistent with its distinct in vivo effects, the activity of ASBNP.1 may not be explained through binding and activation of NPR-A or NPR-B. Thus, the biodesigner peptide ASBNP.1 enhances GFR associated with heart failure while lacking the vasoactive properties of BNP. These findings demonstrate that peptides with unique properties may be designed based on products of alternatively splicing.
Keywords: vasoactive, myocardial, kidney
Genome-wide analyses have revealed the prevalence of alternative splicing of multiexonic genes (1, 2). In fact, much of the complexity of the human proteome is accounted for by alternative splicing of messenger RNA. Identification of these altered forms may allow for unique opportunities to diagnose, understand, and treat human disease. Therefore, we hypothesized that it might be possible to identify splice variants and to design therapeutics based on their unique structure and function.
As an example of the wide potential of this technology to alter disease states, we focused on a peptide with broad mechanistic, diagnostic, and therapeutic importance in cardiovascular disease, B-type natriuretic peptide (BNP). BNP is encoded by a small multiexonic gene, and although discovered in brain (3), is expressed primarily in the heart (4). BNP, like atrial natriuretic peptide and C-type natriuretic peptide, is expressed as a prepro-hormone that is processed to a mature [32-amino acid (aa)] form by extracellular proteases (5). Mature BNP contains short carboxyl and amino termini and a central 17-aa ring. BNP has important autocrine, paracrine, and endocrine actions that are mediated through the NPRA receptor and activation of cGMP in target cells (6). Infusion of a recombinant form of mature BNP (nesiritide) has been used clinically in heart failure for its vasodilatory properties (7), but its use has been limited by hypotension and concerns regarding worsening of renal function (8).
We identified an alternatively spliced transcript of B-type natriuretic peptide resulting from intron 2 retention in failing left ventricular tissue. We further used the unique sequence of the deduced peptide to design a peptide with unique renal protective actions without attendant hypotension.
Results
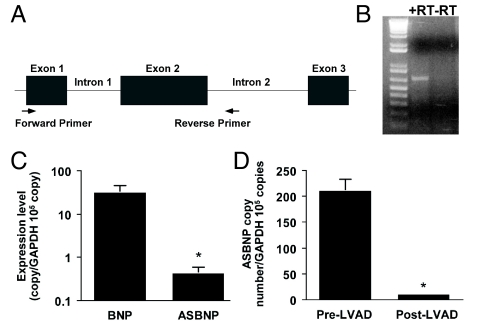
A BLAST search identified 2 ESTs (accession numbers BQ130258 and CN267260), consistent with intron 2 retention of the BNP transcript that would result in a unique carboxyl terminus while maintaining the amino terminus and ring structure of mature BNP (Fig. 1). Using primers specific for this transcript, we identified polyadenylated mRNA containing the first and second exons and second intron (lacking the first intron of the BNP gene) in failing human heart tissue (Fig. 2 A and B). Sequencing of the PCR product confirmed the identity of this transcript. Using left ventricular tissue obtained pre- and post-LVAD placement, we demonstrated that the level of expression of the alternatively spliced transcript is 2 log-fold less than the wild-type transcript pre-LVAD (Fig. 2C). Additionally, expression of the alternatively spliced form was decreased with the mechanical unloading associated with LVAD placement (Fig. 2D).
Fig. 1.
Amino acid sequence of mature ANP, BNP, CNP, DNP, ASBNP, and ASBNP.1. Residues resulting from alternative splicing are highlighted in bold.
Fig. 2.
ASBNP mRNA is present in failing human hearts. (A) Schematic of genetic structure of BNP gene and primer localization for RT-PCR. (B) PCR product ASBNP with and without reverse transcriptase (RT). (C) Comparison of BNP and ASBNP transcripts in failing left ventricles. (D) Comparison of ASBNP transcripts pre- and post-LVAD placement. *, P < 0.05.
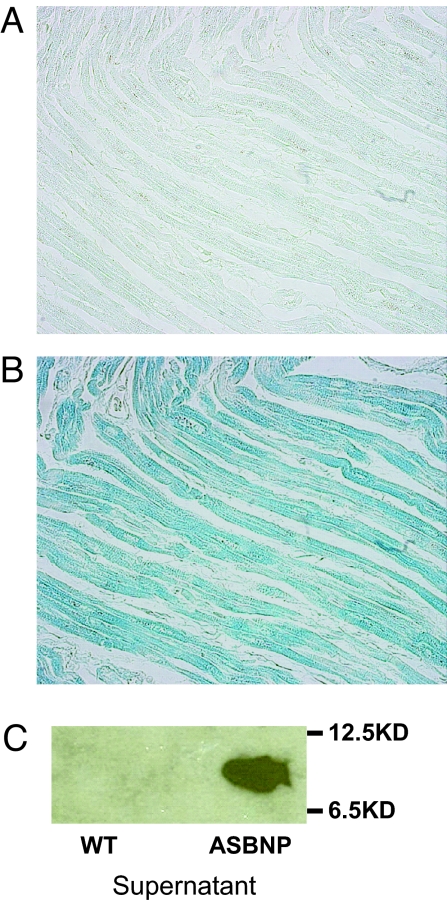
To determine whether this alternatively spliced transcript resulted in expression of the proposed peptide (referred to as ASBNP), we generated a rabbit polyclonal antiserum to the unique carboxyl terminus of the proposed peptide sequences from the alternatively spliced form. This antiserum specifically detected recombinant ASBNP expressed from baculovirus in Sf9 cells compared with uninfected cell supernatants (Fig. 3) as well as ASBNP but not BNP produced by synthesis. Most importantly, we identified immunoreactivity for ASBNP from cardiomyocytes in failing human hearts (Fig. 3).
Fig. 3.
ASBNP carboxyterminus is present in failing human hearts. (A) Section of human left atrium stained with rabbit antiserum generated to peptide encoded by intron 2 (specific for ASBNP) or (B) section stained with preimmune serum. (C) Immunoblot of recombinant full-length peptide (ASBNP) or control stained with specific rabbit antisera.
Since BNP is known to activate cGMP in an NPRA-dependent manner in endothelium, we compared the ability of ASBNP (generated synthetically) to that of BNP in vitro. ASBNP (unlike BNP) lacked the ability to stimulate cGMP in HUVECs (Fig. 4A). Furthermore, we also demonstrated that ASBNP lacked the ability of BNP to vasorelax preconstricted arterial rings (Fig. 4B). Thus, in these in vitro studies, ASBNP lacked the vascular effects of BNP. The potential reasons for the lack of activity in vascular cells and arterial rings would include the length or sequence of the carboxyl terminus which might interfere with receptor binding or activation.
Fig. 4.
ASBNP lacks vasoactive potency of BNP. (A) ASBNP fails to stimulate cGMP in HUVECs compared with BNP. (B) ASBNP lacks potency of BNP to vasorelax preconstricted rabbit carotid arterial rings. Responses were significantly different (P < 0.05) at concentrations > 10−10 M.
As our interest has been focused on developing therapeutic peptides, consideration was given to the length of ASBNP (60 aa) that might limit the ability for large-scale synthesis and the presence of 3 cysteines that might lead to dimerization and risk of aberrant ring formation. Based on these structural considerations, we designed a carboxyl-terminal truncated form of ASBNP referred to as ASBNP.1 (Fig. 1). In vitro, compared with BNP, ASBNP.1 like ASBNP fails to stimulate cGMP in human endothelial or vascular smooth muscle cells at equimolar doses or relax preconstricted human arterial rings (Fig. 5 A–C). However, in freshly isolated canine glomeruli, ASBNP.1 had similar ability to stimulate cGMP at equimolar doses to that of BNP (Fig. 5D). Studies in primary human kidney mesangial cells confirm that ASBNP.1 retained the ability to stimulate cGMP to a similar degree as BNP (Fig. 5E). Additional studies in mesangial cells demonstrate that the cGMP response to ASBNP.1 is inhibited by HS-142-1 (Tokyo Research Laboratories, Kyowa Hakko Kogyo Co.), suggesting that the effect is mediated through a particulate guanylyl cyclase linked receptor (Fig. 5F). These data suggest that ASBNP.1 retained the effects of BNP on renal cells but lacked the effects on vascular cells and intact vascular rings.
Fig. 5.
The biodesigner peptide ASBNP.1 retains renal effects but lacks vascular effects of BNP in vitro. (A) ASBNP.1 differs from BNP in its inability to stimulate cGMP in endothelial cells and (B) vascular smooth muscle cells and (C) in its inability to dilate preconstricted human left internal mammary arteries. (D) ASNP.1 retains the ability to stimulate cGMP in canine glomerular isolates (*, P < 0.001 vs. control; †P < 0.0001 vs. control, BNP vs. ASBNP.1 not significant) and (E) in primary human mesangial cells. (F) The cGMP effect in mesangial cells was inhibited by HS-142–1.
To determine whether ASBNP.1 activates NPR-A or NPR-B, in vitro studies were performed using HEK cells that stably express human NPR-A or human NPR-B (9) (Fig. 6 A and B). For NPR-A, the EC50 for BNP was 33.5 nM, and for ASBNP.1, the EC50 was 3.86 μM. For NPR-B, the EC50 for CNP was 48.4 nM, and for ASBNP.1, the EC50 was >10 μM. Since NPR-C does not activate cGMP, formal binding studies were performed. The IC50 for BNP was 4.52 nM and was 38.8 nM for ASBNP.1. These data suggest that ASBNP.1 is a poor activator of NPR-A and has reduced binding to NPR-C compared with BNP. These data hint at a receptor or receptor subtype in the kidney to which ASBNP.1 is a ligand.
Fig. 6.
ASBNP.1 is a poor activator of human natriuretic peptide receptors. (A) ASBNP is a poor activator of HEK293 cells stably expressing human NPR-A (P value = 0.0007). (B) ASBNP is a poor activator of human NPR-B stably expressed in HEK293 cells (BNP vs. ASBNP.1 not significant).
Based on the distinct in vitro properties of ASBNP.1 including the ability to stimulate cGMP in isolated canine glomeruli and in cultured human mesangial cells without similar effects on vascular cells, we hypothesized that ASBNP.1 might have therapeutic properties in a disease state such as heart failure independent of vasoactivity in vivo. A dose escalation study (2, 10, and 100 pmol/kg/min) of i.v. infusion of ASBNP.1 in a canine rapid-pacing model of overt heart failure (10) demonstrated increased aquaresis (from 0.19 ± 0.04 to 0.32 ± 0.07, 0.46 ± 0.11, and 0.40 ± 0.09 mL/min, P < 0.05) with a trend for urinary sodium excretion to increase (Table S1). Importantly, ASBNP.1 enhanced the glomerular filtration rate (GFR) (from 31 ± 4 to 47 ± 8, 69 ± 10, and 57 ± 9 mL/min, P < 0.05). These renal actions were associated with increases in urinary BNP, ANP, and cGMP (P < 0.05) excretion. ASBNP.1 did not have any systemic or renal vasodilatory action demonstrated by a lack of change in mean arterial blood pressure, renal blood flow, or cardiac-filling pressures even at the highest dose.
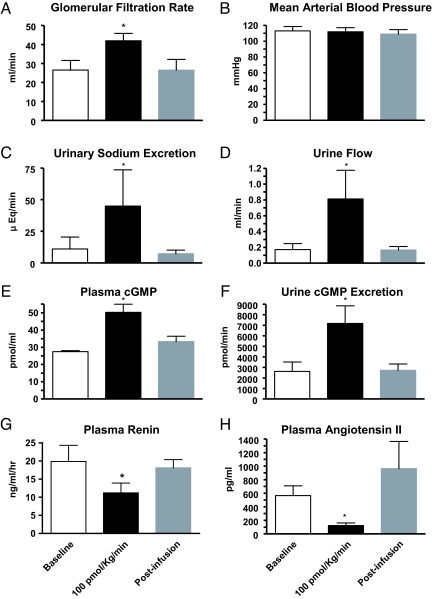
These data led to a further study of longer infusion at the highest dose (n = 5). A sustained IV infusion of ASBNP.1 at 100 pmol/kg/min had no effect on mean arterial pressure (Fig. 7). Importantly, ASBNP.1 markedly enhanced GFR while inducing natriuresis and diuresis. These specific renal actions were associated with increases in plasma and urinary cGMP excretion. ASBNP.1 also significantly suppressed both plasma renin and angiotensin II during the infusion. No change in renal blood flow was noted at any time during the study. Thus, in vitro and in vivo we demonstrated that ASBNP.1 has renal-enhancing actions while lacking any dose-limiting hypotensive effects. Taken together, ASBNP.1 has significant potential as a unique and distinct renal protective and GFR-enhancing therapeutic.
Fig. 7.
The biodesigner peptide ASBNP.1 retains renal effects but lacks vascular effects of BNP in a canine model of congestive heart failure. Infusion of i.v. ASBNP.1 increases GFR (A), urinary sodium excretion (C), urine flow (D), without affecting mean arterial blood pressure (B). Plasma (E) and urine (F) cGMP were increased whereas plasma renin (G) and angiotensin II (H) were decreased. Open bars, baseline; black bars, infusion of 100 pmol/kg/min; gray bars, postinfusion period. *, P < 0.05.
Discussion
The complexity of the human proteome provides a diverse array of peptides that might be used as therapeutic targets or biologic therapeutics. As alternative splicing usually affects coding regions (11), altered functions of resulting proteins would be predicted. We identified an alternatively spliced transcript of BNP resulting from retention of intron 2 in failing human left ventricular tissue. This alternatively spliced transcript is expressed at levels 2 log-fold less than BNP and is down regulated in the setting of ventricular unloading. As BNP expression is up-regulated in heart failure, it is not surprising that alternative forms might exist in this setting where regulated and stochastic mechanisms might exist (12). We further identified protein expression of the unique carboxyl terminus from cardiac myocytes in humans using a specific antiserum, which supports that the alternative transcript is translated in humans. Determining the extent and degree of expression in health and disease will require further study.
The deduced peptide ASBNP was synthesized and shown to lack equipotency of BNP in stimulating cGMP in vascular cells and lacked the ability to vasorelax preconstricted arterial rings. Based on structural considerations regarding the alternative transcript, we then generated a carboxyl terminus-deleted form of the peptide (ASBNP.1) that also lacked vascular effects but importantly possessed renal properties. ASBNP.1 stimulated cGMP in a dose-dependent manner in isolated canine glomeruli and human mesangial cells, although these effects required higher concentrations of either BNP or ASBNP.1. The cGMP effects in mesangial cells were inhibited in a dose-dependent manner by HS-142-1, an inhibitor of particulate guanylyl cyclase receptors. Interestingly, ASBNP.1 is a poor activator of NPR-A and does not activate NPR-B in cells which specifically express human forms. Additionally, ASBNP.1 binds NPR-C less avidly than BNP. These data suggest a mechanism for the effects of ASBNP.1 including through an undefined particulate guanylyl cyclase linked receptor (13). The functional effects of ASBNP.1 should provide the opportunity to define this mechanism.
Furthermore, in vivo studies in experimental heart failure corroborated these effects. ASBNP.1 has distinct renal effects but lacked the dose-limiting hypotensive effects of BNP. As such, ASBNP.1 may have therapeutic potential in patients with the cardiorenal syndrome in whom the GFR enhancement might be achieved without risk of hypotension. These effects would be distinct from BNP and are of great interest given the contraindication of nesiritide in hypotensive patients. In future studies, it would be of interest to determine whether the renal actions of ASBNP.1 may be potentiated by inhibition of neutral endopeptidase which degrades the endogenous natriuretic peptides as it is highly expressed in the kidney.
While the precise mechanism for selective renal actions of ASBNP.1 will require further study, this designer peptide represents a natriuretic peptide analogue totally devoid of systemic hemodynamic actions but with GFR-enhancing properties. Such a biological profile is highly attractive in human disease syndromes of reduced GFR in which most GFR-enhancing therapeutic interventions are associated with reductions in arterial pressure and compromised renal perfusion. Thus, alternatively spliced transcripts may not only provide drug targets and mechanisms of disease but may also provide opportunities to design therapeutics with unique features.
Methods
Cell Culture.
Human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection and cultured in endothelial growth medium (EGM) (Cambrex) containing FBS, bovine brain extract, human epidermal growth factor, hydrocortisone, gentamicin, and amphotericin B at 37 °C in humidified 5% CO2 in air. Primary human glomerular mesangial cells (Cell Systems Inc.) were grown in endothelial growth media. Cells from passages 2–5 were used in this study. Sf9 cells (BD Biosciences Pharmingen) were cultured in Hank's TNM-FH medium supplemented with 10% FBS (Mediatech).
RNA Isolation and Reverse Transcription.
Tissue from explanted failing human hearts was obtained using a human protocol approved by the Institutional Review Boards of the Mayo Foundation and the University of Minnesota. Total RNA was prepared by homogenization of heart tissues using TRIzol (Invitrogen) method and reverse transcription was performed using standard techniques and reagents. (RT-PCR kit, Invitrogen).
Generation of ASBNP cDNA.
The forward primer 5′-AGACATGGATCCCCAGACAG-3′ (start codon with first 4 nucleotides from 5′ ULT region) and reverse primer 5′-GGCTGCCAAATGATAAACAG-3′ (selected from second intron) were used for cloning ASBNP gene. A 2-μL aliquot of the reverse transcription reaction product was used as DNA template in PCR and was carried out in Minicycler (MJ Research). The PCR amplified cDNA fragments were subcloned into the pCR2.1-TOPO vector using TOPO TA Cloning Kit (Invitrogen). The plasmid containing PCR fragments were purified using Miniprep kit (QIAGEN), and the sequence of the insert was conformed at Mayo Sequencing Core Facility.
Quantitative Real-time PCR.
The mRNA levels of BNP and ASBNP were compared by real-time quantitative RT-PCR (PCR) analysis using the Light Cycler thermocycler (Roche Diagnostics Corp.). Details are provided in SI Methods.To quantify the amount of mRNA for ASBNP in failing human hearts, left ventricular tissues (n = 6) from patients were obtained (14). The mRNA levels of BNP and ASBNP in left ventricular tissue were examined in specimens using quantitative real-time PCR.
Antisera Generation and Purification.
The deduced unique 33-aa ASBNP carboxyl terminus (GKHPLPPRPPSPI PVCDTVRVTLGFVVSGNHTL) was synthesized in Mayo Proteomic Research Center. A polyclonal anti-ASBNP c-terminus antiserum was generated using standard techniques (Cocalico Biologicals). The anti-ASBNP rabbit serum was collected, and the IgG was purified using affi-Gel Protein A MAPS II Kit (BIO-RAD) according to the manufacturer's instructions. The antisera detects ASBNP (and ASBNP.1) but does not detect ANP, CNP, DNP, urodilatin, or VNP) (Fig. S1).
Immunohistochemistry.
Failing human heart samples were sectioned into 5-μm thick sections and deparaffinized and incubated 30 min in 10 mM citrate buffer, pH 6.0, at 100 °C for 30 min. The sections were blocked 30 min with 10% normal donkey serum followed with an incubation time for 60 min with rabbit anti-ASBNP antibody at 32 μg/mL. Normal rabbit IgG replaced the primary antibody to service as the negative control. The sections were then incubated with biotinylated donkey anti-rabbit (GE Healthcare Bio-Sciences Corp.) at 1:200 for 30 min followed by alkaline phosphatase streptavidin (Vector Laboratories) at 1:300 for 45 min. The reaction was visualized using Vector Blue (Vector Laboratories).
Construction of pAcGP67B-ASBNP.
The secretory pAcGP67B transfer vector (BD Biosciences) was chosen to match the codon sequence. A forward primer 5′-CGGGATCCAGCCCCAAGATGGTGCAA-3′ containing 18 bases from 2nd exon with a BamHI site and a reverse primer 5′-GAAGATCTTTAAAGAGTGTGGTTCCC-3′ containing 18 nucleotides from 2nd intron with a BglII site were used as PCR primers to amplify cDNA fragments that encoded mature ASBNP. The obtained PCR fragments were purified and subcloned into transfer vector pAcGP67B between the BamHI and BglII sites. The sequence of the insert was confirmed by double-stranded sequencing (Mayo Molecular Core Facility).
Measurements of Total Cellular cGMP from Cells in Culture.
The intracellular cGMP were measured by using BIOTRAK cGMP enzyme immunoassay system Kit (GE Healthcare Bio-Sciences Corp.) as previously described (15). HUVECs were plated in 96-well Falcon plates and incubated 24 h to reach 80% confluence. Then cells were stimulated with various diluted conditioned media from transfected cells and different concentrations of synthetic BNP peptides dissolved in endothelial growth medium containing 0.5 mM IBMX for 30 min at 37 °C with 5% CO2, and cells were subjected to lysis following the instruction of BIOTRAK Kit. The amount of cGMP was calculated according to the standard curve that was generated from parallel reactions within the same experiment. HS-142–1 was used to investigate whether the effect of ASBNP.1 on mesangial cells was dependent upon a particulate guanylyl cyclase receptor.
Studies in Isolated Canine Glomeruli.
Normal canine kidneys were harvested and glomeruli were isolated (16, 17). After 10 min of preincubation (37 °C), aliquots of glomeruli suspended in Krebs buffer were incubated with human BNP (hBNP, Phoenix Pharmaceuticals, Inc.) or ASBNP.1 (final concentration 10−5 M for both hBNP and ASBNP.1) for 10 min in the presence of isobutylmethylxanthine (0.3 mM). The controls consist of the same composition with the exception that Krebs buffer alone was used instead of glomeruli suspended in Krebs buffer. The final assay volume was 500 μL. The reaction was terminated by addition of ice-cold trichloroacetic acid (final concentration 6.6%) (16). Following centrifugation, a supernatant aliquot (800 μL) was extracted with ether for cGMP assay (15, 16), and the remaining supernatant was assayed for protein levels (BCA protein assay, Pierce Biotechnology). Results were corrected for protein levels (mean ± SEM).
Expression of Recombinant ASBNP Mature Peptide and Western Blot Analysis.
Recombinant mature ASBNP peptides were expressed by using BD BaculoGold Bright system (BD Biosciences). Details are provided in SI Methods.
Arterial Vasoactivity Studies.
To determine the arterial and venous vasodilator effects of ASBNP, ASBNP.1 in comparison with BNP, canine carotid artery, and discarded human left internal mammary artery (LIMA) were used as previously described (18, 19). Details are provided in SI Methods.
Whole-cell Binding Assays.
Human embryonic kidney 293 cells stably expressing human NPR-A or NPR-B or NPR-C were previously described (9). Binding of 125I-ANP to HEK 293 cells stably expressing various natriuretic peptide receptors was performed as previously described (9).
In Vivo Animal Studies.
Studies were conducted in anesthetized male mongrel dogs (18–23 kg). Specifically, 2 groups of dogs with overt chronic heart failure produced by rapid ventricular pacing at 240 beats per minute (bpm) for 10 days were used: (i) a dose escalation study (2, 10, and 100 pmol/kg/min) of i.v. infusion of ASBNP.1 (n = 10) and (ii) a sustained IV infusion of ASBNP.1 at 100 pmol/kg/min (n = 5). Studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee. Experimental details are provided in SI Methods.
Statistical Analysis.
Results are reported as mean ± 1 SEM. For the cell culture studies, each experiment was performed in triplicate in 2 or 3 (human NPR-C binding) separate assays. Differences between groups were made using unpaired t-tests. Statistical analysis was performed with the SAS software package. Physiologic parameters in study groups were compared with 1-way ANOVA or repeated measures ANOVA. The normal distribution was tested with univariate analysis. A P value <0.05 was considered significant.
Supplementary Material
Acknowledgments.
This work was funded in part by grants from Anexon, Inc., the Mayo Foundation, and the National Institutes of Health (HL 76611).
Footnotes
Conflict of interest statement: Drs. Pan, Chen, Burnett, and Simari are named inventors on a Mayo Clinic owned patent, which has been licensed to Anexon, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811851106/DCSupplemental.
References
- 1.Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: Towards a cellular code. Nat Rev Mol Cell Biol. 2005;6:386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 2.Blencowe BJ. Alternative splicing: New insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 4.Mukoyama M, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide, and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbers DL, et al. Membrane guanylyl cyclase receptors: An update. Trends Endocrinol Metab. 2006;17:251–258. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. J Am Med Assoc. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 8.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 9.Dickey DM, Burnett JC, Jr., Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HH, Huntley BK, Schirger JA, Cataliotti A, Burnett JC., Jr Maximizing the renal cyclic 3′-5′-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: A novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol. 2006;17:2742–2747. doi: 10.1681/ASN.2006020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Dov C, Hartmann B, Lundgren J, Valcarcel J. Genome-wide analysis of alternative pre-mRNA splicing. J Biol Chem. 2008;283:1229–1233. doi: 10.1074/jbc.R700033200. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Allen PD, Izumo S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Correlation with expression of the Ca(2+)-ATPase gene. Circ Res. 1992;71:9–17. doi: 10.1161/01.res.71.1.9. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford RA, Matsuda Y, Wilkins MR, Polak JM, Wharton J. Identification of renal natriuretic peptide receptor subpopulations by use of the non-peptide antagonist, HS-142–1. Br J Pharmacol. 1994;113:931–939. doi: 10.1111/j.1476-5381.1994.tb17082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall JL, et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004;17:283–291. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 15.Steiner AL, Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- 16.Supaporn T, et al. Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- 17.Chaumet-Riffaud P, Oudinet JP, Sraer J, Lajotte C, Ardaillou R. Altered PGE2 and PGF2 alpha production by glomeruli and papilla of sodium-depleted and sodium-loaded rats. Am J Physiol. 1981;241:F517–524. doi: 10.1152/ajprenal.1981.241.5.F517. [DOI] [PubMed] [Google Scholar]
- 18.Hasdai D, Mathew V, Schwartz RS, Holmes DR, Jr, Lerman A. The effect of basic fibroblast growth factor on coronary vascular tone in experimental hypercholesterolemia in vivo and in vitro. Coron Artery Dis. 1997;8:299–304. doi: 10.1097/00019501-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Hasdai D, et al. Insulin and insulin-like growth factor-I cause coronary vasorelaxation in vitro. Hypertension. 1998;32:228–234. doi: 10.1161/01.hyp.32.2.228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.