Abstract
NK cells use surface NK receptors to discriminate self from non-self. The NK receptor ligand-binding domain (NKD) has been considered the sole regulator of ligand binding. Using a prototypic murine NK receptor, Ly49A, we show that the membrane proximal nonligand binding ecto-domain (the stalk region) is critical to ligand binding and signaling. The stalk region is required for receptor binding to ligand on target cells (trans interaction), but is dispensable for receptor binding to ligand on the same cell (cis interaction). Also, signaling in a trans manner depends on the stalk region mediating the formation of the immunological synapse. Thus, our data modeling receptor function at the cellular level reveal an essential role for the stalk region as a specific mediator of receptor signal integration, by which NKD-ligand interactions at the interface initiate and deliver information to the spatially separated cytoplasmic domain.
Keywords: MHC, NK cells, NK receptor
NK cells are a fundamental component of the innate immune system by virtue of their ability to kill virally infected and transformed tumor cells (1–3). NK cell responses are determined by a balance of inhibitory and activating signals. On ligand binding, NK inhibitory receptors transmit inhibitory signals to NK cells (4–6). Ly49A is a prototypic C-type lectin murine NK inhibitory receptor that recognizes the MHC I ligand, H2Dd, on target cells (7). In addition to conventional receptor-ligand interactions between 2 cells (trans interaction), Ly49 family members and their ligands expressed on the same NK cell surface have been shown to interact with one another (cis interaction) (8, 9). Ly49A constitutively interacts with its ligand on the NK cell surface in a cis manner, rendering Ly49A recognition of ligand on target cells (trans interaction) less efficient.
Biophysical analyses using surface plasmon resonance (SPR) have established that the stalk region is dispensable for optimal NK receptor ligand-binding domain (NKD) conformation and ligand binding, and that the NKD of Ly49A is the sole regulator of ligand specificity and affinity (10, 11). Consistent with this conclusion, the crystallographic structure of Ly49A and H2Dd was determined without the stalk region (12). The noncovalently dimerized Ly49A NKD asymmetrically recognizes one H2Dd interface at a region termed Site 2 (13). These biophysical and crystallographic studies have been used to support the model that the NKD of Ly49 family members solely determines ligand specificity and affinity without involvement of the stalk region. Another model, in which the stalk region has a role in the trans interaction, has also been proposed based on the existing structural data of Ly49 NKDs and ligands (14). It is also speculated that a long stalk region with certain flexible structures is required for Ly49A to cis-interact with its ligand (8, 15). However, neither model has been tested at the functional level of the cell.
Here, we report the function of the stalk region of the Ly49A receptor at the cellular level using mutant Ly49A receptors in primary and in vitro cellular models of NK cellular function. We found that the stalk region is required for ligand binding and signaling in a trans manner, but is dispensable for the cis interaction at the cellular level. Also, the stalk region is critical to NK receptor signaling through its role in regulating immunological synapse (IS) formation. These data at the cellular level demonstrate that the stalk region of the Ly49A receptor mediates signaling by regulating IS formation between NK cells and their targets.
Results
Detection of Ly49A Cis and Trans Interactions with H2Dd.
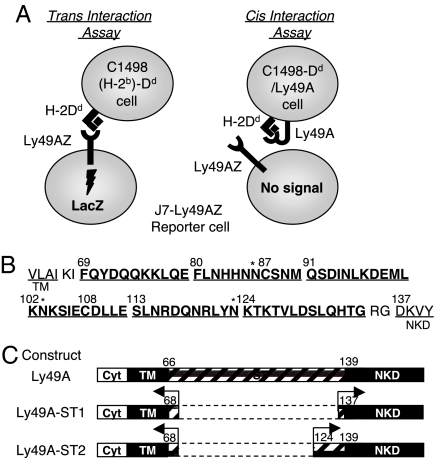
To examine the role of the stalk region in the regulation of trans and cis interactions, we established assays that could specifically detect Ly49A-H2Dd binding in the cis or trans conformations. We designed a human reporter cell line, JLZ-7 (J7), for specific detection of the trans interaction between Ly49A and mouse MHC class I. This reporter assay consists of J7 cells expressing an Ly49A chimeric receptor with a CD3ζ cytoplasmic domain (Ly49AZ) (Fig. 1A Left), in which induced LacZ expression indicates specific Ly49A-H2Dd binding and subsequent signaling in a trans manner (Fig. S1 a and b). Because the reporter cell is human, and it does not express any component of Ly49A ligands, the only signals detected are those in the trans from target cells.
Fig. 1.
Ly49A reporter assays and the stalk-deletion mutant Ly49As. (A) Schematic representation of reporter cell assays to detect cis and trans binding of Ly49A and ligand. In both assays, successful engagement of ligand by reporter cells expressing Ly49AZ or various Ly49AZ mutants induces LacZ expression. (Left) Trans interaction assay. A positive readout indicates reporter cell receptor binding to ligand on a target cell (trans interaction) and subsequent signal transduction. (Right) Cis interaction assay. Ly49A expressed on target cells binds to H2Dd on the same cell (cis interaction); thus, masking the trans-recognition by wild-type reporter cells expressing Ly49A. Various Ly49A mutant receptors were expressed on the target cells. A negative response by reporter cells indicates that the receptor on target cells cis interacts with ligand on the same target cell. (B) The amino acid sequence of the stalk region is arbitrarily divided into 6 segments that correspond to the sites deleted from Ly49A mutant receptors. Two cysteines are located at 87 and 108. Three potential N-glycosylation sites are marked with an asterisk. (C) Schematic representation of stalk-deletion mutant Ly49A-ST1 and Ly49A-ST2 receptors. TM, transmembrane; Cyt, cytoplasmic; ST, stalk region.
We next designed an assay to detect Ly49A-H2Dd binding in a cis manner. It has been shown that H2Dd engages Ly49A in its cis interaction at Site 2 (8). We postulated that target cells expressing both Ly49A and H2Dd would interact with each other in a cis manner. This cis interaction would inhibit the trans recognition by J7-Ly49AZ reporter cells by masking the ligand interface (Fig. 1A Right). To test this conjecture, we prepared target cells expressing H2Dd ligand and Ly49A (C1498-Dd/Ly49A). As a control, we prepared target cells expressing H2Dd ligand and GFP (C1498-Dd/GFP). We observed that Ly49A expression on Dd-expressing target cells blocks J7-Ly49AZ reporter responses, presumably by effective competition for Dd, whereas GFP expression had no such effect (Fig. S1c). We also observed the same masking pattern with KZH cells (H2k haplotype) transduced with Ly49A (Fig. S1d), suggesting that Ly49A, as with H2Dd, utilizes the same interface of H2Dk for the trans and cis recognitions. These data indicate that our xenogeneic reporter systems can uniquely detect either cis or trans binding conformations between receptor and ligand (Fig. 1A).
Using this system and site-directed mutants of Ly49A, we also confirmed that receptor homo-dimerization mediated by disulfide bonds at the stalk region is not required for Ly49A ligand recognition in the trans or cis interaction (Fig. S2 a–e).
Most of Ly49A Stalk Sequence Is Dispensable for NKD Folding and the Cis Interaction.
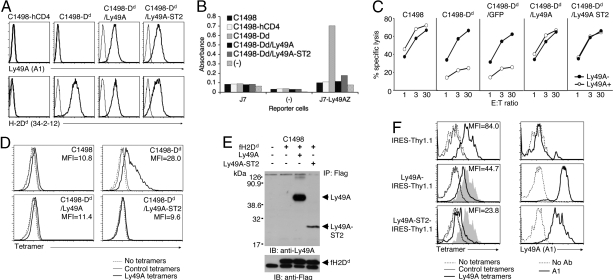
We speculated that for the Ly49A to cis interact with its ligand, a long stalk region with certain flexible structures may be required independent of cysteine-disulfide bonding in the region (8, 15). We prepared 2 stalk-deletion mutant constructs in the Ly49A backbone with an intact Ly49A ITIM cytoplasmic region (Ly49A-ST1 and Ly49A-ST2). Ly49A-ST1 was missing most of the Ly49A stalk sequence, whereas Ly49A-ST2 only contained the NKD proximal sequences of the stalk region (Fig. 1 B and C). We transduced both constructs into C1498-Dd target cells. Ly49A-ST2 expression was comparable with that seen with wild-type Ly49A by FACS analysis with the anti-Ly49A mAb, A1 (Fig. 2A). However, we failed to detect Ly49A-ST1 surface expression by FACS analysis (Image 1 in SI Appendix), which is consistent with previous data that the NKD proximal stalk region is required for appropriate folding of the NKD (10). Therefore, only C1498-Dd/Ly49A-ST2 target cells could be used in reporter cell assays to detect the cis interaction. Ly49A reporter cells were unable to detect target cells expressing H2Dd and Ly49A-ST2, but were able to detect control target cells expressing H2Dd and GFP (Fig. 2B; Fig. S1c). To examine the effect of Ly49A-ST2 on the Dd recognition by native Ly49A, we also used killing assays, where it was observed that Ly49A-positive lymphokine activated killer (LAK) cells killed C1498-Dd/Ly49A-ST2 target cells, presumably because LAK cells failed to receive inhibitory signals from target cells expressing both Ly49A-ST2 and ligand (Fig. 2C). Ly49A tetramers failed to bind to C1498-Dd/Ly49A and C1498-Dd/Ly49A-ST2, whereas the tetramer bound to C1498-Dd target cells (Fig. 2D). Also, Ly49A-ST2 and the H2Dd molecule coimmunoprecipitated from the lysate of C1498-Dd/Ly49A-ST2 cells, although a lower amount of Ly49A-ST2 was coimmunoprecipitated compared with wild-type Ly49A (Fig. 2E; Discussion). To examine the cis-binding ability of Ly49A-ST2 in primary cells, we transduced Ly49A-ST2 into LAK from B10.D2 mice (H2d background) using a bicistronic IRES-Thy1.1 vector. By gating on Thy1.1-positive cells, we examined Ly49A tetramer binding (Fig. 2F). The Ly49A tetramers bound less efficiently to these primary cells expressing H2d and either Ly49A or Ly49A-ST2. We observed the same effects with CD4 T cells from B10.D2 mice (Image 2 in SI Appendix). Together, these data indicate that Ly49A-ST2 constitutively interacts with ligand in a cis manner on the cell surface.
Fig. 2.
Constitutive cis interaction of Ly49A-ST2 and H2Dd. (A) Expression level of Ly49A and Ly49A-ST2 on C1498-Dd target cells. FACS analyses using an anti-Ly49A mAb (A1) and anti-H2Dd (34-2-12) are shown. (B) Cis interaction assays with C1498-Dd/Ly49A-ST2 target cells. Indicated target and reporter cells were incubated overnight and subjected to CPRG assays. The (−) indicates no target or reporter cells. (C) Killing assays with the C1498-Dd/Ly49A-ST2 target cells. Killing assays were performed with indicated target cells and Ly49A-positive or Ly49A-negative LAK cells from B10 mice. Representative data from 4 independent experiments are shown. (D) Failure of Ly49A tetramer binding to C1498-Dd/Ly49A and C1498-Dd/Ly49A-ST2. Indicated cells were stained with streptavidin-phycoerythrin (SA-PE)-conjugated Ly49A or control (H2Dd/hβ2m) tetramers. The quantitative Ly49A tetramer binding to C1498-Dd coexpressing Ly49A or Ly49A-ST2 is assessed by mean fluorescence intensity (MFI). (E) Physical interaction of Ly49A-ST2 and H2Dd. C1498 cells were transduced with flag-tagged H2Dd (fH2Dd) and wild-type Ly49A or Ly49A-ST2. Lysates were immunoprecipitated by anti-flag mAb, and interrogated for Ly49A by Western blotting with anti-Ly49A polyclonal Abs under reducing conditions. Representative data from 3 independent experiments are shown. (F) Reduced Ly49A tetramer binding to primary LAK cells expressing H2d and Ly49A. Indicated retrovirus and lentivirus constructs were transduced into day 3 LAK cells from B10.D2, respectively. Thy1.1-positive cells were gated and stained with SA-PE-conjugated Ly49A or control (H2Dd/hβ2m) tetramers (Left), and with biotinylated A1 mAb followed by SA-PE (Right). Gray shades represent the overlaid histogram of the tetramer binding to the pMX-IRES-Thy1.1-transduced cells. No Ly49A tetramer binding was observed in LAK cells from B10 mice.
Stalk Region of Ly49A Is Required for Trans Interaction in Vitro and in Vivo.
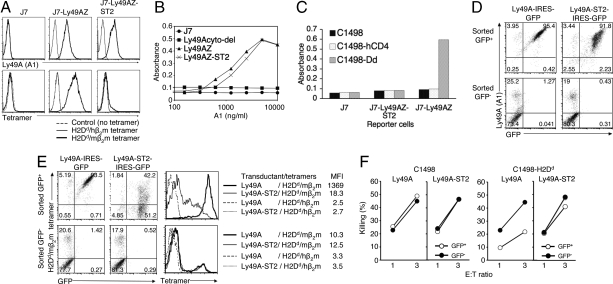
We next examined the requirement of the Ly49A stalk region for the trans interaction with ligand and subsequent signal transduction. We infected J7 reporter cells with a retrovirus encoding the Ly49A-ST2 stalk-deletion mutant within the Ly49AZ reporter construct (Ly49AZ-ST2). As with the wild-type reporter cells, these Ly49AZ-ST2 reporter cells expressed the Ly49A-ST2 stalk mutant fused to a CD3ζ cytoplasmic region. We observed that A1 mAb bound to Ly49AZ-ST2 less efficiently than to wild-type Ly49A on J7 cells, which was confirmed by observing the mAbs binding to J7 cells expressing GFP fusion proteins (Image 3 in SI Appendix). To enhance the expression of the stalk mutant receptor, we used a lentivirus vector containing the elongation factor (EF1α) as a promoter to express Ly49AZ-ST2. We observed a similar level of Ly49A expression using the A1 mAb on J7 reporter cells transduced with Ly49AZ-ST2 (Fig. 3A Upper). Immobilized A1 mAb efficiently stimulated these reporter cells in a dose-dependent manner similar to reporter cells expressing wild-type Ly49AZ, indicating that these receptors have the ability to signal when they are cross-linked by mAb (Fig. 3B). We did not observe a significant difference in the mAb cross-linking assays with J7-Ly49A-ST2 cells regardless of the different promoters (Image 4 in SI Appendix), indicating that differences in ST2 expression levels detected by A1 mAb between the EF1α and pMX promoters is functionally minimal. However, in reporter cell assays with C1498-Dd cells, we observed that the Ly49AZ-ST2 reporter cells failed to recognize H2Dd (Fig. 3C), as indicated by a lack of induced LacZ expression.
Fig. 3.
Essential role of Ly49A stalk region for the trans interaction. (A) Expression level of Ly49A and Ly49A-ST2 on J7 reporter cells, as determined by anti-Ly49A mAb (A1) (Upper), and H2Dd/mβ2m tetramers binding to reporter cells expressing Ly49A-ST2 (Lower). Ly49A-ST2 was transduced with lentivirus, and selected with the same dose of puromycin as for J7-Ly49AZ selection. Indicated reporter cells were stained with SA-PE-conjugated H2Dd/mβ2m or H2Dd/hβ2m tetramers. (B) Signaling ability of Ly49A-ST2 by mAb cross-linking. Indicated doses of A1 mAb (100 μL) were immobilized in a 96-well plate overnight. Cells (1 × 105) of each reporter cell line were plated and incubated overnight. CPRG assays were then performed. (C) Trans interaction assays with reporter cells expressing Ly49A-ST2. Indicated target and reporter cells were incubated overnight and subjected to CPRG assays. (D and E) B6 BM progenitor cells were transduced with retrovirus encoding the indicated constructs to make BM chimera with B6 mice. Forty days after the transplantation, LAK cells were generated. CD3-negative LAK cells were sorted into 2 populations by GFP expression, and stained with anti-Ly49A mAb (A1) for D, and H2Dd/mβ2m tetramers for E. MFI of tetramer bindings in each transductant is shown (E). FACS profiles of LAK cells after FACS-sorting by GFP are shown in D. (F) Killing assays were performed with indicated target and sorted LAK cells.
One explanation for this surprising finding could be that the Ly49A stalk mutants on reporter cells cannot bind to MHC class I ligand on the target cells (trans interaction). To confirm the ligand binding capacity of Ly49A mutant receptors on reporter cells, we prepared H2Dd per mouse β2m (H2Dd/mβ2m) tetramers and control H2Dd/human β2m (H2Dd/hβ2m) tetramers. Although the H2Dd/mβ2m tetramers bound to Ly49AZ and the monomer form of the Ly49AZ receptor (Ly49AZ-C87/108S) on J7 reporter cells, the tetramers showed significantly reduced binding to Ly49AZ-ST2 (Fig. 3A Lower; Fig. S2a).
The requirement of the stalk region for the trans interaction observed in the in vitro assay was consistent with primary NK cells when Ly49A-ST2 was transduced (Fig. 3 D–F; Fig. S3). These data in primary NK cells eliminate other possible explanations for the difference between human reporter cells and mouse primary LAK cells, such as differential O-glycosylation in the NKD. Also, the J7 reporter cells expressing the Ly49A triple mutant in the putative N-glycosylation sites maintained the ability to bind soluble ligand and signal (Fig. S4 a–c), suggesting that the function of the Ly49A stalk region in mediating the receptor-ligand trans interaction is independent of receptor N-glycosylation and ligand glycosylation. Together, these cellular observations indicate that the Ly49A stalk region is required for trans binding independent of N-glycosylation.
Stalk Region Functions to Present the NKD to Soluble Ligand in a Trans Manner.
We were surprised to find that Ly49A-ST2 lost its ability to bind to tetramers (trans interaction), whereas the same receptor retained the ability to interact with ligand in a cis manner. Because Ly49A-ST2 lacks most of the stalk region, we postulated that the NKD may be embedded among other surface molecules on the cell surface, and may not be easily accessible to ligand on target cells or the soluble form of ligand (trans interaction). To further examine the requirement of the stalk region for receptor-ligand trans interaction, we generated a series of stalk-deletion mutant receptors (Fig. S5a). All of these mutant receptors were capable of binding ligand in a cis manner in reporter cell assays, as indicated by a lack, or significantly reduced amount, of induced LacZ expression in Ly49AZ reporter cells (Fig. S5 b and c). In the trans interaction assays, mutant receptors with a longer stalk region were more capable of binding to H2Dd/mβ2m tetramers, regardless of the segment(s) deleted in the stalk region (Fig. S5 d and e). These data suggest that one of the critical functions of the stalk region is to elevate NKD above other cell surface molecules; this elevation allows for more efficient ligand binding. However, even those stalk deletion mutant receptors (Ly49A-ST4, -ST5, -ST6, -ST23, and -ST24) that were fully capable of binding to the tetramers failed to signal (i.e., interact in trans) as efficiently as wild-type Ly49A (Fig. S5f). Also, mutant receptors with the same length of stalk region (Ly49A-ST5 and Ly49A-ST24) showed variability in their signaling capabilities. These data suggest that the stalk region as a whole, not a single segment or residue, is important to signal transduction.
Stalk Chimeric Ly49A Receptor Maintains the Ability to Bind Soluble Ligand, but Fails to Signal in in Vitro Assays and Primary NK Cell Models.
Besides length, the features of the stalk region that facilitate NKD binding to ligand to initiate signaling in a trans manner are unknown. If the stalk region only functions as a stem for presenting NKD to ligand, then it is conceivable that any stalk structure of comparable length could support NKD binding to ligand and initiate subsequent signaling. However, if the stalk region physically interacts with the NKD-ligand complex with specificity, then a homologous stalk structure of similar length with a slightly different composition might impact the signaling. The stalk region of Ly49 family members shows considerable conservation of sequence. Ly49C and Ly49H have the exact same length of stalk region with only 9 differing amino acids out of 76 (Fig. 4A). This homology suggests that the cognate function, motifs, and structure of the stalk region, if any, have been conserved as a requirement for stalk region-mediated ligand binding and signaling.
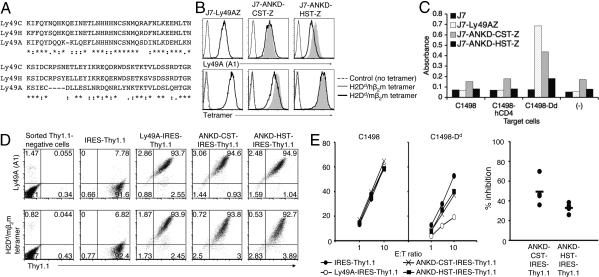
Fig. 4.
Stalk-chimeric Ly49A receptors effectively bind ligand, but show functional discrepancy in signaling. (A) Stalk sequences of Ly49A, Ly49C, and Ly49H. Identical and conserved residues are marked with asterisks and colons, respectively. (B) Surface expression of stalk-chimeric receptors. Ly49AZ, ANKD-CST-Z, and ANKD-HST-Z on J7 reporter cells were stained with anti-Ly49A mAb (A1) and H2Dd/mβ2m tetramers. Gray shades represent the overlaid histogram from Ly49AZ. (C) Trans interaction reporter assays were performed with indicated target and reporter cells. (D) FACS profiles of sorted Thy1.1-positive LAK cells. LAK cells were prepared from B6 mice treated with A1 mAb, which depleted 90–95% of endogenous Ly49A-positive cells when assessed by A1 staining with day 9 LAK cells. LAK cells were infected with lentiviruses encoding wild-type Ly49A and mutant receptors with IRES-Thy1.1 on day 3 and sorted for Thy1.1 expression on day 6. Sorted cells were stained with anti-Ly49A mAb (A1) and H2Dd/mβ2m tetramers on day 9. No binding was observed with control H2Dd/hβ2m tetramers. (E) Killing assays were performed with indicated target cells and sorted Thy1.1-positive LAK cells infected with lenti-virus encoding IRES-Thy1.1 (Left). Thy1.1-negative LAK cells from each transductant killed C1498 and C1498-Dd target cells similarly. Relative inhibitory capabilities of mutant receptors compared with the wild-type Ly49A receptor from 4 experiments are shown (Right). Thy1.1-negative LAK cells from each transductant showed similar killing to C1498 and C1498-Dd target cells. No difference was observed in killing of C1498 target cells between Thy1.1-negative and -positive LAK cells. No statistically significant difference was observed between ANKD-HST and ANKD-CST in the relative inhibitory capabilities in the combined data from 4 separate experiments.
To elucidate the functional relationship between the stalk region and NKD in ligand binding and signaling, we constructed stalk chimeric Ly49AZ reporter constructs, in which the stalk region is replaced with stalk domains from other Ly49 family members. We prepared chimeric receptors consisting of the Ly49A NKD domain with either a Ly49C or Ly49H stalk region (ANKD-CST-Z and ANKD-HST-Z, respectively). We transduced reporter cells with each chimeric receptor, and confirmed that their expression levels were similar to the wild-type Ly49A receptor by FACS with A1 mAb (Fig. 4B). H2Dd/mβ2m tetramers bound to reporter cells expressing ANKD-CST and ANKD-HST at a similar level as the wild-type Ly49AZ-expressing reporter cells (Fig. 4B). Immobilized A1 mAb efficiently stimulated these reporter cells in a dose-dependent manner similar to reporter cells expressing wild-type Ly49AZ (Image 5 in SI Appendix), indicating that these receptors have the ability to signal when they are cross-linked. Interestingly, reporter cells expressing ANKD-HST or ANKD-CST signaled weakly, compared with wild-type Ly49AZ reporter cells (Fig. 4C). The inability of ANKD-HST to signal is not due to the absence of an interaction with DAP12 or a specific feature of the stalk region, because another chimeric receptor with the stalk region from activating Ly49D, Ly49A NKD with wild-type Ly49D stalk (ANKD-DST), responded to H2Dd ligand at similar levels as wild-type Ly49A reporter cells (Fig. S6 a and b). Rather, it is due to specific interactions between NKD and the stalk region that critically influence the signaling capacity of the receptor.
This in vitro observation is consistent with the results of killing assays. Primary LAK cells expressing the ITIM-containing ANKD-HST and ANKD-CST receptors were less capable of inhibiting the killing of target cells expressing H2Dd, as compared with LAK cells expressing wild-type Ly49A (Fig. 4 D and E). Together, these data support our conclusion that the stalk region has an essential role in Ly49A signaling in addition to functioning as a stem to present the NKD to ligand.
Stalk Region Regulates IS Formation.
Accumulation or self-assembly of proteins (receptor clustering) at the intercellular contact is considered the first step of IS formation (16, 17). Given that stalk chimeric receptors maintain the ability to bind soluble ligands and to signal in mAb cross-linking assays at a level comparable with wild-type Ly49A, we postulated that the stalk region facilitates receptor clustering. To elucidate the role of the stalk region in receptor clustering, we examined the functionality of stalk-chimeric receptors in IS formation by confocal microscopy.
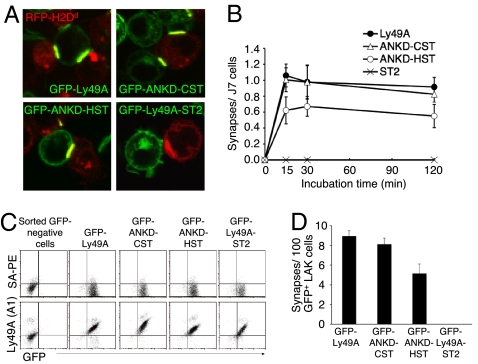
To observe specific interactions of mutant Ly49A receptors with ligand without the involvement of endogenous Ly49A and activating receptors, we used xenogeneic cells in in vitro assays. We first prepared J7 cells expressing ITIM-containing stalk mutant receptors tagged with GFP in the cytoplasmic region, and confirmed that these cells express similar levels of GFP expression (Image 6 in SI Appendix). We also prepared clonal C1498 cells expressing different levels of H2Dd tagged with intracellular red fluorescence protein (RFP; C1498-Dd/RFP) (Fig. S7a). We cocultured these target and J7 cells for 2 h, and examined IS formation by confocal microscopy. C1498-Dd cells expressing higher levels of Dd (C1498-Dd/RFP-high), but not lower levels, allowed us to observe clear clustering of both receptor and ligand (Fig. 5A and Image 7a in SI Appendix). However, even with C1498-Dd/RFP-high cells, J7 cells expressing the stalk deletion mutant Ly49A-ST2 fused with GFP did not form any ISs (Fig. 5A; Table S1). J7 cells expressing the cytoplasmic-deleted Ly49A fused to GFP also formed ISs with C1498-Dd/RFP-high (Image 7b in SI Appendix), indicating that the observed receptor clustering is independent of the ITIM sequence of Ly49A. We also observed ISs between J7 cells expressing either ANKD-CST or ANKD-HST and target cells (Fig. 5A). However, the number of ISs formed by ANKD-HST receptors was significantly decreased (Table S1). We did not observe significant differences in IS formation between Ly49A and ANKD-CST constructs, whereas we observed different responses in reporter and killing assays. The expression level of H2Dd on C1498-Dd cells used for reporter and killing assays was low, compared with the higher expression level on C1498-Dd cells used for the IS formation assay (Fig. S7a). Therefore, the differential results among assays are most likely due to the different level of ligand expression on target cells used for assays and the differential sensitivities among assays. No difference in the size of synapses or GFP-intensity was observed between the receptors (Image 8 in SI Appendix), suggesting that the quality of each synapse was similar once the synapse formed. Time-course studies revealed that a quantitative difference in IS formation was detected as soon as 15 min after coculture, and that the quantitative difference remained the same for the next 2 h (Fig. 5B). Together, these data suggest that the stalk region functions during the very early stages of IS formation and affects subsequent signaling.
Fig. 5.
Regulation of IS formation by the stalk region of the NK receptor. (A) Confocal images of IS formation between H2Dd-expressing target cells and J7 cells expressing mutant Ly49A receptors. C1498 cells expressing RFP-tagged H2Dd (C1498-Dd/RFP-high) were cocultured for 2 h with J7 cells expressing the GFP-tagged Ly49A, Ly49A-ST2, ANKD-CST, or ANKD-HST receptors. Merged images are shown, and yellow indicates IS formation between reporter and target cells. (B) Decreased IS formation in cells with stalk-mutant Ly49A receptors expressed on J7 cells. The average number of synapses for each incubation period was obtained as described for synapse/J7 in Table S1. Data were combined from 9 independent experiments. Total number of synapses was not statistically significant between Ly49A and ANKD-CST at any of the time points. All other comparisons were statistically significant at all of the time points. (C) LAK cells were infected with lentivirus encoding the indicated GFP-fusion receptor. FACS profiles of LAK cells after FACS-sorting by GFP are shown. (D) IS formation (GFP clustering) was counted 30 min after incubation of C1498-Dd/RFP-high cells and LAK cells expressing GFP-fusion receptors. The P of all comparisons except for the combination of Ly49A and ANKD-CST was <0.001 (P for Ly49A and ANKD-CST was 0.087); therefore, the differences were statistically significant. Representative data from 3 independent experiments is shown.
To examine the functionality of the stalk region in IS formation with primary NK cells, we transduced these GFP-chimeric receptors in LAK cells with lentiviral vectors. We observed that the cross-linking of Ly49A fused with GFP did not inhibit IFN-γ production of LAK cells by anti-NK1.1 mAb (Image 9 in SI Appendix), indicating that the GFP-fusion protein does not have inhibitory signaling capabilities. Consistent with our in vitro data with J7 cells, we observed a reduced number of ISs with ANKD-HST receptors (Fig. 5 C and D). Together, these data indicate that stalk-dependent receptor clustering occurs independent of ITIM-mediated receptor signaling.
Discussion
Using a prototypic NK inhibitory receptor, Ly49A, we demonstrate in this study an essential role for the stalk region in NK receptor signaling. The stalk region is required for ligand binding in trans interactions and subsequent signal transduction both in in vitro reporter systems and with primary NK cells. Most of the stalk deletion mutants maintained the capability to bind ligand in a cis manner, whereas mutants with a longer stalk region retained a degree of ligand binding capability in the trans manner. More importantly, however, the stalk region regulates IS formation and, thus, the intensity of receptor signaling. Even though stalk chimeric Ly49A receptors were capable of binding ligand as efficiently as wild-type Ly49A receptors, these mutant receptors were functionally deficient in signaling, killing, and IS formation assays. Our study using mutant Ly49A receptors in functional reporter and killing assays with soluble ligands uniquely distinguished the ligand binding phase from the subsequent specific signaling outcome.
As with all mutation studies, one caveat is that any mutation in the stalk region may cause unintentional conformational changes at the distant ligand-binding interface of NKD. In addition to previously published data in biophysical binding studies (10), several lines of evidence suggest that the phenotypes of these mutations are intrinsic to the stalk region without affecting the binding interface. As observed with Ly49A-ST2, severe ablation of the stalk region did not affect cis-binding ability. Also, various lengths of stalk deletion mutant receptors retained the ability to bind ligand in a cis manner. Regardless of the deleted segments of the stalk region, those receptors with longer stalk regions were better able to bind to H2Dd/mβ2m tetramers. Consistent with these studies with deletion mutant receptors, the stalk chimeric receptors (ANKD-CST and ANKD-HST) also maintained the ability to bind to H2Dd tetramers both in in vitro and primary NK cell assays. These data strongly suggest that the ligand binding interface of NKD in these mutant receptors has minimal, if any, conformational changes.
IS formation by NK cells is a multistep process that is similar to IS formation by TCR (18–20). Our time course study of Ly49A IS formation suggests that the stalk region has a role in signaling in the early steps of IS formation, rather than having a role in stabilizing and maintaining the IS. Receptor sensitivity in the early phase of immuno-receptor ligand interaction is often defined by receptor affinity and avidity. The molecular mechanism of TCR signaling initiation has been a topic of intense debate, spawning many models including TCR oligomerization and conformational changes (21, 22). The molecular mechanism underlying the initiation of NK receptor signaling is also poorly understood. Notably, we observed that mAb cross-linking of Ly49A-expressing reporter cells results in efficient signaling, although the immobilized monomer as well as tetramer forms of H2Dd/mβ2m failed to stimulate reporter cells (Image 8 in SI Appendix). This finding indicates that a simple oligomerization of Ly49A is not enough for signaling. We also observed that higher expression of H2Dd on target cells augmented signaling intensity of the stalk chimeric receptors (ANKD-CST and ANKD-HST) (Fig. S7b), suggesting that the higher avidity condition for the receptor can compensate for the signaling defect by the receptors. Given these findings, an attractive model explaining the mechanism by which the stalk region mediates Ly49 family receptor signaling is through changes in conformation leading to corresponding changes in either affinity or avidity (Fig. S8). Ligand binding to the NKD induces specific interactions between NKD and the stalk region (conformational change of the stalk region), which in turn increases receptor affinity by lowering the off-rate of binding or increases receptor avidity by transforming from a closed to an open conformation of the NKD that is capable of interacting with 2 ligands (23). This model could explain why inhibitory receptors cluster independently of actin polymerization and ATP metabolism (24). The conformational changes of a stalk region pull in the ligand to reduce the distance between the effector and target cells, which then facilitates bindings between neighboring Ly49A receptors and ligands resulting in IS formation. Our hypothetical mechanism of augmented ligand binding by the stalk region could also explain why we observed less ST2 coimmunoprecipitated with flag-tagged H2Dd (Fig. 2E). We believe that the immunoprecipitation assay does not reflect actual cis conformation of receptor and ligand, because on lytic removal of the cell membrane with a detergent, the mode of receptor and ligand binding may shift from a stalk-independent cis interaction to a stalk-dependent trans interaction.
Our model, the augmentation of the ligand binding induced by large conformational changes of the stalk region, is strongly argued by SPR studies of Ly49A and Ly49C (10, 25), with the caveat that the stalk region of the bacterially prepared proteins may or may not be folded and coupled appropriately. Although it is not known if the stalk region is a natively folded or unfolded protein, the stalk region may interact with lipids of the transmembrane region and change structure, analogous to the conformational changes of the cytoplasmic CD3ζ chain induced by lipids (26). Such an induced conformational change of the stalk region in the presence of membrane lipids may initiate an interaction between the stalk region and ligand-binding complex, leading to increased receptor-ligand binding. Molecular binding studies employing SPR or the sedimentation velocity method may fail to model the functionality of the stalk region because of the absence of cell membrane lipid layers in the system. The crystal structure and/or NMR analysis of the whole extracellular soluble portion of Ly49A (meaning the NKD with the stalk region), and its complex form with ligand will also provide valuable information to elucidate the initiation of NK receptor signaling. Further studies of other Ly49 family members are also required to understand the general features of the stalk region in the family members.
A previous study of the cis interaction of NK receptor and ligand focused on the effector function of NK cells (8). It has not been examined how such a cis interaction may affect the status of NK cells as target cells. Our data suggest that NK cells with NK inhibitory receptors interacting with their MHC class I molecules in a cis manner should be susceptible to NK cell killing. Such a mechanism may counterregulate the augmented proliferation of licensed NK cells in vivo (27). Further studies with the mutant receptor exclusively and specifically interacting with the ligand in a cis manner will help to define the consequence of the receptor-ligand cis interaction in NK cell development, function, and homeostasis.
Materials and Methods
For the reporter assay, a total of 1 × 105 reporter cells were cultured overnight with 1 × 105 target cells. LacZ activity was determined with the substrate chlorophenol red-D-galactoside (CPRG), as described before (28). Other methods and materials are detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank H. Matsuo and S. Jameson for technical support in production of tetramers; D. Margulies, O. Naidenko, B. Binstadt, and E. Peterson for critical comments on the manuscript; R. Tsien for the RFP cDNA (University of California at San Diego, La Jolla); and M. Franklin for editorial assistance with this manuscript. The K.I. laboratory is located in a facility that was constructed with support from National Center for Research Resources National Institutes of Health Research Facilities Improvement Program Grant CO6 CA062526-01. K.I. was supported by National Institutes of Health Grant R21AI064270, a scholar award from the American Society of Hematology, an American Cancer Society Institutional Research Grant, and the Minnesota Medical Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900664106/DCSupplemental.
References
- 1.Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 2.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 3.Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;16:344–347. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 7.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 8.Doucey MA, et al. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 9.Scarpellino L, et al. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–1284. doi: 10.4049/jimmunol.178.3.1277. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan K, et al. Interaction of the NK cell inhibitory receptor Ly49A with H-2Dd: Identification of a site distinct form the TCR site. Immunity. 1999;11:591–601. doi: 10.1016/s1074-7613(00)80134-x. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, et al. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd AND beta 2-microglobulin. J Biol Chem. 2002;277:1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- 12.Tormo J, Natarajan K, Margulies DH, Mariuzza RA. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402:623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto N, Mitsuki M, Tajima K, Yokoyama WM, Yamamoto K. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp Med. 2001;193:147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibson PJ, Pease LR. Having it both ways: MHC recognition in cis and trans. Nat Immunol. 2004;5:237–238. doi: 10.1038/ni0304-237. [DOI] [PubMed] [Google Scholar]
- 16.Davis DM. Assembly of the immunological synapse for T cells and NK cells. Trends Immunol. 2002;23:356–363. doi: 10.1016/s1471-4906(02)02243-3. [DOI] [PubMed] [Google Scholar]
- 17.Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–327. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–314. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee PP, et al. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochran JR, Aivazian D, Cameron TO, Stern LJ. Receptor clustering and transmembrane signaling in T cells. Trends Biochem Sci. 2001;26:304–310. doi: 10.1016/s0968-0004(01)01815-1. [DOI] [PubMed] [Google Scholar]
- 22.Janeway CA., Jr Ligands for the T-cell receptor: Hard times for avidity models. Immunol Today. 1995;16:223–225. doi: 10.1016/0167-5699(95)80163-4. [DOI] [PubMed] [Google Scholar]
- 23.Dam J, et al. Variable dimerization of the Ly49A natural killer cell receptor results in differential engagement of its MHC class I ligand. J Mol Biol. 2006;362:102–113. doi: 10.1016/j.jmb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Davis DM, et al. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dam J, et al. Variable MHC class I engagement by Ly49 natural killer cell receptors demonstrated by the crystal structure of Ly49C bound to H-2K (b) Nat Immunol. 2003;4:1213–1222. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- 26.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.