Abstract
Asparagine-linked glycosylation is a common posttranslational modification of diverse secretory and membrane proteins in eukaryotes, where it is catalyzed by the multiprotein complex oligosaccharyltransferase. The functions of the protein subunits of oligoasccharyltransferase, apart from the catalytic Stt3p, are ill defined. Here we describe functional and structural investigations of the Ost3/6p components of the yeast enzyme. Genetic, biochemical and structural analyses of the lumenal domain of Ost6p revealed oxidoreductase activity mediated by a thioredoxin-like fold with a distinctive active-site loop that changed conformation with redox state. We found that mutation of the active-site cysteine residues of Ost6p and its paralogue Ost3p affected the glycosylation efficiency of a subset of glycosylation sites. Our results show that eukaryotic oligosaccharyltransferase is a multifunctional enzyme that acts at the crossroads of protein modification and protein folding.
Keywords: crystal structure, mass spectrometry, N-glycosylation, protein folding, thioredoxin-like protein
Asparagine linked (N-)glycosylation is a common and essential co- and posttranslocational modification of proteins in eukaryotes, and also occurs in archaea and some bacteria (1). In eukaryotes, the presence of N-glycans assists protein folding in the endoplasmic reticulum (ER) (2), and subsequent modification in the Golgi results in a diverse range of glycan structures on mature proteins. The specific glycan structures present are important in modulating protein function in processes including development, inflammation, cancer, and the immune response (3–5).
In eukaryotes, N-glycosylation is catalysed in the ER lumen by the multiprotein complex oligosaccharyltransferase (OTase), which consists of up to 8 protein subunits (6). Glycan from the mature lipid-linked oligosaccharide donor (typically Glucose3Mannose9N-acetylglucosamine2-pyrophosphate-dolichol) (7) is transferred en bloc to asparagines in selected glycosylation sequons (N-x-S/T; x≠P) in protein substrates (6). In some divergent eukaryotes, archaea and N-glycosylating bacteria, OTase is a single protein, homologous to the catalytic STT3 protein subunit of the eukaryotic enzyme (8).
The protein subunits of OTase, in addition to the catalytic STT3 protein, are possibly involved in regulation of activity, including influencing glycan and protein substrate specificity. It is emerging that several protein subunits of the eukaryotic OTase are required for efficient glycosylation of particular subsets of protein substrates. For example, mammalian ribophorin I and II are required for glycosylation of selected membrane proteins and P-glycoprotein, respectively (9–11). The catalytic STT3 and the homologous OST3/6 proteins are also present in multiple isoforms in many organisms (STT3A/B and OST3/6). This results in the presence of OTase complexes with different protein isoform compositions, which in turn have been reported to affect OTase glycan and protein substrate-specific activities. The mammalian STT3A/B isoforms are differentially expressed in various tissues, and result in OTases with altered kinetic characteristics (12) and preferences for co- or posttranslational glycosylation (13). The OTase of trypanosomatids consists of a single STT3 protein, but often multiple isoforms are present. These single protein OTase isoforms have different substrate specificities in Trypanosoma brucei (14, 15) and Leishmania major (16). These organisms therefore appear to have increased the range of glycoprotein substrates through duplication and divergence of STT3 protein activity, rather than addition of accessory protein subunits to OTase. In yeast, Ost3p and Ost6p are products of paralogous genes, and have the same predicted topology of an N-terminal domain located in the ER lumen followed by 4 transmembrane helices. Incorporation of either Ost3p or Ost6p results in the presence of 2 alternative OTase complexes (17–19), which have different protein substrate-specific activities at the level of individual glycosylation sites (20). Additionally, the human homologues of Ost3/6p (IAP and N33) are required for correct brain development (21, 22), suggesting that they also facilitate glycosylation of a subset of protein substrates.
The ER lumenal domain of Ost3/6p is predicted to have a thioredoxin-like fold with a characteristic CxxC active-site motif (23). Thioredoxin-like folds are commonly present in proteins with disulfide oxidoreductase activity and in the ER are specifically involved in disulfide oxidation and isomerization during protein oxidative folding (24). Oxidative protein folding and N-glycosylation are linked processes in eukaryotic cells. Disulfide bond formation can compete with glycosylation in some substrates (25), in accordance with the requirements of eukaryotic OTase for a flexible substrate. Somewhat in contradiction to this, N-glycans play an essential role in the oxidative folding of proteins by locally recruiting the protein disulfide isomerase ERp57 through the lectin calnexin to regions of proteins requiring disulfide isomerase activity for correct folding (26–29). The presence of a thioredoxin-like fold in a subunit of the eukaryotic OTase suggested a multifunctional enzyme, in which disulfide oxidoreductase activity could directly influence the folding of protein substrates and affect their glycosylation.
Here, we investigated the mechanisms of Ost3/6p-dependent glycosylation site selection by the yeast OTase. We showed that the thioredoxin-like CxxC active-site motif of Ost3/6p was important for efficient glycosylation of a subset of sites. We performed in vitro biochemical analyses of the ER lumenal domain of Ost6p (Ost6L), and showed that it was an active oxidoreductase. Crystal structures of this domain revealed a thioredoxin-like fold with a redox-dependent peptide-binding groove formed by a loop adjacent to its CxxC active-site motif. Together, our results show that the oxidoreductase and redox-dependent peptide binding activities of Ost3/6p increase the glycosylation efficiency of defined sites in protein substrates by OTase.
Results
The predicted thioredoxin-like fold of Ost3p/Ost6p includes a CxxC putative active-site motif. To investigate the function of this CxxC motif on selection of N-glycosylation sites, we made use of our recently described method for measuring N-glycan occupancy at many sites (20). This method involves enrichment of glycoproteins covalently bound to the yeast polysaccharide cell wall via glycosylphosphatidylinositol anchor remnants or alkali-sensitive linkages (30), digestion with endoglycosidase H (leaving a single N-acetylglucosamine on previously glycosylated asparagines), protease digestion, and liquid chromatography electrospray ionization tandem mass spectometry (LC-ESI-MS/MS) detection. The intensities of N-acetylglucosamine-modified and unmodified versions of the same peptide were compared to determine site-specific N-glycan occupancy for 24 N-x-S/T sequon-containing peptides.
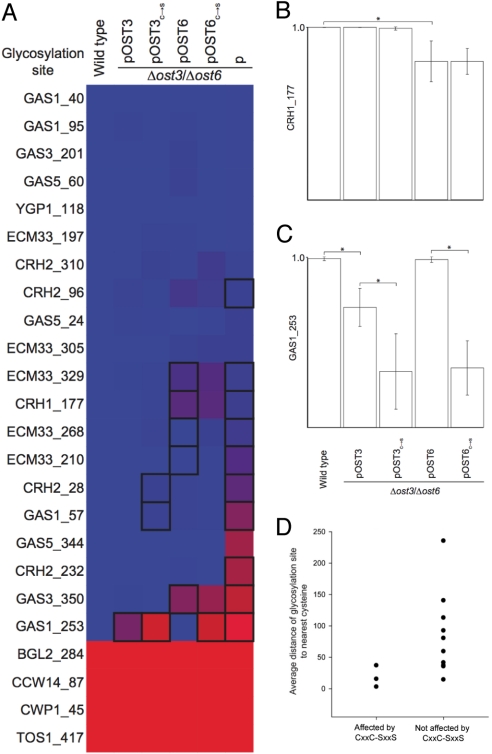
To determine the role of the CxxC motif in N-glycosylation, we replaced both active-site cysteines with serines in Ost3p and Ost6p. As yeast OTase contains either Ost3p or Ost6p, we could generate strains with genomic deletion of both OST3 and OST6 loci (Δost3/Δost6), and expressing plasmid-encoded variant Ost3p or Ost6p. This led to cells that expressed OTase at a wild-type level containing only variant Ost3p or Ost6p (18). Analysis of these cells showed that mutations in the CxxC motif affected the glycosylation efficiency of 3 out of 24 sites, albeit to different extents [Fig. 1 and Table S1]. A strong reduction of glycosylation was observed for site N253 in Gas1p, while weak but statistically significant reduction was observed for sites N28 in Crh2p and N57 in Gas1p.
Fig. 1.
The CxxC active-site motif of Ost3p and Ost6p affect in vivo site-specific OTase activity. (A), Site-specific N-glycosylation occupancy is mapped from blue (100%) to red (0%). Table S1 shows the values. Boxes indicate P < 0.05 in a 2-sided Mann-Whitney test, n > 5, comparing: wild-type cells with cells containing ost3 and ost6 deletions but expressing high copy number plasmid-encoded Ost3p (pOST3), Ost6p (pOST6), or neither Ost3p nor Ost6p (p); pOST3 cells with pOST3c→s cells (with active-site cysteines replaced by serines); or pOST6 cells with pOST6c→s cells. Data from wild-type, pOST3, pOST6 and p cells are as previously reported (20). [Reproduced with permission from ref. 21 (Copyright 2008, The American Society for Biochemistry and Molecular Biology). All rights reserved.] (B and C) Examples of specific sites requiring Ost3/6p function for efficient glycosylation. Error bars indicate SEM; *, P < 0.01 in a 2-sided Mann-Whitney test, n > 5. (D) Average distance from glycosylation sites to the nearest cysteine residues in substrate proteins, for sites affected or not affected by mutations in the Ost3/6p CxxC motif.
Ost6p Thiol Oxidoreductase Activity.
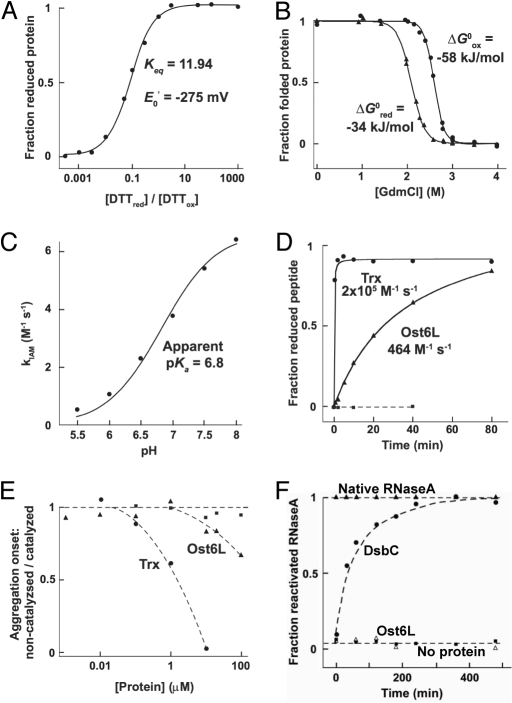
The relevance of the CxxC motif on glycosylation in vivo suggested that the predicted thioredoxin-like domain of Ost3/6p had oxidoreductase activity. To evaluate this activity by in vitro experiments, we expressed the N-terminal thioredoxin domain (residues 1–166 of the predicted mature protein) of Ost6p in Escherichia coli and purified the protein (Ost6L). Based on the N-terminal protein sequence of the mature Ost3p (31) and the high similarity to Ost3p (32), this domain is proposed to be located in the lumen of the ER (6), but alternative topological models based on hybrid fusion proteins have been proposed (33). The redox potential of Ost6L was found to be –275 mV (Fig. 2A), highly reducing and equivalent to that of E. coli cytoplasmic thioredoxin (Trx) (34). Supporting this reducing redox potential, Ost6L was more stable when oxidised (Fig. 2B), and showed an apparent pKa of 6.8 (Fig. 2C) similar to the N-terminal, nucleophilic cysteine C32 of Trx (35). However, the reaction of Ost6L with iodoacetamide, with a maximum rate constant (kIAM) of ≈7 M−1 s−1, was much slower than that of Trx (kIAM = 155 M−1 s−1) (35). In contrast to reduced Trx, both active-site cysteines of Ost6Lred were accessible to modification with iodoacetamide. To verify that Ost6L is oxidized in the ER, we measured the oxidation state of Ost6L in the presence of GSH:GSSG ratios reported for the ER lumen (36). In agreement with the strongly reducing redox potential of Ost6L, only the oxidized form could be detected under these conditions (Fig. S1).
Fig. 2.
In vitro characterization of Ost6L. (A) Determination of the redox potential of Ost6L from its redox equilibrium with DTTred/DTTox. (B) Guanidinium-induced equilibrium unfolding incidated that Ost6Lox (circles) was more stable than Ost6Lred (triangles). (C) The apparent pKa of the active-site cysteines of Ost6L as determined from its reactivity with iodoacetamide. (D) Stoichiometric reduction of an oxidized model peptide [NRCSQGSCWN (44)] by reduced Ost6L (triangles), E. coli thioredoxin (circles), or no protein (squares). (E) Reduction of insulin (115 μM) by various concentrations of Ost6L (triangles), E. coli thioredoxin (circles), or no protein (squares). (F) Disulfide isomerase activity as determined by reactivation of scRNAse by the E. coli disulfide isomerase DsbC (circles), Ost6L (squares), or no protein (open triangles), relative to native RNaseA (triangles).
To determine if Ost6L was a redox-active protein, we tested its activity toward several in vitro model substrates. Indeed, Ost6Lred stoichiometrically reduced a disulfide bond in a model peptide (Fig. 2D), and showed activity as a catalyst of insulin reduction at ratios of Ost6p:insulin near unity (Fig. 2E). In both cases, the reductase activity of Ost6L was lower than that of Trx. Ost6L had no detectable disulfide isomerase activity (Fig. 2F).
Ost6L Structural Characterization.
We determined 3 crystal structures of the Ost6L monomer in different oxidation states: Ost6Lox at 2.21 Å resolution; an oxidized high-resolution structure with the active-site disulfide partially photo-reduced by synchrotron radiation (Ost6Lox*) at 1.3 Å resolution; and the reduced state (Ost6Lred) at 1.96 Å resolution. In agreement with our biochemical data, Ost6L indeed exhibited a thioredoxin-like core fold, but several additional features unexpected for a protein belonging to the thioredoxin-like superfamily were observed (Fig. 3B): an N-terminal αβα-motif (D8-L30); a conspicuous loop preceding the CxxC active-site motif (R46GTNSNGMS54); two 310-helices between β-strands 4 and 5 (S114-Q119, 310A), and between β-strand 5 and α-helix 5 (P134-N136, 310B); and a C-terminal linker connecting the lumenal domain with the first predicted transmembrane helix of Ost6p.
Fig. 3.
Ost6L has a thioredoxin-like fold with unique features. (A) Schematic overview of the yeast OTase complex. The ER lumenal domain of Ost6p (Ost6L) is boxed. (B) Ribbon diagram of the structure of Ost6L. The active-site cysteines are shown in yellow. (C) Superimposed active-site regions of Ost6Lox (brown) and Ost6Lred (cyan). In Ost6Lox, the loop was stabilized by hydrogen bonds (black dashed lines); and in Ost6Lred, helix α3 was extended by S54 and C55, and the loop was flexibly disordered (dotted cyan line, no electron density was visible). (D) Surface representation of Ost6Lox* showing the proposed peptide-binding groove adjacent to the exposed active-site C55, colored by electrostatic surface potential (red, acidic; white, neutral; blue, basic).
In contrast to classical thioredoxin-like proteins, Ost6L had a distinctive loop adjacent to its CxxC motif (see Fig. 3 and Fig. S2). A similar insertion before the active-site motif corresponding to the Ost6L loop is present in all Ost3/6p sequences, suggesting that this is a general, functionally important feature of this protein subclass (Fig. S3). In both Ost6Lox and Ost6Lox*, this active-site loop adopted an essentially fixed conformation, stabilized by several main-chain hydrogen bonds (see Fig. 3C). The main-chain amide of C55 hydrogen-bonded the carbonyl group of G47 via a well-ordered water molecule. Other stabilizing interactions were provided by hydrogen bonds formed by the main-chain amide of T48 with the carbonyl of M53, the carbonyl of N49 with the main-chain amide of N51, and by the carboxamide of N49 with the main-chain amide moieties of G52 and M53.
Upon reduction of the Ost6L disulfide, the active-site loop became flexible, with no electron density observed for residues G47 to G52 (see Fig. 3C). The loop flexibility allowed the active-site cysteine C55 to adopt a conformation different from other reduced thioredoxin-like proteins (37, 38). In classical thioredoxin-like proteins, reduction mainly leads to variation in the χ1 torsion angle of the N-terminal cysteine C55, with only marginal main chain rearrangements. In Ost6L, reduction instead led to conformational changes in both the C55 side and main chain, resulting in an elongated active-site helix. Extension of the active-site helix forced the main chain amino group of C55 to point away from G47, and thus prevented the formation of the hydrogen bond network that presumably kept the loop ordered in the oxidized form.
The ordered loop in Ost6Lox created a distinct groove near the active-site motif (see Fig. 3D). This groove was a strong candidate for a peptide-binding site, as it possessed slightly basic and hydrophobic regions, an acidic patch, and contained several residues that could easily adapt to various substrate polypeptides (M45, N49, M53 and N89). The surface of Ost6L also featured a distinctive hydrophobic patch (Fig. S4). The residues in the patch were also the only continuous area of the surface of Ost6L under strong purifying selection (see Fig. S4B) and the corresponding residues in Ost3p are also hydrophobic (see Fig. S3). This hydrophobic patch may be involved in interactions with substrate polypeptides or with other proteins in a multiprotein complex.
Previous genetic experiments (18, 20) and our in vivo and structural data (see Figs. 1 and 3) suggested that Ost3/6p might be involved in substrate recognition mediated by the redox-dependent active-site groove. We therefore tested if Ost6Lox and Ost6Lred could bind peptides noncovalently in vitro. A complex mixture of peptides from yeast cell-wall proteins (a rich source of N-glycoproteins, and hence potential Ost6L substrates) was applied to columns containing covalently bound Ost6Lox or Ost6Lred. Retained peptides were then identified by mass spectrometry. Ost6Lox but not Ost6Lred differentially enriched specific peptides (Fig. 4, Fig. S5, and Table S2), in accordance with the putative peptide-binding site present only in Ost6Lox (see Fig. 3).
Fig. 4.

Noncovalent peptide binding by Ost6Lox. Relative enrichment of the Cwp1p21−31 versus the HS150p338–345 peptide, by columns with covalently attached Ost6Lox (circles), Ost6Lred (p), or no protein (squares). Data are average of triplicates. Error bars indicate the range.
Discussion
The thioredoxin-like fold of the Ost3p/Ost6p subunit predicted a CxxC active-site motif essential for oxidoreductase activity. This prompted us to hypothesize that a potential oxidoreductase activity of Ost3/6p directly affects the oxidative folding of OTase protein substrates to assist their efficient glycosylation. We made use of our recently described method to measure the N-glycan occupancy of many sites in proteins covalently linked to the yeast polysaccharide cell wall (20). Glycosylation site occupancy in cells with both active-site cysteines of Ost3p and Ost6p replaced by serines revealed that these replacements did not completely abolish the function of Ost3/6p, as there was still a subunit-specific glycosylation phenotype of these variant forms: a subset of sites requiring Ost3p was not affected in the Ost3p CxxC variant strain. However, we observed site-specific underglycosylation in the SxxS variants, pointing to a functional role of the CxxC motif in specific glycosylation site selection. These 2 classes of sites were exemplified by N253 in Gas1p (GAS1_253) and N177 in Crh1p (CRH1_177) (see Fig. 1 B and C). CRH1_177 required Ost3p, either wild-type or CxxC variant, for complete glycosylation, while GAS1_253 required the presence of Ost6p as well as a CxxC motif. Interestingly, the GAS1_253 glycosylation site is present in a domain with 3 predicted disulfide bonds. Furthermore, sites that were affected by mutations in the CxxC motif of either Ost3p or Ost6p (such as GAS1_253) were more proximal in primary sequence to cysteines than sites that were not affected (see Fig. 1D). Our analysis monitored the glycosylation status of multiple N-glycosylation sites and only a subset of these was affected by mutation in the CxxC motif. We therefore conclude that the CxxC motif has an impact on efficient glycosylation. Irrespective of the number of sites affected, our results were best explained by the hypothesis that these OTase subunits have a dual function in site recognition: one function required the CxxC motif, the other was independent of this motif.
The CxxC-dependent glycosylation of defined sites in substrate proteins supported the hypothesis that the potential oxidoreductase activity of the Ost3p/Ost6p subunit affected OTase function in vivo. To investigate this putative oxidoreductase activity, we performed various in vitro assays using the purified ER lumenal thioredoxin-like domain of Ost6p (Ost6L). Surprising for a protein present in the oxidizing environment of the ER, the redox potential, apparent active-site cysteine pKa, and relative stability of the oxidized and reduced forms of Ost6L were similar to cytoplasmic Trx. Ost6Lred could stoichiometrically reduce a disulfide bond in a model peptide (see Fig. 2D) and showed activity as a catalyst of insulin reduction (see Fig. 2E), supporting oxidreductase activity of this OTase subunit. However, these activities were lower than that of Trx. Although it is possible that the reductase activity of Ost6L allows Ost3/6p to locally reverse oxidative folding in substrate polypeptides, thereby providing unfolded substrate to the catalytic site of OTase, we favor an alternative model that is based on our finding that Ost6L is oxidized under the redox conditions of the ER (see Fig. S1). We propose that reduced cysteines of nascent polypeptide chains can perform a nucleophilic attack on the active-site disulfide bond of Ost6L, forming a mixed disulfide (Fig. 5). This mixed disulfide could tether the nascent polypeptide to Ost6p and prevent oxidative folding of the protein, thereby allowing efficient glycosylation of nearby N-x-S/T sequons by the catalytic center of OTase. The tethered nascent polypeptide chain would be released after resolution of the mixed disulfide by the free cysteine of the Ost6p CxxC motif, leaving the polypeptide reduced and Ost6L oxidised. The slow kinetics we observed for Ost6L redox reactions relative to Trx may act to prolong substrate tethering to Ost6p, while the exceptionally reducing redox potential of the Ost6L active-site cysteines would thermodynamically favor release of reduced substrate polypeptide and re-oxidized Ost6L. Folding of the glycosylated polypeptide chain could continue after its release, including N-glycan/ERp57-mediated oxidative folding.
Fig. 5.
Proposed model of Ost3/6p function in N-glycosylation. See text for details. (A) Nascent polypeptide chain with reduced cysteine residues passes through the translocon into the ER lumen. (B) Nucleophilic attack performed by the reduced polypeptide cysteine residue leads to (C) binding of the polypeptide to Ost6p via a mixed disulfide. Alternatively, polypeptide may be bound through noncovalent interactions with the peptide-binding groove. Translocation of the nascent polypeptide continues, with folding inhibited because of Ost6p binding constraints. (D) The catalytic site of OTase transfers glycan to the asparagine of a glycosylation sequon. (E) Resolution of the mixed disulfide releases the captured polypeptide chain. (F) Translocation and folding of the glycosylated polypeptide chain continues. (G) Glycosylated and folded protein.
The structural change in the loop and groove close to the CxxC active-site motif of Ost6L (see Fig. 3C), and accompanying redox-state dependent peptide binding (see Fig. 4), could provide an additional, noncovalent, mechanism controlling temporary recruitment of specific polypeptide stretches. Specific polypeptide binding, both noncovalent and disulfide-mediated, may thus slow the early stages of substrate protein folding, thereby increasing the accessibility of specific sites and, hence, N-glycosylation efficiency. Bacterial OTase, a single protein homologous to the catalytic subunit of the eukaryotic enzyme, glycosylates sites in intrinsically flexible regions of folded substrate protein (39). Ost3/6p may thus allow eukaryotic OTase to actively maintain substrate protein in an accessible, unfolded state to increase the range of potential glycosylation sites.
To our knowledge, Ost6L is unique among thioredoxin-like proteins in showing such a dramatic redox-dependent structural change. Polypeptide substrate recognition mediated by the redox-dependent active-site groove could also explain the presence of Ost3/6p homologues in plants lacking a CxxC active-site motif, but containing a loop insertion at this position (see Fig. S3). The high-resolution structures we present here are unique in representing soluble, biochemically active domains of the eukaryotic OTase. In combination with the previously reported structures of the small transmembrane protein Ost4p from yeast (40), the soluble C-terminal domain of archaeal Stt3p from Pyrococcus furiosus (41), and the cryo-EM structure of the yeast OTase complex (42), our results provide a significant step toward a unified structural understanding of the mechanisms controlling N-glycosylation activity and substrate selection by the eukaryotic OTase.
Materials and Methods
Yeast Strains.
Yeast strains used: YG889 (MATa ade2–101 ura3–52 his3Δ200 tyr1 Δost3::HIS3 Δost6::kanMX4) (18) and SS330 (MATa ade2–101 ura3–52 his3Δ200 tyr1) (43). Standard protocols were followed for yeast manipulation. Cells were grown as described (20).
Cell-Wall Protein Sample Preparation and Mass Spectrometry.
Analysis of N-glycan site-specific occupancy was performed as described (20).
Plasmid Constructs.
For expression in E. coli, the DNA sequence corresponding to the lumenal domain of Ost6p (from the signal peptidase cleavage sites to the beginning of the first transmembrane helix as predicted in the UniProt Knowledgebase: residues 1–166 of mature Ost6p) was cloned into a derivative of the pBAD E. coli expression vector with the sequence coding for a myc-His6 tag replaced by one coding for a His10 tag, using PCR primers incorporating NcoI and BamHI restriction sites (pBAD-Ost6L-His-10). For expression in yeast, the following plasmids were used: pOST3 (18), pOST6 (18), pOST3c→s, pOST6c→s. Variants with amino acid replacements were constructed from the previously reported pOST3 and pOST6 plasmids using overlap PCR, using oligonucleotide pairs incorporating the desired sequence changes and PstI-SalI restriction endonucleases.
Protein Expression and Purification.
Protein expression in E. coli cells harboring the vector pBAD-ost6L-His-10 was induced by addition of L-arabinose. Ost6L was purified from cell extracts by anion exchange-, immobilized metal ion- and size exclusion chromatography. See the SI Text for full details.
In Vitro Protein Analysis. The redox potential of the active-site disulfide of Ost6L was determined through equilibration with an excess of DTTox and DTTred at different ratios. The free energies of stabilization (ΔG0) of oxidized and reduced Ost6L were determined from guanidinium-induced equilibrium unfolding curves based on the 2-state model of protein folding. The pH-dependent reactivity of the active-site thiols of Ost6L with iodoacetamide (35) was used to determine their apparent pKa. A previously described synthetic peptide (NRCSQGSCWN) (44) was used as substrate in an in vitro reductase assay. The peptide contains 2 cysteines, and served as a model of an unfolded, oxidized polypeptide. Ost6L-catalysed reduction of insulin by DTT was determined essentially as described (45). Protein disulfide isomerase activity was measured by reactivation of disulfide-scrambled RNaseA upon catalyzed disulfide shuffling. See the SI Text for full details.
Protein Crystallization and Structure Determination.
Purified Ost6Lox was crystallized using the sitting drop vapor diffusion method with 50 mM Na-citrate pH 3, 18% PEG 3350 as the precipitation solution. Crystals were incubated with 10 mM Tris(2-carboxyethyl)phosphine to obtain Ost6Lred. For structure solution, crystals of Ost6Lox were soaked for 45 s in a cryo-protecting solution containing 0.5 M NaBr and a single wavelength anomalous dispersion dataset at the Br-peak wavelength (λ = 0.9193 Å; Ost6Lpeak) to 2.0 Å resolution was collected and used for phasing. Three other datasets were collected: native protein (2.21 Å resolution, Ost6Lox), protein with the active-site disulfide partially reduced by synchrotron radiation (1.3 Å resolution, λ = 0.8551 Å; Ost6Lox*), and TCEP-reduced protein (1.96 Å resolution; Ost6Lred). Crystallographic statistics are given in Table S3. See the SI Text for full details.
Sequence and Structural Analyses.
Electrostatic surface calculations at 310 K were carried out solving the linearized Poisson-Boltzmann equation with APBS [dielectric constants: 2 (protein) and 80 (solvent); 0.144 M monovalent salt; surface probe radius: 1.4 Å] (46) integrated in Pymol v0.99. Electrostatic surface potential representations were interpolated between acidic, –45 kT and basic, +45 kT.
Ost6L Peptide-Binding Determination.
Tryptic peptides from deglycosylated cell wall proteins (20) were applied to CNBr-activated Sepharose 4B beads coupled with Ost6Lred, Ost6Lox or no protein, and eluted in PBS with 1 mM EDTA. Peptides in each fraction were analyzed by MALDI-TOF/TOF-MS. See the SI Text for full details.
Supplementary Material
Acknowledgments.
Data collection was performed at the Functional Genomics Center Zurich (FGCZ) and at Swiss Light Source, Paul Scherrer Institut, Villigen, Switzerland. We thank the staff of FGCZ and of the beamline X06SA for support in MS and X-ray data collection, respectively. This project was funded by the Swiss National Science Foundation, the Eidgenössische Technische Hochschule Zürich, and the Universität Zürich within the framework of the National Center of Competence in Research Structural Biology program and the GlycoInit program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Atomic coordinates and structure factors have been deposited with the Protein Data Bank, www.pdb.org (PDB ID codes 3G7Y, 3G9B, and 3GA4).
This article contains supporting information online at www.pnas.org/cgi/content/full/0812515106/DCSupplemental.
References
- 1.Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3(3):225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- 2.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Sharon N, Lis H. Lectins–proteins with a sweet tooth: functions in cell recognition. Essays Biochem. 1995;30:59–75. [PubMed] [Google Scholar]
- 5.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16(4):47R–62R. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol. 2007;177(1):29–37. doi: 10.1083/jcb.200611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacker M, et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298(5599):1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 9.Honma K, et al. RPN2 gene confers docetaxel resistance in breast cancer. Nat Med. 2008;14(9):939–948. doi: 10.1038/nm.1858. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CM, High S. Ribophorin I acts as a substrate-specific facilitator of N-glycosylation. J Cell Sci. 2007;120(4):648–657. doi: 10.1242/jcs.000729. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CM, Roebuck Q, High S. Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core. Proc Natl Acad Sci USA. 2008;105(28):9534–9539. doi: 10.1073/pnas.0711846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12(1):101–111. doi: 10.1016/s1097-2765(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136(2):272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DC, Mehlert A, Güther ML, Ferguson MA. Deletion of the glucosidase II gene in Trypanosoma brucei reveals novel N-glycosylation mechanisms in the biosynthesis of variant surface glycoprotein. J Biol Chem. 2005;280(43):35929–35942. doi: 10.1074/jbc.M509130200. [DOI] [PubMed] [Google Scholar]
- 15.Manthri S, Guther MLS, Izquierdo L, Acosta-Serrano A, Ferguson MAJ. Deletion of the TbALG3 gene demonstrates site-specific N-glycosylation and N-glycan processing in Trypanosoma brucei. Glycobiology. 2008;18(5):367–383. doi: 10.1093/glycob/cwn014. [DOI] [PubMed] [Google Scholar]
- 16.Parsaie Nasab F, Schulz BL, Gamarro F, Parodi AJ, Aebi M. All in one: Leishmania major STT3 proteins substitute for the whole oligosaccharyltransferase complex in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19(9):3758–3768. doi: 10.1091/mbc.E08-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz M, Knauer M, Lehle L. Yeast oligosaccharyltransferase consists of two functionally distinct sub-complexes, specified by either the Ost3p or Ost6p subunit. FEBS Lett. 2005;579(29):6564–6568. doi: 10.1016/j.febslet.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 18.Spirig U, Bodmer D, Wacker M, Burda P, Aebi M. The 3.4-kDa Ost4 protein is required for the assembly of two distinct oligosaccharyltransferase complexes in yeast. Glycobiology. 2005;15(12):1396–1406. doi: 10.1093/glycob/cwj025. [DOI] [PubMed] [Google Scholar]
- 19.Yan A, Lennarz WJ. Two oligosaccharyl transferase complexes exist in yeast and associate with two different translocons. Glycobiology. 2005;15(12):1407–1415. doi: 10.1093/glycob/cwj026. [DOI] [PubMed] [Google Scholar]
- 20.Schulz BL, Aebi M. Analysis of glycosylation site occupancy reveals a role for Ost3p and Ost6p in site-specific N-glycosylation efficiency. Mol Cell Proteomics. 2009;8(2):357–364. doi: 10.1074/mcp.M800219-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Garshasbi M, et al. A defect in the TUSC3 gene is associated with autosomal recessive mental retardation. Am J Hum Genet. 2008;82(5):1158–1164. doi: 10.1016/j.ajhg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molinari F, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am J Hum Genet. 2008;82(5):1150–1157. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fetrow JS, et al. Genomic-scale comparison of sequence- and structure-based methods of function prediction: does structure provide additional insight? Protein Sci. 2001;10(5):1005–1014. doi: 10.1110/ps.49201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3(11):836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 25.Allen S, Naim HY, Bulleid NJ. Intracellular folding of tissue-type plasminogen activator. Effects of disulfide bond formation on N-linked glycosylation and secretion. J Biol Chem. 1995;270(9):4797–4804. doi: 10.1074/jbc.270.9.4797. [DOI] [PubMed] [Google Scholar]
- 26.Daniels R, Kurowski B, Johnson AE, Hebert DN. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell. 2003;11(1):79–90. doi: 10.1016/s1097-2765(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 27.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4(3):181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 28.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver JD, Roderick HL, Llewellyn DH, High S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol Biol Cell. 1999;10(8):2573–2582. doi: 10.1091/mbc.10.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin QY, et al. Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls: identification of proteins covalently attached via glycosylphosphatidylinositol remnants or mild alkali-sensitive linkages. J Biol Chem. 2005;280(21):20894–20901. doi: 10.1074/jbc.M500334200. [DOI] [PubMed] [Google Scholar]
- 31.Karaoglu D, Kelleher DJ, Gilmore R. Functional characterisation of Ost3p. Loss of the 34-kD subunit of the Saccharomyces cerevisiae oligosaccharyltransferase results in biased underglycosylation of acceptor substrates. J Cell Biol. 1995;130:567–577. doi: 10.1083/jcb.130.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knauer R, Lehle L. The oligosaccharyltransferase complex from Saccharomyces cerevisiae. Isolation of the OST6 gene, its synthetic interaction with OST3, and analysis of the native complex. J Biol Chem. 1999;274(24):17249–17256. doi: 10.1074/jbc.274.24.17249. [DOI] [PubMed] [Google Scholar]
- 33.Yan A, Wu E, Lennarz WJ. Studies of yeast oligosaccharyl transferase subunits using the split-ubiquitin system: Topological features and in vivo interactions. Proc Natl Acad Sci USA. 2005;102(20):7121–7126. doi: 10.1073/pnas.0502669102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause G, Lundström J, Barea JL, Pueyo de la Cuesta C, Holmgren A. Mimicking the active site of protein disulfide-isomerase by substitution of proline 34 in Escherichia coli thioredoxin. J Biol Chem. 1991;266(15):9494–9500. [PubMed] [Google Scholar]
- 35.Mossner E, Huber-Wunderlich M, Glockshuber R. Characterization of Escherichia coli thioredoxin variants mimicking the active-sites of other thiol/disulfide oxidoreductases. Protein Sci. 1998;7(5):1233–1244. doi: 10.1002/pro.5560070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 37.Jeng MF, et al. High-resolution solution structures of oxidized and reduced Escherichia coli thioredoxin. Structure. 1994;2(9):853–868. doi: 10.1016/s0969-2126(94)00086-7. [DOI] [PubMed] [Google Scholar]
- 38.Stirnimann CU, et al. High-resolution structures of Escherichia coli cDsbD in different redox states: A combined crystallographic, biochemical and computational study. J Mol Biol. 2006;358(3):829–845. doi: 10.1016/j.jmb.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Kowarik M, et al. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science. 2006;314(5802):1148–1150. doi: 10.1126/science.1134351. [DOI] [PubMed] [Google Scholar]
- 40.Zubkov S, Lennarz WJ, Mohanty S. Structural basis for the function of a minimembrane protein subunit of yeast oligosaccharyltransferase. Proc Natl Acad Sci USA. 2004;101(11):3821–3826. doi: 10.1073/pnas.0400512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igura M, et al. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2008;27(1):234–243. doi: 10.1038/sj.emboj.7601940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Chavan M, Schindelin H, Lennarz WJ, Li H. Structure of the oligosaccharyl transferase complex at 12 Å resolution. Structure. 2008;16(3):432–440. doi: 10.1016/j.str.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Vijayraghavan U, Company M, Abelson J. Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev. 1989;3(8):1206–1216. doi: 10.1101/gad.3.8.1206. [DOI] [PubMed] [Google Scholar]
- 44.Ruddock LW, Hirst TR, Freedman RB. pH-dependence of the dithiol-oxidizing activity of DsbA (a periplasmic protein thiol:disulphide oxidoreductase) and protein disulphide-isomerase: studies with a novel simple peptide substrate. Biochem J. 1996;315(Pt 3):1001–1005. doi: 10.1042/bj3151001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254(19):9627–9632. [PubMed] [Google Scholar]
- 46.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.