Abstract
In today's angiosperm-dominated terrestrial ecosystems, leptosporangiate ferns are truly exceptional—accounting for 80% of the ≈11,000 nonflowering vascular plant species. Recent studies have shown that this remarkable diversity is mostly the result of a major leptosporangiate radiation beginning in the Cretaceous, following the rise of angiosperms. This pattern is suggestive of an ecological opportunistic response, with the proliferation of flowering plants across the landscape resulting in the formation of many new niches—both on forest floors and within forest canopies—into which leptosporangiate ferns could diversify. At present, one-third of leptosporangiate species grow as epiphytes in the canopies of angiosperm-dominated tropical rain forests. However, we know too little about the evolutionary history of epiphytic ferns to assess whether or not their diversification was in fact linked to the establishment of these forests, as would be predicted by the ecological opportunistic response hypothesis. Here we provide new insight into leptosporangiate diversification and the evolution of epiphytism by integrating a 400-taxon molecular dataset with an expanded set of fossil age constraints. We find evidence for a burst of fern diversification in the Cenozoic, apparently driven by the evolution of epiphytism. Whether this explosive radiation was triggered simply by the establishment of modern angiosperm-dominated tropical rain forest canopies, or spurred on by some other large-scale extrinsic factor (e.g., climate change) remains to be determined. In either case, it is clear that in both the Cretaceous and Cenozoic, leptosporangiate ferns were adept at exploiting newly created niches in angiosperm-dominated ecosystems.
Keywords: divergence-time estimates, diversification, ecological opportunistic response, epiphytes, modern tropical rain forests
Through the 80 million years composing the Cretaceous period (145.5–65.5 Ma; time scale follows ref. 1), the Earth's vegetation changed dramatically from a landscape populated by gymnosperms and seed-free vascular plants to one dominated by angiosperms (2–8). As flowering plants rose to prominence, other vascular plant lineages were largely relegated to the sidelines, if not driven completely to extinction. Today, angiosperms account for about 96% of vascular plant diversity, whereas nearly all of the 12 remaining major vascular plant lineages comprise just a few—or perhaps a few hundred—species [supporting information (SI) Table S1]. Leptosporangiate ferns are the only exception. Although not as diverse as flowering plants, this group comprises more than 9,000 living species—4 times the number of extant species in all other nonflowering lineages combined.
Leptosporangiate ferns originated near the start of the Carboniferous period (359.2 Ma) (9, 10)—about 200 million years before the evolution of angiosperms (11). Based on the fossil record, this group of ferns is thought to have undergone 3 successive radiations (12–14): an initial radiation in the Carboniferous, giving rise to 6 now-extinct families; a second radiation in the late Paleozoic and early Mesozoic, resulting in several families with extant representatives; and a third radiation beginning in the Cretaceous, primarily within what is now referred to as the “polypod” clade. An analysis combining fossil and living data confirmed the timing of this third radiation, demonstrating that the bulk of polypod diversity arose following the rise of flowering plants (15). Subsequent divergence-time estimates suggested that this pattern of recent diversification—in the shadow of angiosperms—might be echoed in other leptosporangiate orders (16). Thus, it appears that the remarkable diversity of leptosporangiate ferns on Earth today is not simply the result of being adept at holding on in the face of angiosperm domination. Rather, it seems that ferns may have somehow been able to capitalize upon it.
One plausible explanation for the success of leptosporangiates involves an ecological opportunistic response to the rise of angiosperms (12, 15, 17). In such a scenario, the proliferation of angiosperms across the landscape and the ensuing establishment of more complex ecosystems would have resulted in a plethora of new niches into which leptosporangiate ferns could have diversified. But why were leptosporangiates able to flourish as other nonflowering vascular plant lineages floundered? In part, their success may be linked to acquiring a unique photoreceptor that enhanced their sensitivity to light (in orienting leaves and chloroplasts) (18) and likely allowed them to better occupy the shady floors of angiosperm-dominated forests (15). Traits associated with the evolution of epiphytism—a capacity to reside on an above-ground plant surface while not extracting water or nutrients from the host plant or the ground (19)—may also have played an important role. Desiccation tolerance has been documented both in fern sporophytes and (especially) gametophytes (20), and many epiphytic ferns also possess features (e.g., leathery leaves and thick cuticles) that allow them to withstand dry conditions (21, 22). Some are even able to absorb water directly into their leaves or stems (23), while others have specialized in the impoundment of leaf litter to form suspended soils (24).
With this suite of adaptations, leptosporangiate ferns have shown an extraordinary ability to colonize the canopies of modern, angiosperm-dominated, tropical rain forests (21, 25–29). Although these ferns account for just 3% of the world's vascular plant diversity, they comprise more than 10% of the epiphytic species (see Table S1 and ref. 30). Unfortunately, we know too little about the evolutionary history of epiphytic leptosporangiates to assess whether or not their diversification was in fact linked to the establishment of angiosperm-dominated tropical rain forests, as would be predicted by the ecological opportunistic response hypothesis. There is not even a consensus as to how many times epiphytism has arisen within leptosporangiates. In this study, we combine the best-sampled molecular dataset for ferns to date with an expanded set of age constraints from the fossil record to obtain a more complete picture of leptosporangiate diversification. We then reconstruct habit across the resulting phylogenetic chronogram (timetree) to more fully understand the evolution of epiphytism and the timing of epiphytic radiations. This allows us to recognize what factors may have been responsible for epiphytic diversification—ultimately providing further insight into the leptosporangiate success story.
Results and Discussion
Leptosporangiate Phylogeny.
Phylogenetic analysis of our 3-gene dataset yielded a well-resolved and well-supported evolutionary framework (80% of the nodes received maximum likelihood bootstrap support ≥ 70%), while also providing an unprecedented picture of relationships across leptosporangiate ferns. The tree topology is presented in Fig. S1; branch lengths, support values, and a thorough discussion of relationships are provided elsewhere (31).
Leptosporangiate Diversification.
Divergence-time estimates, resulting from the integration of our molecular phylogeny (see Fig. S1) with age constraints from the fossil record (see Table S2), suggest that there was very little accumulation of extant fern diversity in the Permian, Triassic, or even Jurassic periods (see Figs. 1 and 2). Most divergences among extant lineages, especially within the polypod clade (node 86), took place in the Cretaceous and Cenozoic. Overall, this pattern is consistent with the fern fossil record (12–14), as well as previous integrative analyses (15, 16) that suggested a link between the diversification of leptosporangiates and the Cretaceous rise of angiosperms. However, the limited sampling of the earlier studies and the incomplete nature of the fern fossil record have precluded a detailed understanding of leptosporangiate evolution. Here, by combining a broad (and proportional; see Materials and Methods) sample of extant ferns with numerous fossil age constraints, we are better able to identify the contributions of specific leptosporangiate clades to the overall pattern. But while our new analysis does provide improved insight into fern diversification, it should be emphasized that our results are a function of several factors, including taxonomic sampling, selection and application of fossil age constraints, and the method used to combine the living and fossil information. Our findings, like those of any study relying on divergence-time estimates, should therefore be considered tentative and subject to future revision. Nonetheless, current best age estimates for all nodes resolved in the most likely phylogeny (see Fig. 1 and Fig. S1) are provided in Table S3. Ages for the major clades also appear in Table S4, along with confidence intervals that account for phylogenetic uncertainty. In the paragraphs that follow, we summarize the deepest leptosporangiate divergences (citing our best age estimates) (see Tables S3 and S4).
Fig. 1.
Leptosporangiate fern timetree, showing ancestral reconstructions of habit. Phylogenetic chronogram results from maximum likelihood analysis of 3 plastid genes sequenced for each of 400 taxa (taxon numbers correspond to those in Fig. S1), followed by penalized likelihood analysis incorporating 24 fossil age constraints (see Table S2). Maximum likelihood reconstructions of habit (see key, Upper Left) across this timetree are shown. Important nodes are indicated in tree; names, as well as age, diversification rate, and other statistics for these nodes are provided in Table S4. Statistics for all nodes (see Fig. S1) appear in Table S3. Geologic timescale and subdivisions follow ref. 1: Ci, Cisuralian; Eo, Eocene; Gu, Guadalupian; L, Lower; Lo, Lopingian; M, Middle; Mi, Miocene; Ol, Oligocene; Pa, Paleocene; U, Upper; Pliocene, Pleistocene, and Holocene are not labeled because of space constraints. The Cretaceous/Tertiary boundary (K/T; solid vertical line) and Paleocene/Eocene thermal maximum (PETM; dashed vertical line) are indicated (see Results and Discussion for significance). Thumbnail silhouettes correspond to major epiphytic clades (silhouettes result from modification of illustrations by B. Manara, in ref. 32, with permission).
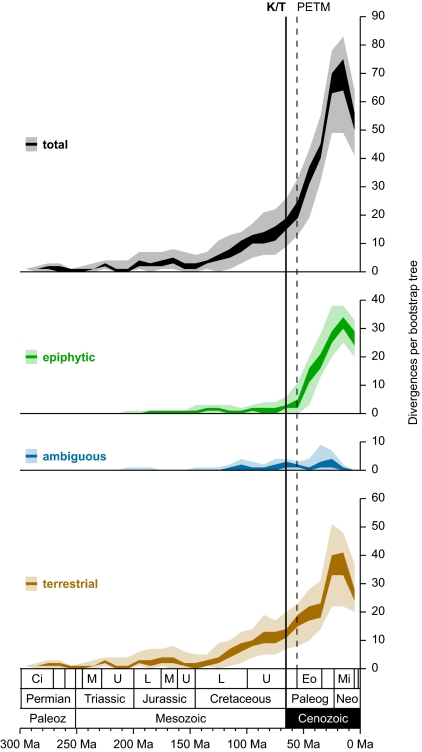
Fig. 2.
Leptosporangiate fern divergences through time, according to habit. Plots summarize the results of penalized likelihood analyses of, and maximum likelihood reconstructions across, 100 bootstrap trees (see Materials and Methods). For each 10-million-year interval, the interquartile range (dark colors) and the complete span (light colors) of observed divergences are provided. Geologic timescale, subdivisions, and abbreviations follow Fig. 1; several additional subdivisions are not labeled here because of space constraints. The Cretaceous/Tertiary boundary (K/T; solid vertical line) and Paleocene/Eocene thermal maximum (PETM; dashed vertical line) are indicated (see Results and Discussion for significance). The decline in the number of divergences observed in the most recent time intervals is not indicative of a change in diversification rate, but rather is merely an artifact of incomplete taxonomic sampling (which preferentially captures deeper divergences).
Based on evidence from the fossil record (see Table S2), the first split in the leptosporangiate crown group (node 0) occurred near the Carboniferous/Permian boundary (299.0 Ma), and our integrative analysis reveals that all 7 extant leptosporangiate orders originated in the Permian and Triassic periods (299.0–199.6 Ma) (see Fig. 1). The osmundaceous ferns (Osmundales sensu ref. 33; node 1), which are sister to the remaining leptosporangiates, trace their history to the start of this interval (originating at 299.0 Ma). The filmy ferns (Hymenophyllales; node 4), gleichenioids (Gleicheniales; node 32), and schizaeoids (Schizaeales; node 43) are also estimated to have originated in the Permian (at 280.1 Ma, 276.4 Ma, and 264.6 Ma, respectively). In the Triassic, the lineages belonging to the core leptosporangiates (node 49) emerged: the heterosporous ferns (Salviniales; node 50) at 234.7 Ma, and the tree ferns (Cyatheales; node 55) and polypods (Polypodiales; node 86) at 223.2 Ma.
For each of the 7 extant fern orders, the time of lineage origination was almost always substantially decoupled from the onset of crown group diversification (i.e., the initial divergence resulting in 2 extant lineages). For the osmundaceous ferns (Osmundales; node 1) (see Fig. 1), diversification was delayed until near the Triassic/Jurassic boundary (199.6 Ma) (see Table S4), the time at which the earliest crown group fossils appear (see Table S2). For filmy ferns (Hymenophyllales; node 4), we estimate the initial divergence yielding the 2 major extant lineages—hymenophylloids and trichomanoids—to have occurred somewhat later, in the Lower Jurassic (185.1 Ma). But although the trichomanoids (node 5) began to diversify soon thereafter (147.3 Ma), the hymenophylloids (node 18) did not begin their diversification until the Eocene (41.9 Ma). Gleichenioid ferns (Gleicheniales; node 32) are rather exceptional, having begun to diversify before the end of the Paleozoic (262.2 Ma)—only 14.2 Ma after their inferred origin. For the schizaeoid ferns (Schizaeales; node 43), we again see a substantial lag between origin (264.6 Ma) and crown group diversification (218.4 Ma); however, this latter date is still much older than the oldest crown group fossils for this clade (see Table S2). Based on our estimates, crown group diversification for both the heterosporous ferns (Salviniales; node 50) and the tree ferns (Cyatheales; node 55) began in the Lower Jurassic (186.8 Ma and 186.7 Ma, respectively).
Polypods (Polypodiales; node 86) (see Fig. 1), with more than 7,000 extant species (33), compose what is by far the largest leptosporangiate order. According to our analysis, the initial divergence in this clade (resulting in 2 extant lineages) occurred in the Lower Jurassic (191.0 Ma) (see Table S4)—well before the earliest unequivocal fossil evidence for this clade appears in the Lower Cretaceous (145.5–99.6 Ma) (see Table S2), but in agreement with the Lower Jurassic age estimate from an earlier integrative analysis focused on this group (15). The Lower Jurassic also witnessed the start of diversification for the smaller of the 2 earliest-diverging polypod clades (at 179.9 Ma). Within the other primary polypod clade, 2 successive divergences in the Middle Jurassic (at 165.6 Ma and 163.2 Ma) quickly gave rise to the dennstaedtioids, pteroids, and eupolypods (nodes 95, 106, and 158, respectively) (see Fig. 1). However, crown group diversification within each of these 3 lineages was delayed until the Cretaceous period (see Table S4).
Epiphytic Origins.
Although there is some ambiguity regarding the precise number of transitions to and from the epiphytic habit (see Fig. 1), our maximum likelihood reconstructions are unambiguous with regard to the ancestral-state for leptosporangiate ferns (see Table S4)—epiphytism is clearly a derived condition. Within filmy ferns (node 4), epiphytism is inferred to have evolved sometime before the initial crown group split, followed by at least 3 losses within the trichomanoid subclade (node 5; see ref. 34 for an alternative hypothesis). Epiphytism was also reconstructed as the ancestral state for the vittarioid ferns (node 151), essentially all of which grow as epiphytes (35). Within asplenioids (node 163), the evolutionary history of epiphytism is somewhat more complex: one scenario involves a transition to epiphytism relatively early in the clade, followed by several losses and perhaps a secondary transition back to epiphytism; the other scenario implies several independent gains of the epiphytic habit. For elaphoglossoids (node 295), the situation is again unambiguous—the trait evolved before the first divergence within the clade but was lost at least twice. The most recent common ancestor of the polygrammoids (node 340) was also epiphytic, although epiphytism clearly evolved somewhat earlier (see Fig. 1). The few other transitions to the epiphytic habit reconstructed elsewhere in the leptosporangiate phylogeny were apparently not followed by substantial diversification (see Fig. 1).
Epiphytic Diversification.
The pattern of epiphytic fern diversification uncovered by our analysis was markedly different from that observed for terrestrial leptosporangiates (see Figs. 1 and 2). Although terrestrial divergences contributing to extant fern diversity were relatively common in the Cretaceous (145.5–65.5 Ma), essentially all epiphytic diversification was restricted to the Cenozoic (65.5–0 Ma). Hymenophylloids (node 18), vittarioids (node 151), asplenioids (node 163), elaphoglossoids (node 295), and polygrammoids (node 340) all experienced what we interpret to be explosive radiations during the Cenozoic (see Fig. 1), contributing to a rapid accumulation of extant epiphytic lineages beginning soon after the Cretaceous/Tertiary (K/T) boundary (65.5 Ma) (see Fig. 2). Only the trichomanoids (node 5) began to diversify in the Mesozoic. Thus, it would seem that the diversification of epiphytic ferns was not closely coupled to the rise of angiosperms in the Cretaceous, but rather spurred on by another factor.
Today, epiphytic ferns are virtually restricted to the canopies of angiosperm-dominated tropical rain forests (21, 25–29). Therefore, it seems reasonable to hypothesize that the establishment of these forests was responsible for the diversification of epiphytic ferns. Tropical rain forest trees provide a range of substrates, from giant trunks of every texture to tiny twigs and leaves, and the closed canopies they compose considerably stratify humidity and light levels (28, 29, 36). Thus, the origin of modern tropical rain forests—with closed, multistratal, angiosperm-dominated canopies—resulted in an extraordinary, and likely unprecedented, diversity and abundance of niches for epiphytic ferns to colonize. Unfortunately, the evolutionary history of this rain forest biome remains somewhat contentious.
Despite the first evidence for flowering plants appearing in the early Cretaceous (11), large angiosperm trunks and seeds [potentially indicative of a closed, angiosperm-dominated canopy (37, but see ref. 38)], as well as angiosperm leaves typical of everwet climates, are not abundant in the fossil record until after the K/T boundary (65.5 Ma) (39–47). This preponderance of fossil data has led to the prevailing view that the origin of modern tropical rain forests was a Cenozoic (65.5–0 Ma) phenomenon. However, some fossil floras have been interpreted as evidence for the existence of tropical rain forests in the Upper Cretaceous (42, 44, 47), and one recent study that estimated divergence times for a diverse clade of angiosperms (Malphigiales) (48) also supported this possibility.
Regardless of whether modern tropical rain forests were established in the Upper Cretaceous or the early Cenozoic, our findings (see Figs. 1 and 2) are consistent with the hypothesis that epiphytic ferns diversified within angiosperm-dominated canopies. And, if the broad consensus from the fossil record is correct, it would seem that modern tropical rain forest establishment in the Cenozoic triggered the epiphytic radiation. However, our results suggest that the increase in epiphytic fern diversification was not synchronous with the inferred origin of modern rain forests near the K/T boundary, but instead with the Paleocene/Eocene thermal maximum (PETM) almost 10 Ma later (see Fig. 2). The same appears to be true for the most diverse epiphytic clades within the Orchidaceae (49)—home to two-thirds of epiphytic angiosperms (30). The sudden rise in temperature and precipitation associated with the PETM (50–52) probably facilitated the invasion of angiosperm-dominated canopies. This change in climate most likely also resulted in a rapid expansion of the rain forest biome (42, 51, 53), which in turn led to a sudden increase in available canopy niche space. Notably, there appears to be no signature of the PETM in the diversification curve for terrestrial ferns (see Fig. 2). This, however, may be a result of the fact that increased exposure to the elements and the absence of a soil connection make epiphytic ferns more sensitive to climatic conditions than their terrestrial counterparts (21, 54).
Of the major epiphytic leptosporangiate lineages, only the trichomanoids (node 5) began to diversify in the Mesozoic (before both the PETM and K/T) (see Fig. 1). Interestingly, the initial divergences among the epiphytic clubmosses (see Table S1) were also estimated to have occurred in this era (55). These exceptions suggest that late Mesozoic conditions were somehow conducive to epiphyte growth and diversification, yet they were clearly not sufficient for the most prolific epiphytic radiations (see Figs. 1 and 2) (49). The earlier forests almost certainly lacked the closed, multistratal, angiosperm-dominated canopies typical of modern tropical rain forests (39–47), and the climate was not as warm or humid as it was at the PETM (50–52). While a Mesozoic analog might have allowed for the early diversification of epiphytic trichomanoids (see Fig. 1) and clubmosses (55), and even some understory trees (48), it was not the cradle of biodiversity that the tropical rain forest biome is today (28, 29, 42).
Relative Rates of Diversification.
Under a constant rate of diversification (speciation minus extinction), the number of lineages through time, and (by extension) the number of divergences through time, should increase exponentially (56). But although our plots of terrestrial divergences (see Fig. 2) generally conformed to this expectation, those for leptosporangiates as a whole did not (see Table S5). Therefore, it would seem that the evolution of epiphytism resulted in a shift in the diversification rate for leptosporangiate ferns. Our plots of epiphytic divergences (see Fig. 2) are themselves not exponential (see Table S5)—apparently because of the rapid increase in divergences at the PETM.
The suggestion that the evolution of epiphytism in ferns was associated with a shift in diversification rate was reinforced by our diversification-rate estimates. For epiphytic nodes, absolute rates of diversification are generally higher than those for terrestrial nodes, regardless of background extinction rate (see Tables S3 and S4). In fact, the median rate for epiphytic nodes is about 70% greater than the median rate for terrestrial nodes (see Table S6). Even if one only considers nodes after the K/T boundary or the PETM, the pattern is the same, although the increase (roughly 30%) is somewhat less pronounced. In every case, these differences are significant (P < 0.01), but it is important to note that because of the nested nature of a phylogeny, the diversification rate estimates for all nodes are not independent.
For leptosporangiates as a whole (node 0; see Fig. 1 and Fig. S1), the rates of diversification we recover (0.0281 and 0.0226 net speciation events per million years, depending on the relative extinction rate) (see Table S4) are only about one-third those calculated for the angiosperm clade in Magallón and Sanderson (56) (0.0893 and 0.0767 net speciation events per million years)—not surprising considering the substantially older crown group age for leptosporangiates and their lower estimated species diversity. It is interesting, however, that the diversification rates calculated for nearly all of the major epiphytic fern lineages (and even a few major terrestrial lineages) are on par with, or even exceed, the overall estimates for angiosperms. For example, the epiphytic hymenophylloids (node 18), asplenioids (node 163), elaphoglossoids (node 295), and polygrammoids (node 340) all have exceptionally high rates of diversification (relative to other leptosporangiate clades), and the rates for vittarioids (node 151) are not far behind (see Table S4). Rates for major terrestrial clades are somewhat more variable, ranging from very low in the osmundaceous ferns (node 1), gleichenioids (node 32), schizaeoids (node 43), and heterosporous ferns (node 50) to quite high in the lindsaeoids (node 90), pteridoids (node 112), thelypteroids (node 192), and tectarioids (node 327).
Conclusions
The fossil record suggests that the evolutionary history of leptosporangiates included 3 distinct pulses of diversification, the most recent of which is understood to be responsible for the exceptional diversity of polypod ferns we find on the Earth today (12–14). In our study, as in ref. 15, we see the signature of this third fern radiation in the Cretacaeous beginning soon after the rise of flowering plants. This radiation was likely an ecological opportunistic response to the establishment of more complex angiosperm-dominated ecosystems and, based on our current findings, was at its inception almost entirely restricted to niches on the forest floor, the occupation of which was possibly facilitated by the evolution of a unique photoreceptor in polypods (18). In this study, however, we also find evidence for what we interpret as a fourth leptosporangiate radiation in the Cenozoic, which appears to be driven by the evolution of epiphytism and the subsequent invasion of the angiosperm-dominated canopies of modern tropical rain forests soon after their origin. Whether this latter radiation was triggered simply by the establishment of these forests or spurred on by some other factor (e.g., climate change) remains to be determined. Nonetheless, it is now clear that in both of the more recent pulses of diversification, leptosporangiate ferns—unlike other nonflowering vascular plant lineages—were able to successfully exploit newly created niches in angiosperm-dominated ecosystems.
Materials and Methods
Sampling and Sequencing.
To gain a realistic approximation of the leptosporangiate fern tree of life, 400 species (of about 9,000) were sampled proportionally according to habit (about two-thirds of the sampled species are terrestrial, one-third epiphytic) and clade size (more species were sampled from larger clades, fewer from smaller clades). Names for all sampled species appear in Fig. S1; voucher information is provided elsewhere (31). To ensure a robust phylogeny, 3 plastid protein-coding genes (rbcL, atpA, and atpB; totaling more than 4,000 base pairs) were sequenced for each of the 400 species plus 5 (eusporangiate) outgroups. For detailed methods regarding DNA isolation, amplification, sequencing, and alignment, as well as GenBank accession numbers, see ref. 31.
Phylogenetic Analyses.
The combined 3-gene dataset was phylogenetically analyzed using RAxML-VI-HPC (Randomized Axelerated Maximum Likelihood for High Performance Computing) 2.2.1 (57) with the GTRMIX model of nucleotide substitution and model parameters estimated and optimized separately for each gene. One-thousand alternative runs from distinct randomized maximum parsimony starting trees were conducted, each using the rapid hill-climbing algorithm. To assess support and obtain a pool of alternative trees (with branch lengths) to account for phylogenetic uncertainty in the subsequent analyses, a nonparametric bootstrap analysis (with 100 replicates) was also conducted using RAxML-VI-HPC.
Divergence-Time Estimates.
Divergence times were estimated for all ingroup nodes in the most likely tree (see Fig. S1), as well as the 100 bootstrap trees, using penalized likelihood in r8s 1.71 (58), incorporating 23 minimum-age constraints and a single fixed-calibration point from the fossil record (see Table S2). For each of the 101 trees, the appropriate smoothing value was independently identified using cross validation (smoothing values from 1 to 10,000 were considered; for most trees, including the most likely tree, a value of 100 was found to be the most appropriate). Searches for solutions that optimized the penalized-likelihood function were conducted using the truncated Newton algorithm with 10 random starts, each with 10 random perturbations.
Ancestral State Reconstructions.
Using regional floras, we scored each of the 400 sampled species as either epiphytic or terrestrial (for scorings, see Fig. S1). Following ref. 19, we scored as epiphytic those species that typically root in another plant. Species that root in soil (even if scandent), rocks, or water were scored as terrestrial (i.e., not epiphytic). None of the sampled taxa were scored as polymorphic. Habit was reconstructed across the 101 dated phylogenies using maximum likelihood in Mesquite 1.12 (59). An asymmetrical 2-parameter Markov k-state model was used, with rates of change estimated. Ancestral-state decisions were made using a threshold of 2 log-likelihood units.
Tests for Differential Rates of Diversification.
To determine whether or not rates of diversification were constant through geologic time, we fit exponential curves to the plots of terrestrial, ambiguous, epiphytic, and total leptosporangiate divergences drawn from our 101 dated phylogenies, assessing goodness-of-fit with the nonparametric Kolmogorov–Smirnov test in JMP 7.0.1 (SAS Institute Inc.). We then calculated absolute rates of diversification for all nodes resolved in our most likely timetree (see Fig. 1 and Fig. S1) using equation 7 of ref. 56. Species numbers for 32 key nodes (see Table S4) were estimated from the literature (primarily ref. 33). Counts for all other nodes (see Table S3) were based on our proportional sampling approach: for each node, we multiplied the number of sampled descendants by our sampling factor of 22.5 (9,000 total leptosporangiate species divided by 400 sampled species). We used our best age estimates (see Tables S3 and S4) and made calculations both in the absence of extinction and under a high relative extinction rate (0.9; following ref. 56). We assessed the similarity of epiphytic and terrestrial diversification rates using the nonparametric Wilcoxon rank-sum test in JMP 7.0.1.
Supplementary Material
Acknowledgments.
We are grateful for the generous contributions of numerous collectors, botanical gardens, herbaria, and government agencies; without their assistance, this project and the earlier studies upon which it is built would not have been possible. For helpful comments, criticism, and advice at various stages in the completion of this project, we thank J.B. Beck, J.G. Burleigh, A.L. Grusz, R.L. Huiet, F. Lutzoni, P.S. Manos, J.S. Metzgar, C.J. Rothfels, E.M. Sigel, D.L. Swofford, J.L. Thorne, and J.E. Watkins; J.M. Mercer, N.S. Nagalingum, V.L. Roth, H. Schneider, M.D. Windham, and the two anonymous reviewers were especially helpful. At our request, A. Stamatakis was kind enough to modify RAxML to optimize and save branch lengths for bootstrap trees. This research was supported in part by an American Society of Plant Taxonomists Rogers McVaugh Graduate Student Research Grant (to E.S.), a Duke University Department of Biology AW Mellon Plant Systematics Program Award (to E.S.), a Lawrence Memorial Award (to E.S.), a National Science Foundation CAREER Award DEB-0347840 (to K.M.P.), a National Science Foundation Doctoral Dissertation Improvement Grant Award DEB-0408077 (to K.M.P. and E.S.), and a Society of Systematic Biologists Graduate Student Research Award (to E.S.). This article is part of a doctoral dissertation completed at Duke University by E.S.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811136106/DCSupplemental.
References
- 1.Gradstein FM, Ogg JG, Smith AG. A Geologic Time Scale. Cambridge: Cambridge Univ Press; 2004. [Google Scholar]
- 2.Crane PR. In: The Origin of Angiosperms and Their Biological Consequences. Friis EM, Chaloner WG, Crane PR, editors. Cambridge: Cambridge Univ Press; 1987. pp. 107–144. [Google Scholar]
- 3.Crane PR, Friis EM, Pederson KR. The origin and early diversification of angiosperms. Nature. 1995;374:27–33. [Google Scholar]
- 4.Lidgard S, Crane PR. Quantitative analyses of the early angiosperm radiation. Nature. 1988;331:344–346. [Google Scholar]
- 5.Lidgard S, Crane PR. Angiosperm diversification and Cretaceous floristic trends: a comparison of palynofloras and leaf macrofloras. Paleobiology. 1990;16:77–93. [Google Scholar]
- 6.Lupia R, Lidgard S, Crane PR. Comparing palynological abundance and diversity: implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology. 1999;25:305–340. [Google Scholar]
- 7.Nagalingum NS, Drinnan AN, Lupia R, McLoughlin S. Fern spore diversity and abundance in Australia during the Cretaceous. Rev Palaeobot Palynol. 2002;119:69–92. [Google Scholar]
- 8.Niklas KJ, Tiffney BH, Knoll AH. Patterns in vascular land plant diversification. Nature. 1983;303:614–616. [Google Scholar]
- 9.Galtier J, Phillips TL. In: Pteridology in Perspective. Camus JM, Gibby M, Johns RJ, editors. Kew: Royal Botanic Gardens; 1996. pp. 417–433. [Google Scholar]
- 10.Galtier J, Scott AC. Diversification of early ferns. Proc R Soc Edinburgh B Biol Sci. 1985;86:289–301. [Google Scholar]
- 11.Brenner GJ. In: Flowering Plant Origin, Evolution, and Phylogeny. Taylor DW, Hickey LJ, editors. New York: Chapman and Hall; 1996. pp. 91–115. [Google Scholar]
- 12.Lovis JD. Evolutionary patterns and processes in ferns. Adv Bot Res. 1977;4:229–415. [Google Scholar]
- 13.Rothwell GW. Complex Paleozoic Filicales in the evolutionary radiation of ferns. Am J Bot. 1987;74:458–461. [Google Scholar]
- 14.Rothwell GW, Stockey RA. In: Biology and Evolution of Ferns and Lycophytes. Ranker TA, Haufler CH, editors. Cambridge: Cambridge Univ Press; 2008. pp. 332–366. [Google Scholar]
- 15.Schneider H, et al. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:553–557. doi: 10.1038/nature02361. [DOI] [PubMed] [Google Scholar]
- 16.Pryer KM, et al. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am J Bot. 2004;91:1582–1598. doi: 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- 17.Smith AR. Comparison of fern and flowering plant distributions with some evolutionary interpretations for ferns. Biotropica. 1972;4:4–9. [Google Scholar]
- 18.Kawai H, et al. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature. 2003;421:287–290. doi: 10.1038/nature01310. [DOI] [PubMed] [Google Scholar]
- 19.Moffett MW. What's “up”? A critical look at the basic terms of canopy biology. Biotropica. 2000;32:569–596. [Google Scholar]
- 20.Watkins JE, Jr., Mack MC, Sinclair TR, Mulkey SS. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytol. 2007;176:708–717. doi: 10.1111/j.1469-8137.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 21.Benzing DH. Vascular Epiphytes. Cambridge: Cambridge Univ Press; 1990. [Google Scholar]
- 22.Dubuisson J-Y, Schneider H, Hennequin S. Epiphytism in ferns: diversity and history. C R Biol. 2009;332:120–128. doi: 10.1016/j.crvi.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Schneider H. Morphology and anatomy of roots in the filmy fern tribe Trichomaneae H. Schneider (Hymenophyllaceae, Filicatae) and the evolution of rootless taxa. Bot J Linn Soc. 2000;132:29–46. [Google Scholar]
- 24.Janssen T, Schneider H. Exploring the evolution of humus collecting leaves in drynarioid ferns (Polypodiaceae, Polypodiidae) based on phylogenetic evidence. Plant Syst Evol. 2005;252:175–197. [Google Scholar]
- 25.Gentry AH, Dodson C. Contribution of nontrees to species richness of a tropical rain forest. Biotropica. 1987;19:149–156. [Google Scholar]
- 26.Gentry AH, Dodson CH. Diversity and biogeography of neotropical vascular epiphytes. Ann Mo Bot Gard. 1987;74:205–233. [Google Scholar]
- 27.Nieder J, Engwald S, Barthlott W. Patterns of neotropical epiphyte diversity. Selbyana. 1999;20:66–75. [Google Scholar]
- 28.Richards PW. The Tropical Rain Forest: An Ecological Study. Cambridge: Cambridge Univ Press; 1996. [Google Scholar]
- 29.Whitmore TC. An Introduction to Tropical Rain Forests. Oxford: Oxford Univ Press; 1998. [Google Scholar]
- 30.Kress WJ. The systematic distribution of vascular epiphytes: an update. Selbyana. 1986;9:2–22. [Google Scholar]
- 31.Schuettpelz E, Pryer KM. Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes. Taxon. 2007;56:1037–1050. [Google Scholar]
- 32.Berry PE, Holst BK, Yatskievych K, editors. Flora of the Venezuelan Guayana, Vol. 2, Pteridophytes and Spermatophytes (Acanthaceae to Araceae) Timber Press, Portland: Missouri Botanical Garden Press, St. Louis; 1995. [Google Scholar]
- 33.Smith AR, et al. A classification for extant ferns. Taxon. 2006;55:705–731. [Google Scholar]
- 34.Hennequin S, Schuettpelz E, Pryer KM, Ebihara A, Dubuisson J-Y. Divergence times and the evolution of epiphytism in filmy ferns (Hymenophyllaceae) revisited. Int J Plant Sci. 2008;169:1278–1287. [Google Scholar]
- 35.Crane EH. A revised circumscription of the genera of the fern family Vittariaceae. Syst Bot. 1997;22:509–517. [Google Scholar]
- 36.Lowman MD, Rinker HB, editors. Forest Canopies, Second Edition. Amsterdam: Elsevier; 2004. [Google Scholar]
- 37.Grime JP. Plant Strategies and Vegetation Processes. Chichester: John Wiley and Sons; 1979. [Google Scholar]
- 38.Grubb PJ, Metcalfe DJ. Adaptation and inertia in the Australian tropical lowland rain-forest flora: contradictory trends in intergeneric and intrageneric comparisons of seed size in relation to light demand. Funct Ecol. 1996;10:512–520. [Google Scholar]
- 39.Burnham RJ, Johnson KR. South American palaeobotany and the origins of neotropical rainforests. Philos Trans R Soc Lond B Biol Sci. 2004;359:1595–1610. doi: 10.1098/rstb.2004.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs BF. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Philos Trans R Soc Lond B Biol Sci. 2004;359:1573–1583. doi: 10.1098/rstb.2004.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson KR, Ellis B. A tropical rainforest in Colorado 1.4 million years after the Cretaceous-Tertiary boundary. Science. 2002;296:2379–2383. doi: 10.1126/science.1072102. [DOI] [PubMed] [Google Scholar]
- 42.Morley RJ. Origin and Evolution of Tropical Rain Forests. Chichester: John Wiley and Sons; 2000. [Google Scholar]
- 43.Tiffney BH. Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Ann Mo Bot Gard. 1984;71:551–576. [Google Scholar]
- 44.Upchurch GR, Wolfe JA. In: The Origins of Angiosperms and Their Biological Consequences. Friis EM, Chaloner WG, Crane PR, editors. Cambridge: Cambridge Univ Press; 1987. pp. 75–105. [Google Scholar]
- 45.Wheeler EA, Baas P. A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA Bull New Ser. 1991;12:275–332. [Google Scholar]
- 46.Wing SL, Boucher LD. Ecological aspects of the Cretaceous flowering plant radiation. Annu Rev Earth Planet Sci. 1998;26:379–421. [Google Scholar]
- 47.Wolfe JA, Upchurch GR. North American nonmarine climates and vegetation during the Late Cretaceous. Palaeogeogr Palaeoclimatol Palaeoecol. 1987;61:33–77. [Google Scholar]
- 48.Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ. Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. Am Nat. 2005;165:E36–E65. doi: 10.1086/428296. [DOI] [PubMed] [Google Scholar]
- 49.Ramírez SR, Gravendeel B, Singer RB, Marshall CR, Pierce NE. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature. 2007;448:1042–1045. doi: 10.1038/nature06039. [DOI] [PubMed] [Google Scholar]
- 50.Bowen GJ, Beerling DJ, Koch PL, Zachos JC, Quattlebaum T. A humid climate state during the Palaeocene/Eocene thermal maximum. Nature. 2004;432:495–499. doi: 10.1038/nature03115. [DOI] [PubMed] [Google Scholar]
- 51.Wing SL, et al. Transient floral change and rapid global warming at the Paleocene-Eocene boundary. Science. 2005;310:993–996. doi: 10.1126/science.1116913. [DOI] [PubMed] [Google Scholar]
- 52.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 53.Willis KJ, McElwain JC. The Evolution of Plants. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 54.Lüttge U, editor. Vascular Plants as Epiphytes. Berlin: Springer-Verlag; 1989. [Google Scholar]
- 55.Wikström N, Kenrick P. Evolution of Lycopodiaceae (Lycopsida): estimating divergence times from rbcL gene sequences by use of nonparametric rate smoothing. Mol Phylogenet Evol. 2001;19:177–186. doi: 10.1006/mpev.2001.0936. [DOI] [PubMed] [Google Scholar]
- 56.Magallón S, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 57.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 58.Sanderson MJ. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. [DOI] [PubMed] [Google Scholar]
- 59.Maddison WP, Maddison DR. Mesquite: A Modular System for Evolutionary Analysis, Version 1.12. 2006 http://mesquiteproject.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.