Abstract
Ecological responses to climate change may occur gradually with changing conditions, or they may occur rapidly once some threshold or “tipping point” has been reached. Here, we use a high-resolution, 30-year data set on the upper vertical limit of a high intertidal alga to demonstrate that distributional shifts in this species do not keep pace with gradual trends in air temperature or sea level, but rather occur in sudden, discrete steps. These steps occur when unusually warm air temperatures are associated with unusually calm seas and are contingent in the sense that neither atmospheric nor sea conditions by themselves were sufficient to generate the underlying physiological challenge. Shifts in the upper limit did not correlate with large environmental perturbations such as El Niños; rather, they appeared to be associated with stochastic departures from otherwise gradual environmental trends. Our results exemplify the view that multiple environmental factors should be considered when attempting to understand ecological responses to climate change. Furthermore, punctuated responses such as those we have identified urge caution when attempting to infer causal mechanisms and project future distributional shifts using data of limited temporal resolution or scope.
Keywords: Mazzaella parksii, intertidal zonation, range shift, sea level, temperature
Distributional limits, especially as determined along steep physiological gradients as found on mountain slopes and rocky intertidal shores, provide ecologists with model systems against which to evaluate the relationship between the biota and the environment. Studies of such systems are particularly instructive with regard to the effects of anthropogenic climate change. Globally, the planet's surface warmed by ≈0.74 °C during the 20th century, and is expected to rise another 1.8−4.0 °C during the current one (1). Ecological responses to climate forcing are already evident in the form of shifting patterns of species distribution and abundance (2–4). Although a wide range of ecological changes has been attributed to climate forcing, the mechanistic relationships between shifts in the abiotic environment and ecological responses are not straightforward.
Temporal shifts in the distribution and abundance of species may be gradual or punctuated; each of these patterns suggests different mechanisms and has different implications. Distributional limits or local abundance may change gradually because the underlying forcing agent(s) is (are) changing gradually or because populations are buffered in ways that “smooth out” stochastic environmental fluctuations (e.g., sublethal effects and time lags between changes in the environment and changes in population size). Conversely, punctuated ecological change may be the result of sudden changes in forcing agents (e.g., climatic regime shifts) or the effects of gradual or stochastic changes that periodically, either acting independently or together, exceed some critical threshold or “tipping point.” In the case of tipping points, ecological change is rapid, temporally nonlinear, and difficult to anticipate, even when the underlying climatic change is gradual and temporally linear (5, 6). Evidence for the importance of tipping points has been accumulating in a wide range of ecological systems and in organisms ranging from diatoms to copepods to salmon (6), and tipping points are often well described in ecological models (e.g., ref. 7).
Although distributional shifts along gradients of latitude, elevation, and depth are considered to be the “fingerprint” of anthropogenic climate change (3, 4), there is remarkably little information on the extent to which distributional shifts occur as sudden changes associated with tipping points. Most of the data on range shifts consist of very few time points—often just a beginning and an end. Thus, many range shifts can be correlated to general trends, but not attributed to specific climatic circumstances that allow (or prevent) the existence of a species in a particular place. Although broad-brush patterns encourage broad-brush predictions, they do not permit identification of important thresholds or make specific mechanistically based predictions regarding future range shifts in species of ecological or socioeconomic concern. This is especially true when ecological change is driven by rare combinations of environmental conditions.
Here, we investigate the patterns and underlying causes in changes in the vertical distribution of an intertidal red alga, Mazzaella parksii, in a high-resolution data set from Tatoosh Island, Washington State. (See Methods for life history details). Like many intertidal organisms, M. parksii already lives near its environmental tolerance limits, particularly with regard to temperature (8). Once disturbed, limited dispersal (generally <50 cm) and extremely poor survivorship of recruits result in long recovery times even if source populations are nearby. M. parksii zonation thus serves as a model system for understanding larger-scale distributional shifts across gradients of latitude, elevation, and depth. In this study, we compare multidecadal change (1978–2008) in the upper limit, measured as frequently as every 2 weeks during the summer, to variation in abiotic stress. Specifically, we ask: (i) how has the intertidal environment changed, (ii) how has the vertical distribution of M. parksii changed, and (iii) if distributional change has not followed a temporally gradual trend, what are the potential mechanistic linkages between the environmental forcing and the ecological response?
Results and Discussion
Changes in the Abiotic Environment.
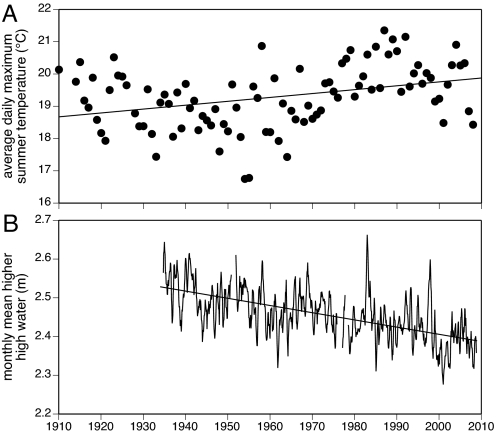
Air temperatures in northwest Washington State are influenced by both global warming trends and regional climatic cycles such as the Pacific Decadal Oscillation (PDO). The PDO alternates between warm and cool phases every 2 to 3 decades and underwent a well-known phase shift (cool to warm) in 1976 (9, 10); this shift is apparent in the increase in average summertime (April–September) daily maximum air temperatures near Tatoosh Island in the mid-1970s (Fig. 1A). In a multiple regression analysis, the effect of year (i.e., the effect of the anthropogenic signal which is, to a first approximation, temporally linear) and the effect of summertime PDO index (a cyclical signal) were both significantly related to summertime average daily maximum air temperature (year effect: F1,87 = 9.16, P = 0.0033; summertime PDO effect: F1,87 = 26.3, P < 0.0001). Together, these 2 variables accounted for approximately one-third of the variation in average daily maximum summer air temperatures over the past 99 years (multiple regression R2 = 0.32). Overall, the mean daily high temperature during the summer months has increased at an average rate of 0.131 °C per decade over the past century (Fig. 1A).
Fig. 1.
Long-term environmental change in the vicinity of Tatoosh Island, WA. (A) Average daily maximum air temperature for the summer months recorded at Forks, WA. (B) Trend in sea level (raw monthly MHHW plotted as a 5-month running average) at Neah Bay, Washington.
Relative sea level is also changing in the vicinity of Tatoosh Island, although the net effect is not in the expected direction. While global sea level has been rising an average of 1.8 mm per year since 1960 and as fast as 3.1 mm per year more recently (1), northwest Washington is being thrust upward at an even greater rate by the accumulation of strain associated with the subduction of the Juan de Fuca tectonic plate (11). The net result is relative sea level fall at Neah Bay, located 9 km east of the study site. The rate of this fall, in terms of the height of mean higher high water (MHHW), is ≈1.88 mm per year over the past 75 years (Fig. 1B), although brief anomalies (e.g., the 1982/1983 and 1997/1998 El Niño events) are readily apparent. The overall negative trend in the height of MHHW, using monthly data corrected for the annual cycle in tidal amplitude, was highly significant (F1,873 = 216, P < 0.0001, R2 = 0.20). From an organism's perspective, the net decrease in sea level should increase the physiological stresses at its upper limit and be reflected in a gradual decline of that limit.
Shifts in the Upper Limit of M. parksii.
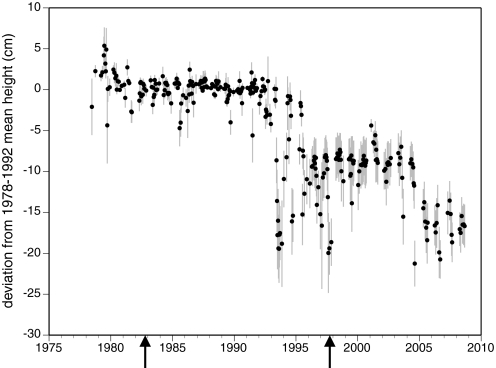
Despite ongoing environmental change, the upper limit of M. parksii remained remarkably stable from 1978 until 1993 (Fig. 2). (See Methods for details of upper limit assessment). In the summers of 1993 and 1994, and again in 1997, there were marked declines, although the upper limit recovered to its former position by the following spring. These recoveries were likely enabled by regrowth from the encrusting basal phase of this alga, which can survive even after the more easily observed upright fronds have been removed by disturbance (12). During the summers of 1995 and 2004, downward displacements of the upper limit occurred from which M. parksii has not recovered. Our data suggest that temperature shocks in these 2 years were sufficiently severe to kill the encrusting basal system (see Causes of Distributional Shifts below). Neither of the 2 major El Niños (1982–1983 and 1997–1998) that occurred during our study appeared to have had any lasting effect (Fig. 2), suggesting that the pronounced and sustained shifts in the upper limit had other causes.
Fig. 2.
Long-term change in the position of the upper limit of Mazzaella parksii on Tatoosh Island. Data are the deviation from the 1978–1992 mean, with negative deviations representing downshore shifts in the upper limit. Error bars are standard error (n = 8 sampling transects). The 2 strong El Niños that occurred during the study period (arrows) had no discernible effect on the upper limit.
Within the pronounced interannual changes in the position of the upper limit, there were repeated seasonal patterns. The upper limit of visible M. parksii fronds generally advanced upshore during the winter and retreated downshore during the summer (see Table S1). When comparing the position of the upper limit in April/May to that in August/September, summertime retreats occurred in 23 of 30 years. The average magnitude of this change (mean ± SE) was −3.01 ± 0.75 cm (where negative means downshore), which was significantly different from zero (Wilcoxon signed rank test, df = 29, t = −171.5, P < 0.001). The change from late summer to the following spring was on average upward (2.56 ± 0.69 cm) and was also significantly different from zero (Wilcoxon signed rank test, df = 28, t = 156.5, P < 0.001). Again, the apparent winter recovery of the upper limit in most years was likely accomplished by regrowth of upright fronds from a surviving encrusting basal system (12). Temporal variation in the position of the upper limit—seasonal or otherwise—did not change among the 3 relatively long periods of stasis (i.e., 1978–1992, 1998–2003, and 2005–2008; O'Brien's test for heterogeneity of variance P = 0.70). The apparent increase in variability after 1992 (see error bars in Fig. 2) arose because the post-1992 upper limit changed by different amounts in each of the 8 transects, leading to spatial variation in the displacement from the 1978–1992 means to which the data in each transect were standardized.
Causes of the Distributional Shifts.
Algal thallus temperature is determined by a variety of factors, including air temperature and the duration of emersion (13). M. parksii is known to be vulnerable to thermal stress associated with south-facing slopes and prolonged emersion during low tide (8). Waves have the potential to interrupt stress events by rehydrating organisms and by resetting body temperatures to that of sea water (14). Thus, the upper limits of sessile species, including M. parksii, are generally higher on the shore in areas of increasing wave exposure (14–16).
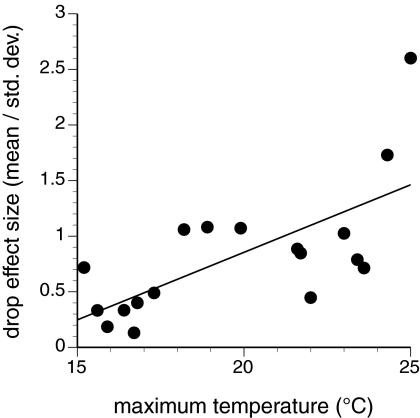
Given that stress will be positively related to air temperature unless ameliorated by waves (see also ref. 17), we compared the magnitude of the drop in the upper limit of M. parksii within a 4-week period, expressed as the effect size (mean/standard deviation), to the highest temperature recorded on days when seas were calm (significant wave height <1.0 m) (Fig. 3). The relationship was highly significant (F1,17 = 13.9, P = 0.0018, R2 = 0.47), with maximum temperature explaining nearly half of the variation in the magnitude of the downward shift in the upper limit. The 2 drops from which there was no recovery (in 1995 and 2004) coincided with the 2 hottest days with calm seas (24.3 °C and 25 °C, respectively). Interestingly, the only hotter day on record at Tatoosh was 25.6 °C on July 11, 2007. Although the buoy west of Tatoosh was not functioning during 2007, the next closest buoy (≈250 km south of Tatoosh, near the mouth of the Columbia River) recorded significant wave heights in excess of 2 m on that day. No change in the upper limit of M. parksii was associated with this high-temperature, high-wave event.
Fig. 3.
Relationship between maximum air temperature and the effect size (mean/standard deviation) of the largest drop in the upper limit over 4 weeks for all years with available data. Only days with calm seas (significant wave heights <1.0 m) were used in the determination of maximum air temperature in each year. The 2 largest effect-size values correspond to the upper limit shifts in 2004 and 1995. See text for details.
Despite many years of more moderate conditions, recovery from the 1995 and 2004 disturbances is not evident. One possible explanation is that the relative decline in sea level has made formerly occupied shore levels uninhabitable. This seems unlikely, however, since the rate of sea level fall (5.6 cm in 30 years) is much less than the downward shift in the algal turf's upper limit (roughly 16.8 cm in the 30 years since 1978). Alternatively, postdisturbance recovery may be hampered by the life history characteristics of M. parksii; dispersal is very limited (tens of centimeters), and isolated individuals, such as new recruits, are exceptionally vulnerable to desiccation, loss of photosynthetic capacity, and bleaching (18). Experimental clearings in M. parksii beds suggest that recovery from disturbance is usually achieved by vegetative regrowth (C.D.G.H., unpublished data), a process that occurs at rates of 3–8 mm per year (19). Applying that range of growth rates to our system, we can calculate that M. parksii would regain 16.8 vertical cm in 21 to 56 years if it were growing straight up a vertical wall. However, because our study site has an average slope of 18°, not 90°, and therefore the actual distance to recover is ≈54 cm, we predict it would actually take between 68 and 181 years for M. parksii to reclaim 16.8 vertical cm along our sloping shoreline by vegetative growth alone.
Implications of Future Warming Scenarios.
In the case of M. parksii on Tatoosh Island, the long-term trend in air temperature is temporally nonlinear, but this nonlinearity does not appear to influence the timing of shifts in the upper limit of the alga. Rather, the changes are more akin to sudden shifts associated with linear stochastic environmental trends (i.e., temporally linear trends with stochastic variation around the trend line), as has been found in a variety of marine ecological time series (6). Indeed, another turf-forming red alga (Endocladia muricata) also exhibits punctuated change in the position of its upper limit (20), although the exact causes remain unknown. The potentially widespread occurrence of punctuated change presents particular challenges for conservation biologists and resource managers. For one, it is difficult to use short time series to estimate long-term trends. For example, if M. parksii had been monitored only from 1978 to 1992, we would have concluded that the upper limit of this species was stationary. Conversely, if we had data only from 1992 to 2004, we would conclude that it is shifting more rapidly than it likely is. Although punctuated ecological responses are difficult to anticipate, they can be modeled and, in a probabilistic sense, anticipated if sufficient information exists regarding the environmental thresholds that induce ecological change and the resilience of a population or community following the stress event (17). Data on patterns of change at sufficient resolution are rare, but are invaluable in helping to pinpoint important abiotic thresholds. In this case, air temperatures above 24 °C, coupled with calm seas, appear to be the trigger for complete mortality at and near the upper limit of M. parksii. However, interannual variation in the upper limits of other marine species could have other explanations (21). Lengthy time series, as advocated by Biggs et al. (22), are required to identify both the magnitude and especially the likely causes of these shifts.
Our results suggest that occasional extreme high air temperatures, when coupled with low tides and calm seas, result in severe disturbance to M. parksii beds and that recovery from such disturbance is extremely slow. Given the anticipated changes in air temperature during the current century, we can make some basic predictions regarding the return time of severe stress events. We estimated average “return time” by dividing the total time sampled by the number of stress events (see ref. 17); this metric provides a measure of the frequency of extreme events. Taking only those days with verified calm seas, the 24 °C threshold was exceeded in only 2 (11%) of 18 years of observations (average return time = 9 years). If we take the conservative IPCC scenario for 21st century warming (+1.8 °C) and apply it to observed temperature records from Tatoosh Island, there would be 5 summers out of 18 with stress events that exceeded 24 °C (28% annual likelihood, average return time = 3.6 years). Using the high end IPCC scenario (+4.0 °C), the threshold would be exceeded in 8 of 18 years (44% annual likelihood, average return time = 2.25 years).
This increased frequency of disturbance would have important consequences for the distribution of intertidal species, including M. parksii. Our survey site is on a very wave-exposed promontory, which may make it especially vulnerable to calm weather as the M. parksii zone is above the extreme high tide line (15). However, even lower shore populations would be vulnerable to stressful air temperatures during low tides and during periods of neap high tides. Thus, rising temperatures should result in a stepwise decline in the upper limit of M. parksii at most if not all sites. It is important to note that this may not result in a concurrent downward shift in the lower limit. The lower limit is determined by molluscan herbivores, and this limit is independent of temperature across a gradient of substratum orientation (8). Thus, the M. parksii zone may eventually collapse to zero (a local extinction event) as conditions warm through time, as has been observed across local and regional scale temperature gradients in space (8). Furthermore, in the southern portions of its range, or at localized “hotspots” (23, 24), site-scale local extinctions should become more frequent, resulting in step-like range contractions that reflect the stochastic occurrence of lethal stress events.
Although the “tipping point” and the mechanisms underlying the responses of this high intertidal alga may seem minor on a global scale, they clearly identify the challenge to managers of anticipating, let alone reacting to more catastrophic events. When rare and uncorrelated stressors, generally harmless when alone, coincide, their compounded impact can have dramatic population consequences (17, 25). Such contingencies are potentially manageable when the stressors are of biological origin (e.g., anthropogenic nutrient input, fishing pressure; ref. 22). We are less optimistic when the forcing involves physical parameters (e.g., extreme ambient temperatures, wind velocity, wave height) such as we have identified. It remains to be determined whether the responses to our changing climate will be reflected in regime shifts involving integrated ecosystems or most clearly identified in the responses of individual species. Both are certain to be characterized by surprises, driven by contingencies, and dominated by complex patterns of temporal change.
Methods
Study Site and Species.
M. parksii (formerly Iridaea cornucopia) is a turf-forming red alga composed of upright fronds up to 4 cm in length and an encrusting basal system. M. parksii is common on exposed shores from northern California through Alaska (26); we studied M. parksii near the center of its latitudinal range on Tatoosh Island, located on the outer coast of Washington State (48°24′N 124°44′W). On the Tatoosh Island archipelago, M. parksii occupies a broad zone on north-facing slopes (8). Our focal population was located along the northern shore of North Island, which is a very wave-exposed bench subjected directly to northwesterly swell. Within the M. parksii zone, the bench slopes downward at a 10–25° (mean 18°) angle until it reaches a vertical cliff >4 m high. The lower boundary of the M. parksii zone coincides with the discontinuity in the slope of the substratum, below which molluscan grazers have a refuge from avian predators.
Environmental Data Collection.
Air temperature records for Tatoosh Island (1985 to 2007) were obtained from the National Data Buoy Center (NDBC) (station TTIW1). Wave height data (1987–2007) were obtained from NDBC buoy 46041 (115 km south of Tatoosh Island). Long-term monthly sea level data for Neah Bay (1934 to 2008) were obtained from the National Oceanographic and Atmospheric Administration (station 9443090). To best approximate sea level change relevant to a high intertidal species, we used mean higher high water (MHHW) rather than mean sea level. The tidal amplitude (MHHW–MLLW) varies on an annual cycle by ≈33 cm at Neah Bay; this variation was removed before statistical analysis by calculating each month's average deviation from the annual mean and subtracting this deviation from the raw data. Long-term air temperature records (1910–2008) were obtained from the Western Regional Climate Center for Forks, Washington (station 452914), located 55 km south southeast of Tatoosh Island. We used summertime (April–September) average daily maximum temperature as our variable of interest. This time period corresponds with daylight low tides on Tatoosh and with the season during which Mazzaella experiences thermal stress. It should be noted that Forks is several km inland relative to Tatoosh, and daily maximum air temperatures at Forks are higher by ≈4–5 °C. However, there is a strong correlation between average daily maximum summertime temperatures at Forks and those of the longest available historical (1931–1965) data set from Tatoosh Island (F1,34 = 24.8, P < 0.0001, R2 = 0.43). Data collected on Tatoosh in the mid-1900s were from a different location on the island, and are thus not directly comparable with the more recent (1985–present) Tatoosh time series. Monthly values of the Pacific Decadal Oscillation were obtained from http://jisao.washington.edu/data_sets/pdo/#data. As with air temperature, we calculated the summertime mean of the PDO index for each year since 1910 using data from April through September.
Mazzaella Surveys.
Series of 2, 3, and 3 permanent stainless steel bolts (n = 8) were drilled in the rock above the upper limit of M. parksii in 1978. Bolts were 1–2 m apart; the first 2 series were about 3 m apart, while the third was 20 m to the east. The upper limit of the M. parksii zone was measured as the distance from each bolt directly downshore to the uppermost edge of the continuous turf. Small outlying patches were ignored. The slope of the shore was surveyed with a rotating laser level along the 8 downshore transects below each of the 8 marker bolts. These measurements allowed us to translate measured distances from upper limit to bolt into absolute vertical positions relative to mean lower low water (MLLW).
Because the study bench varied slightly in its exposure to surge and spray, the upper limit of M. parksii was ≈30 vertical cm higher at the more westerly bolts than at the more easterly bolts. To standardize across this variation, we present upper limit data scaled relative to a long-term mean at each bolt. This mean was chosen to be the time period from 1978–1992, which coincided with the longest period of relative stasis in the data.
To facilitate comparisons of environmental conditions and biological responses, we calculated a simple metric for each. As a proxy for environmental stress, we recorded the highest temperature of each summer that occurred on a day when seas were calm (defined as an average significant wave height <1.0 m at buoy 46041). Average significant wave heights <1.0 m occurred on 14.9% of days for which wave data were available during the summer (April–September). The maximum drop in the upper limit of M. parksii was described as the maximum drop to occur over a 4-week period (which reflects the 2- to 4-week sampling interval during the summer) divided by the standard deviation of this drop across the 8 transects. This is equivalent to calculating an effect size and helps to downweight apparent drops, which are driven by changes at a small subset of the 8 sites. Note that, as defined, the maximum drop associated with a particular stress event could be greater than or less than the total change in the upper limit over the course of the summer (see Table S1). To reduce the possibility of coincidental (i.e., noncausal) associations, care was taken to ensure that drops occurring before the highest temperature of the summer were omitted (none of these drops were large). Statistical analyses were performed using JMP 7.0.1 (SAS Institute).
Supplementary Material
Acknowledgments.
Ideas presented in this manuscript benefitted from discussions with L. Hunt, and the manuscript itself was greatly improved by the input of M. Denny, B. Menge, and R. Kordas. C.D.G.H. thanks Natural Sciences and Engineering Research Council for funding a portion of this work. R.T.P. thanks Egbert G. Leigh for suggesting that the upper limit of Mazzaella should be assessed, the National Science Foundation for financing decades of underappreciated monitoring, and the Andrew W. Mellon Foundation for support. We both express our deep appreciation to the Makah Tribal Council for permission to work on their lands.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904946106/DCSupplemental.
References
- 1.IPCC. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. Climate Change 2007: The Physical Science Basis. [Google Scholar]
- 2.Barry JP, Baxter CH, Sagarin RD, Gilman SE. Climate-related, long-term faunal changes in a California rocky intertidal community. Science. 1995;267:672–675. doi: 10.1126/science.267.5198.672. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh C, Glaser SM, Lucas AJ, Sugihara G. Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature. 2005;435:336–340. doi: 10.1038/nature03553. [DOI] [PubMed] [Google Scholar]
- 7.van Nes EH, Scheffer M. Large species shifts triggered by small forces. Am Nat. 2004;164:255–266. doi: 10.1086/422204. [DOI] [PubMed] [Google Scholar]
- 8.Harley CDG. Abiotic stress and herbivory interact to set range limits across a two-dimensional stress gradient. Ecology. 2003;84:1477–1488. [Google Scholar]
- 9.Ware DM. A century and a half of change in the climate of the NE Pacific. Fish Oceanogr. 1995;4:267–277. [Google Scholar]
- 10.Francis RC, Hare SR, Hollowed AB, Wooster WS. Effects of interdecadal climate variability on the oceanic ecosystems of the NE Pacific. Fish Oceanogr. 1998;7:1–21. [Google Scholar]
- 11.Mitchell CE, Vincent P, Weldon II RJ, Richards MA. Present-day vertical deformation of the Cascadia margin, Pacific Northwest, United States. J Geophys Res B Solid Earth. 1994;99(B6):12,257–12,277. [Google Scholar]
- 12.Olson AM. Corvallis, OR: Oregon State University; 1985. Early succession in beds of the red alga, Iridaea cornucopiae Post. & Rupr. (Gigartinaceae): Alternate pathways. MS thesis. [Google Scholar]
- 13.Bell EC. Environmental and morphological influences on thallus temperature and desiccation of the intertidal alga Mastocarpus papillatus Kutzing. J Exp Marine Biol Ecol. 1995;191:29–55. [Google Scholar]
- 14.Harley CDG, Helmuth BST. Local- and regional-scale effects of wave exposure, thermal stress, and absolute vs. effective shore level on patterns of intertidal zonation. Limnol Oceanogr. 2003;48:1498–1508. [Google Scholar]
- 15.Leigh EG, Jr, Paine RT, Quinn JF, Suchanek TH. Wave energy and intertidal productivity. Proc Natl Acad Sci USA. 1987;84:1314–1318. doi: 10.1073/pnas.84.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JR. The Ecology of Rocky Shores. London: English Universities Press LTD; 1964. [Google Scholar]
- 17.Denny MW, Hunt LJH, Miller LP, Harley CDG. Ecol Monogr. 2009. On the prediction of extreme ecological events. in press. [Google Scholar]
- 18.Scrosati R, DeWreede RE. The impact of frond crowding on frond bleaching in the clonal intertidal alga Mazzaella cornucopiae (Rhodophyta, Gigartinaceae) from British Columbia, Canada. J Phycol. 1998;34:228–232. [Google Scholar]
- 19.Olson AM. Corvallis, OR: Oregon State University; 1992. Evolutionary and ecological interactions affecting seaweeds. PhD thesis. [Google Scholar]
- 20.Hunt LJH. Palo Alto, CA: Stanford University; 2006. The rise of Endocladia: punctuated change at an abrupt range edge. PhD thesis. [Google Scholar]
- 21.Denny MW, Paine RT. Celestial mechanics, sea-level changes, and intertidal ecology. Biol Bull. 1998;194:108–115. doi: 10.2307/1543040. [DOI] [PubMed] [Google Scholar]
- 22.Biggs R, Carpenter SR, Brock WA. Turning back from the brink: Detecting an impending regime shift in time to avert it. Proc Natl Acad Sci USA. 2009;106:826–831. doi: 10.1073/pnas.0811729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmuth B, et al. Mosaic patterns of thermal stress in the rocky intertidal zone: Implications for climate change. Ecol Monogr. 2006;76:461–479. [Google Scholar]
- 24.Helmuth B, et al. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2002;298:1015–1017. doi: 10.1126/science.1076814. [DOI] [PubMed] [Google Scholar]
- 25.Paine RT, Tegner MJ, Johnson EA. Compounded perturbations yield ecological surprises. Ecosystems. 1998;1998:535–545. [Google Scholar]
- 26.Abbott IA, Hollenberg GJ. Marine Algae of California. Palo Alto, CA: Stanford Univ Press; 1976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.