Abstract
Cardiac hypertrophy is a growth response of the heart to increased hemodynamic demand or damage. Accompanying this heart enlargement is a remodeling of Ca2+ signaling. Due to its fundamental role in controlling cardiomyocyte contraction during every heartbeat, modifications in Ca2+ fluxes significantly impact on cardiac output and facilitate the development of arrhythmias. Using cardiomyocytes from spontaneously hypertensive rats (SHRs), we demonstrate that an increase in Ca2+ release through inositol 1,4,5-trisphosphate receptors (InsP3Rs) contributes to the larger excitation contraction coupling (ECC)-mediated Ca2+ transients characteristic of hypertrophic myocytes and underlies the more potent enhancement of ECC-mediated Ca2+ transients and contraction elicited by InsP3 or endothelin-1 (ET-1). Responsible for this is an increase in InsP3R expression in the junctional sarcoplasmic reticulum. Due to their close proximity to ryanodine receptors (RyRs) in this region, enhanced Ca2+ release through InsP3Rs served to sensitize RyRs, thereby increasing diastolic Ca2+ levels, the incidence of extra-systolic Ca2+ transients, and the induction of ECC-mediated Ca2+ elevations. Unlike the increase in InsP3R expression and Ca2+ transient amplitude in the cytosol, InsP3R expression and ECC-mediated Ca2+ transients in the nucleus were not altered during hypertrophy. Elevated InsP3R2 expression was also detected in hearts from human patients with heart failure after ischemic dilated cardiomyopathy, as well as in aortic-banded hypertrophic mouse hearts. Our data establish that increased InsP3R expression is a general mechanism that underlies remodeling of Ca2+ signaling during heart disease, and in particular, in triggering ventricular arrhythmia during hypertrophy.
Keywords: calcium, ECC, IP3, SHR, signalling
In response to increased hemodynamic requirements or damage the heart undergoes a hypertrophic growth response. Hypertrophy is induced by physiological stimuli, such as exercise or pregnancy and by pathological conditions such as hypertension and ischemic heart disease. Although hypertrophy can initially be an adaptive compensatory response, chronically it may become decompensated. As a result, cardiac function is decreased and the heart exhibits an increased propensity for arrhythmias that together ultimately lead to heart failure and death (1).
Ca2+ is a fundamental regulator of cardiac function causing myocyte contraction via excitation-contraction coupling (ECC) (2), and stimulating the gene transcription that underlies hypertrophy (3). Accompanying cardiac hypertrophy and failure is a remodeling of Ca2+ signaling (4). Whilst enhanced Ca2+ transients facilitate greater myocyte contraction during adaptive hypertrophy, Ca2+ fluxes are diminished during heart failure and thereby contribute to decreased cardiac output (5). Remodeling of the Ca2+ signaling proteome also underlies the increased arrhythmias associated with hypertrophy and heart failure (6).
In addition to the RyRs that mediate ECC-dependent Ca2+ fluxes, cardiomyocytes also express InsP3Rs, albeit outnumbered by RyRs at approximately 50:1 (7). Mammals have 3 InsP3R isoforms (types 1–3) (8), with InsP3R2 being the main isoform in cardiomyocytes (9, 10). Although Ca2+ flux via these InsP3Rs is relatively small in comparison to the large Ca2+ transients occurring during every heartbeat, recent data suggests that InsP3Rs have an important role in cardiac physiology. We, and others have shown, that Ca2+ release through InsP3Rs contributes to the inotropic, arrhythmogenic, and hypertrophic effect of Gαq-coupled agonists such as the vasoactive peptide ET-1 (11–16). Whether altered InsP3R signaling also contributes to remodeling of Ca2+ homeostasis during cardiac hypertrophy is not yet determined. An increase in InsP3R expression has however been reported during heart failure in humans (17). Moreover, InsP3-induced Ca2+ release (IICR) is increased in SR microsomes prepared from hypertrophic myocytes (18).
Here, we hypothesized that enhanced Ca2+ release via InsP3Rs contributes to remodeling of ECC-mediated Ca2+ transients, and to the increased arrhythmogenic Ca2+ signals observed in ventricular cardiomyocytes during compensated hypertrophy. To test these hypotheses, in a model that reflects the slow development of hypertrophy in humans, Ca2+ fluxes and contractility were investigated in hypertrophic ventricular myocytes isolated form SHRs (19). We found that the increase in amplitude of ECC-mediated Ca2+ transients and propensity for extra-systolic spontaneous Ca2+ signals, characteristic of hypertrophic myocytes, was caused by augmented InsP3R signaling. This profound effect of enhanced InsP3R activity in hypertrophic myocytes was due to an increase in InsP3R expression, specifically in the junctional SR membrane in close proximity to RyRs. At this location, Ca2+ release via InsP3Rs acted to sensitize RyRs, thereby enhancing Ca2+ release during ECC and inducing spontaneous elementary Ca2+-release events and extra-systolic Ca2+ transients. InsP3R2 expression was also increased in hypertrophic cardiomyocytes isolated from aortically banded mice and in human hearts displaying ischemic dilated cardiomyopathy. We propose that InsP3Rs play a fundamental role in the physiology of hypertrophic hearts contributing to remodeled cardiac function and triggering ventricular arrhythmia.
Results
SHR Cardiomyocytes Develop Hypertrophy.
As previously described, at 6 months, cardiomyocytes from SHRs are hypertrophic (20). Cardiomyocyte width was increased in SHRs compared to WKY controls (Table S1) resulting in a decrease in the cellular length/width ratio (WKY: 3.41 ± 0.11 vs. SHR: 2.71 ± 0.12; P < 0.001). Messenger RNA levels of the hypertrophic marker atrial natriuretic factor (ANF) (21) were also greater in SHR cardiomyocytes at the age of 6 months than in WKY controls (12.68 ± 4.04 vs. 0.69 ± 0.32, P < 0.05). In 12-week-old animals, cell size and ANF mRNA levels were not different between the 2 strains (Table S1).
Ca2+ Release via InsP3Rs Is Increased During Hypertrophy and Remodels ECC.
Consistent with previous observations (20), the amplitude of electrically evoked systolic Ca2+ transients was greater in cardiomyocytes from 6-month-old SHRs than in WKY controls (SHR: 0.70 ± 0.07 mM, WKY: 0.50 ± 0.06 mM; n = 48 and 42, respectively, P < 0.05). Fractional shortening of myocytes under basal conditions was however not significantly different between the 2 strains (WKY: 10.35 ± 1.50%, SHR: 7.59 ± 0.98%).
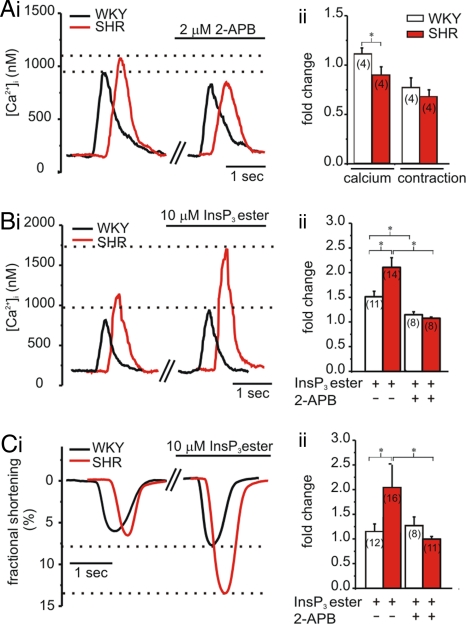
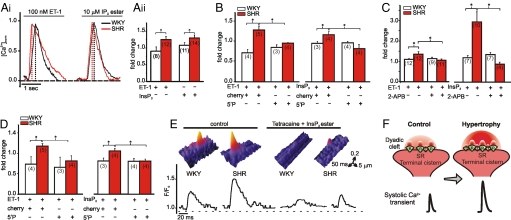
To reveal whether increased Ca2+ release via InsP3Rs contributes to remodeling of Ca2+ signaling and myocyte function during hypertrophy, we measured global Ca2+ transients and cellular contraction under conditions where InsP3Rs were either inhibited or activated. As Ca2+ transients are greater under basal conditions in hypertrophic SHR than in WKY myocytes, the systolic Ca2+ amplitude was normalized to that before treatment. Inhibition of InsP3Rs with 2-APB (2 μM) (11) caused a greater reduction in ECC-mediated Ca2+ transient amplitude in SHR myocytes compared with WKY controls (Fig. 1A). Concurrently, 2-APB also decreased the magnitude of contraction in SHRs (Fig. 1Aii). These data suggested that Ca2+ release via InsP3Rs contributes to the greater basal ECC-associated Ca2+ transients observed in SHR myocytes.
Fig. 1.
Systolic Ca2+ transient amplitude and cellular contraction recorded from indo-1 AM-loaded ventricular myocytes isolated from 6-month-old WKY rats and SHRs. Data presented were obtained 20 min after application of 2-APB or InsP3 ester. Representative traces are shown in i; values normalized to pre-application are shown in ii. For better comparison, single traces have been time-shifted. (A) Effect of 2 μM 2-APB on Ca2+ transient amplitude and fractional shortening. (B) Effect of 10 μM InsP3 ester ± 2 μM 2-APB on Ca2+ transient amplitude. (C) As in B on fractional shortening. N numbers are indicated. *, P < 0.05; Student's t test.
Direct activation of InsP3Rs with InsP3 ester (11) promoted a greater increase in Ca2+transient amplitude and inotropy in SHR compared to WKY myocytes (Fig. 1 B and C), which was abrogated in both strains by 2-APB (Fig. 1 Bii and Cii). No difference in ECC-mediated Ca2+ transient amplitude or cellular contraction was observed between myocytes isolated from 12-week-old WKY rats or SHRs (Fig. S1A).
InsP3R2 Expression Is Increased in Hypertrophic Cardiomyocytes.
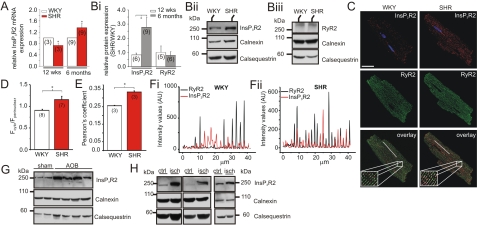
Next, we analyzed whether an increase in InsP3R expression underlies altered InsP3 signaling during hypertrophy. At 6 months, InsP3R2 mRNA and protein levels were higher in SHR than in WKY myocytes, whereas at 12 weeks, InsP3R2 mRNA and protein levels were lower in SHRs compared to WKY controls (Fig. 2 A and B). RyR2 protein levels were not different between the 2 strains at the age of 12 weeks or 6 months (Fig. 2B).
Fig. 2.
Expression of InsP3R2 during hypertrophy. (A) Relative InsP3R2 mRNA levels. Values for SHRs have been normalized to age-matched WKY rats. (Bi) Relative InsP3R2 and RyR2 protein levels. SHR/WKY ratios have been determined for 12-week- and 6-month-old rats. Representative immunoblots for InsP3R2 (ii) and RyR2 (iii) are shown. Seventy-five micrograms membrane proteins from ventricular cardiomyocytes were loaded per lane. (C) Immunofluorescent staining for InsP3R2 and RyR2. DAPI was used to stain the nuclei. (Scale bar, 30 μm.) Pixels positive for InsP3R2 and RyR2 are shown in white. (D) Ratio of cytosolic/perinuclear fluorescence intensity in a 3-μm ring in these 2 regions. (E) Pearson's coefficient for co-localization of RyR2 and InsP3R2. (F) Profiles of RyR2 and InsP3R2 fluorescence intensity sampled along the longitudinal axis of a WKY (i) or SHR (ii) myocyte, as depicted by the white lines on the overlay images in C. (G) Representative immunoblots detecting InsP3R2 in hearts from mice that have undergone aortic banding (AOB) and control mice. (H) As in G in human disease hearts from patients showing ischemic cardiomyopathy (isch) as well as control hearts (ctrl). Forty micrograms membrane proteins from left ventricles were loaded per lane. Calnexin and calsequestrin were used as loading controls. N numbers are indicated. *, P < 0.05; Student's t test.
Immunofluorescent labeling revealed that in WKY myocytes, InsP3R2 was predominantly expressed in the perinuclear regions with weaker staining along the SR membrane, where RyRs are localized (Fig. 2C). InsP3R2 was also expressed in the perinuclear regions of SHR cardiomyocytes, but compared to WKY cells its expression was significantly greater along the RyR2-stained striations outside the nuclear region. Thus, the ratio of cytosolic/nuclear InsP3R2 immunofluorescence was increased (Fig. 2D). No difference in RyR2 immunostaining between the 2 strains was observed (Fig. 2C). In both WKY and SHR myocytes, InsP3R2 co-localized with RyR2s (intensity profile along indicated line, Fig. 2 C and F) and Pearson's coefficient (Fig. 2E), indicating that like RyR2s, InsP3R2s are located at dyadic junctions alongside T-tubule membranes (Fig. 2C). The co-localization of these 2 channels was markedly increased in hypertrophic myocytes (Fig. 2 E and F). We concluded that, as a result of hypertrophic remodeling, the number of InsP3R2s located in the junctional SR membrane is increased, thereby mediating their greater co-localization with RyR2s.
InsP3R2 expression was also increased in hearts from mice after aortic banding (Fig. 2G) and in human patients with ischemic dilated cardiomyopathy (Fig. 2H). These data suggested that increased InsP3R2 expression is a general hallmark of hypertrophy.
Increased InsP3R Expression in the Junctional SR Causes a Spatially Restricted Remodeling of ECC-mediated Ca2+ Transients during Hypertrophy.
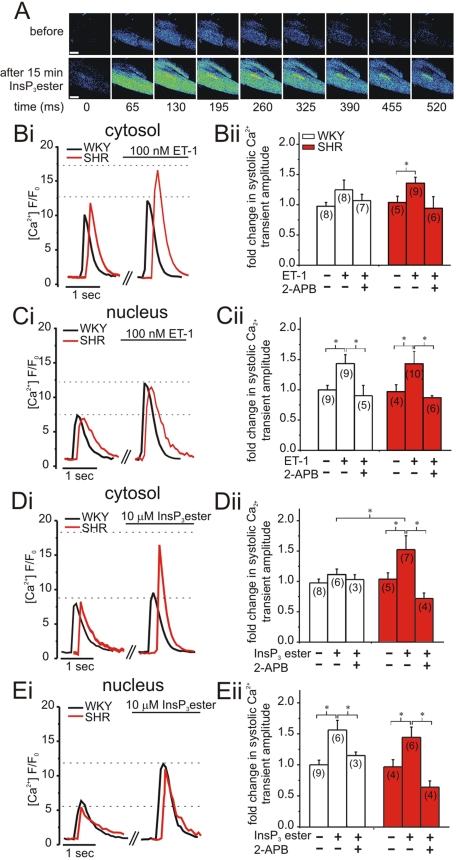
To establish how increased InsP3R expression in the junctional SR membrane impacted on ECC-mediated Ca2+ signals, we performed confocal Ca2+ imaging experiments. In addition to directly stimulating InsP3Rs with a membrane-permeant InsP3 ester, we tested the effect of physiologically activating InsP3Rs with InsP3 generated following ET-1 stimulation (11, 22). In SHR myocytes, stimulation with ET-1 and InsP3 ester increased the amplitude of nuclear and cytosolic Ca2+ transients during electrical pacing (Fig. 3 A–E). Contrastingly, in WKY myocytes, only nuclear systolic Ca2+ transients were augmented (Fig. 3 C and E). The enhancement of systolic Ca2+ transients by ET-1 and InsP3 ester was sensitive to 2-APB, further indicating that this effect was mediated by InsP3Rs (Fig. 3 Bii–Eii). The increase in nuclear Ca2+ transient amplitude in SHRs was comparable to that observed in WKY myocytes (Fig. 3 C and E). To accommodate for variation between cells in the absolute magnitude of Ca2+ changes, the ratio of nuclear to cytosolic Ca2+ transient amplitude was calculated. This ratio was increased in WKY myocytes following ET-1 or InsP3 ester stimulation whereas no change was observed in SHR myocytes (Fig. S2A). The difference in ratio between the 2 strains is explained by restriction of the ET-1- and InsP3 ester-stimulated increase in Ca2+ transient amplitude to the nuclear compartment in WKY myocytes, whereas, in SHR myocytes nuclear and cytosolic Ca2+ transient amplitude were both increased. These data indicate that in non-hypertrophied myocytes, Ca2+ release via InsP3Rs impacts more profoundly on nuclear Ca2+ transients, whereas in hypertrophic myocytes, increased junctional InsP3R expression specifically augments the cytosolic Ca2+ transients. There was no difference in the ratio of nuclear to cytoplasmic Ca2+ transients between 12-week-old WKY and SHRs under basal conditions, or during ET-1 or InsP3 ester stimulation (Fig. S1B).
Fig. 3.
Confocal analysis of systolic nuclear and cytosolic Ca2+ transient amplitude in fluo-4 AM-loaded ventricular myocytes. Data presented were determined 15 min after application of ET-1; InsP3 ester or 2-APB. Representative traces are shown in i; values normalized to pre application are shown in (ii). For better comparison, single traces have been slightly time-shifted. (A) Representative SHR myocyte displaying Ca2+ transients at a series of time points before and after application of 10 μM InsP3ester. (Scale bar, 10 μm.) (B) Effect of 100 nM ET-1 ± 2 μM 2-APB on cytosolic peak amplitude. (C) As in B for nuclear peak amplitude. (D) Effect of 10 μM InsP3 ester ± 2 μM 2-APB on cytosolic peak amplitude. (E) as in D for nuclear peak amplitude. N numbers are indicated. *, P < 0.05; Student's t test.
Maximal Ca2+ release from nuclear and cytosolic Ca2+ stores induced by 10 mM caffeine (RyR agonist) was not significantly different between WKY and SHR myocytes (Fig. S2B), indicating that differences in Ca2+ store content do not underlie the changes in ECC-associated Ca2+ transients during ET-1 and InsP3 ester stimulation.
Extra-Systolic InsP3-dependent Ca2+-release Events Are Increased in SHR Myocytes.
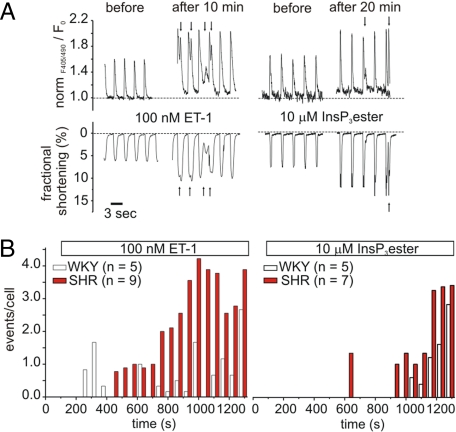
In atrial cardiomyocytes, which express approximately 6 fold more InsP3Rs than ventricular myocytes, Ca2+ release via InsP3Rs underlies the induction of extra-systolic Ca2+ transients (11, 12). We therefore investigated whether the increased InsP3R expression and activity observed in SHR ventricular myocytes caused them to exhibit more extra-systolic Ca2+-release events. Extra-systolic events were determined as rises in Ca2+ concentration that were temporally distinguished from signals induced by field stimulation and that also impacted on contraction (see arrows Fig. 4A). Stimulation with ET-1 or InsP3 ester caused a 2-APB-sensitive increase in the number of extra-systolic Ca2+ transients in both SHR and WKY myocytes (Fig. 4B and Table S2). However, the number of cells that exhibited extra-systolic Ca2+ transients and the frequency of events per cell were greater in SHRs than WKYs (ET-1: WKY: 26% vs. SHR: 50%; InsP3 ester: WKY: 29% vs. SHR: 57%, Table S2 and Fig. 4B). In both strains, extra-systolic Ca2+-release events began to occur within a few minutes of InsP3 ester or ET-1 stimulation and increased throughout the time-course of the experiment (Fig. 4B). The rate at which the frequency of the extra-systolic Ca2+ transients increased following InsP3 ester or ET-1 stimulation was greater for SHR myocytes than WKY myocytes. After 1,000 s, the incidence of extra-systolic Ca2+ transients was significantly higher in SHRs than WKY cells (Fig. S3). No difference in the frequency of extra-systolic Ca2+ transients was observed between the 2 strains at 12 weeks (Fig. S1 C and D). These data indicate that activation of InsP3Rs was responsible for the initiation of the extra-systolic Ca2+ transients and provides an explanation for the increased frequency of extra-systolic Ca2+ transients during hypertrophy.
Fig. 4.
Analysis of extra-systolic Ca2+ release events in indo-1 AM-loaded ventricular myocytes during hypertrophy. (A) Representative traces for global Ca2+ transients and cellular contraction recorded from SHR myocytes before and after stimulation with 100 nM ET-1 or 10 μM IP3 ester. Arrows indicate extra-systolic events. (B) Extra-systolic Ca2+ release events per cell during stimulation with 100 nM ET-1 or 10 μM InsP3 ester.
Enhanced Ca2+ Release via InsP3Rs Increases the Rate of Rise of Systolic Ca2+ Transients and Elevates Diastolic [Ca2+] in SHRs.
A hypertrophy-associated increase in InsP3R-mediated Ca2+ flux via junctional InsP3Rs acting to induce Ca2+ release via neighboring RyRs could provide a mechanism to accentuate Ca2+ signaling during ECC. To test this hypothesis, the effect of ET-1 and InsP3 ester on the rate of rise of pacing-evoked systolic Ca2+ transients was measured. During both ET-1 and InsP3 ester stimulation, the rate of rise of the Ca2+ transient was faster in hypertrophic SHR than in WKY cells (Fig. 5A). The effects of ET-1 and InsP3 ester were abrogated by adenoviral-mediated expression of a cherry fluorescent protein-tagged InsP3 5′-phosphatase, which disrupts InsP3 signaling (5′P; Fig. 5B) (16). There was no difference in the rate of rise of the systolic Ca2+ transient in myocytes from 12-week-old WKY and SHRs (Fig. S1E).
Fig. 5.
Analysis of global Ca2+ transient kinetics, diastolic [Ca2+], and elementary Ca2+ release during hypertrophy. (A) Rate of rise of Ca2+ transient (peak amplitude/time to peak) after 20 min stimulation with 100 nM ET-1 or 10 μM InsP3 ester (InsP3). Representative traces are shown in i; values normalized to pre application are shown in ii. Cells have been loaded with indo-1 a.m. and electrically paced at 0.3 Hz. (B) As in Aii for ventricular myocytes infected with control cherry virus or InsP3 5′-phosphatase (5′P) virus. (C) Changes in diastolic [Ca2+] during application of 100 nM ET-1 ± 2 μM 2-APB or 10 μM InsP3 ester ± 2 μM 2-APB, normalized to before application. (D) As in C for ventricular myocytes that have been infected with control cherry virus or 5′P virus. (E) Surface plot of representative elementary Ca2+ release events during normal pacing (control) and during pacing in the presence of 1 mM tetracaine + 10 μM InsP3 ester. F/F0 traces are shown below. (F) Schematic indicating how InsP3R-mediated Ca2+ release augments systolic Ca2+ transients during hypertrophy. (I) InsP3R2; R, RyR2. N numbers are indicated: *, P < 0.05; Student's t test.
As RyR opening is controlled by [Ca2+]i, we next tested whether Ca2+ release via InsP3Rs modulated the efficiency of Ca2+-induced Ca2+ release (CICR) by changing diastolic [Ca2+] levels. Under basal conditions, diastolic Ca2+ levels were not different between strains (WKY: 103.39 ± 8.86 nM vs. SHR: 95.74 ± 14.21 nM). Following stimulation with ET-1 or InsP3 ester, diastolic [Ca2+] was increased in SHR myocytes (ET-1: 96.1 ± 5.6 nM to 132.6 ± 15.2 nM, InsP3 ester: 69.5 ± 19.9 nM to 184.5 ± 36.7 nM, Fig. 5C), whereas no change was seen in WKY cells (Fig. 5C). 2-APB or 5′P expression abrogated the increase in diastolic [Ca2+] caused by InsP3 ester or ET-1 in SHRs (Fig. 5 C and D) without effecting diastolic [Ca2+] under basal conditions. At 12 weeks of age, there was no difference in diastolic [Ca2+] during stimulation of SHR myocytes with ET-1 or InsP3 ester (Fig. S1F).
Frequency of Elementary InsP3-dependent Ca2+-release Events Is Increased during Hypertrophy.
To further resolve the consequences of increased InsP3R expression for Ca2+ signaling, elementary Ca2+-release events were analyzed. Under normal paced conditions, Ca2+ events during the diastolic period were of greater amplitude in the hypertrophic SHR myocytes than in WKY cells (WKY: ΔF/F0 = 0.26 ± 0.01 vs. SHR: 0.34 ± 0.04, Table S3 and Fig. 5E). Under conditions where RyRs were blocked with 1 mM tetracaine, InsP3 ester application stimulated elementary Ca2+-release events (Fig. 5E) that occurred at a greater frequency in hypertrophic myocytes (WKY: 2.12 ± 0.46 vs. SHR: 6.84 ± 0.65, Table S3). These data suggest that InsP3R-mediated Ca2+ signals contribute to the greater amplitude of diastolic Ca2+ events observed in SHR myocytes and may underlie the elevated diastolic [Ca2+] observed in SHR myocytes stimulated with InsP3 or ET-1.
Our data suggest a model to explain the enhanced ECC-mediated Ca2+ signals and increased extra-systolic Ca2+-release events observed during hypertrophy (Fig. 5F). Key to this model is a hypertrophy-associated increase in InsP3R expression in the dyadic region. Thus, more InsP3Rs are in close proximity to RyRs in the SR membrane (Fig. 2 E and F). Ca2+ released via these InsP3Rs sensitizes their adjacent RyRs, bringing them closer to threshold for activation. Under conditions of increased [InsP3], elementary InsP3-dependent Ca2+-release events are increased in frequency and diastolic [Ca2+] is elevated. Consequently, RyRs are triggered to generate extra-systolic Ca2+ signals and to accelerate the rate of rise of pacing-evoked Ca2+ transients (Fig. 5F).
Discussion
Here we demonstrate that enhanced Ca2+ signaling via InsP3Rs located in the dyadic cleft remodels Ca2+ signaling during hypertrophy.
In agreement with previous data, we found that the amplitude of ECC-mediated Ca2+ transients under basal conditions was significantly greater as a result of hypertrophy in SHR myocytes (in the absence of any other stimulation) (20). Significantly, we determined that this increased amplitude of basal ECC-mediated Ca2+ transients was due to augmented Ca2+ release via InsP3Rs. These data demonstrated that InsP3Rs could contribute to ECC-mediated Ca2+ fluxes without additional neurohormonal input, thereby modifying myocyte Ca2+ signaling.
A greater role for InsP3Rs in regulating ECC-mediated Ca2+ transients during hypertrophy was revealed following their direct activation with cell-permeant InsP3 ester. These data showed that increased activation of InsP3Rs could augment the amplitude of ECC-mediated Ca2+ transients mediated via RyRs even further. Consistent with previous reports (20, 23, 24), no increase in SR releasable Ca2+ was observed in hypertrophic SHR myocytes, thereby indicating that the enhancement of ECC-associated Ca2+ flux by InsP3Rs was not due to an increase in store loading. InsP3R2 expression was elevated as a result of hypertrophy thereby providing a mechanism for increased Ca2+ release via InsP3Rs. InsP3R expression in the heart has previously been reported to be modified following disease. In particular, InsP3R expression is increased in atrial myocytes of humans and dogs during atrial fibrillation (AF) (25, 26). Furthermore, elevated InsP3R levels and increased InsP3 binding was reported in the left ventricle during human heart failure (17). Consistent with these reports and our observations in rats, we found that InsP3R2 expression was significantly elevated in cardiac tissue from aortically-banded hypertrophic mice and from human hearts showing ischemic dilated cardiomyopathy. Due to its very low expression and insensitivity to hypertrophy in rat cardiac fibroblasts (Fig. S4), we considered that the changes in InsP3R2 expression detected in human and mouse cardiac tissue was due solely to InsP3R2 in cardiac myocytes. Our findings in rats, mice, and humans therefore suggested that increased InsP3R expression is a general feature of cardiac disease, raising the possibility that increased Ca2+ release via InsP3Rs contributes to pathological changes in Ca2+ signaling.
The enhanced InsP3R2 expression had a striking spatial aspect in that InsP3R expression was specifically increased in the junctional SR. Detailed analysis showed that these junctional InsP3Rs co-localized with RyRs, which reside primarily in the dyadic cleft. This profound remodeling in InsP3Rs expression and distribution had significant functional consequences. In particular, the increased number of dyadic InsP3Rs augmented the amplitude of the cytosolic ECC-mediated Ca2+ transients and enhanced the positive inotropic effect of InsP3 ester. Similarly, cytosolic ECC-mediated Ca2+ transient amplitude and contraction were enhanced when InsP3Rs were engaged by InsP3 generated following application of ET-1. Given that ET-1 is a potent pro-hypertrophic agonist, and its levels are elevated during heart failure, these findings have significant implications for cardiac function during hypertrophy (16, 22, 27). The activation of InsP3Rs in SHR myocytes by ET-1 is in agreement with data from our laboratory and elsewhere showing that stimulation of the InsP3 signaling cascade in cardiomyocytes with ET-1 modifies Ca2+ fluxes and contractility (11, 13, 28). The increase in nuclear Ca2+ transient amplitude during ECC by ET-1 and InsP3 ester was not altered during hypertrophy reflecting the lack of a change in InsP3R expression in this region. Together, these data suggested that Ca2+ release via InsP3Rs in the dyadic region primed ECC-mediated Ca2+-induced Ca2+ release via RyRs (see Fig. 5F). Specifically, Ca2+ release via InsP3Rs could elevate diastolic [Ca2+] closer to the threshold for activation of RyRs. Thus, we established that increased Ca2+ release via InsP3Rs in hypertrophic myocytes can significantly contribute to remodel ECC-mediated Ca2+ signals.
At the most fundamental level, in the absence of RyR activity, SHR myocytes exhibited an increased frequency of elementary InsP3-dependent Ca2+-release events. Interestingly, the amplitudes of those events were no different between WKY and SHR myocytes. This is not surprising given that Ca2+ puffs are fundamental Ca2+ signals that are conserved between cells as diverse as Xenopus oocytes and HeLa human epithelial cells (29). At the molecular level, Ca2+ puffs arise via the stochastic recruitment of neighboring InsP3Rs (a cluster) until a threshold number required for puff generation is reached (30). Thus, it is plausible that greater InsP3R expression in SHR myocytes simply increases the probability of recruiting this puff-generating threshold number of receptors without altering the properties of puffs. As a result, only the frequency of elementary events is increased in SHRs. The greater abundance of these elementary events may explain the elevated diastolic [Ca2+] observed in SHR myocytes stimulated with InsP3 ester and ET-1. These data are consistent with the requirement for InsP3Rs for the ET-1-stimulated increase in diastolic [Ca2+] observed in atrial myocytes (which have ≈6-fold greater InsP3R expression than ventricular myocytes) (10, 12). As elementary Ca2+-release events (Ca2+ sparks and puffs) are the building blocks of higher order Ca2+ transients, it was not surprising that SHR myocytes also exhibited an increased frequency of extra-systolic Ca2+ transients. Similarly, stimulation of atrial myocytes with InsP3 or InsP3-generating agonists such as ET-1, potently induced arrhythmogenic Ca2+-release events that were dependent on InsP3R2 expression (11–13, 28).
By bringing Ca2+ levels closer to the threshold for activation of RyRs, InsP3-mediated sensitization of RyRs also served to increase the rate of rise of ECC-mediated Ca2+ transients. This may remediate the deterioration in Ca2+ signaling that occurs as hypertrophy progresses to failure. In particular, extra Ca2+ release via dyadic InsP3Rs may compensate for the decreased coupling efficiency between L-type Ca2+ channels and RyRs due to a deterioration in the T-tubular network and increased width of the dyadic cleft that occurs during disease (31).
The arrhythmogenic effect of InsP3R activity in the ventricles may have profound consequences. Coupled with increased systemic levels of InsP3-generating agonists, such as ET-1 during hypertension and heart failure, it provides a possible mechanistic explanation why hypertrophic hearts are more likely to develop potentially lethal ventricular arrhythmias (32).
As InsP3R2 is increased during cardiac hypertrophy, yet is dispensable for the normal physiological function of the healthy heart (12), it may represent an ideal target to which pharmacological modulators could be developed to intervene in both the induction of the hypertrophic gene program and the generation of arrhythmias.
Materials and Methods
Detailed methods for myocyte isolation, adenoviral infection, photometric, and confocal measurements of [Ca2+]i, immunoblotting, immunofluorescence, quantitative RT-PCR, and cell length measurements are provided elsewhere (14, 33) and in SI Methods and Fig. S5.
Animal Models.
Male SHRs and normotensive Wistar-Kyoto (WKY) rats were obtained from Harlan and were housed under control conditions with ad libitum food and water. All experiments were performed in accordance with the guidelines from the code of practice for humane killing under Schedule 1 of the Animals (Scientific Procedures) Act 1986. Constriction of the transverse thoracic aorta was performed on 3-month-old male mice as described in SI Methods. The sham procedure was identical but without aortic ligation.
Patients.
Left ventricular tissue samples of human failing hearts were from individuals undergoing heart transplantation due to end-stage heart failure. All samples were obtained from male caucasians, aged 41–62. Samples from non-failing donor hearts were provided by the U.K. Human Tissue Bank. After cardiectomy, left ventricular samples were frozen in liquid nitrogen and stored at −80 °C. Detailed information about the patients can be found in SI Methods. All experiments involving human tissue samples have been approved by the Cambridgeshire Research Ethics Committee.
Recordings of Myocyte Contraction and [Ca2+]i.
All experiments, unless otherwise stated, were performed at 22 °C on myocytes electrically paced with field electrodes at 0.33 Hz. This condition is referred to as the basal condition. Detailed procedures can be found in SI Methods.
Statistics.
Data are expressed as mean ± SEM. Statistical comparisons were carried out with Student's t test or 2-way ANOVA. Statistically significance was accepted at P < 0.05.
Supplementary Material
Acknowledgments.
We thank V. Sorrentino (University of Sienna) for the RyR2 Ab, the BI antibody facility for generating the rat anti-InsP3R2 antibodies, Anne Segonds-Pichon for statistical analysis, and Reinhard Seifert (Caesar, Bonn) for his help with the analysis of the data. D.H. also thanks Wolfson College for a Junior Research Fellowship. The work was supported by the British Heart Foundation (PG/06/034/20637). H.L.R. is a Royal Society Research Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905485106/DCSupplemental.
References
- 1.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 5.Gomez AM, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 6.Kahan T, Bergfeldt L. Left ventricular hypertrophy in hypertension: Its arrhythmogenic potential. Heart. 2005;91:250–256. doi: 10.1136/hrt.2004.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moschella MC, Marks AR. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. J Cell Biol. 1993;120:1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudhof TC, et al. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez PJ, Ramos-Franco J, Fill M, Mignery GA. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J Biol Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 10.Lipp P, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–943. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie L, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 13.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proven A, et al. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci. 2006;119:3363–3375. doi: 10.1242/jcs.03073. [DOI] [PubMed] [Google Scholar]
- 15.Kockskämper J, et al. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higazi DR, et al. Endothelin-1-stimulated insP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009;33:472–482. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Go LO MM, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi HSH, et al. Increased calcium release from sarcoplasmic reticulum stimulated by inositol trisphosphate in spontaneously hypertensive rat heart cells. Mol Cell Biochem. 1993;119:51–57. doi: 10.1007/BF00926853. [DOI] [PubMed] [Google Scholar]
- 19.Bing OH, et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol. 1995;27:383–396. doi: 10.1016/s0022-2828(08)80035-1. [DOI] [PubMed] [Google Scholar]
- 20.Shorofsky SR, et al. Cellular mechanisms of altered contractility in the hypertrophied heart: Big hearts, big sparks. Circ Res. 1999;84:424–434. doi: 10.1161/01.res.84.4.424. [DOI] [PubMed] [Google Scholar]
- 21.Masciotra S, Picard S, Deschepper CF. Cosegregation analysis in genetic crosses suggests a protective role for atrial natriuretic factor against ventricular hypertrophy. Circ Res. 1999;84:1453–1458. doi: 10.1161/01.res.84.12.1453. [DOI] [PubMed] [Google Scholar]
- 22.Russell FD, Molenaar P. The human heart endothelin system: ET-1 synthesis, storage, release, and effect. Trends Pharmacol Sci. 2000;21:353–359. doi: 10.1016/s0165-6147(00)01524-8. [DOI] [PubMed] [Google Scholar]
- 23.Keller E, Bond M, Schomisch Moravec C. Progression of left ventricular hypertrophy does not change the sarcoplasmic reticulum calcium store in the spontaneously hypertensive rat heart. J Mol Cell Cardiol. 1997;29:461–469. doi: 10.1006/jmcc.1996.0288. [DOI] [PubMed] [Google Scholar]
- 24.McCall E, et al. Ca flux, contractility, and excitation-contraction coupling in hypertrophic rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 1998;274:H1348–1360. doi: 10.1152/ajpheart.1998.274.4.H1348. [DOI] [PubMed] [Google Scholar]
- 25.Zhao ZH, et al. Inositol-1,4,5-trisphosphate and ryanodine-dependent Ca2+ signaling in a chronic dog model of atrial fibrillation. Cardiology. 2007;107:269–276. doi: 10.1159/000095517. [DOI] [PubMed] [Google Scholar]
- 26.Yamada J, et al. Up-regulation of inositol 1,4,5 trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation. J Am Coll Cardiol. 2001;37:1111–1119. doi: 10.1016/s0735-1097(01)01144-5. [DOI] [PubMed] [Google Scholar]
- 27.Sugden PH, Clerk A. Endothelin signalling in the cardiac myocyte and its pathophysiological relevance. Curr Vasc Pharmacol. 2005;3:343–351. doi: 10.2174/157016105774329390. [DOI] [PubMed] [Google Scholar]
- 28.Kockskamper J, et al. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci. 2008;121:186–195. doi: 10.1242/jcs.021386. [DOI] [PubMed] [Google Scholar]
- 29.Tovey SC, et al. Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J Cell Sci. 2001;114:3979–3989. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- 30.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, et al. Intermolecular failure of L-type Ca2+ channel and Ryanodine receptor signaling in hypertrophy. PLoS Biol. 2007;5:e21. doi: 10.1371/journal.pbio.0050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Versailles JT, Verscheure Y, Le Kim A, Pourrias B. Comparison between the ventricular fibrillation thresholds of spontaneously hypertensive and normotensive rats–investigation of antidysrhythmic drugs. J Cardiovasc Pharmacol. 1982;4:430–435. doi: 10.1097/00005344-198205000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Bootman MD, Harzheim D, Smyrnias I, Conway SJ, Roderick HL. Temporal changes in atrial EC-coupling during prolonged stimulation with endothelin-1. Cell Calcium. 2007;42:489–501. doi: 10.1016/j.ceca.2007.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.