Abstract
T cell receptors (TCRs) on T lymphocytes in an individual bind foreign peptides bound to major histocompatibility complex (MHC) molecules expressed in that individual (designated MHCA). Results from radiation bone marrow chimeras and TCR transgenic mice indicate that this complex form of antigen recognition is the result of positive selection of clones with low affinity for self peptide:MHCA complexes during development. Here we used a sensitive peptide:MHC tetramer enrichment method to quantify the role of positive selection in the generation of the preimmune polyclonal T cell repertoire in normal individuals. We made the surprising observation that mouse and human naive T cells capable of binding to foreign peptide:MHCA were present at the same frequency in hosts that expressed MHCA or a different MHC isoform (MHCB). However, most of the clones in MHCB hosts also recognized self peptide:MHCA complexes. When these “alloreactive” T cells were removed from the MHCB repertoire via negative selection in an MHCA host, the number of foreign peptide:MHCA-binding T cells was reduced to one fifth and many of the remaining cells did not respond to the peptide. Therefore, although positive selection on MHCA was not required to produce foreign peptide:MHCA-binding clones, it had a large effect on selecting responsive clones.
Keywords: CD4+ T lymphocyte, MHC restriction, negative selection, T cell receptor, tetramer
Each T cell expresses a unique T cell receptor (TCR) capable of binding to a short peptide embedded in a major histocompatibility complex (MHC) molecule on the surface of host cells (1, 2). The repertoire of mature T cells found in a given individual contains clones capable of responding to foreign peptides bound to the type of MHC encountered in the thymus of that individual. This property of T cell antigen recognition is known as MHC restriction (3). The T cell repertoire is also self-tolerant, due in large part to intrathymic negative selection via apoptosis of clones that express TCRs with a high affinity for self peptides bound to self MHC molecules (4).
T cell clones with these features are thought to be selected from a large pool of immature cells based on the affinity of their TCRs for the MHC molecules encountered in the thymus (5). Evidence supporting this hypothesis comes from bone marrow chimera experiments in which mice homozygous for MHC allele A (MHCA) are irradiated and transplanted with F1 bone marrow cells co-expressing MHCA and a different allele, MHCB. Immunization of these chimeras with a foreign antigen produces T cell responses specific for immunogen-derived foreign peptide:MHCA that are at least 50 times larger than those specific for immunogen-derived foreign peptide:MHCB, despite the fact that antigen-presenting cells in these mice express both MHCA and MHCB (6, 7).
This restricted recognition of foreign peptide:MHCA by mature T cells has been explained by the phenomenon of positive selection (5). It has been shown that low affinity TCR binding to self peptide:MHCA on radio-resistant thymic epithelial cells is required for a T cell clone to complete development in the MHCA thymus (8, 9). Low affinity for a self peptide:MHCA is thought to predispose a T cell clone to high affinity recognition of a foreign peptide:MHCA.
Analyses of transgenic mice expressing a single TCR have also yielded evidence for positive selection. For almost all of the TCR transgenic lines studied to date, mature T cells only develop in the presence of the form of MHC expressed by the strain from which the original T cell clone was derived (5, 10). Although only a relatively small number of different TCR transgenic lines have been tested (5), results from these lines indicate that foreign peptide:MHCA-specific T cells survive selection in MHCA but not MHCB hosts.
The aforementioned studies relied on monoclonal T cells or postimmune polyclonal T cells. Thus, the question still remains: how does positive selection shape the polyclonal preimmune repertoire in normal individuals? Assessment of the normal situation would require analysis of a foreign peptide:MHCA-specific T cell population in the preimmune polyclonal repertoires of normal individuals that do or do not express MHCA. We performed this experiment in mice and humans using the sensitive method of peptide:MHC tetramer/magnetic bead-based enrichment.
Results
Detection of Naive Foreign Peptide:MHCA-Binding T Cells in Mice Expressing MHCB.
Positive selection had not been studied in the preimmune polyclonal T cell repertoire because individual foreign peptide:MHC-specific populations are extremely small. Recently, however, we (11) and then others (12–14) overcame this problem using peptide:MHC tetramers and magnetic bead-based enrichment (15) to identify naive foreign peptide:MHC-specific T cells in mice. This technique has the capacity to identify most if not all of the relevant T cells in a mouse with a limit of detection of about 5 cells per mouse (11).
Tetramer enrichment was initially used to study the T cell repertoires of inbred mouse strains. Two peptide:murine MHCII tetramers were used for this purpose. Both tetramers contained the I-Ab MHCII molecule expressed by the C57BL/6 (B6) strain. One contained a peptide called 2W1S, which is a variant of an I-Ab-binding peptide from the α chain of the murine I-Ed MHCII molecule (16). The other tetramer contained peptide 427–441 from the FliC protein of Salmonella typhimurium (17). Both peptides are immunogenic in B6 mice (11, 16, 17).
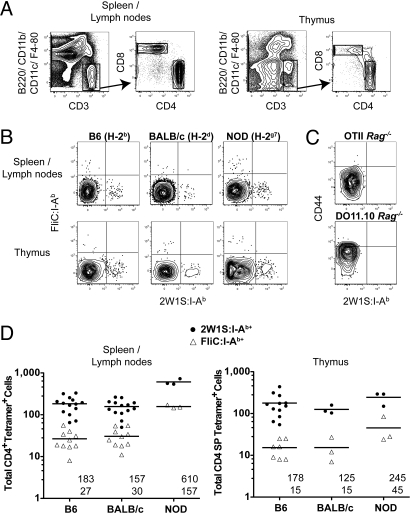
These tetramers were used with magnetic enrichment to enumerate peptide:I-Ab-specific naive T cells in mouse strains expressing I-Ab (B6) or other MHCII molecules (BALB/c and NOD). As expected based on past work (11), each naive B6 mouse contained small populations of CD4+ (Fig. 1A) CD44low 2W1S:I-Ab+ and FliC:I-Ab+ T cells in the spleen and lymph nodes (Fig. 1B). Remarkably, 2W1S:I-Ab+ and FliC:I-Ab+ CD4+ T cells were also detected in the spleen and lymph nodes of BALB/c and NOD mice (Fig. 1B), which express I-Ad and I-Ed, or I-Ag7 molecules. The T cells in all strains were detected via specific binding of the tetramers to the relevant TCRs as evidenced by the finding that the 2 tetramers detected mutually exclusive populations (Fig. 1B). In addition, each tetramer stained very few CD8+ T cells [≈5 per mouse (11)], or CD4+ T cells from TCR transgenic lines (18, 19) that could only recognize ovalbumin peptide:MHCII ligands (Fig. 1C). B6 and BALB/c mice contained about 170 2W1S:I-Ab+ and 30 FliC:I-Ab+ naive CD4+ T cells in the spleen and lymph nodes (Fig. 1D). C.B10-H2b and B6.C-H2d mice had 339 ± 14 (n = 3) and 84 ± 12 (n = 3) 2W1S:I-Ab+ CD4+ T cells, respectively. In contrast, NOD mice contained about 600 2W1S:I-Ab+ and 160 FliC:I-Ab+ CD4+ T cells (Fig. 1D). FVB (H-2q) mice contained twice as many 2W1S:I-Ab+ naive CD4+ T cells (586 ± 113, n = 5) as B6 mice.
Fig. 1.
Detection of naive foreign peptide:MHCA-binding T cells in mice expressing MHCB. (A) Gates used to identify T cells in peptide:I-Ab tetramer-enriched spleen and lymph node or thymus samples. T cells were identified as CD3+ B220- CD11b- F4/80- CD11c- events (Left), which were further gated into CD4+ and CD8+ populations (Right). (B) FliC:I-Ab tetramer versus 2W1S:I-Ab tetramer staining for CD4+ T cells from 2W1S:I-Ab and FliC:I-Ab tetramer-enriched spleen and lymph node or thymus samples from naive mice. (C) CD44 versus 2W1S:I-Ab tetramer staining on CD4+ T cells from 2W1S:I-Ab tetramer-enriched spleen and lymph node samples of an OTII Rag2−/− or a DO11.10 Rag1−/− TCR transgenic mouse. (D) Total CD4+ 2W1S:I-Ab+ (circles) or FliC:I-Ab+ (triangles) cells from the spleen and lymph nodes (Left) or thymus (Right) of individual mice with the mean values for each group indicated as horizontal bars and numerically under each set of values (Upper: 2W1S:I-Ab; Lower: FliC:I-Ab).
A similar situation was observed in the thymus. Tetramer enrichment was performed on all of the thymocytes from individual 6–8 week old mice. Mutually exclusive 2W1S:I-Ab- and FliC:I-Ab-binding cells were detected in the CD4SP but not CD8SP thymocyte populations (Fig. 1A) in B6, BALB/c, and NOD mice (Fig. 1B). The average sizes of the 2W1S:I-Ab+ CD4SP populations in B6, BALB/c, and NOD mice were 180, 130, and 245 cells per mouse, respectively, whereas the FliC:I-Ab+ CD4SP populations in both B6 and BALB/c strains were 15 cells per mouse and 45 cells per NOD mouse (Fig. 1D). Therefore, foreign peptide:I-Ab-binding CD4+ T cells were generated in the thymuses and exported to the periphery in mice that expressed I-Ad and I-Ed at the same frequency as in mice that expressed I-Ab molecules, and at an even higher frequency in mice that expressed I-Ag7.
Foreign Peptide:I-Ab-Specific T Cell Populations in B6 and BALB/c Mice Have Different Compositions.
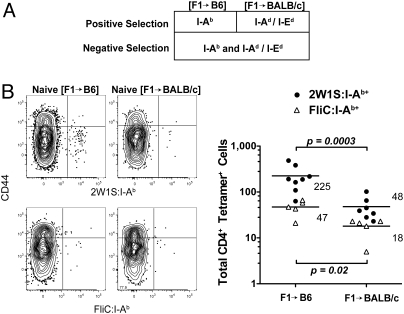
Although the foreign peptide:I-Ab-binding CD4+ populations in B6 and BALB/c mice were numerically similar, the fine specificity of their constituent clones could have been different. This was a likely possibility because the population in B6 but not BALB/c mice was subjected to negative selection by self peptide:I-Ab complexes. Radiation bone marrow chimeras were used to address this point. (B6 × BALB/c) F1 bone marrow cells were injected into lethally irradiated BALB/c (I-Ad/I-Ed) or B6 (I-Ab) hosts to produce F1 > BALB/c or F1 > B6 chimeras. Because negative selection is caused mainly by radio-sensitive bone marrow-derived cells (20) and positive selection by the radio-resistant thymic epithelium (21) (Fig. 2A), the T cell repertoires in these mice would be expected to be devoid of clones with high affinity for self peptides bound to I-Ab, I-Ad, and I-Ed molecules, and positively selected for clones with low affinity for self peptides bound to either I-Ab or I-Ad and I-Ed molecules.
Fig. 2.
Foreign peptide:I-Ab-specific T cell populations in F1 > parent chimeras. (A) Summary showing the MHCII molecules responsible for positive and negative selection of CD4+ T cells in F1 > parent chimeras. (B) CD44 versus 2W1S:I-Ab (Upper) or FliC:I-Ab (Lower) tetramer staining for CD4+ T cells from the spleen and lymph nodes of a naive F1 > B6 (Left) or F1 > BALB/c (Right) chimera and total CD4+ 2W1S:I-Ab+ (circles) or FliC:I-Ab+ (triangles) cells from the spleen and lymph nodes of individual F1 > B6 or F1 > BALB/c chimeras with the mean values for each group indicated as horizontal bars. P values are indicated on the plot.
F1 > B6 chimeras had significantly more naive 2W1S:I-Ab+ or FliC:I-Ab+ CD4+ T cells in the spleen and lymph nodes than F1 > BALB/c chimeras (225 versus 48, and 47 versus 18, respectively) (Fig. 2B). Similarly, the number of 2W1S:I-Ab+ CD4SP thymocytes in F1 > B6 chimeras (139 ± 39, n = 4) was significantly greater (P value = 0.001) than the number in F1 > BALB/c chimeras (15 ± 3, n = 4). Therefore, when negative selection was induced by self peptides bound to I-Ab, I-Ad, and I-Ed molecules, naive foreign peptide:I-Ab-specific CD4+ T cell populations were larger when positively selected in thymuses expressing I-Ab than in thymuses expressing I-Ad/I-Ed molecules. This finding indicated that many clones in the foreign peptide:I-Ab-specific populations in intact BALB/c mice were also specific for self peptide:I-Ab complexes. The presence of these “alloreactive” clones indicated that the 2W1S:I-Ab+ and FliC:I-Ab+ CD4+ T cell populations in BALB/c mice had a different clonal composition than the comparable populations in B6 mice.
Foreign Peptide:I-Ab-Specific T Cell Populations in BALB/c Mice Were Less Functional Than Those in B6 Mice.
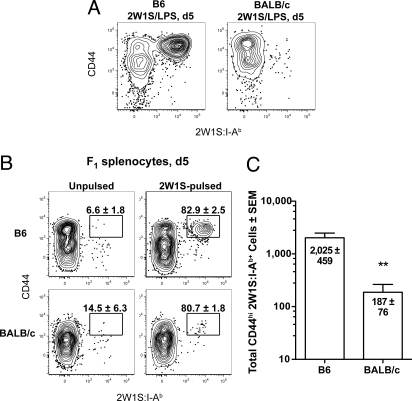
The differences in fine specificity raised the possibility that the T cell populations in B6 and BALB/c mice might respond differently to immunization. The activation of 2W1S:I-Ab-binding CD4+ T cells in B6 and BALB/c mice was assessed 5 days after i.v. injection of 2W1S peptide plus the adjuvant lipopolysaccharide (LPS) to test this possibility. As shown in Fig. 3A, this injection induced the naive 2W1S:I-Ab+ population in B6 but not BALB/c mice to undergo robust clonal expansion and conversion to the CD44high phenotype. However, this was probably not a true test of the function of the 2W1S:I-Ab-binding T cells in BALB/c mice because I-Ad or I-Ed molecules would present the 2W1S peptide in this case.
Fig. 3.
Foreign peptide:I-Ab-specific T cell populations in BALB/c mice were less functional than those in B6 mice. (A) CD44 versus 2W1S:I-Ab tetramer on CD4+ T cells from 2W1S:I-Ab tetramer-enriched spleen and lymph node cells from a B6 (Left) or BALB/c (Right) mouse 5 days after i.v. injection of 50 μg 2W1S peptide and 5 μg LPS. (B) CD44 versus 2W1S:I-Ab tetramer plots as in A from a B6 (Upper) or BALB/c (Lower) mouse 5 days after i.v. injection of unpulsed (Left) or 2W1S peptide-pulsed (Right) F1 splenocytes. The mean percentage (± SEM, n = 3) of CD44high cells in the 2W1S:I-Ab+ population is indicated on each plot. (C) Mean CD44high 2W1S:I-Ab+ cells (± SEM, n = 3) from B6 or BALB/c mice 5 days after i.v. injection of 2W1S peptide-pulsed F1 splenocytes. **, P value < 0.01.
To circumvent this problem, activation was measured 5 days after i.v. injection of F1 spleen cells that were pulsed in vitro with 2W1S peptide. In this case, the 2W1S:I-Ab-binding T cells in both strains would be exposed to 2W1S:I-Ab complexes on the F1 antigen-presenting cells. As shown in Fig. 3B, injection of 2W1S-pulsed F1 spleen cells into B6 mice stimulated the naive 2W1S:I-Ab+ population to undergo marked clonal expansion and conversion to the CD44high phenotype, whereas injection of unpulsed F1 spleen cells did not. 2W1S-pulsed but not unpulsed F1 spleen cells also stimulated the naive 2W1S:I-Ab+ population in BALB/c mice to convert to the CD44high phenotype and undergo some expansion (Fig. 3 B and C). The expansion was much less than that observed in B6 mice (Fig. 3C, P value < 0.01), suggesting that the naive 2W1S:I-Ab+ population in BALB/c mice was less functional than that in B6 mice. Again, however, it is possible that this experiment was not a fair test of the functionality of the BALB/c population. The F1 spleen cells were certainly rejected by both B6 and BALB/c recipients. This process could have released the 2W1S peptide from the F1 cells such that it could be bound by I-Ab molecules on recipient antigen-presenting cells and re-presented to the naive 2W1S:I-Ab+ T cells. Because this type of re-presentation may not occur in BALB/c mice, the naive 2W1S:I-Ab+ population in BALB/c mice would be at a disadvantage.
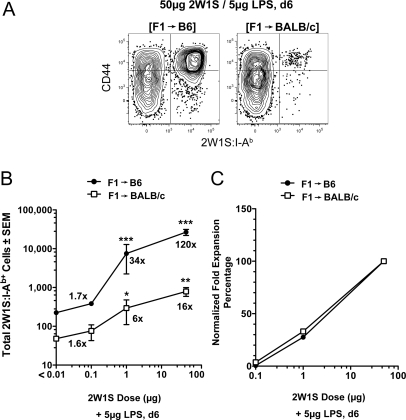
Radiation bone marrow chimeras were again useful for addressing this issue. F1 > B6 and F1 > BALB/c chimeras both contained F1 antigen-presenting cells capable of producing peptide:I-Ab complexes, and contained T cell repertoires that were tolerant of self peptide:I-Ab complexes. The only difference was that one repertoire was positively selected on I-Ab and the other on I-Ad/I-Ed (Fig. 2A). The capacity of peptide:I-Ab-specific T cells in these mice to respond to an antigen was tested by injection of 50 μg of 2W1S peptide plus LPS. The population of ≈200 2W1S:I-Ab+ T cells in naive F1 > B6 chimeras increased 120-fold to 24,000 cells 6 days after injection, whereas the initial population of 40 cells in naive F1 > BALB/c chimeras increased only 16-fold to 640 cells (Fig. 4 A and B). Thus, 2W1S:I-Ab-binding T cells that were positively selected by I-Ad/I-Ed and negatively selected by I-Ab were capable of responding to 2W1S, but 7-fold less well on a per cell basis than those that were positively and negatively selected by I-Ab.
Fig. 4.
2W1S:I-Ab-specific T cell populations in F1 > BALB/c chimeras are less functional than those in F1 > B6 chimeras. (A) CD44 versus 2W1S:I-Ab tetramer on CD4+ T cells from 2W1S:I-Ab tetramer-enriched spleen and lymph node cells from a F1 > B6 (Left) or F1 > BALB/c (Right) mouse 6 days after i.v. injection of 2W1S peptide and LPS. (B) Mean CD4+ 2W1S:I-Ab+ cells (± SEM, n ≥ 3 at each dose) from F1 > B6 (filled symbols) or F1 > BALB/c (open symbols) chimeras 6 days after i.v. injection of the indicated amounts of 2W1S peptide and LPS. Values that are significantly greater than the naive values are indicated by asterisks: ***, P value < 0.001; **, P < 0.01; *P < 0.05. The numbers near each data point represent the fold increase of CD4+ 2W1S:I-Ab+ cells calculated by dividing the mean number of CD4+ 2W1S:I-Ab+ cells from immunized mice by the mean number of CD4+ 2W1S:I-Ab+ cells from the naive chimeras shown in Fig. 2. (C) Normalized fold expansion percentage values = [(mean number at indicated dose − mean naive number)/(mean number at 50 μg dose − mean naive number)] × 100.
The antigen dose used for immunization was reduced to assess the functional avidity of the T cells in the chimeras. The 2W1S:I-Ab+ T cells in F1 > B6 chimeras increased 34-fold following injection of 1 μg of 2W1S (Fig. 4B). Notably, the cells in F1 > BALB/c chimeras were also capable of responding to this low dose of 2W1S as evidenced by a 6-fold expansion over the starting number. When the fold expansions were set to 100% at the 50 μg dose, it became clear that antigen dose-response curves for the cells in each population that were capable of expansion were very similar (Fig. 4C). Together, the results lead to 2 conclusions: (i) 5- to 7-fold more of the 2W1S:I-Ab-binding CD4+ T cells that were positively selected on I-Ab were capable of responding to 2W1S than was the population that was selected on I-Ad/I-Ed, and (ii) the functional avidities of the cells that were capable of responding in both populations was similar.
Detection of Naive Foreign Peptide:MHCA-Binding T Cells in Humans Expressing MHCB.
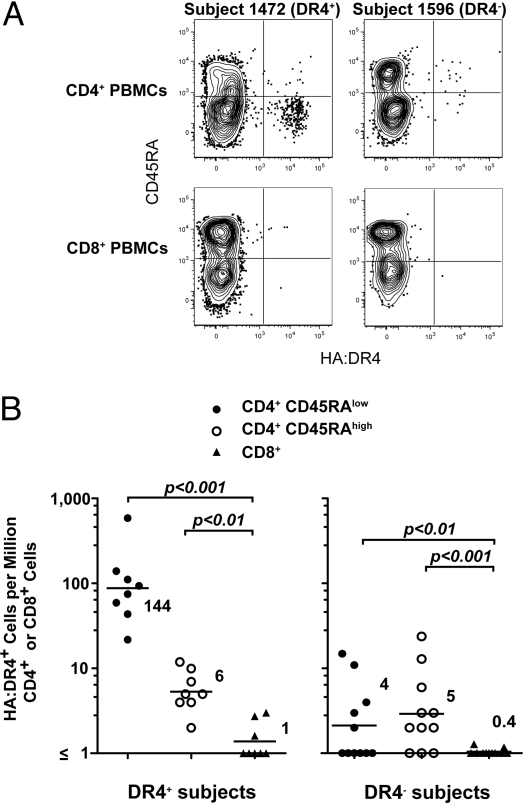
The experiments comparing the T cell repertoires in mice indicated that naive foreign peptide:MHCA-binding T cells were present in expressing MHCB hosts. We sought to confirm this conclusion in humans. Magnetic bead enrichment was performed on peripheral blood mononuclear cells (PBMCs) from influenza virus vaccinated people using a tetramer containing the influenza hemagglutinin (HA) peptide 307–319 (22) bound to HLA-DR4 (DR4).
All vaccinated individuals who expressed DR4 contained a CD45RAlow HA:DR4-binding CD4+ T cell population at a frequency of about 140 cells per million CD4+ T cells (Fig. 5 A and B). The lack of CD45RA (Fig. 5A) suggested that these were the memory cell progeny of naive cells that had responded to the vaccine (23). DR4+ individuals also had much smaller (6 cells per million CD4+ T cells) populations of CD45RAhigh naive HA:DR4-binding CD4+ T cells (Fig. 5 A and B). These cells may not have been activated because they did not enter the draining lymph nodes during the time that the vaccine antigen was presented there. HA:DR4 tetramer binding to both CD45RAlow and CD45RAhigh CD4+ populations was TCR-specific, as it bound to less than 1 cell per million CD8+ T cells (Fig. 5 A and B).
Fig. 5.
Detection of naive foreign peptide:MHCA-binding T cells in humans expressing MHCB. (A) Contour plots of CD45RA versus HA:DR4 tetramer fluorescence intensity for CD19− CD4+ (Upper) or CD8+ (Lower) cells from tetramer-enriched samples from a subject who expresses DR4 (Left) or a different subject who expresses non-DR4 alleles (Right), 1 year after influenza virus vaccination. (B) The number of CD45RAlow (filled circles) or CD45RAhigh (open circles) CD4+ tetramer+ cells per million total CD4+ T cells and CD8+ tetramer+ cells per million total CD8+ T cells (triangles). The mean values indicated as horizontal bars are shown for each group.
Remarkably, CD45RAhigh naive HA:DR4-binding CD4+ T cells were detected in most DR4− individuals at a similar frequency (5 cells per million total CD4+ cells) as in DR4+ individuals (Fig. 5A and B). In contrast to DR4+ individuals, DR4− individuals had very few CD45RAlow HA:DR4-binding CD4+ memory T cells despite having been vaccinated (Fig. 5 A and B). This finding could have been related to the fact that the antigen-presenting cells in DR4− individuals do not express DR4, and thus would be incapable of presenting the DR4-restricted HA peptide after vaccination. In any case, the presence of naive phenotype HA:DR4-binding CD4+ T cells in people lacking DR4 indicated that positive selection on DR4 was not absolutely required for the generation of this population.
Discussion
The presence of 2W1S:I-Ab-binding T cells in I-Ab− mice and HA:DR4-binding T cells in DR4− individuals would appear to be at odds with the theory of positive selection. However, several pieces of evidence indicated that these T cells differed from the 2W1S:I-Ab-binding T cells in B6 mice or HA:DR4-binding T cells in DR4+ individuals. First, the unlawful 2W1S:I-Ab-binding T cells in BALB/c mice or HA:DR4-binding T cells in DR4− individuals did not become activated following injection of 2W1S or HA. This finding could have been related to the fact that these peptides cannot bind to I-Ad/I-Ed or non-DR4 HLA molecules. Alternatively, 2W1S:I-Ad/I-Ed or HA:non-DR4 complexes may have formed but were unrecognizable by 2W1S:I-Ab- or HA:DR4-binding T cells. Thus, unlike the 2W1S:I-Ab- or HA:DR4-binding T cells in B6 mice or DR4+ people, the 2W1S:I-Ab- or HA:DR4-binding T cells in BALB/c mice or DR4− humans were not useful participants in the immune responses to these peptides.
The second piece of evidence indicating that the 2W1S:I-Ab-binding T cells in BALB/c mice differed from those in B6 mice related to fine specificity. The finding that the number of 2W1S:I-Ab-binding cells in F1 > BALB/c chimeras was 5-fold lower than the number in intact BALB/c hosts indicated that many of the cells in BALB/c mice were also specific for unknown self peptide:I-Ab complexes and would have been deleted in an I-Ab-expressing thymus. Huseby et al. (24) described similar cells in transgenic mice expressing a single self peptide:MHC complex. These cells recognized peptides bound to many allelic forms of MHC and were tolerant of many TCR contact residue substitutions in the peptide. The absence of these peptide:MHC degenerate/promiscuous clones in normal hosts indicates that although they may be positively selected, they are then normally removed by negative selection via recognition of a diverse set of self peptide:MHC ligands.
Because many of the 2W1S:I-Ab-binding T cells in BALB/c mice were also specific for unknown self peptide:I-Ab complexes, they could be thought of as alloreactive T cells. It should be noted, however, that these cells did not show signs of activation following injection of F1 spleen cells that were not pulsed with 2W1S peptide. Thus, it is possible that the self peptide:I-Ab complexes that caused the deletion of such cells in F1 > BALB/c chimeras were different from those displayed by B6 spleen cells. Alternatively, the TCRs on these cells may have had a high enough affinity for certain self peptide:I-Ab complexes to cause deletion of immature T cells but not high enough to activate mature cells as described in another system (25).
Notably, some foreign peptide:I-Ab-binding naive CD4+ T cells remained in F1 > BALB/c chimeras. This finding indicates that some genuine foreign peptide:I-Ab-specific, self peptide:I-Ab-tolerant T cells can be positively selected by I-Ad and/or I-Ed. The alternative explanation that these clones were positively selected by I-Ab molecules expressed on the F1 bone marrow derived cells was ruled out by earlier work showing bone marrow-derived cells cannot mediate positive selection of CD4+ T cells (26). However, it is important to note that the 2W1S:I-Ab-binding populations in F1 > BALB/c chimeras were 5-times smaller than the comparable ones in F1 > B6 chimeras. Thus, positive selection on self MHC (in this case defined as the I-Ab of the tetramer) had a beneficial effect on naive population size. This effect was smaller than the 40- to 600-fold effects observed in classic experiments by Bevan (6) and Sprent (7) for cytotoxic and helper T cell responses in immunized mice.
The discrepancy was explained by our finding that a smaller fraction of the 2W1S:I-Ab-binding population in F1 > BALB/c mice responded to antigen injection compared to the comparable population in F1 > B6 mice. Notably, the responsive cells in both groups had the same functional avidity. These results are consistent with the possibility that the 40 naive 2W1S:I-Ab tetramer-binding cells in F1 > BALB mice contained a small subpopulation that responded to 2W1S:I-Ab with exactly the same sensitivity as the majority of cells in F1 > B6 mice, and a larger subpopulation that did not respond even at the highest dose of antigen. Therefore, although positive selection on MHCA was not absolutely required to produce foreign peptide:MHCA-binding clones, it had a large effect on selecting responsive clones.
Materials and Methods
Mice.
Six- to 8-week-old C57BL/6 (B6), BALB/c, C.B10-H2b, B6.C-H2d, (B6 × BALB/c) F1, NOD, and FVB mice were purchased from the National Cancer Institute or from The Jackson Laboratory. DO11.10 Rag1−/− and OT II Rag2−/− mice were bred in facilities at the University of Minnesota. All mice were housed in specific pathogen-free conditions in accordance with University of Minnesota and National Institutes of Health guidelines.
Bone-Marrow Chimeras.
T cell depleted bone marrow cells obtained from (B6 × BALB/c) F1 mice were injected intravenously into lethally irradiated (1,000 rad) B6 or BALB/c mice. Chimeras were used for experiments 8 to 12 weeks after bone marrow transfer. Greater than 97% of the blood cells in the chimeras at this time were of F1 origin as evidenced by co-expression of H-2Db and H-2Dd.
Peripheral Blood Analyses.
Sixty milliliters of heparinized blood was drawn from volunteers from whom informed consent was obtained according to a protocol approved by the University of Minnesota institutional review board. Five milliliters of blood was used for HLA typing by the University of Minnesota Immunology/Histocompatibility Lab. Peripheral blood mononuclear cells (PBMCs) were isolated from the remaining 55 mL by Histopaque (Sigma) density gradient centrifugation according to the manufacturer's protocol. PBMCs were then analyzed for HA:DR4-specific CD4+ T cells by tetramer enrichment as described below.
Antibodies.
Fluorochrome-labeled antibodies specific for murine or human molecules were purchased from eBioscience, Caltag, or BD PharMingen.
Immunizations.
Mice were injected intravenously with 50, 1, or 0.1 μg of 2W1S or FliC peptides (GenScript Corp.) with 5 μg of LPS (List Biologicals) or 2 × 106 T cell depleted (B6 × BALB/c) F1 splenocytes that were first incubated with 100 μg/mL 2W1S peptide at 37 °C for 2 h. Human subjects received the inactivated influenza vaccine containing an H3N2 A virus, an H1N1 A virus, and a B virus via intramuscular injection.
Tetramer Production.
Soluble 2W1S (EAWGALANWAVDSA):I-Ab and FliC (VQNRFNSAITNLGNT):I-Ab molecules were produced as described by Moon et al. (11). A similar approach was used to produce soluble HA:DR4 molecules. A pRMHa-3 vector was constructed to encode the DRA*0101 chain followed by an acidic leucine zipper and a BirA biotinylation signal sequence at the C terminus. A separate pRMHa-3 vector was constructed to encode the influenza A virus hemagglutinin 307–319 peptide (PYKVKQNTLKLAT) (22) fused to DRB1*0401 by a flexible polyglycine linker followed by a basic leucine zipper and a His epitope tag at the C terminus.
Tetramer-based Enrichment.
Tetramer-based enrichment was performed as previously described by Moon et al. (11, 15).
Statistical Methods.
Standard error of the mean and P values were determined using Prism software (GraphPad Software, Inc.). P values were calculated using appropriate 2-tailed t test or ANOVA analysis followed by Bonferroni posttest with a 95% confidence interval.
Acknowledgments.
This work was supported by an American Heart Association predoctoral fellowship (to H.H.C.), by National Institutes of Health Grant R01 AI39614 (to M.K.J.), and by National Center for Research Resources Grant M01 RR00400.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 4.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 5.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Bevan MJ. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- 7.Sprent J. Restricted helper function of F1 leads to parent bone marrow chimeras controlled by K-end of H-2 complex. J Exp Med. 1978;147:1838–1842. doi: 10.1084/jem.147.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Alam SM, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 10.Berg LJ, Davis MM. T-cell development in T cell receptor alpha beta transgenic mice. Semin Immunol. 1989;1:105–116. [PubMed] [Google Scholar]
- 11.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotturi MF, et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009 doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon JJ, et al. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees W, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 18.Murphy DB, et al. Monoclonal antibody detection of a major self peptide. MHC class II complex. J Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- 19.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P, Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989;338:74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- 21.Vukmanovic S, et al. Positive selection of T-lymphocytes induced by intrathymic injection of a thymic epithelial cell line. Nature. 1992;359:729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wedderburn LR, et al. Mapping T cell recognition: The identification of a T cell receptor residue critical to the specific interaction with an influenza hemagglutinin peptide. Eur J Immunol. 1995;25:1654–1662. doi: 10.1002/eji.1830250627. [DOI] [PubMed] [Google Scholar]
- 23.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 24.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markowitz JS, Auchincloss H, Jr, Grusby MJ, Glimcher LH. Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice. Proc Natl Acad Sci USA. 1993;90:2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]