Abstract
Membrane depolarization activates voltage-dependent Ca2+ channels (VDCCs) inducing Ca2+ release via ryanodine receptors (RyRs), which is obligatory for skeletal and cardiac muscle contraction and other physiological responses. However, depolarization-induced Ca2+ release and its functional importance as well as underlying signaling mechanisms in smooth muscle cells (SMCs) are largely unknown. Here we report that membrane depolarization can induce RyR-mediated local Ca2+ release, leading to a significant increase in the activity of Ca2+ sparks and contraction in airway SMCs. The increased Ca2+ sparks are independent of VDCCs and the associated extracellular Ca2+ influx. This format of local Ca2+ release results from a direct activation of G protein-coupled, M3 muscarinic receptors in the absence of exogenous agonists, which causes activation of Gq proteins and phospholipase C, and generation of inositol 1,4,5-triphosphate (IP3), inducing initial Ca2+ release through IP3 receptors and then further Ca2+ release via RyR2 due to a local Ca2+-induced Ca2+ release process. These findings demonstrate an important mechanism for Ca2+ signaling and attendant physiological function in SMCs.
Keywords: muscarinic receptors, ryanodine receptors
Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyRs) in response to membrane depolarization drives the mechanical force and other cellular responses in skeletal and cardiac muscle cells. The underlying molecular processes in these 2 types of muscle cells differ greatly. In skeletal muscle cells, membrane depolarization activates voltage-dependent Ca2+ channels (VDCCs) in the plasma membrane, which can open RyR1 via their physical interaction with plasmalemmal VDCCs without an extracellular Ca2+ influx, inducing massive Ca2+ release from the SR. This process is termed voltage-induced Ca2+ release (VICR). In cardiac myocytes, activation of VDCCs by depolarization results in a small amount of extracellular Ca2+ influx. This Ca2+ influx subsequently causes the opening of RyR2, and then induces large Ca2+ release from the SR, a process of Ca2+-induced Ca2+ release (CICR) (1).
All 3 subtypes of RyRs (RyR1, RyR2, and RyR3) are expressed in smooth muscle cells (SMCs). However, there is no electrophysiological study showing skeletal VICR in SMCs. Cardiac-like CICR has been observed in bladder myocytes; however, unlike in cardiac cells, VDCCs and RyRs in these SMCs are “loosely coupled,” by which activation of VDCCs following depolarization produces non-obligate Ca2+ release from the SR; as such, sufficient Ca2+ influx through VDCCs is required to activate CICR (2, 3). In contrast, voltage depolarization does not evoke Ca2+ release in SMCs from numerous tissues including the trachea (4).
We here report that long voltage or chemical depolarization induces local Ca2+ release from the SR in airway SMCs without the involvement of VDCCs and associated extracellular Ca2+ influx. This form of local Ca2+ release results from a direct activation of native M3 muscarinic receptors (M3Rs) in the absence of exogenous agonists. Moreover, depolarization-evoked excitation of M3Rs turns on their downstream G protein−phospholipase C (PLC)-IP3 signaling axis, leading to initial Ca2+ release through IP3Rs and then further Ca2+ release via RyRs due to a local IP3R/RyR interaction-mediated CICR mechanism. The GPCR- and IP3R-dependent, depolarization-induced local Ca2+ release is specifically mediated by RyR2, but not RyR1 and RyR3. This M3R-dependent, depolarization-induced Ca2+ release evokes airway muscle contraction, showing an important physiological significance in SMCs.
Results
Membrane Depolarization Induces Ca2+ Release Through Ryanodine Receptors in SMCs.
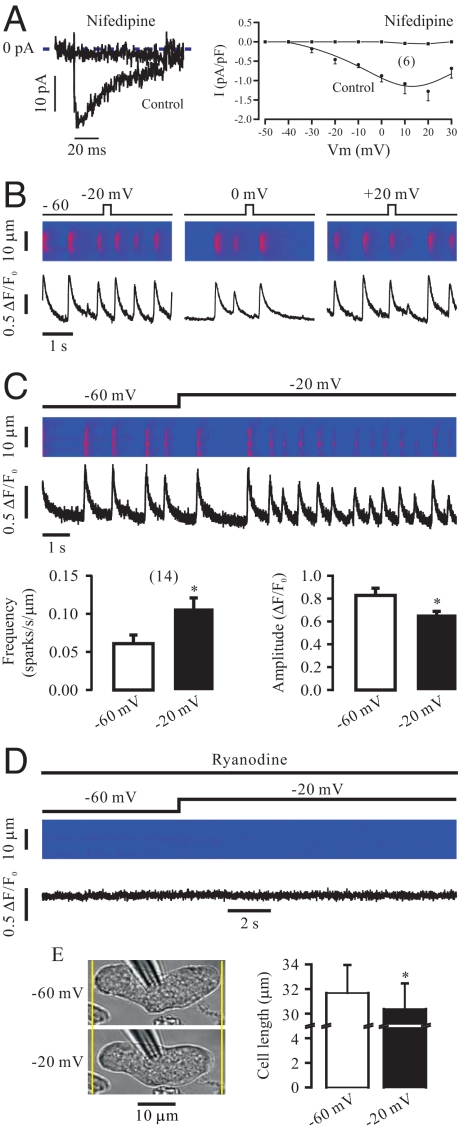
Voltage depolarization from a holding potential of −60 mV to various potentials between −40 mV and 30 mV for 250 ms generated VDCC currents that were selectively blocked by the channel blocker nifedipine (10 μM) for 5 min (Fig. 1A). As examples shown in Fig. 1B, voltage depolarization in such duration did not induce detectable, instantaneous Ca2+ release in 12–16 cells tested. Long depolarization for 5 s also did not evoke Ca2+ release in the initial first second. After that, however, local Ca2+ release was evident, leading to a large increase in the frequency of Ca2+ sparks (Fig. 1C). The mean frequency of Ca2+ sparks was increased from 0.061 ± 0.011 to 0.107 ± 0.014 sparks/s/μm (n = 14, P < 0.05). Thus, there is a latency of >1 s for depolarization-induced Ca2+ release. We also observed that the amplitude of Ca2+ sparks was reduced from 0.829 ± 0.062 to 0.659 ± 0.028 ΔF/F0 (P < 0.05). On the other hand, there were no changes in the Ca2+ spark rise time (31.9 ± 1.7 vs. 29.7 ± 0.9 ms), full duration at half maximal amplitude (29.7 ± 2.2 ms vs. 27.6 ± 1.3 ms), decay time (129.5 ± 15.1 vs. 116.3 ± 7.4 ms), and full width at half maximal amplitude (5.2 ± 0.4 vs. 4.9 ± 0.2 μm). As an example shown in Fig. 1D, application of ryanodine (100 μM) for 8 min to specifically inhibit RyRs abolished spontaneous Ca2+ sparks and subsequent depolarization-evoked Ca2+ sparks as well in 5 cells tested. In agreement with prominent Ca2+ release, voltage depolarization for 5 s caused significant contraction, determined by cell shortening, in isolated cells (Fig. 1E).
Fig. 1.
Voltage depolarization for a long, but not short duration induces local Ca2+ release in airway SMCs. (A) Original recordings (left) show VDCC currents generated by voltage depolarization from a holding potential of −60 mV to 10 mV for 250 ms in a cell before and after application of nifedipine (10 μM) for 5 min. The cell was voltage-clamped using nystatin-perforated patch clamp technique. Graph (right) illustrates the current-voltage curves for Ca2+ currents obtained from 6 different cells. (B) Voltage depolarization from −60 mV to −20, 0, and 20 mV for 250 ms did not induce detectable Ca2+ release in 12, 14, and 16 cells, respectively. (C) Voltage depolarization from −60 mV to −20 mV for over 1 s significantly increased the frequency of Ca2+ sparks, and decreased the amplitude of Ca2+ sparks. *, P < 0.05 compared with −60 mV. (D) Application of ryanodine (100 μM) for 8 min to specifically block RyRs abolished spontaneous and depolarization-induced local Ca2+ release. (E) Voltage depolarization from −60 mV to −20 mV for 5 s caused significant cell contraction, determined as the difference in cell length before and after voltage depolarization under perforated patch-clamp conditions.
Similar to voltage depolarization, chemical depolarization by elevating extracellular K+ from 5 to 60 mM for 1 min also caused local Ca2+ release in the absence of exogenous agonists, resulting in an increase in Ca2+ spark frequency and a decrease in Ca2+ spark amplitude without affecting other spatiotemporal characteristics (Fig. 2A). Chemical depolarization failed to induce local Ca2+ release in cells that were voltage-clamped at −60 mV (Fig. 2A) or pretreated with ryanodine (100 μM) for 8 min (Fig. 2B).
Fig. 2.
Chemical depolarization with high K+ exposure for 1 min also induces local Ca2+ release in airway SMCs. (A) Application of high K+ (60 mM) for 1 min significantly increased the frequency of Ca2+ sparks, and reduced the amplitude of Ca2+ sparks in cells that were not voltage-clamped; however, high K+ had no effect in cells voltage-clamped at −60 mV using the perforated patch clamp technique. (B) Application of ryanodine (Rya, 100 μM) prevented high K+-induced local Ca2+ release.
Membrane Depolarization-Induced Ca2+ Release Can Be Independent of Voltage-Dependent Ca2+ Channels and Associated Extracellular Ca2+ Influx in SMCs.
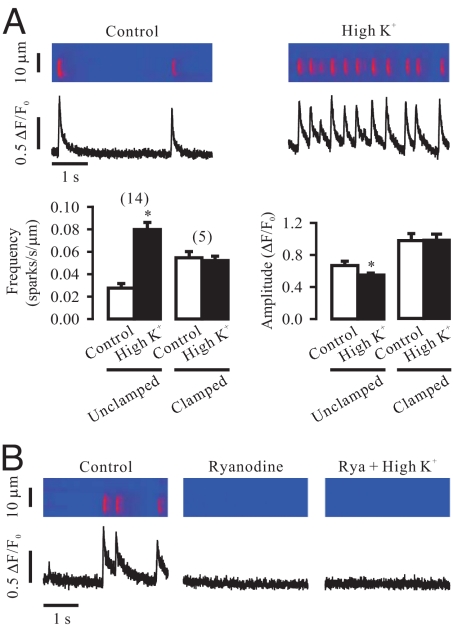
The selective VDCC blocker nifedipine (10 μM) or D600 (100 μM) for 5 min did not prevent the effect of chemical depolarization on the frequency and amplitude of Ca2+ sparks (Fig. 3A). Removal of extracellular Ca2+ (equilibration with nominally Ca2+-free plus 0.25 mM EGTA for 30 s) did not block the effect of chemical depolarization, either. This effect was not due to a significant Ca2+ contamination, as the free Ca2+ concentration in the recording chamber was found to be 1.87 ± 0.02 nM (n = 6). Moreover, chemical depolarization was able to induce significant contraction in isolated airway smooth muscle cells and tissues after treatment with nifedipine or removal of extracellular Ca2+ (Fig. 3B). Taken together, these data reveal that VDCCs and associated extracellular Ca2+ influx are not required for RyR-mediated local Ca2+ release and attendant contraction in SMCs.
Fig. 3.
Membrane depolarization-induced local Ca2+ release is independent of VDCCs and associated extracellular Ca2+ influx in airway SMCs. (A) Application of nifedipine (Nif, 10 μM) for 5 min to inhibit VDCCs or incubation with nominally Ca2+-free plus 0.25 mM EGTA bath solution (0 Ca2+) for 30 s to remove extracellular Ca2+ did not prevent chemical depolarization-evoked local Ca2+ release. (B) High K+ exposure was able to cause significant shortening in isolated airway SMCs (left) and muscle contraction (tension) in tracheal rings (normalized to tissue weight; right) after treatment with nifedipine (10 μM) for 5 min and incubation with nominally Ca2+-free plus 0.25 mM EGTA bath solution for 30 s.
Direct Activation of G Protein-Coupled Receptors and Their Downstream Signaling Axis Are an Initial, Indispensible Event for Depolarization-Induced Local Ca2+ Release in SMCs.
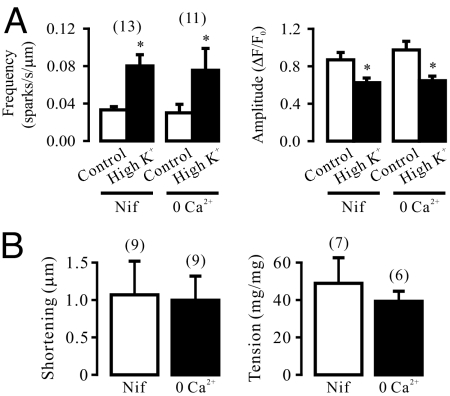
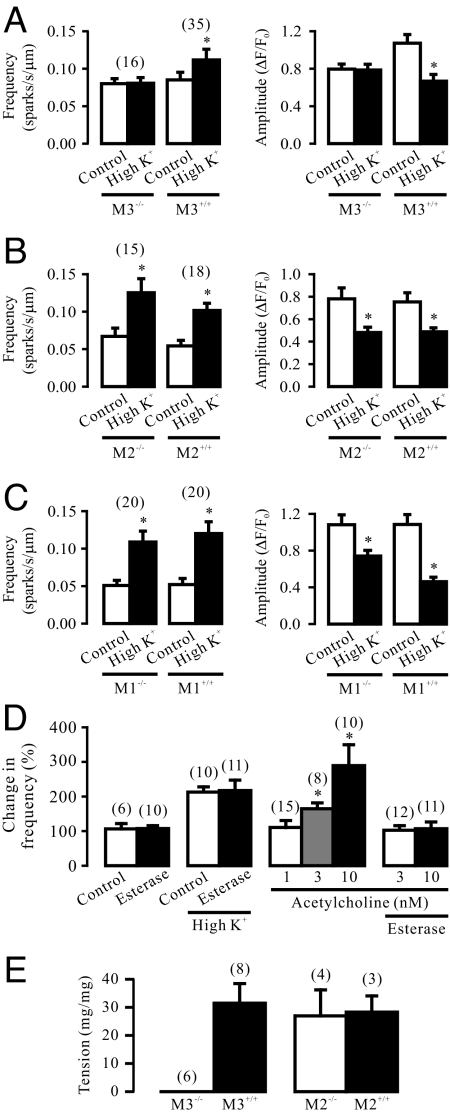
The long latency for depolarization-induced Ca2+ release in airway SMCs is similar to that for agonist-induced Ca2+ release, which is generally considered to represent the time course of the GPCR-dependent biochemical cascade reactions in SMCs (6). It has also been reported that membrane depolarization causes charge movement (gating current) of heterologously expressed GPCRs, M1Rs and M2Rs, in Xenopus oocytes (7). Thus, we wondered whether membrane depolarization might result in a direct activation of native GPCRs and their downstream signaling axis to trigger RyR-mediated local Ca2+ release in the absence of exogenous agonists. Our results indicate that targeted gene deletion of M3Rs, functionally important GPCRs expressed in SMCs, could prevented depolarization causing an increase in the frequency of Ca2+ sparks and a reduction in the amplitude of Ca2+ sparks (Fig. 4A). On the contrary, M2R and M1R gene deletion did not significantly affect chemical depolarization-triggered local Ca2+ release (Fig. 4 B and C).
Fig. 4.
Membrane depolarization-induced local Ca2+ release is a direct activation of G protein-coupled, M3 muscarinic receptors in airway SMCs. (A) Depolarization with high K+ increased Ca2+ spark frequency and decreased Ca2+ spark amplitude in M3R+/+, but not in M3R−/− cells. (B) Effect of high K+ on Ca2+ sparks were comparable in M2R−/− and M2R+/+ cells. (C) M1R gene deletion did not affect chemical depolarization-induced local Ca2+ release, either. (D) Treatment with acetylcholine esterase (85 U/mL) for 10 min had no effect on the activity of spontaneous and high K+-evoked Ca2+ sparks, but fully blocked acetylcholine (3 and 10 nM)-evoked response. (E) M3R, but not M2R, gene deletion prevented high K+-induced muscle contraction in isolated tracheal rings.
Membrane depolarization can significantly enhance muscarinic agonist-induced Ca2+ release by affecting multiple signaling cascade sites in several types of cells (8), and muscarinic receptors provide a predominant neural control of Ca2+ signaling in airway SMCs (9). Thus, to exclude the potential involvement of the endogenous muscarinic agonist acetylcholine, we examined the effect of acetylcholine esterase, an endogenous enzyme known to rapidly metabolize acetylcholine, on depolarization-induced Ca2+ release. Treatment with this enzyme (85 U/mL) for 10 min, as used in lung slice preparations (10), did not affect the activity of spontaneous and depolarization-induced Ca2+ sparks in isolated cells (Fig. 4D). We also found that application of acetylcholine at 1 nM had no effect on Ca2+ sparks, but at 3 and 10 nM significantly increased the activity of Ca2+ sparks (Fig. 4D). The acetylcholine (3 and 10 nM)-evoked responses were completely blocked by pretreatment with acetylcholine esterase (85 U/mL) for 10 min. These results suggest that the muscarinic agonist acetylcholine is unlikely to be required for depolarization-induced Ca2+ release in single isolated airway SMCs.
Chemical depolarization with high K+ could result in large muscle contraction in M3R+/+ tracheal rings, but had not effect in M3R−/− tracheal rings (Fig. 4E). In contrast, high K+ caused similar muscle contraction in M2R+/+ and M2R−/− tracheal rings. Therefore, M3Rs are the important sites of the primary voltage sensor to mediate the initial phase of depolarization-induced local Ca2+ release in SMCs.
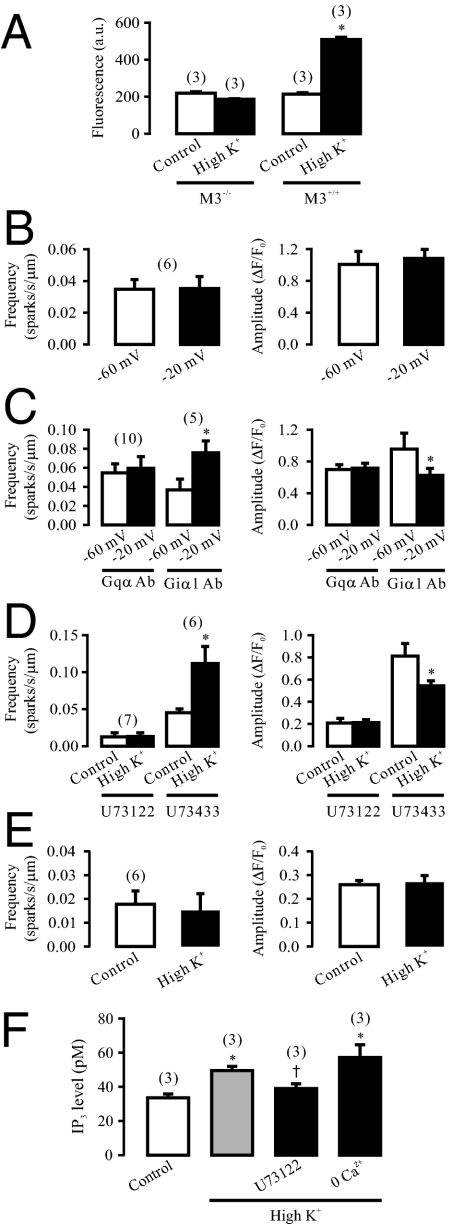
In line with the critical role of M3Rs as the loci of the primary voltage sensor, chemical depolarization significantly increased the activity of Gq proteins, determined using a newly-developed, europium-labeled GTP binding assay, in M3R+/+, but not in M3R−/− mouse airway smooth muscle tissues (Fig. 5A).
Fig. 5.
Gq protein/PLC/IP3/IP3R signaling axis is involved in depolarization-induced local Ca2+ release in airway SMCs. (A) high K+ exposure significantly increased the activity of Gqα proteins in M3R+/+, but not in M3R−/− mouse airway smooth muscle tissues. The activity of Gqα proteins was determined by measuring europium (Eu)-derived fluorescence, which is expressed as arbitrary units (a.u.), with an Eu-labeled GTP binding assay. (B) Intracellular dialysis of GDP-β-S to specifically inhibit G proteins prevented the effect of voltage depolarization on Ca2+ sparks. Cells were voltage-clamped at −60 mV using the classic patch clamp technique. Ca2+ sparks were recorded after dialysis of GDP-β-S (1 mM) for 5 min. (C) Dialysis of anti-Gqα antibodies (Gqα Ab), but not anti-Giα1 antibodies (Giα1 Ab) (4 μg/mL) for 5 min abolished voltage depolarization-induced local Ca2+ release. (D) Application of the selective PLC inhibitor U73122 (2.5 μM), but not its inactive analog U73433 (2.5 μM) for 8 min blocked high K+-induced increase in Ca2+ spark frequency and reduction in Ca2+ spark amplitudes. (E) Application of the IP3R antagonist 2-APB (100 μM) for 8 min completely inhibit the effect of high K+ on Ca2+ sparks. (F) High K+ increased in IP3 production (level) in tracheal muscle tissues. Treatment with U73122 (2.5 μM) for 8 min blocked high K+-induced IP3 production, but incubation with nominally Ca2+-free plus 0.25 mM EGTA bath solution for 30 s had no effect.
Consistent with depolarization-induced activation of Gq proteins, intracellular dialysis of GDP-β-s (1 mM) for 5 min to specifically inhibit G proteins prevented voltage depolarization-elicited Ca2+ release (Fig. 5B). Furthermore, dialysis of specific anti-Gqα antibodies (4 μg/mL) for 5 min to block Gq protein abolished the effect of voltage depolarization as well (Fig. 5C). However, dialysis of anti-Giα1 antibodies (4 μg/mL) had no effect (Fig. 5C).
The selective PLC inhibitor U73122 (2.5 μM) for 8 min fully blocked the effect of depolarization on the frequency and amplitude of Ca2+ sparks (Fig. 5D). In contrast, the structurally similar, inactive analog U73433 (2.5 μM) was without effect.
Similar to our recent report (5), application of 2-aminoethoxydiphenyl-borate (2-APB, 100 μM) for 8 min to block IP3Rs could significantly inhibit spontaneous Ca2+ sparks in airway SMCs. However, in the continued presence of 2-APB, chemical depolarization failed to affect either Ca2+ spark frequency or amplitude (Fig. 5E). Bath application of the IP3R antagonist xestospongin-C (10 μM) for 8 min and intracellular dialysis of the selective IP3R blocker heparin (5 mg/mL) for 5 min prevented high K+ from inducing local Ca2+ release as well.
We also found that chemical depolarization significantly increased IP3 production, measured using a [H3]IP3 radioreceptor assay, in tracheal muscle tissues. The increased production of IP3 by chemical depolarization was blocked by treatment with U73122 (2.5 μM) for 8 min, but not by elimination of extracellular Ca2+ (Fig. 5F). Collectively, depolarization-induced, RyR-mediated local Ca2+ release results from the initial Ca2+ release from IP3Rs due to a local CICR process through the GPCR-dependent PLC−IP3 signaling pathway in SMCs.
Ryanodine Receptor 2, But Not Ryanodine Receptor 1 and 3 Is Responsible for Membrane Depolarization-induced Local Ca2+ Release in SMCs.
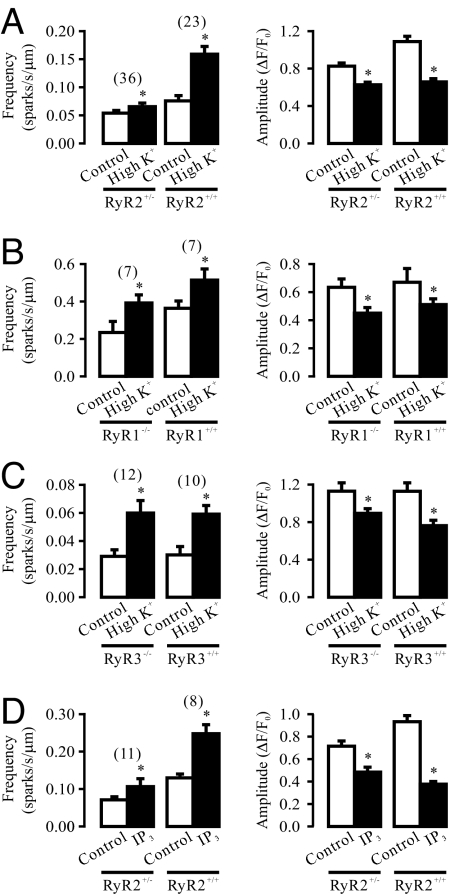
RyR2 is known to mediate CICR in cardiac cells; thus, we examined whether these Ca2+ release channels might also underlie depolarization-induced local CICR due to the IP3R/RyR interaction in SMCs using RyR2+/− mice [RyR2−/− mice die at early embryonic stage (11)]. Compared with RyR2+/+ cells, spontaneous Ca2+ spark frequency and amplitude were both reduced in RyR2+/− cells (Fig. 6A). Moreover, depolarization-induced local Ca2+ release was almost prevented in RyR2+/− cells. After high K+ exposure, the frequency of Ca2+ sparks was increased by 20.4% (from 0.054 ± 0.005 to 0.065 ± 0.006 sparks/s/μm) in RyR2+/− cells (n = 36), and 109.2% (from 0.076 ± 0.010 to 0.159 ± 0.014 sparks/s/μm) in RyR2+/+ cells (n = 23). In addition, the effect of chemical depolarization with high K+ on the amplitude of Ca2+ sparks was blocked in RyR2+/− cells as well.
Fig. 6.
RyR2 mediates depolarization-induced local Ca2+ release in airway SMCs. (A) Depolarization by high K+ exposure almost failed to affect Ca2+ sparks in RyR2+/− cells; the frequency and amplitude of spontaneous Ca2+ spark were significantly lower in RyR2+/− cells as well. (B) RyR1 gene deletion did not affect depolarization-induced Ca2+ sparks, although it inhibited spontaneous Ca2+ sparks. (C) RyR3 gene deletion neither inhibited spontaneous nor high K+-induced Ca2+ sparks. (D) Intracellular dialysis of IP3 (1 μM) for 5 min significantly increased Ca2+ spark frequency and decreased Ca2+ spark amplitude in RyR2+/+, but not in RyR2+/− cells.
We next examined the role of RyR1 in depolarization-induced local Ca2+ release using RyR1−/− and RyR1+/+ mice at embryonic day 17, as RyR1−/− mice die at or just before birth (12). We found that the frequency and amplitude of spontaneous Ca2+ sparks were decreased in RyR1−/− cells, relative to RyR1+/+ cells. However, depolarization with high K+ caused a similar increase in the activity of Ca2+ sparks and reduction in the amplitude of Ca2+ sparks in airway SMCs from embryonic RyR1−/− and RyR1+/+ mice (Fig. 6B).
RyR3 gene deletion did not affect either spontaneous or depolarization-induced local Ca2+ release (Fig. 6C). Following high K+ exposure, the mean increase in the frequency of Ca2+ sparks and the mean reduction in the amplitude of Ca2+ sparks were both comparable in RyR3−/− and RyR3+/+ cells.
Finally, we examined and compared IP3R-dependent local Ca2+ release in RyR2+/+ and RyR2+/− cells. Intracellular dialysis of IP3 to directly activate IP3Rs resulted in a large increase in the frequency of Ca2+ sparks and decrease in the amplitude of Ca2+ sparks in RyR2+/+cells. In contrast, the effect of dialysis of IP3 was strongly inhibited in RyR2+/− cells (Fig. 6D). These findings provide compelling evidence supporting the importance of RyR2 in depolarization-induced local Ca2+ release as a result of the activation of IP3Rs in SMCs.
Discussion
As membrane depolarization normally leads to an obligatory Ca2+ release through RyRs within a few milliseconds, the so-called instantaneous Ca2+ release, in cardiac and skeletal myocytes (1), we wondered whether an analogous Ca2+ release mechanism could exist in SMCs. Unlike in cardiac and skeletal myocytes, voltage depolarization for 250 ms does not induce instantaneous Ca2+ release in freshly isolated airway myocytes. After voltage depolarization for over one second, however, prominent local Ca2+ release occurs, manifesting itself in a great increase in the frequency of RyR-mediated Ca2+ sparks and significant contraction. Long chemical depolarization with high K+ exposure has similar effects. Both long voltage and chemical depolarization can decrease the amplitude of Ca2+ sparks. This effect is likely to be secondary to a reduction in local Ca2+ content in the release sites due to the increased activity of Ca2+ sparks (5). In agreement with our results, long voltage and chemical depolarization also induce Ca2+ release in basilar artery myocytes (13). Voltage and chemical depolarization-evoked local Ca2+ release may result from the opening of RyRs, as they are prevented by specific block of RyRs with ryanodine.
VDCCs are loosely coupled to RyRs in bladder SMCs, by which sufficient extracellular Ca2+ influx through VDCCs following depolarization is required to activate RyRs leading to SR Ca2+ release (2). However, depolarization-evoked Ca2+ release can be independent of extracellular Ca2+ influx in basilar artery SMCs (13). Accordingly, we sought to test whether VDCCs, extracellular Ca2+ influx or both would be involved in depolarization-triggered Ca2+ release in airway myocytes. Our data reveal that chemical depolarization causes a significant increase in the activity of Ca2+ sparks and associated contraction in the presence of the specific VDCC blocker nifedipine or D600. Similarly, depolarization-induced local Ca2+ release and contraction occur under extracellular Ca2+-free conditions. These data demonstrate a form of depolarization-induced Ca2+ release and contraction in SMCs, which are independent of VDCCs and the associated Ca2+ influx.
The long latency for depolarization-induced Ca2+ release in airway SMCs suggests the involvement of GPCR-dependent biochemical cascade reactions. In addition, membrane depolarization causes charge movement (gating currents) in heterologously expressed M1Rs and M2Rs in Xenopus oocytes (7). As a consequence, we hypothesized that the form of VDCC- and Ca2+ influx-independent, depolarization-induced Ca2+ release could result from a direct activation of native GPCRs in the absence of exogenous agonists. In support of this hypothesis, we have discovered that targeted gene deletion of M3Rs, functionally important GPCRs in airway, bladder, gastric, intestinal, and other SMCs (9), completely inhibits depolarization-induced local Ca2+ release in airway SMCs. In contrast, neither M2R nor M1R gene deletion produces an effect. Furthermore, depolarization-induced muscle contraction is abolished in M3R−/−, but not in M2R−/− airway tissues. These findings are consistent with the well-known fact that M3Rs, but not M2Rs and M1Rs, normally provide predominant neuronal control of Ca2+ and contractile responses in SMCs. Billups et al. have shown that voltage depolarization significantly increases the muscarinic agonist oxotremorine-evoked Ca2+ release by augmenting IP3 production in neurons (14). It is also known that membrane depolarization can affect other multiple GPCR-dependent signaling cascade sites to enhance muscarinic agonist-induced Ca2+ release in a variety of cells (8). Presumably, if the muscarinic agonist acetylcholine could be endogenously present, even at a low concentration, membrane depolarization might amplify the role of endogenous acetylcholine, causing significant Ca2+ release. However, our data do not favor this assumption, because acetylcholine esterase which rapidly degrades acetylcholine (10) has no effect on spontaneous and depolarization-induced local Ca2+ release in isolated cells. Supportively, application of exogenous acetylcholine (3 or 10 nM) leads to a large increase in the activity of Ca2+ sparks in cells untreated, but not treated with acetylcholine esterase. Therefore, M3Rs are the important loci of the primary voltage sensor in airway SMCs, similar to expressed M1Rs and M2Rs in Xenopus oocytes (7). However, depolarization-induced activation of native M3Rs results in Ca2+ release in airway SMCs (in this study), whereas depolarization-induced charge movement in expressed M1Rs and M2Rs, respectively, enhances and reduces agonist binding in Xenopus oocytes (7).
Since native M3Rs serve as the primary voltage sensor, we expect that target gene deletion of these receptors can prevent depolarization-induced activation of their downstream signaling molecules. In agreement with this view, M3R gene deletion fully blocks depolarization-caused increase in the activity of Gqα proteins in airway smooth muscle tissues. Specific inhibition of the activation of Gq proteins by intracellular dialysis of GDP-β-s or anti-Gqα antibodies, PLC with U73122 and IP3Rs with 2-APB, xestospongin-C, and heparin all prevent depolarization causing local Ca2+ release. Dialysis of GDP-β-s or application of U73122 fully blocks depolarization-evoked Ca2+ release as well in basilar artery SMCs (13). Moreover, our studies also disclose that membrane depolarization increases IP3 production in airway muscle tissues; the increased production of IP3 production is completely inhibited by U73122, but not affected by elimination of extracellular Ca2+. These data provide convincing evidence that membrane depolarization induces RyR-mediated local Ca2+ release due to the GPCR signaling-dependent activation of IP3Rs in SMCs.
We further examined which subtype of RyRs would locally interact with IP3Rs to mediate depolarization-triggered local Ca2+ release using receptor gene deletion mice. The frequency and amplitude of spontaneous Ca2+ sparks are both decreased in RyR2+/− airway SMCs. Similar inhibition of Ca2+ sparks has been observed in RyR2+/− bladder SMCs (15). More importantly, depolarization-evoked local Ca2+ release is almost totally blocked in RyR2+/− cells. Local Ca2+ release (Ca2+ spark) is due to the instantaneous opening of functionally or structurally congregated RyRs; as such, the decreased expression of RyR1 by heterozygous gene deletion may lead to the inability of the remaining channels to assemble the functional or structural constitution necessary to generate a Ca2+ spark. This may explain the failure of depolarization to induce local Ca2+ release in RyR2+/− cells. Consistent with our findings, depolarization-induced local Ca2+ release is reduced as well in RyR2+/− bladder myocytes (15) and RyR2 gene knocked-down portal vein SMCs (16). RyR1 gene deletion greatly inhibits spontaneous Ca2+ release in airway SMCs. However, depolarization-induced local Ca2+ release is comparable in RyR1+/+ and RyR1−/− cells. RyR3 gene deletion neither affects spontaneous nor depolarization-evoked local Ca2+ release. Consonant with our data, suppression of RyR3 gene expression has no effect on spontaneous and/or depolarization-induced Ca2+ sparks in rat portal vein myocytes (16) and mouse cerebral artery myocytes (17). Thus, depolarization-evoked local Ca2+ release via the GPCR/PLC/IP3/IP3R signaling axis may be specifically mediated by RyR2, but not RyR1 and RyR3 in SMCs.
Direct activation of IP3Rs by intracellular dialysis of IP3 results in a large increase in the activity of Ca2+ sparks in RyR2+/+, but not in RyR2+/− cells. These results provide additional evidence for the specific role of RyR2 in depolarization-induced, GPCR/PLC/IP3/IP3R-dependent local Ca2+ release in SMCs. The basal activity of IP3Rs significantly enhances spontaneous Ca2+ sparks in airway (5) and portal vein myocytes (18), downregulates Ca2+ sparks in vas deferens myocytes (19), and has no effect in pulmonary artery SMCs (20). Despite the effects of IP3Rs on Ca2+ release in different types of SMCs, stimulation of IP3Rs by neurotransmitters, hormones and growth factors can activate adjacent RyRs leading to further Ca2+ release through RyRs in airway and other SMCs (21–26). Collectively, our findings suggest that RyR2 may also be essential for agonist-induced, IP3R-mediated global Ca2+ release in SMCs.
Experimental Procedures
Measurement of Ca2+ Sparks.
Single mouse airway SMCs from Swiss Webster, M1R−/−, M2R−/−, M3R−/−, RyR1−/−, RyR2+/−, RyR3−/−, and corresponding control (wild-type) mice were obtained using a 2-step enzymatic digestion method (5, 27). M1R−/−, M2R−/−, M3R−/−, RyR1−/−, RyR2+/−, and RyR3−/− mice were generated and maintained, as reported previously (11, 12, 28–31).
Ca2+ sparks were measured using a LSM-510 laser scanning confocal microscope (Carl Zeiss) in the line-scan mode (5, 27). Freshly isolated cells were incubated in physiological saline solution (PSS) containing 2.5 μM fluo-4/AM. The composition of PSS was (mM): 125 NaCl, 5 KCl, 2.0 CaCl2, 1 MgSO4, 10 glucose, and 10 Hepes (pH 7.4). Spatiotemporal characteristics of Ca2+ sparks were analyzed using the Physiology Analysis software (Carl Zeiss) and Interactive Data Language software (Research Systems).
Patch Clamp Recordings.
Cells were loaded with fluo-4/AM and voltage clamped using the nystatin-perforated or classic patch clamp technique with the EPC-9 patch clamp system (HEKA) (5, 27). The intracellular solution contained (mM): 125 KCl, 1 MgSO4, 10 HEPS, 0.5 CaCl2, and 0.85 EGTA (pH 7.4). Fluo-4 free acid (50 μM) was included in the patch pipette solution to avoid a potential reduction in fluorescent intensity due to dialysis effect. To record VDCC currents, the pipette solution was composed of (mM): 130 CsCl, 1 MgCl2, 2 CaCl2, 3 EGTA, and 10 Hepes (pH 7.4), and bath solution contained (mM): 130 TEACl, 1 MgCl2, 2 CaCl2, 10 Hepes, and 10 Glucose (pH 7.4).
Measurement of Cell Contraction.
Single cell contraction was measured by assessing cell length shortening (32). Transmitted-light x-y images were taken using Zeiss LSM510 confocal microscope. Cell length in each image was determined at its longest axis.
Muscle contraction in isolated tracheal rings was measured using the organ bath technique (25). Tracheal rings were removed off the connective tissue and epithelia, and then mounted in 2-ml organ bath chambers containing PSS at 37 °C. Muscle tension was determined in the presence of nifedipine (10 μM) and Y27632 (10 μM) to prevent VDCC-mediated Ca2+ influx and RhoA kinase-linked Ca2+ sensitivity.
Measurement of IP3 Production.
Mouse tracheal tissues were dissected free of the epithelium, cartilage and connective tissue, and then homogenized in ice-cold, 1 M trichloroacetic acid (TCA). After centrifugation at 1,000 × g for 10 min at 4 °C, the supernatant was incubated at room temperature for 15 min. TCA was removed from the supernatant by adding 1-ml 1,1,2-trichlorotrifluoroethane and tri-n-octlyamine (3:1), followed by vigorous shaking and centrifugation at 2,000 × g for 1 min. The upper phase of the reaction buffer was used to assay IP3 production using an [H3]IP3 radioreceptor assay kit (PerkinElmer). The radioactivity of the reaction samples was determined in an LS6500 β-counter (Beckman Instruments). IP3 concentrations were calculated from a standard curve that was generated by plotting the percentage binding (bound/free ratio) as a function of the logarithm of known IP3 concentrations.
Measurement of Gqα-protein Activity.
Gqα-protein activity was determined using DELFIA GTP Binding Kit (Perkin-Elmer), according to the manufacturer's instruction. Isolated mouse tracheas were homogenized and centrifuged to obtain cell membrane fractions. Samples were incubated with specific anti-Gqα antibodies with protein-A beads for 3 h at room temperature and then centrifuged 3 times at 2,000 × g for 3 min to collect the beads. Proteins were eluted from the beads using eluting buffer containing 1% SDS, incubated in reaction buffer containing 2.5 mM MgCl2, 0.5 μM GDP, 50 mM NaCl, and 15 μg/mL saponin for 30 min, and then added with 1 μM GTP-europium reagent followed by incubation for 30 min. After 3 washes on manifold vacuum, europium-derived fluorescence was measured at 615 nm emission with 340 nm excitation on a FlexStation-III microplate reader (Molecular Devices).
Reagents.
2-APB, U73122, U73433 and xestospongin-C were purchased from Calbiochem; ryanodine, D600, heparin, GDP-β-s, and Y27632 from Sigma; fluo-4/AM and fluo-4 free acid from Invitrogen; nifedipine from Tocris; and anti-Gqα and anti-Giα1 antibodies from Santa Cruz Biotechnology. All experiments were conducted at room temperature (≈22 °C), unless indicated otherwise.
Data Analysis.
Data are presented as mean ± SEM. Statistical comparisons between groups were performed with Student's t test or 1-way ANOVA with an appropriate posthoc test. Differences at P < 0.05 were considered statistically significant.
Acknowledgments.
This work was supported by National Institutes of Health (NIH) R01HL071000 (Y.-X.W.), NIH Intramural Research Program (J.W.), American Lung Association Research Grant 19994 (Q.-H.L.), American Heart Association (AHA) Scientist Development Grant 0630236N (Y.-M.Z.) and 0730242N (Q.-H.L.), and AHA Established Investigator Award 0340160N (Y.-X.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 2.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: Loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115:653–662. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji G, Feldman M, Doran R, Zipfel W, Kotlikoff MI. Ca2+-induced Ca2+ release through localized Ca2+ uncaging in smooth muscle. J Gen Physiol. 2006;127:225–235. doi: 10.1085/jgp.200509422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann BK, Wang YX, Pring M, Kotlikoff MI. Voltage-dependent calcium currents and cytosolic calcium in equine airway myocytes. J Physiol. 1996;492:347–358. doi: 10.1113/jphysiol.1996.sp021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu QH, Zheng YM, Wang YX. Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells. Pflugers Arch. 2007;453:531–541. doi: 10.1007/s00424-006-0130-1. [DOI] [PubMed] [Google Scholar]
- 6.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Chaim Y, et al. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- 8.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29:421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 10.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002;119:187–198. doi: 10.1085/jgp.119.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeshima H, et al. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshima H, et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- 13.del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Ca2+ channel-sarcoplasmic reticulum coupling: A mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J. 2003;22:4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billups D, Billups B, Challiss RA, Nahorski SR. Modulation of Gq-protein-coupled inositol trisphosphate and Ca2+ signaling by the membrane potential. J Neurosci. 2006;26:9983–9995. doi: 10.1523/JNEUROSCI.2773-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotta S, et al. Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol. 2007;582:489–506. doi: 10.1113/jphysiol.2007.130302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J Biol Chem. 2000;275:9596–9603. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- 17.Lohn M, et al. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- 18.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White C, McGeown JG. Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol Cell Physiol. 2003;285:C195–C204. doi: 10.1152/ajpcell.00374.2002. [DOI] [PubMed] [Google Scholar]
- 20.Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L433–L444. doi: 10.1152/ajplung.00468.2001. [DOI] [PubMed] [Google Scholar]
- 21.Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- 22.Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WM, et al. ET-1 activates Ca2+ sparks in PASMC: Local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 24.Wang YX, et al. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol. 2004;286:C538–C546. doi: 10.1152/ajpcell.00106.2003. [DOI] [PubMed] [Google Scholar]
- 25.Zheng YM, et al. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du W, Stiber JA, Paul RB, Gerhard M, Eu JP. Ryanodine receptors in muscarinic receptor-mediated Bronchoconstriction. J Biol Chem. 2005;280:26287–26294. doi: 10.1074/jbc.M502905200. [DOI] [PubMed] [Google Scholar]
- 27.Liu QH, et al. Protein kinase C-ε regulates local calcium signaling in airway smooth muscle cells. Am J Respir cell Mol Biol. 2009;40:663–671. doi: 10.1165/rcmb.2008-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisahn A, et al. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- 29.Takeshima H, et al. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem. 1996;271:19649–19652. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- 30.Gomeza J, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada M, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 32.Rathore R, et al. Mitochondrial ROS-PKCε signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]