Abstract
The mechanisms that generate itch are poorly understood at both the molecular and cellular levels despite its clinical importance. To explore the peripheral neuronal mechanisms underlying itch, we assessed the behavioral responses (scratching) produced by s.c. injection of various pruritogens in PLCβ3- or TRPV1-deficient mice. We provide evidence that at least 3 different molecular pathways contribute to the transduction of itch responses to different pruritogens: 1) histamine requires the function of both PLCβ3 and the TRPV1 channel; 2) serotonin, or a selective agonist, α-methyl-serotonin (α-Me-5-HT), requires the presence of PLCβ3 but not TRPV1, and 3) endothelin-1 (ET-1) does not require either PLCβ3 or TRPV1. To determine whether the activity of these molecules is represented in a particular subpopulation of sensory neurons, we examined the behavioral consequences of selectively eliminating 2 nonoverlapping subsets of nociceptors. The genetic ablation of MrgprD+ neurons that represent ≈90% of cutaneous nonpeptidergic neurons did not affect the scratching responses to a number of pruritogens. In contrast, chemical ablation of the central branch of TRPV1+ nociceptors led to a significant behavioral deficit for pruritogens, including α-Me-5-HT and ET-1, that is, the TRPV1-expressing nociceptor was required, whether or not TRPV1 itself was essential. Thus, TRPV1 neurons are equipped with multiple signaling mechanisms that respond to different pruritogens. Some of these require TRPV1 function; others use alternate signal transduction pathways.
Keywords: itch, PLCb3, scratching
Itch can be induced by a variety of chemical stimuli generated within or applied to the skin, and this reliably produces a characteristic scratching reflex (1). It is significant that in humans, itch is never felt in muscle, joints, or internal organs (2). In contrast, itch is readily evoked from skin, which is innervated by primary afferents that respond directly to itch-producing stimuli, or are activated indirectly, via keratinocytes, mast cells, and Langerhans cells, which release itch-producing compounds., Schmelz and colleagues (3) used iontophoresis of histamine to show that the unmyelinated fibers that convey information producing histamine-evoked itch comprise 5% of the afferent C-fibers in human skin. Based on conduction velocities, responses to sensory modalities (heat vs. mechanical stimuli) and innervation territories, these histamine-sensitive fibers appear to be distinct from the polymodal C-fiber nociceptors that mediate pain (3, 4).
These findings are the basis of the specificity theory of itch, which posits that itch is transmitted by neurons distinct from those that transmit pain. In fact, electrophysiological studies of spinothalamic tract (STT) neurons in the cat have provided evidence for the existence of specific itch neurons in the central nervous system (5). Other reports (6, 7), however, found that histamine-responsive STT neurons were also activated by both noxious mechanical and heat stimuli. Histamine-sensitive primary afferents in human also respond to capsaicin, a painful stimulus (4). These results argue against the specificity theory. However, a gastrin-releasing peptide receptor (GRPR) expressed in the spinal dorsal horn has been specifically implicated in itch, but not pain (8). Clearly, the extent to which itch is exclusively mediated by specific, nonnociceptive neurons remains controversial.
A major difficulty in discerning the extent to which the specificity view holds for the generation of itch is the lack of molecular markers of pruritogen-responsive afferents or of itch-specific genes that distinguish itch-related dorsal root ganglion (DRG) neurons from others. Recently, we found that histamine induces itch responses (scratching) via the action of a histamine H1 receptor that is expressed by a small subset of C-fibers. We also showed that intracellular signal transduction via PLCβ3 is critical to the histamine-induced scratching in mice (9). Other studies reported that TRPV1-deficient mice also showed diminished scratching to histamine or trypsin (10, 11). Taken together, these results suggest that TRPV1+PLCβ3+ neurons are histamine-responsive itch neurons.
The TRPV1 channel is directly activated by pain-producing stimuli such as capsaicin, heat and acid but not by pruritogens. Activation of TRPV1+ neurons by pruritogens appears, instead, to require an intracellular signal-transducing mechanism that lies downstream of G protein-coupled receptors (10, 11). Given that a number of different pruritogens can trigger distinct and specific intracellular signaling pathways to activate itch-sensing neurons, it is possible that there are different subsets of TRPV1-expressing neurons specialized to process signals from specific groups of pruritogens. Alternatively, each of the TRPV1-expressing neurons may be equipped with multiple mechanisms to process responses to different pruritogens and to generate an itch response. One approach to assessing these different possibilities is to ablate the DRG neurons that express TRPV1 and to examine the behavioral consequences of applying different groups of pruritogens that may signal through different mechanisms. Using this approach, we now report that TRPV1+ neurons, as a population, respond to pruritogens through multiple and distinct intracellular mechanisms.
Results
Behavioral Responses to Innocuous or Noxious Thermal, Mechanical Stimuli Are Normal in PLCβ3-Deficient Mice.
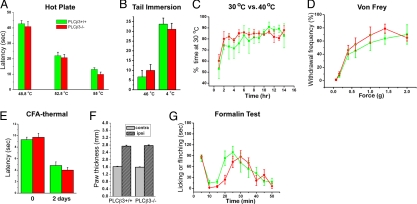
Many of the pruritogenic stimuli that produce scratching can also produce behaviors indicative of pain. In fact, our previous finding that the PLCβ3 protein is predominantly expressed in C-fiber nociceptors (9) raised the possibility that PLCβ3 is part of a signaling pathway that contributes to noxious stimulus-evoked pain behavior. We specifically addressed this question by studying the contribution of PLCβ3 in a variety of assays of pain behavior (Fig. 1). In the hot plate and Hargreaves tests, the paw is stimulated by contact with a hot plate or radiant heat source, respectively, and the time to paw licking or withdrawal is measured. In the tail immersion test, the distal portion of the tail is immersed in a hot or cold water bath, and the time to tail flick is recorded. PLCβ3-mutant mice were indistinguishable from their wild-type littermates in the hot plate (48, 52.5, and 55 °C), Hargreaves, and tail immersion (46 °C and 4 °C) tests (Fig. 1 A, B, and E). Thus, acute noxious heat or cold responsiveness is intact in the absence of PLCβ3.
Fig. 1.
Temperature, mechanical, and pain behavioral analysis in PLCβ3-deficient mice and their littermate controls. (A) Latency to exhibit evidence of discomfort (paw shaking and licking) on a hot plate (n = 8 per genotype). (B) Latency to tail withdrawal from a hot or cold water bath (n = 6 per genotype). (C) Percentage of time spent at 30 °C when given a choice between 30 °C and 40 °C (n = 4 per genotype). (D) Paw withdrawal frequency to von Frey filaments (n = 8 per genotype). (E) Latency to paw withdrawal from radiant heat before (“0”) and 2 days after CFA injection (n = 8 per genotype). (F) Paw thickness of the CFA-injected (ipsi) and uninjected (contra) hindpaw (n = 8 per genotype). (G) Time spent for paw licking and shaking after formalin injection into a hind paw during each 5-min interval of a 50-min test period (n = 8 per genotype). There were no significant differences at any point between 2 groups (P > 0.05, two-way ANOVA with Bonferroni posttests) for any of the behavioral tests. All data are presented as means ± SEM.
C-fiber nociceptors have also been implicated in sensitivity to innocuous temperature (12–14). We therefore also asked whether PLCβ3-deficient mice could discriminate between 2 innocuous temperatures (30 vs. 40 °C). Mice were placed in an apparatus in which 2 chambers were maintained at different temperatures. We allowed the mice free access to the 2 chambers and measured the time spent at the different temperatures. Fig. 1C illustrates that both wild-type and PLCβ3-mutant mice equally favor the 30 °C chamber, spending ≈80% of their time at this temperature. Finally, we tested mice for mechanical sensitivity. The mechanical withdrawal threshold in the PLCβ3-mutant mice, tested with calibrated von Frey filaments, did not differ from wild-type mice (Fig. 1D). These results indicate that the appreciation of both innocuous and noxious temperature and the ability to perceive noxious mechanical stimuli are preserved in the absence of PLCβ3.
We also did not find any differences in the magnitude of the thermal hypersensitivity that occurs in the setting of inflammation of the hindpaw produced by injection of Complete Freund's Adjuvant (CFA) (Fig. 1E). Importantly, the magnitude of paw edema produced by CFA did not differ in PLCβ3-deficient mice when compared with their wild-type littermates (Fig. 1F), which indicates that the afferent terminals are capable of generating a normal neurogenic inflammatory response in the absence of PLCβ3.
Next, we examined the mice in the formalin test, which is considered to model tissue-injury induced postoperative pain. We monitored the flinching and licking behavioral responses produced by hindpaw injection of formalin and again, we did not find significant differences between wild-type and mutant mice, in either the first (0–10 min) or in the second phase (10–60 min) of the test (Fig. 1G). Taken together, these data suggest that PLCβ3 is not required for the normal appreciation of innocuous or noxious mechanical or thermal stimuli, both in the absence and presence of tissue injury/inflammation. Based on these observations, we believe that any changes in scratching behavior observed in response to various pruritogens in the PLCβ3-deficient mice likely reflects the fact that different mechanisms underlie the generation of itch and pain in response to noxious stimulation.
PLCβ3 Is Required for the Induction of Scratching in Response to Some, but Not All Pruritogens.
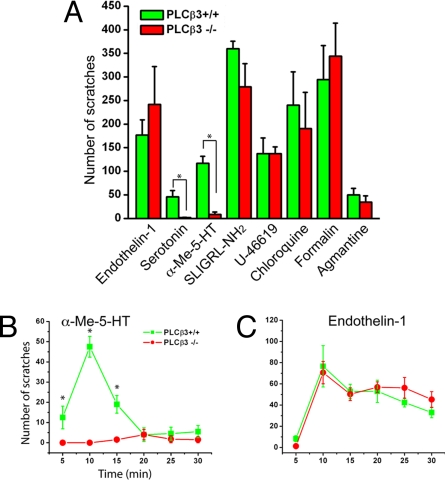
We reported that PLCβ3 contributes to the itch produced by histamine and its related chemical compounds (9). Here, we asked how general is the contribution of PLCβ3 signaling to the itch that is produced by a variety of pruritogens. To this end, we tested the effects of 8 different pruritic compounds in PLCβ3-deficient mice and in their wild-type littermates. Each pruritic compound was injected s.c. into the skin of the nape of the neck and we recorded the resultant scratching responses over the next 30 min (Fig. 2A). Generally, hindpaw scratching was directed toward the injection site after a lag time of several minutes. The scratching peaked between 5 to 10 min.
Fig. 2.
The scratching elicited by serotonin and α-Me-5-HT, but not other pruritogens, is eliminated in PLCβ3-deficient mice. (A) The total number of “bouts” of scratching per 30 min in response to s.c. injections into the nape of the neck of 8 different pruritic compounds, in a volume of 100 μL. Compounds tested: 5-HT (100 nmol), α-Me-5HT (30 μg), ET-1 (10 pmol), chloroquine (200 μg), formalin (100 μL of 0.6%), U-46619 (3.5 μg), SLIGRL-NH2 (100 nmol), and agmantin (160 μg). At least 4 and up to 10 pairs of the mice were used for each compound. (B and C) Time course of scratching after injection of α-Me-5HT and ET-1 in wild-type (filled rectangle) versus PLCβ3−/− (filled circle) mice. Compared with wild-type mice (n = 8), PLCβ3-deficient mice (n = 8) show a complete abrogation for α-Me-5HT but no effect for ET-1. Data represent means ± SEM. Asterisks denote significant differences compared with littermate controls. *, P < 0.01, Mann–Whitney U test.
Among the various pruritogens tested, 5-HT and the selective agonist, α-Me-5-HT, which targets 5-HT2 receptors, evoked scratching that was almost completely abolished in PLCβ3-deficient mice. However, the scratching produced by a variety of other pruritic agents (including ET-1 and chloroquine) neither decreased nor increased in the mutant mice (Fig. 2A). This distinction is further illustrated in Fig. 2 B and C, which show the time course of scratching during the 30-min test period, at 5-min intervals after injection of 2 representative pruritogens: α-Me-5-HT, which is a PLCβ3-dependent pruritogen and ET-1, which is clearly PLCβ3-independent. We conclude that PLCβ3's contribution as an intracellular itch mediator is highly selective.
Primary sensory neurons express both metabotropic and ionotropic serotonin receptors (15). The 5-HT3 receptor, a serotonin gated ion channel receptor, is the dominant isoform expressed in sensory neurons (16). We asked whether 5-HT or α-Me-5HT elicit scratching by activating the 5-HT3 receptor. In fact, neither 5-HT- nor α-Me-5HT-evoked scratching was altered in 5-HT3 receptor-deficient mice (5-HT: 83.0 ± 41 for WT and 87.6 ± 38.4 for KO, n = 5 per genotype; α-Me-5-HT: 154.3 ± 17.2 for WT and 207.0 ± 38.1 for KO mice, n = 6 per genotype). These results are in agreement with the conclusion that serotonin-evoked scratching involves a PLCβ3-coupled metabotropic receptor.
The Magnitude of Scratching Responses Correlates with Pruritogen-Induced Fos Expression in the Dorsal Horn.
Among the various pruritic compounds tested, we chose 2 (α-Me-5HT and ET-1) for further study. These pruritogens differ in their requirement for PLCβ3 and show robust scratching in WT mice. The total number of “bouts” of scratching induced by α-Me-5-HT was approximately double that induced by 5-HT at the same dose. Based on the dose required to evoke scratching, ET-1 was the most potent of the pruritogens tested. Thus, most pruritogens, including the 5-HT related agonists induce scratching at micromole doses, but ET-1 is effective in the picomole range, suggesting that ET-1 acts directly on its cognate receptor to induce itch.
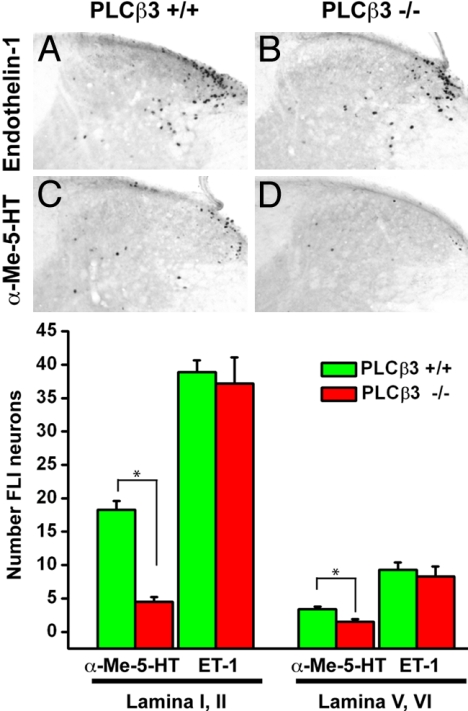
To test the hypothesis that the magnitude of scratching is reflected by increased activity of spinal dorsal horn neurons, we examined Fos induction in upper cervical spinal cord segments. We studied PLCβ3-deficient mice and wild-type controls after s.c. injection of α-Me-5-HT or ET-1. Fig. 3 illustrates that both pruritogens induced a comparable pattern of Fos expression in the lateral aspect of the superficial dorsal horn (laminae I and II), and consistent but much less Fos expression in the region of laminae V and VI (Fig. 3 A and C). The mean number of Fos-like immunoreactive (FLI) neurons induced by ET-1 was approximately double that recorded after injection of α-Me-5HT (Fig. 3 A and C), which is comparable to the relative magnitude of the scratching induced by these 2 compounds. Furthermore, the α-Me-5-HT- induced FLI was profoundly decreased in the PLCβ3-deficient mice, by 75% in laminae I and II and 56% in laminae V and VI. In contrast, ET-1-induced FLI did not change in the mutant mice. Again, the persistence of Fos expression perfectly parallels our finding that ET-1 scratching is not reduced in the PLCβ3-deficient mice. Because the magnitude of the behavioral phenotype correlated with a marker of activity in neurons located immediately downstream of the primary afferents, these results indicate that differential activity of the primary afferent in the mutant and wild-type mice likely accounts for the differences between α-Me-5HT and ET-1 induced scratching.
Fig. 3.
Fos-like immunoreactivity (FLI) in superficial dorsal horn of the rostral cervical spinal cord in wild-type and PLCβ3−/− mice after s.c. injection of α-Me-5HT or ET-1 into the nape of the neck. (A–D) There is a dense cluster of FLI neurons in the lateral part of laminae I and II, where primary afferents from the nape of the neck terminate. ET-1 induced FLI was not altered in the PCLβ3−/− mice compared with wild-type mice, but α-Me-5-HT-evoked FLI was greatly reduced in PCLβ3−/− mice (n = 4 to 8 per genotype for each compound). The graphs indicate number of FLI neurons in laminae I, II, V, and VI. The statistical difference between the 2 genotypes was significant for α-Me-5-HT-evoked FLI (*, P < 0.0001, Mann–Whitney U test), but not for ET-1. Error bars, means ± SEM.
PLCβ3 Mutants Exhibit Deficient Nocifensive Responses to Hindpaw Injections of Pruritogen.
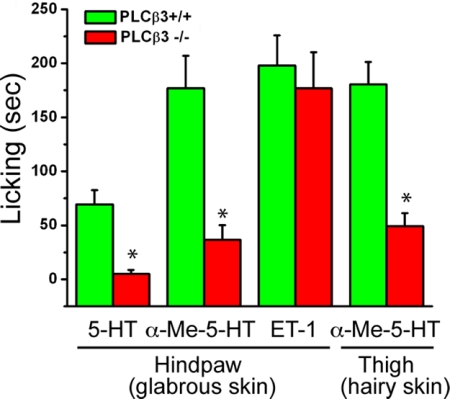
In addition to their scratching responses, serotonin and ET-1 induce licking of the hindpaw after s.c. injection into the plantar footpad. This “nocifensive” behavior has been thought to reflect a pain response (17, 18). Because PLCβ3 is dispensable for behavioral responses to noxious thermal and mechanical stimuli, it was of interest to assess whether PLCβ3 is required for the licking behavioral responses induced by pruritogens. We made injections into the glabrous skin of the hindpaw and monitored the animals' behavior after injection of 5-HT, α-Me-5-HT or ET-1. We used the same amount of pruritogen as for the nape of the neck and tested the animals every 10 min for 30 min.
As expected, injection of the pruritogens produces licking rather than scratching. In wild-type mice, 5-HT, α-Me-5-HT, and ET-1 elicited robust licking of the paw (Fig. 4). In contrast, in PLCβ3-deficient mice we found significant deficits in the magnitude of licking produced by injection of either 5-HT or α-Me-5-HT. However, the licking produced by hindpaw injection of ET-1 was not altered (Fig. 4).
Fig. 4.
PLCβ3-deficient mice show impaired licking behavioral responses to hindpaw injection of 5-HT (n = 4 per genotype) and α-Me-5-HT (n = 9 per genotype), but not to ET-1 (n = 8 per genotype). Note that the licking responses produced by thigh injection of α-Me-5-HT (n = 5 per genotype) are also significantly impaired in PLCβ3-deficient mice. *, P < 0.05, Student's t test for both 5-HT and α-Me-5-HT compared with wild-type mice. All data presented as means ± SEM.
Whether the licking represents a response to a painful or an itch-producing stimulus or both is unclear and cannot be determined from our analysis. On the one hand, pruritogens injected into the hindpaw may evoke a sensation of itch rather than pain, but licking behavior may occur because mice are not able to scratch the footpad. However, pruritogens injected into the hindpaw may evoke a sensation of pain rather than itch, leading to licking behavior. It is also possible that the different behavior reflects the type of skin (i.e., hairy vs. glabrous) injected. To address this question, we next injected the pruritogens subcutaneouly into the hairy skin of the thigh. Licking of the thigh is possible, but scratching is difficult. As for the hindpaw, we found that PLCβ3 was required for the licking behavior produced by α-Me-5-HT (Fig. 4). These results indicate that PLCβ3 is required for behaviors evoked by injection of 5-HT or α-Me-5-HT (either scratching or licking), and that this requirement is independent of the region stimulated (hairy vs. glabrous skin). Whether these different behaviors reflect a common sensation (itch) producing location-specific behavioral responses, or location-specific percepts that evoke sensation-specific behaviors remains to be determined.
TRPV1-Expressing Primary Afferents Are Essential for the Behavioral Responses to Both α-Me-5-HT and ET-1.
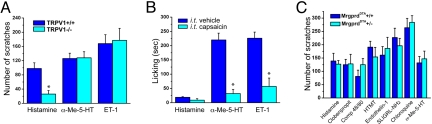
As noted above, there is increasing evidence for a central role of the TRPV1 channel in itch and pain. To determine how generalized this contribution is, we examined whether TRPV1 function is also required for the activity of the pruritic compounds studied in our analyses. As shown in Fig. 5A, TRPV1-deficient mice showed significant deficits in histamine induced scratching responses, which is consistent with a report in ref. 10. In contrast, neither α-Me-5-HT- nor ET-1-evoked scratching was reduced in the mutant mice, indicating that these pruritogens do not require TRPV1 function. However, these results do not rule out the possibility that TRPV1-expressing neurons use an alternate signal transducing mechanism, independent of TRPV1 itself, to respond to a variety of pruritogens.
Fig. 5.
TRPV1+ neurons are required for the behavioral responses to different pruritogens. (A) Scratching in response to histamine (n = 4 per genotypes), α-Me-5-HT (n = 5 per genotypes), and ET-1 (n = 4 per genotypes) in TRPV1-mutant mice. In contrast to histamine, scratching in responses to α-Me-5-HT and ET-1 was not altered in the mutant mice. *, P < 0.05, Student's t test for histamine comparing the 2 genotypes. (B) Licking in response to histamine, α-Me-5-HT, and ET-1, 1 d after intrathecal capsaicin or vehicle injection. Ablation of the central terminals of TRPV1-expressing neurons led to significant loss of the behavioral responses to both α-Me-5-HT and ET-1. *, P < 0.01, two-way rmANOVA followed by Sheffé's test; n = 3 for capsaicin treated or vehicle-treated). (C) Mice lacking MrgprD+ neurons do not show altered scratching behavior in response to 8 different itch-provoking compounds. Data represent means ± SEM; n = 4–8 for all tests.
To address this question, we used intrathecal injection of capsaicin to selectively kill the central terminals of TRPV1-expressing nociceptors and then studied the behavioral consequences (licking) in response to hindpaw injection of different pruritic compounds. We showed that intrathecal injection of capsaicin (10 μg) kills the central terminals of TRPV1-expressing neurons but spares DRG cell bodies and the peripheral terminals of the TRPV1-positive afferents (33). Fig. 5B illustrates that compared with vehicle-treated mice, intrathecal capsaicin-pretreated mice showed greatly reduced licking responses after hindpaw injection of both α-Me-5-HT and ET-1. The magnitude of licking was reduced by 86 and 75%, respectively in response to the 2 pruritogens (Fig. 5B). Because hindpaw injection of histamine produced very weak responses compared with those observed after its injection in the nape of the neck, we were not able to study the effects of deleting the TRPV1-expressing nociceptors (Fig. 5B). Nevertheless, based on our previous observations, it appears that histamine-induced itch requires both the PLCβ3 (9) and the TRPV1 gene (10). Thus, we conclude that TRPV1-expressing neurons carry the input that drives the behavioral responses to both PLCβ3-dependent and -independent pruritogens.
Finally, we investigated whether a major subset of TRPV1-negative neurons are also implicated in itch. In the mouse, MrgprD (MrgD)-positive primary afferents account for >90% of all cutaneous nonpeptidergic neurons and represent ≈60% of free nerve endings in the skin (19). In particular, MrgprD+ neurons in the mouse represent a major subpopulation of cutaneous C-fiber nociceptors that do not express TRPV1. To determine whether MrgprD neurons are involved in normal itch, we used MrgprD cell-ablated mice (MrgprDDTA), in which the MrgprD locus was replaced with the diphtheria toxin (33), allowing for the selective elimination of MrgprD-expressing neurons. Using heterozygous MrgprDDTA mice and their wild-type littermates, we examined scratching behavior in response to 8 different pruritic compounds (Fig. 5C). Strikingly, we found no significant differences between MrgprDDTA and WT mice, for all pruritogens tested, indicating that MrgprD-expressing neurons are not involved in itch. These negative results must be interpreted with caution, because there is evidence for functional compensation when MrgprD+ neurons are ablated from birth (33). Nevertheless, the data are consistent with the idea that TRPV1-expressing neurons are the major, if not exclusive contributors to the sensation of itch produced by a number of pruritogens.
Discussion
In this study, we selectively ablated 2 nonoverlapping populations of primary sensory neurons that represent most of the cutaneous nociceptors and observed that TRPV1-expressing neurons are required for the behavioral responses to several different pruritic compounds. We also demonstrated that different pruritic compounds use different intracellular pathways to induce the scratching reaction, some PLCβ3-dependent, others PLCβ3-independent or both PLCβ3- and TRPV1-function dependent. Most importantly, we conclude that TRPV1-expressing neurons have multiple intracellular mechanisms that generate or mediate itch signals.
A major caveat in these experiments is that our conclusions depend on different behavioral “read-outs” of the “itch response.” One set of experiments relies on licking to stimuli injected into the hindpaw and the other to scratching in response to ligands injected in the nape of the neck. Licking is generally thought to be a behavioral response generated only by painful noxious stimuli. For example, it is the most pronounced behavior produced by injection of the algogen, capsaicin. However, the characteristics of the licking produced by capsaicin differ from those seen with the pruritic compounds, α-Me-5HT and ET-1. Licking begins immediately after capsaicin injection, and is completed within 5–10 min. Another painful stimulus, bradykinin (BK) also induces only a brief bout of licking immediately after its injection (20). In contrast, ET-1- or α-Me-5-HT-induced licking is prolonged, lasting from 20 to 30 min, which is similar to the time course observed when scratching is induced. Thus, licking resulting from these compounds may predominantly reflect an itch-associated response, rather than a response to a painful stimulus. Our preliminary results using a behavioral “cheek-pouch” assay (21), which reportedly distinguishes itch and pain responses, also showed that there are no obvious pain behaviors after injection of α-Me-5-HT. This is consistent with our conclusion that α-Me-5-HT is a pure itch-evoking compound in the mouse.
The Molecular Specificity of Gαq-PLCβ Intracellular Signaling and Itch.
We identified PLCβ3 as a critical intracellular mediator in C-fiber nociceptors that link the histamine H1 receptor to histamine-evoked itch. Here, we show that PLCβ3 also mediates serotonin-evoked itch. In fact, PLCβ3-deficient mice exhibit an almost complete loss of serotonin- or α-Me-5-HT-induced scratching. By contrast, the PLCβ3-deficient mutant mice respond normally to ET-1, SLIGRL-NH2 (a protease-activated receptor 2 agonist) and U-46619 (a thromboxane A2 receptor agonist), despite the fact that the cognate receptors corresponding to these ligands also signal via Gαq-PLCβ (22–24). Therefore, we conclude that Gαq-coupled pruritic compounds elicit an itch response through at least 2 different Gαq pathways: one is PLCβ3-dependent and the other is PLCβ3-independent.
In our study (9), we found that PLCβ3 is necessary for histamine induced cellular responses, whereas in the same neuron, the responses to bradykinin, a “painful” stimulus, were independent of PLCβ3. Here, we find that PLCβ3 is not required for the behavioral responses in various tests of mechanosensation, temperature responsiveness, and pain. Thus, the PLCβ3-dependent pathway is dedicated to signal itch in response to specific pruritogens, including histamine and serotonin, but this pathway does not contribute to noxious stimulus-evoked pain.
Multiple Mechanisms of Pruritic Signaling in TRPV1-Expressing Neurons.
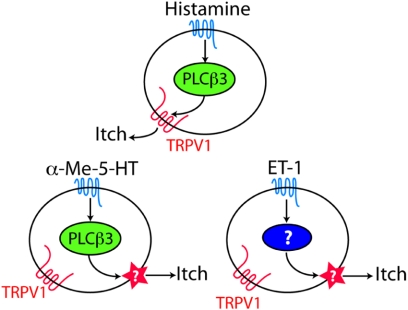
The results after intrathecal capsaicin-induced destruction of the TRPV1-expressing neurons indicate that both PLCβ3-dependent and -independent itch responses occur within the population of TRPV1-expressing neurons. It follows that TRPV1-expressing neurons are heterogenous with respect to the expression of PLCβ3. In fact, double immunolabeling experiments in DRG neurons with TRPV1 and PLCβ3 antibodies indicate that only 35% (160/458) of TRPV1+ neurons express PLCβ3 (Fig. S1). It is likely, therefore, that α-Me-5-HT exerts its itch producing effects via the activity of PLCβ3+TRPV1+ neurons. By contrast, our observation that ET-1-evoked itch requires TRPV1 neurons, but does not require PLCβ3 or TRPV1 protein indicates that the itch evoked by ET-1 is generated by a different mechanism from that which mediates the response to serotonin (Fig. 6), even though TRPV1-expressing neurons are involved. However, histamine-induced itch appears to require both PLCβ3 and TRPV1, which suggests that TRPV1 can function as an effector molecule downstream of PLCβ3. In support of this proposal, a recent electrophysiological study (25) demonstrated that TRPV1 is directly activated by diacylglycerol (DAG), which is produced by hydrolysis of PIP2, a substrate of PLCβ3. There may be other mechanisms as well for TRPV1 activation in histamine-mediated signaling (10). Finally, although there was no diminution of serotonin or ET-1 evoked scratching in TRPV1-mutant mice, ablation of the TRPV1-expressing neurons markedly decreased the behavioral response to these compounds. The difference must reflect the functional differences between deleting the TRPV1 channel and eliminating the TRPV1-expressing afferents.
Fig. 6.
This schematic illustrates 3 distinct and likely independent pathways through which itch can be induced by a histamine, α-Me-5-HT or ET-1 action upon TRPV1-expressing neurons.
The existence of multiple subsets of neurons responding to itch-provoking stimuli is supported by recent reports that histamine- and cowhage-induced itch involve different sensory circuits (26–29). Histamine-induced calcium responses were observed in 23% of IB+ and 7% of the IB4− neurons (9). Moreover, our results suggest that histamine, serotonin, and ET-1 generate itch signals by activating TRPV1+ neurons through at least 3 different intracellular signal transducing pathways (Fig. 6). The disposition of these molecular signal transducing pathways among the TRPV1+ neurons is, however, unclear. Whether these subsets correspond to nonoverlapping populations of TRPV1-expressing neurons remains to be determined.
Materials and Methods
Mice.
Two- to three-month-old PLCβ3-deficient and MrgprdDTA mice (backcross-ed to C57BL/6J) were used. Two-month-old male C57BL/6J mice were purchased from Charles River Laboratories. HTR3a receptor-deficient and TRPV1-mutant mice were purchased from The Jackson Laboratory. The mice for behavioral testing were obtained by mating heterozygous animals. All experiments were approved by the Institutional Animal care and Use Committees and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioral Studies.
PLCβ3−/−, HTR3a−/− TRPV1−/−, MrgprDDTA, and their wild-type littermate controls were individually housed at least 3 days before testing, which was performed blind to genotype and to the particular chemical compounds injected. Hot plate, tail immersion, temperature preference assay, CFA-induced inflammation, and formalin tests were carried out as described (30–32). See SI Text for a detailed description of the behavioral methods.
Intrathecal Injection.
Mice were anesthetized with 1.5% isoflurane before and for 30 min after intrathecal (i.t.) injection of capsaicin (10 μg; Sigma) or vehicle (10% ethanol and 10% Tween-80 in saline) in a volume of 5.0 μL. The injection was made at the level of the pelvic girdle with a luer-tipped Hamilton syringe to which a 30-gauge needle was attached.
Histology.
Fos immunohistochemistry and its quantification were performed as described in SI Text.
Data Analysis.
Data are expressed as the mean ± SE. Statistical comparisons were made using Student's t test, two-way ANOVA with Bonferroni post tests, Mann–Whitney U test, or two-way rmANOVA followed by Sheffé's test. When the P value was <0.05, the difference was considered to be significant.
Supplementary Material
Acknowledgments.
We thank Mark Zylka for invaluable help, Heeju Kim and Valeria Mancino for help with the behavioral assays, and Timothy M. Kim for video analyses. This work was supported by National Institutes of Health Grant PO1-NS048499 (to M.I.S., D.J.A., and A.I.B.). H.S.L. is supported by an award from the Christopher and Dana Reeve Foundation. D.J.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905605106/DCSupplemental.
References
- 1.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiogy of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Twycross R, et al. Itch: Scratching more than the surface. Q J Med. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 3.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmelz M, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 5.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: A central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 6.Davidson S, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simone DA, et al. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 8.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 9.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Shim WS, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa R, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. 2009;33:205–212. doi: 10.1016/j.neubiorev.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson R, Small J, Dixon AK, Spanswick D, Lee K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett. 2003;337:119–122. doi: 10.1016/s0304-3940(02)01256-9. [DOI] [PubMed] [Google Scholar]
- 16.Zeitz KP, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer C. Serotonin in pain and analgesia: Actions in the periphery. Mol Neurobiol. 2004;30:117–125. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- 18.Hans G, Deseure K, Adriaensen H. Endothelin-1-induced pain and hyperalgesia: A review of pathophysiology, clinical manifestations, and future therapeutic options. Neuropeptides. 2008;42:119–132. doi: 10.1016/j.npep.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Katanosaka K, et al. Contribution of TRPV1 to the bradykinin-evoked nociceptive behavior and excitation of cutaneous sensory neurons. Neurosci Res. 2008;62:168–175. doi: 10.1016/j.neures.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Shimada SG, Lamotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: Scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura T, et al. Biological properties of a specific Galpha q/11 inhibitor, YM-254890, on platelet functions and thrombus formation under high-shear stress. Br J Pharmacol. 2006;148:61–69. doi: 10.1038/sj.bjp.0706711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endoh M, et al. Endothelin: Receptor subtypes, signal transduction, regulation of Ca2+ transients and contractility in rabbit ventricular myocardium. Life Sci. 1998;62:1485–1489. doi: 10.1016/s0024-3205(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 25.Woo DH, et al. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG) Mol Pain. 2008;4:42–56. doi: 10.1186/1744-8069-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johanek LM, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johanek LM, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namer B, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao YQ, et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 31.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Avad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.