Abstract
Fibrogenesis is an often-deadly process with increasing world-wide incidence and limited therapeutic options. Pulmonary fibrogenesis involves remodeling of the distal airspace and parenchyma of the lung, and is characterized by excessive extracellular matrix deposition and accumulation of apoptosis-resistant myofibroblasts. Recent studies have added significantly to our understanding of the complex mechanisms involved in lung fibrogenesis. Emerging concepts in this field include the critical role of the epithelium, particularly type II pneumocytes, in the initiation and perpetuation of fibrosis in response to either endogenous or exogenous stress; a growing awareness of alternative activation of macrophages in tissue remodeling; growing appreciation of the alternative origins and phenotypic plasticity of fibroblasts; the roles of epigenetic reprogramming and context-dependent signaling in profibrotic phenotype alterations; and recognition of the importance of cross talk and convergence of intracellular signaling pathways. In vitro, in vivo, and in silico approaches support a paradigm of “disordered re-development” of the lung. Designing effective antifibrotic interventions will require accurate understanding of the complex interactions among the genetic, environmental, epigenetic, biochemical, cellular, and contextual abnormalities that promote pulmonary fibrogenesis.
Lung fibrosis occurs in interstitial lung diseases (ILDs) and idiopathic interstitial pneumonias (IIPs), as part of several systemic connective tissue diseases and childhood interstitial lung disease syndrome, and in response to many types of lung injury, including radiation and some chemotherapeutic drugs. Idiopathic pulmonary fibrosis (IPF) is perhaps the most pernicious and enigmatic form of the greater problem of lung fibrogenesis. IPF claims more lives annually in the United States than many types of cancer1; however, effective therapy is lacking. Recent evidence indicates that both IPF prevalence and mortality are growing in the United States and elsewhere.2 The increasing burden of IPF is not simply reflective of an aging population, as age-adjusted mortality for IPF is increasing as well.2
In much of the latter half of the 20th century, perhaps as the result of successful use of anti-inflammatory therapies such as corticosteroids for some of the IIPs, fibrosis was believed to be initiated and propagated by persistent lung inflammation.3,4 However, corticosteroids and other anti-inflammatory therapies have been uniformly unhelpful for IPF, and may in fact be harmful.5 This fact, combined with absence of classic inflammatory biomarkers in IPF, and evidence from animal models in which fibrosis proceeds in the absence of inflammation, led to reassessment of the inflammatory paradigm.
The view that fibrotic remodeling, both of the lung parenchyma (eg, in IPF) and airways (eg, in asthma) instead represents a form of disordered wound healing, in which epithelial-mesenchymal interactions are predominant, has become canonical in the literature of the past two decades.6,7,8 Selman et al accordingly proposed reconsideration of IPF as a disorder of epithelial-fibroblast interaction.8 Interactions between the epithelium and mesenchyme have been shown to be critical for developmental morphogenesis of the lung,9 and are also prominent in lung fibrogenesis. Recent microarray data from IPF and experimental models of fibrosis demonstrated recapitulation of expression patterns and signaling pathways critical during lung development.10,11 Thus it appears that many of the cellular and molecular events critical to “modeling” of the lung during development are recapitulated during the “re-modeling” that occurs during fibrogenesis. Lung fibrosis may thus not only be conceptualized as a form of aberrant repair,6,12,13,14 but also as a “disordered re-development” of the lung.
Because nearly 45% of all deaths in the developed world are attributed to some type of chronic fibroproliferative disease,15 it is important periodically to review new developments in the study of fibrogenesis. This review focuses on recent experimental findings with regard to the pulmonary epithelium and fibroblasts, which include novel observations regarding the role of the epithelium in the initiation and maintenance of pulmonary fibrogenesis, as well as support newer concepts regarding the origins and differentiation of fibroblasts in fibrotic processes. In addition, it will review the latest data on intracellular signaling pathways and cross talk within and between the fibroblasts and epithelial cells that mediate pulmonary fibrosis. Understanding the emerging concepts discussed in this review and their relative roles in lung fibrosis will facilitate development of novel therapeutic approaches to ameliorate the global burden of fibroproliferative disease.
Role of Epithelium in Genesis and Perpetuation of Fibrosis
Epithelium as a Mediator of ILD
The pulmonary alveolar epithelium is the final barrier interface to inhaled substances. Together with sentinel macrophages, the epithelium controls pulmonary homeostasis by responding to environmental challenges through continuous reciprocal interactions with mesenchymal and vascular cells. Chronic epithelial cell stress, due to either intrinsic cellular defects or extrinsic insults such as infection, can promote epithelial cell death, impair normal re-epithelialization and alter epithelial–mesenchymal interactions, leading to fibroproliferation.16
Defined etiologies for pulmonary fibrosis secondary to intrinsic cellular defects include genetic deficiency of the pulmonary surfactant protein C (SP-C) and ATP binding cassette protein (ABC)A3. Persistent infection by viruses (specifically Herpesviridae) targeting the respiratory epithelium have also been associated with a fibrotic response. These distinct origins of human fibrosis have related mouse models that share many features of human disease, thus better delineating epithelial-based mechanisms of lung fibrosis.
Abnormalities of Surfactant Protein C in Idiopathic and Familial Pulmonary Fibrosis
Pulmonary SP-C is a highly hydrophobic protein that enhances surface activity and contributes to innate immune defense of the lung. SP-C is synthesized and secreted only by alveolar type II cells, which are the site of initial injury in SP-C dysfunction disease. A variety of mutations in the SFTPC gene have since been identified that strongly associate this gene with disease. SFTPC mutations include point mutations that alter single amino acid residues, frame shift mutations that change or terminate downstream translation, and splice site mutations that delete entire exons to produce a truncated proSP-C protein.17,18 The natural history and severity of SP-C-associated lung disease is highly variable and may reflect the cellular response to the distinct mutations in the SFTPC gene coupled with the action of undefined modifier genes.
Alterations of a proprotein structure due to mutations can potentially inhibit proper folding to achieve correct conformation and function (loss of function). Likewise, impaired processing can lead to accumulation of nonfunctional protein that the cell must now eliminate (toxic gain of function). Maturation of newly translated proteins occurs as a series of folding events as proteins transit the endoplasmic reticulum and Golgi for release into cellular compartments for secretion. Cells have adapted a series of endoplasmic reticulum-based responses to restore proper folding or guide elimination of terminally misfolded proteins to relieve further stress. The cascade of responses is termed the unfolded protein response (UPR). If the UPR does not ultimately relieve cellular stress, then caspase-dependent cellular apoptosis pathways can be activated to eliminate cells entirely. The presence of aberrant forms of the SP-C precursor protein is a particular threat to homeostasis due to the high levels of SP-C normally synthesized, processed, and stored for secretion by the type II epithelial cells.
UPR marker immunostaining is increased in the lungs of individuals with both SP-C mutations and IPF.19 These observations have been extended to non-SP-C-related sporadic IPF. Increased expression of UPR mediators and caspase 3 were demonstrated by immunoblotting in IPF but not in chronic obstructive pulmonary disease. Immunostaining co-localized markers of UPR and apoptosis to type II cells in regions of dense fibrosis.20 Collectively, these findings are consistent with a generalized endoplasmic reticulum stress response of epithelial cells in fibrotic tissue.
In vitro expression of SP-C constructs encoding an SFTPC mutation found in patients resulted in SP-C aggregation, impaired epithelial cell growth, increased expression of an endoplasmic reticulum stress response and epithelial cell apoptosis.21,22 Co-chaperones precipitated with the truncated SP-C, indicating association of UPR pathway proteins with the truncated and potentially misfolded SP-C. Expression of the SFTPC exon-four deletion mutation in the lungs of transgenic mice was lethal at birth, and analysis of the lungs demonstrated severe hypoplasia, reduced branching morphogenesis, and epithelial cell death.23 These and additional studies are consistent with the overproduction of non-native proSP-C eliciting a misfolded protein stress response and epithelial cytotoxicity that contributes to the progressive lung disease in affected individuals.
Usual interstitial pneumonia, the hallmark histopathological lesion in IPF, has also been described in two reports of SP-C deficiency without identified mutations in the SFTPC gene, in which no mature SP-C and greatly diminished proSP-C were detected.24,25 Fibrotic disease in these affected individuals implies that the SP-C null condition may also produce altered type II cell or alveolar function that results in disease. When SP-C deficient mice were generated by gene targeting techniques, the mice developed a strain-specific ILD that progressed with age to irregular fibrosis.26 The severity of bleomycin-induced lung fibrosis was increased in the lungs of SP-C deficient mice, suggesting that the absence of SP-C predisposes the lung to fibrosis.27 The SP-C deficient mice do not produce any proSP-C that could stimulate an UPR. Taken together, the origins of SP-C-related lung disease may be multifactorial, resulting from cumulative epithelial cell injury initiated by the lack of SP-C in the airspace, abnormality of cellular SP-C processing events, or the presence of aberrant forms of proSP-C and an UPR.
ATP Binding Cassette Family Member ABCA3 and Interstitial Lung Disease
The ABC transporter family is a diverse group of large transmembrane proteins that use ATP to translocate substrates. The ABCA subfamily facilitates the movement of cholesterol or specific phospholipids. ABCA3 is highly expressed in the lung relative to other organs. The localization of ABCA3 to the limiting membrane of lamellar bodies in type II cells implicated ABCA3 in the assembly of the intracellular storage form of pulmonary surfactant.28,29
ABCA3 mutations are recessive and associated with a highly variable disease phenotype. Infants homozygous for mutations in the ABCA3 gene generally develop severe and usually fatal neonatal respiratory distress; however, a subset has been described with ABCA3 mutations presenting as ILD in later childhood, some of whom had transient neonatal symptoms.30,31,32 Histopathology indicates alveolar proteinosis and interstitial thickening, with ultrastructural evidence of small lamellar bodies with dense eccentrically positioned membranes, consistent with a surfactant lipid transport function for ABCA3.30
Data are limited regarding disease progression in older individuals and the potential to develop clinical fibrosis. However, an adolescent heterozygous for three novel variants of ABCA3 was the first well-documented case of a child with usual interstitial pneumonia, which was not previously thought to occur in children.32 A recent study identifies a modifier effect of ABCA3 mutation in individuals with a single specific SFTPC point mutation. The presence of the ABCA3 mutation with the SFTPC mutation increased the severity of clinical disease in comparison to individuals with only the SFTPC point mutation.33 This study highlights the possibility that idiopathic lung disease may result either from single mutations altering epithelial cell integrity or multiple gene mutations that disrupt different components of a common process.
Distinct mutations have been identified that alter ABCA3 structure, including nonsense mutations that prevent any ABCA3 production.34 Abca3−/− mice die of neonatal respiratory failure. Analysis of lungs of Abca3−/− mice demonstrates no detectable surfactant with impaired lamellar body formation, similar to lamellar body defects of patients.35 ABCA3 missense mutations have also been identified that likely generate a UPR.34 Thus the mechanisms of ABCA3-related ILD may also be multiple and complex, involving decreased surfactant production and cellular stress from UPR responses, similar to SFTPC-related ILD.
Relationship of Pulmonary Fibrosis to Herpesvirus Infection
Viral infection of the pulmonary epithelium is traditionally viewed as a transient injury. However, the capacity of some viruses to establish latent infection has been hypothesized to mediate a state of chronic or repetitive damage to the infected epithelium that eventually results in fibrosis. Herpesvirus family members can establish latency after acute infection, and various herpesviruses have been detected in tissue of patients with IPF. In one study, Epstein-Barr virus was found in lung tissue of almost half of patients with IPF, and a more recent study identified either Epstein-Barr virus, cytomegalovirus, human herpesvirus 7, human herpesvirus 8 (also known as Kaposi’s sarcoma herpesvirus) or combinations of these herpesviruses in the lungs of IPF patients.36,37,38 Herpesviruses were detected in the lungs of non-IPF patients at a lower prevalence. Herpesvirus infection (Epstein-Barr virus, cytomegalovirus, or human herpesvirus 8/Kaposi’s sarcoma herpesvirus) was detected by immunohistochemistry in the lungs of 15/23 individuals with IPF, with similar prevalence in sporadic, non-SFTPC-associated familial, and SFTPC-associated cases, but none was detected in controls. Notably, herpesvirus colocalized with cells expressing increased UPR markers. These results support the hypothesis that herpesvirus infections may be a modifier that exacerbates the severity of SP-C dysfunction disease, as well as an independent cause of disease.
The concept of persistent herpesvirus infection-induced IPF is supported by reports of gamma herpesvirus in equine pulmonary fibrosis and murine gamma herpesvirus (MHV-68)-induced lung fibrosis in interferon γ-deficient mice.39,40 Gamma herpesvirus infection also accentuates the severity of chemically-induced lung fibrosis in mice.41 Fibrosis following chronic viral exposure may also result in part from altered function of alveolar macrophages.
In murine MHV-68 infection models and human IPF lung tissue, macrophages found in fibrotic regions expressed markers of alternatively activated macrophages (AAMs).42,43 AAMs have reduced phagocytic and bactericidal activity and express genes consistent with a repair and remodeling phenotype. Markers associated with the AAM phenotype are better defined in mice, but both mouse and human AAMs express increased arginase 1 activity compared with classically activated macrophages.42,43 Arginine turnover by arginase 1 activity limits nitric oxide production and enhances production of collagen precursors used in wound healing processes. Arginase 1 activity is similarly induced in interstitial fibroblasts from the lungs of bleomycin-treated mice.42,44 Both tissue arginase 1 and AAM arginase 1 activity are increased by chemically induced and herpesvirus-associated fibrosis and may contribute to the aberrant accumulation of matrix.
The pathology of infants with SFPTC and ABCA3-related disease is frequently classified as desquamative interstitial pneumonia (DIP), indicative of a macrophage-dominated histopathology.34,45,46 Sftpc−/− mice have been reported to have an abnormal macrophage phenotype and impaired microbial killing and express markers consistent with an AAM phenotype.47 The relationship between macrophage activation patterns and epithelial dysfunction, and their role in the generation of disease is still unclear.
Section Summary
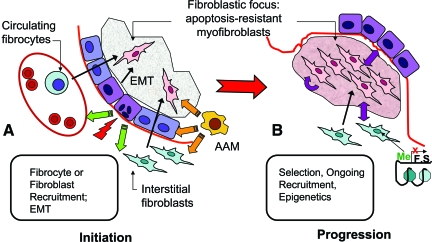
Disease arising from mutations and deficiencies of SP-C or ABCA3 is uniquely alveolar type II cell in origin, emphasizing the role of the epithelium in ILD/IPF. Type II cell injury from either SP-C or ABCA3 dysfunction may be sufficient to drive pathogenesis; alternatively, either of these deficiencies may render the alveolar epithelium more vulnerable to additional insults such as infection. Chronic herpesvirus infection linked to familial and SFTPC-related IPF supports the concept of an infectious process promoting ILD/IPF progression in the setting of alveolar epithelial compromise. Activation of macrophages in a manner identified with tissue repair rather than microbial protection suggests that epithelial cell stress may signal to the innate macrophage population in an effort to restore alveolar integrity. Thus signaling by impaired epithelial cells may be multidirectional, altering function of both fibroblasts and macrophages (Figure 1).
Figure 1.
Schematic representation of cellular and contextual abnormalities in pulmonary fibrogenesis (IPF fibroblastic focus). A: Fibrogenesis is initiated by injury to, infection of, or intrinsic abnormalities (eg, surfactant dysfunction mutations) within alveolar epihtelial cells. Capillary disruption results in deposition of fibrin-rich provisional matrix, into which fibroblasts (either resident interstitial fibroblasts, fibrocytes, or EMT-derived) migrate. Signals for perpetuation of fibrogenesis (eg, fibroblast proliferation, myofibroblastic differentiation, extracellular matrix remodeling) may arise from the altered epithelium, alternatively activated macrophages (AAM), composition and/or biomechanical properties of ECM, or the fibroblasts themselves, recapitulating developmental pathways and resulting in progression of fibrogenesis (B). Epigenetic alterations, such as DNA methylation or histone modifications, lead to silencing of fibrosis supressor (FS) genes, further promoting an apoptosis-resistant myofibroblast phenotype.
Origins and Phenotypic Regulation of Fibroblasts
Central Role of the Fibroblast in Fibrosis
Although evidence is mounting that the epithelium in many cases initiates and perpetuates fibrogenic signaling, the fibroblast is by definition the principal effector cell in fibrosis; its very name implies fibrogenesis. Persistence of fibroblasts in the altered extracellular matrix within damaged airspaces, and their excessive matrix deposition, exemplified in IPF by the histopathological lesions termed “fibroblastic foci,” are the features that correlate most clearly with poor outcomes.48,49
Fibroblasts are somewhat enigmatic cells in that they lack a universal marker. They are present in most tissues, particularly those with prominent epithelial and microvascular components (eg, skin, lung, liver, kidney). Often they are defined by their location in interstitial/mesenchymal compartments, and their elongated morphology. In tissue culture, they are often defined morphologically and by the absence of other specific markers.
Fibroblasts have significant roles in three principal areas in normal biology: development, tissue homeostasis, and wound healing. In organs and tissues which have a prominent epithelial derivation, such as the lung, kidney, liver and skin, fibroblasts are critical to normal development and function. Injury and repair in such tissues follows a fairly defined pathway. Epithelial and/or endothelial damage results in exudation of platelet-rich plasma into procoagulant-rich tissue spaces, where it forms a fibrinous clot (provisional matrix).50 Fibroblasts migrate into the provisional matrix, proliferate, and produce additional extracellular matrix components, such as fibronectin and collagen, resulting in fibroblast-populated granulation tissue. Simultaneously, fibroblasts acquire a myofibroblastic phenotype. The role of myofibroblastic differentiation, the acquisition by fibroblasts of smooth muscle-like phenotypic features such as expression of α-smooth muscle actin, in wound healing and fibrosis has been extensively reviewed recently.51,52 Normal repair depends on re-epithelialization, remodeling of provisional matrix, and eventual removal of fibroblasts, probably through apoptosis.53 Recent findings regarding the origins of fibroblasts in fibrotic tissues, and regulation of their differentiation will be considered in greater detail here.
Circulating Fibroblast Precursors
Multiple studies in the past decade have considered alternative origins of lesional fibroblasts in fibrosis in the lung and other tissues besides differentiation, proliferation, or migration of resident tissue fibroblasts. Bucala popularized the term fibrocyte to refer to circulating fibroblast precursors.54 The idea of circulating fibroblast precursors participating in wound healing dates to the 19th century,55 but the characteristics of this population of cells, their differentiation, and mechanisms of their recruitment have only recently been defined in numerous elegant studies.56,57,58,59
The contribution of fibrocytes to hypertrophic scars and keloids,60 scleroderma, renal fibrosis,61 airway remodeling in asthma,62,63 and experimental models of lung fibrosis59 have been well-described. A recent study demonstrated fibrocytes, defined predominantly by co-expression of CXCR4, a fibrocyte-associated chemokine receptor, with myofibroblast markers such as procollagen I, α-smooth muscle actin, and prolyl-4-hydroxylase, in lung tissue of 8/9 patients with lung fibrosis, but none in normal lungs.64 There was a positive correlation between the abundance of fibroblastic foci and the number of lung fibrocytes (r = 0.79; P < 0.02). However, the exact role of bone marrow-derived fibroblasts in IPF has not been definitively established.
Although cultured fibrocytes can be induced to differentiate into myofibroblasts in vitro, it is not clear in vivo that the bone marrow-derived cells recruited to the lung contribute to pathological fibrosis.59,65,66 In the setting of fibrogenic injury, bone-marrow derived mesenchymal stem cells may promote repair and thus ameliorate fibrosis.67,68 Previous studies had indicated that bone marrow-derived cells can become type II pneumocytes after hematopoietic stem cell transplantation.69 A large number of studies have followed, using different stem cell pools and different animal models, with varying results (reviewed in70). This phenomenon has been suggested to occur in humans undergoing sex-mismatched lung transplants as well, by demonstration of epithelial cells of recipient origin in donor lungs.71 Different populations of fibroblast precursors may be recruited to the lung after injury (eg, fibrogenic fibrocytes versus repair-promoting mesenchymal stem cells). Factors present in the local environment are likely to influence the differentiation of fibroblastic cells from multiple sources, as discussed further below. Additional studies are needed to clarify some of these controversies.
Epithelial-Mesenchymal Transition
During development, cells show remarkable phenotypic plasticity. In gastrulation, endodermal epithelial cells differentiate into mesenchyme, which subsequently contributes to the formation of germ layers.72 After this primary epithelial–mesenchymal transition (EMT), there are additional instances of EMT and also of mesenchymal–epithelial transition during development.72,73 The molecular changes that occur in EMT—loss of cell–cell adhesion, increased motility, cytoskeletal and morphological changes, and resistance to apoptosis—are also changes that occur during wound healing. Because there is similarity among phenotypic characteristics of epithelial and mesenchymal cells in development, wound healing, and fibrosis, it is not surprising that the molecular pathways which regulate EMT overlap significantly with those associated with fibrogenesis.
Numerous studies have characterized EMT in vitro. A549 cells, primary human and rat alveolar epithelial cells, and human bronchial epithelial cell lines can all be induced to undergo EMT in vitro.74,75 There is in vivo evidence of EMT in fibrogenesis as well. In both bleomycin-induced fibrosis and in the transforming growth factor (TGF)-β-overexpressing model, up-regulation of α-smooth muscle actin and vimentin in E-cadherin and surfactant-protein C-expressing cells, respectively, has been observed, suggesting EMT.75,76 In obliterative bronchiolitis following lung transplantation, localization of S100A4/fibroblast-specific protein, a mesenchymal marker, has been observed in bronchial epithelium; explanted bronchial epithelial cells have increased expression of matrix metalloproteinases 2 and 9 and are collagen-invasive, all consistent with EMT.77 Two studies have demonstrated colocalization of epithelial markers, such as thyroid transcription factor-1 or pro-surfactant protein B or C, with α-smooth muscle actin or N-cadherin in cells overlying fibroblastic foci in IPF.78,76 However, others have failed to detect dual expression of epithelial and mesenchymal markers in vivo in either clinical samples or the bleomycin model.79
An intriguing recent study of 30 cases of IPF and multiple disease controls demonstrates a unique and novel finding of cells with a bronchiolar basal cell phenotype in a layer between myofibroblasts and overlying alveolar or bronchial epithelium, perhaps introducing a novel cellular player into the field.80 These cells co-express markers associated with increased cellular motility (laminin-5 γ-2 and facsin), as well as markers of activation of the Wnt-β-catenin pathway, suggesting they may be undergoing EMT. However, the relative contribution of EMT to the pathogenesis of IPF is by no means clear. As fibrogenesis is considered to be a displaced or dysregulated repair process and many of the cellular and molecular events necessary for lung modeling during development are recapitulated during remodeling, the weight of evidence suggests that EMT is an important contributor to pulmonary fibrogenesis. Data from microarray analyses bear this out further, with enrichment of genes representative of multiple developmental pathways in IPF, including Wnt-β catenin, the TGFβ-bone morphogenetic protein family, and multiple developmental transcription factors.10
Fibroblast Heterogeneity
In addition to (or perhaps as a result of) the different possible origins of fibroblasts, it is clear that they exist in a remarkable range of phenotypes. Fibroblast heterogeneity has been the subject of extensive review in the past.81 Differences among subsets of normal lung fibroblasts have been characterized on the basis of size and shape, surface markers, cytoskeletal composition, lipid content, cytokine profile, and expression of cyclooxygenase 2 and telomerase.81,82 Fibroblasts from lungs with active fibrosis have increased proliferation, display anchorage-independent growth, and are morphologically distinct.83,84 Fibroblasts from fibrotic tissue also demonstrate enhanced migration and invasion of matrices, processes critical to their entering damaged airspaces.85,86
One of the most extensively studied in vitro models of fibroblast heterogeneity is based on the surface expression of the lipid raft glycoprotein Thy-1. Thy-1(−) and Thy-1(+) mouse lung fibroblasts are morphologically distinct, with Thy-1(−) being more rhomboid and compact, and Thy-1(+) having the characteristic spindle shape of normal fibroblasts with more abundant intracellular lipid and rough endoplasmic reticulum. The two subpopulations differ in cytokine and growth factor production, cytokine receptor expression, and expression of major histocompatibility complex (MCH) class II.87,88,89,90,91,92 Recent studies demonstrate that Thy-1 is an important modulator of latent TGF-β activation, myofibroblast differentiation, and survival.93,94 Thy-1 thus appears to function as a “fibrosis suppressor,” analogous to tumor suppressors. Another candidate fibrosis suppressor, which like Thy-1 affects lipid raft-associated signaling, is caveolin-1, which modulates multiple aspects of fibrogenic signaling. Its role in pulmonary fibrosis and scleroderma has been recently reviewed.95
Emerging Mechanisms of Regulation of Fibroblast Phenotype
Recently, clinical and laboratory data have generated renewed interest in the role of telomerase in fibroblasts.96 Telomeres are guanine-rich repeat sequences on the ends of chromosomes and are regulated by telomerase, a ribonucleoprotein complex with an RNA component (Terc, hTR) that serves as a template for addition of repeat sequences, and a reverse transcriptase catalytic subunit (Tert).97 The absence of telomerase leads to progressive telomere shortening with each round of cellular replication, resulting in a eventual loss of cellular viability characteristic of replicative senescence.
Telomerase is up-regulated in many malignancies. Terc null mice are initially phenotypically normal, but subsequent generations develop loss of fertility, a number of premature aging phenotypes, and decreased longevity.97 Induction of telomerase activity has been noted in rat lung fibroblasts in bleomycin-induced fibrosis.98 Dyskeratosis congenita is a rare disorder associated with mutations in telomerase genes, with skin manifestations, bone marrow failure, and some cases, usual interstitial pneumonia, often at an early age.99,100 Two recent studies demonstrated telomerase mutations (hTERT, hTR) in families with IPF and in some sporadic cases, associated with abnormal telomere length.101,102 Furthermore, telomerase (TERT)-deficient mice are protected from bleomycin-induced fibrosis; transplantation of wild-type bone marrow into TERT-null mice restores sensitivity to bleomycin, and transplantation of TERT-null bone marrow into wild-type mice is protective.103 This set of studies indicates that telomerase expression is required for the profibrotic fibroblast phenotype, and that bone marrow-derived cells have a critical role in bleomycin-induced fibrosis. Interestingly, patients with IIPs, including IPF, have shorter telomeres than age-matched controls even in the absence of telomerase mutations.104
Epigenetic alterations result in heritable changes in gene function without changes in the DNA sequence, thus offering an extra layer of transcriptional control regarding how, when, and where genes are expressed.105 Epigenetic regulation is important for the diversity of cell types arising during development, and is critical to maintaining the stability and integrity of expressed gene profiles. DNA methylation and chromatin modifications have been extensively studied in cancer research. Other epigenetic mechanisms, such as microRNAs and chromatin structural alterations, are increasingly recognized as critical to defining and maintaining cell phenotype.
There is growing evidence for epigenetic alterations in fibrotic diseases. Methylation of FLI1 is associated with increased collagen expression in scleroderma fibroblasts106; histone deacetylase 4 is required for TGF-β-induced myofibroblastic differentiation of skin fibroblasts.107 However, the extent to which epigenetic reprogramming is responsible for the multiple cellular phenotypic alterations in fibroblasts (or for that matter in other cell types) associated with pulmonary fibrosis has yet to be determined. A recent study demonstrated epigenetic silencing of Thy-1 by DNA hypermethylation specifically within fibroblastic foci in IPF, suggesting that this may be an important mechanism for pathogenic fibroblast alterations.108
MicroRNAs are single-stranded RNA molecules of 21 to 23 nucleotides in length that can be complementary to multiple mRNAs and induce silencing of multiple transcripts. They have been found to regulate reprogramming of gene expression in several types of cancer and in fibrosis in other organs, such as the heart.109 Specific microRNA genes are selectively expressed in the lung, implicating them as candidate regulatory factors in development or disease. Lung epithelial specific overexpression of the miR-17-92 microRNA cluster in transgenic mice stimulated epithelial cell proliferation, while impairing distal lung alveolarization.110 When the miR-223 gene was deleted in vivo, the mice developed a progressive pulmonary inflammation consisting of neutrophils that have an increased oxidative response to challenge.111 These findings indicate that microRNAs are an important component of pulmonary gene regulation; however, the role of microRNA in pulmonary fibrogenesis has yet to be determined.
Mechanisms controlling cellular phenotype such as epigenetic programming, in addition to being regulated temporally (as in development or aging) or environmentally (such as by nutrition or exposure to toxicants) may also be regulated contextually (such as by local biochemical or mechanical signals). A recent study supporting contextual programming applied microarrays and hierarchical clustering to characterize fibroblasts derived from 43 different anatomical sites, demonstrating marked heterogeneity of gene expression.112 Although there were general groupings indicating compartmentalization among anteroposterior, proximal–distal, and dermal–nondermal locations, there were also strong and specific location-restricted profiles such that, for example, forearm dermal fibroblasts had expression profiles more similar to adult lung fibroblasts than to lower leg dermal fibroblasts. Within organs there are also likely to be significant positional differences based on structural localization.113 Epigenetic mechanisms are believed to drive this context-dependent programming.112 Local biochemical signals are critical in contextual programming; for example, regional differences in oxygen tension are important in airway branching.114 Significant influence may also come from the composition or biophysical properties of the extracellular matrix, both of which in turn are altered by the cells themselves.
A large and growing literature, largely from the cancer field, demonstrates the effects of matrix composition and stiffness on cell behavior (reviewed in115,116). A dramatic example of the role of cell–matrix biophysical interactions in regulating phenotype demonstrated that constraining individual mesenchymal stem cells on either a large (10,000 μm2) or small (1024 μm2) surface area of fibronectin promoted differentiation into osteoblast or adipocyte lineage, respectively, even in the absence of classic differentiation-promoting factors.117 Prostaglandin E2 was recently shown to inhibit TGF-β1-induced myofibroblast differentiation through cell shape and adhesion-dependent signaling.118 In addition, fibroblasts from IPF patients elude the normal antiproliferative effects of polymerized collagen through alterations in integrin-dependent signaling.119 Normal myofibroblasts undergo apoptosis within contracting collagen gels; fibroblasts lacking Thy-1 escape this mechanism, which is restored on transfection of Thy-1.93
Myofibroblast differentiation has been demonstrated to be maximized in the presence of both active TGFβ and mechanical tension.51 A related study showed that myofibroblast contraction mediates latent TGF-β1 activation through a direct biophysical alteration of the matrix where it is sequestered.120 Focal adhesion kinase and focal adhesion kinase-related non-kinase, which are critical regulators of cytoskeletal organization downstream of matrix binding, have opposing effects on TGFβ-induced myofibroblastic differentiation.121 A recent intriguing finding is that of genome-wide alterations in translational control in IPF fibroblasts, downstream of aberrant integrin signaling.122
Section Summary
The origin and regulation of fibrogenic myofibroblasts in IPF and other fibrotic disorders are complex and variable. Local structural fibroblasts, those arising through transdifferentiation of other cells, and those recruited from bone marrow or circulating progenitors, are all subject to reprogramming by a number of mechanisms, resulting in developmental or wound-healing expression repertoires that may self-perpetuate, resulting in aberrant fibrogenesis (Figure 1). The immediate biochemical/biomechanical context within fibrotic lesions, and the cell’s ability both to respond to and alter that context, significantly affect fibroblast differentiation, persistence, and survival.
Signaling Pathways and Programming Paradigms
Fibrogenic Signaling: Lessons from Animal Models
Multiple rodent models have been established that recapitulate mechanisms leading to pulmonary fibrosis. Most involve the administration of drugs, chemical compounds, irradiation, or infections.123 Administration of bleomycin through a number of routes to rodents is among the earliest and most widely published methods, causing a transient, inhomogeneous fibrotic response associated with early inflammatory infiltration of macrophages and lymphocytes.124 The use of this model to test antifibrotic interventions has been recently reviewed, demonstrating its very limited ability to predict clinical responses in humans, and underscoring the important differences between bleomycin-induced fibrosis and human IIPs.125 Instillation of other chemicals, including fluorescein isothiocyanate, asbestos fibers, or silica particles, into rodent lungs also results in chronic and progressive inflammation with fibrosis.126,127 In addition, radiation-induced pulmonary fibrosis results in a more uniform cellular injury compared to drug- or chemical-induced lung injury. Within 1 month following irradiation, inflammatory cells are recruited into the air spaces and are closely associated with fibrotic lesions.128 Also, mice defective in interferon-gamma receptor signaling chronically infected with the murine γ-herpesvirus 68, a virus that is closely related to Epstein-Barr virus and human herpesvirus 8, develop progressive interstitial lung fibrosis associated with a robust inflammatory response, as well as enhanced fibrotic responses to bleomycin or fluorescein isothiocyanate.123,129
Limited Role of Inflammation per se and Pre-Eminence of TGF-β
Injury models use agents known to cause pathological disease in humans, and have identified many cells, pathways, and a vast number of chemokines, cytokines, and growth factors that mediate pulmonary fibrosis. The disadvantage of these models is their reliance on acute injury leads to a broad inflammatory response, which is not characteristic of IPF or many other IIPs.
Replication-deficient adenoviral vectors containing cDNAs of specific genes have been transferred to the lung epithelium of rodents to mimic these diseases. Expression of the chemokines macrophage inflammatory protein-2, RANTES, IP-10, monokine induced by gamma interferon and lymphotactin all resulted in marked increases in bronchoalveolar lavage inflammatory cells and tissue pneumonitis, but did not induce lung fibrosis or residual remodeling.130,131,132,133 The cytokines tumor necrosis factor-α, granulocyte macrophage colony-stimulating factor, and interleukin-1β also induced an acute inflammatory response with variable degrees of alveolar destruction,134,135,136 along with a graded fibrotic response ranging from marginal (tumor necrosis factor α) to severe (interleukin-1β). The degree of fibrosis in these models was found to correlate directly to both the amount and duration of expression of active TGFβ1.137 Confirmation of the critical role of TGFβ1 in fibrogenesis was demonstrated by overexpression of active TGFβ1 in rat lung, resulting in prolonged and severe interstitial and pleural fibrosis.138 TGFβ1-induced fibrosis developed and progressed without extensive inflammation, and was not induced by expression of the latent form.138 Together these studies demonstrate that induction of lung fibrosis is not directly dependent on the degree or characterization of the inflammatory response, but rather, the amount and length of TGFβ1 induced downstream of cytokine signaling.
Further evidence supporting the important role of TGFβ1 in modulating the fibrotic response has been demonstrated by other transgenic models. Overexpression of the cytokine IL-13 using the Clara cell secretory protein regulatable airway epithelial promoter led to a marked inflammatory response with airway and parenchymal fibrosis.139 IL-13-induced fibrosis was significantly ameliorated by treatment with a TGFβ antagonist, demonstrating that the fibrotic effects of IL-13 are mediated to a great extent through the TGFβ1 pathway.140 In addition, there is a balance between TGFβ and bone morphogenetic proteins, which appears to be disrupted in some animal models and in human IPF by expression of Gremlin, a bone morphogenetic protein antagonist that is up-regulated by TGFβ in a MAPK-dependent manner. Restoration of bone morphogenetic protein-7 signaling inhibits asbestos-induced fibrosis in mice.141
TGF-β Downstream Signaling
Collectively, studies in both viral-delivered and pulmonary-specific transgenic models demonstrate that TGFβ is a key modulator of lung fibrosis and support targeting TGFβ pathways to prevent or reverse fibrosis. Responses elicited by TGFβ are dependent on and specific for the target cell lineage and are classically mediated by intracellular signaling via Smad proteins. In mesenchymal cells, the fibrotic effects of TGFβ depend on active TGFβ release from the matrix-bound latent complex. Factors which activate latent TGFβ include thrombospondin and the integrin αvβ6.142,143 Once activated, TGFβ family members initiate signaling by interacting with and complexing the TGFβ type II receptors and the TGFβ Type I receptor (ALK5). Smad2 and Smad3 proteins then associate with the activated receptor and become phosphorylated, allowing the formation of an oligomeric complex with Smad4. This complex translocates into the nucleus and binds to specific nucleotide motifs to regulate transcription of target genes. Multiple studies in fibroblasts have established that the Smad pathways modulate TGFβ-induced cellular processes associated with lung fibrosis including enhanced collagen synthesis, proliferation, migration, adhesion, and transdifferentiation into myofibroblasts.144,145 The role of Smad signaling in TGFβ-driven fibrosis has been demonstrated in vivo using Smad3 null mice, which are resistant to TGFβ1 mediated pulmonary fibrosis.146 Furthermore, administration of a selective kinase inhibitor of Alk5 prevents the induction of fibrosis from adenovirus-mediated gene transfer of active TGFβ1, and also blocks progressive fibrosis when administered transiently to rats with established fibrosis.147
Although the Smad pathway is believed to be the primary conduit for signals from the TGFβ1 receptors, emerging evidence highlights the importance of non-Smad pathways. Fibroblasts stimulated with TGFβ1 demonstrate Smad-independent proliferation and matrix protein production which are regulated by mitogen-activated protein kinase and phosphoinositide 3-kinase (PI3K) pathways.145,148,149 c-Albelson is a proto-oncogene regulated by PI3K; TGFβ stimulates c-Albelson kinase activity in fibroblasts independent of Smad2 and Smad3 phosphorylation.150 Loss of c-Albelson kinase prevents TGFβ-mediated stimulation of extracellular matrix gene expression and cell proliferation. In vivo, transgenic mice that conditionally over express TGFβ1 in the lung epithelium develop epithelial apoptosis and extensive inflammation followed by progressive lung fibrosis.151 Mice that are deficient in the semaphorin 7A, a protein believed to function in angiogenesis, apoptosis, and immune responses, when crossed with TGFβ1 overexpressing mice, develop markedly less fibrosis and alveolar remodeling, despite TGFβ1 activation of Smad proteins.152 Together, these findings indicate that TGFβ1-initiated cellular responses are regulated by relative contributions and cross talk between both Smad-dependent and Smad-independent signaling pathways.
Non-TGF-β Pathways to Fibrogenesis
Recent studies have shown that fibrosis and lung remodeling may also develop independently of TGFβ1 signaling. Mice exposed to house dust mite antigen and concurrently treated with a pan-neutralizing anti-TGFβ antibody developed airway remodeling comparable with mice exposed to house dust mite and treated with irrelevant antibody control.153 Similarly, house dust mite-exposed Smad 3 knockout mice developed remodeling to the same extent as house dust mite-exposed wild-type littermate control mice.
TGFα binds to the epidermal growth factor receptor. Overexpression of TGFα using the regulatable Clara cell secretory protein promoter causes progressive and extensive pulmonary fibrosis characterized by pleural, perivascular, and peribronchial matrix deposition. Fibrosis in this transgenic model develops and progresses in the absence of detectable inflammation or activation of TGFβ.154 Oncostatin-M, an inflammatory cytokine that is elevated in IPF BALF, causes exuberant inflammation and fibrosis in an animal model. Interestingly, the oncostatin-M-mediated fibrosis appears independent of both inflammation and TGF-β signaling.155
Potential Downstream Confluence of Signaling Pathways
As fibrosis is likely heterogeneous in etiology and molecular pathophysiology, attempts to block or counteract single pathways may not be sufficient to inhibit cellular processes associated with fibrosis. Ongoing studies have identified potential points of confluence, where multiple inputs eventually converge to elicit the cellular response of mesenchymal proliferation and matrix deposition.
PI3K is a signal transduction enzyme that catalyzes the phosphorylation of phosphatidylinositol (4,5)-biphosphate to form phosphatidylinositol (3,4,5)-triphosphate in response to activation of receptor tyrosine kinase, G-protein coupled receptors or cytokine receptors. Activation of PI3K is essential to a number of cellular processes associated with fibrogenesis including cell growth, proliferation, migration, survival, and collagen gene expression.156 The tumor suppressor phosphatase and tensin homolog is a negative growth regulator of the PI3K-Akt pathway, for which baseline activity is believed to be constitutively high. Both cell proliferation and collagen production are up-regulated in lung fibroblasts deficient in phosphatase and tensin homolog.157 Moreover, in phosphatase and tensin homolog-haploinsufficient mice, using both cutaneous wound healing and bleomycin-induced lung injury models, deficiency in phosphatase and tensin homolog results in a more durable fibroproliferative response.119 Further evidence supporting the PI3K pathway mediating lung fibrosis is demonstrated in transgenic models. Pulmonary fibrosis in the regulatable TGFβ1 transgenic model was significantly attenuated when mice were treated with an Akt inhibitor.152 The PI3K-Akt pathway is also activated in the regulatable TGFα transgenic model, and lung fibrosis in this model is prevented when mice are treated with a PI3K-specific inhibitor (WH, unpublished observations). The platelet derived growth factor family, another profibrotic cytokine group implicated in inflammatory models of lung fibrosis,158,159 also activates PI3K.160 These data further support the PI3K as a common pathway where multiple cytokines synergistically function or become confluent.
While emerging data supports targeting common signaling pathways such as PI3K-Akt, the plethora of cellar processes mediated and potentially affected by broad signaling inhibitors poses significant challenges.161 Therefore, more specific downstream effectors involved in matrix synthesis and proliferation may be better therapeutic targets. The mammalian target of rapamycin (mTOR) is a highly conserved intracellular serine/threonine kinase and a major downstream component of the PI3K pathway.162 Inhibitors of mTOR, such as rapamycin, bind to an intracellular cytoplasmic receptor, the FK506-binding protein-12.162 The complex formed then interacts and disrupts mTOR function and leads to cell cycle arrest in the G1 phase. In addition to blocking cell proliferation, mTOR inhibitors have been identified with anti-inflammatory, anti-tumor, and anti-fibrotic properties.
The role of mTOR in fibrosis has been demonstrated in vivo in rodent models of renal fibrosis and cirrhosis where rapamycin treatment has provided effective in either reversing or preventing fibrosis.149,163 In the lung, the rapamycin analog SDZ RAD reduced bleomycin-induced pulmonary fibrosis by 75% compared to vehicle control.164 In the TGFα transgenic model, rapamycin prevented development of epidermal growth factor receptor-induced fibrosis as assessed by lung histology, lung collagen content, and changes in lung mechanics.165 Activation of mTOR leads to interaction with downstream effectors such as p70 ribosomal S6 kinase (S6K) and eukaryotic initiation factor-4E-binding protein-1. Through these pathways, mTOR controls cellular growth, proliferation and translation. Both S6K and 4E-binding protein-1 possess several phosphorylation sites that are points of convergence of several pathways including not only mTOR, but also PI3K and MAPK.162,166 Future research targeting S6K and 4E-binding protein-1 phosphorylation in fibrosis models will be needed to determine whether these proteins represent effective and specific therapeutic targets.
Recent studies have underscored the role of transcription factors in the regulation of fibrogenesis. The transcription factors Fra-2/AP-1 have been shown to be profibrogenic in lung, in part by regulating coordinated expression of non-TGF-β fibrogenic mediators.167 Tumor necrosis factor-α induces expression and DNA binding of AP-1 in fibroblasts, resulting in increased transcription of the TGF-beta 1 gene.168 Additional studies are needed to further define the roles and hierachies of transcription factors in regulating the “fibrogenic transcriptome” IPF and other IIPs.
Section Summary
Animal models of pulmonary fibrosis have revealed multiple cytokines and growth factors leading to fibrosis through a number of different pathways including TGFβ (Smad dependent and non-Smad dependent) and non-TGFβ pathways. However, to date there is no single model that duplicates the correct temporal, spatial, and dynamic aspects of human fibrotic disease.169 Currently, methods to identify specific pathways causing fibrosis in human disease are limited to “footprints” of molecular activation, such as immunostaining for phosphorylated signaling intermediates. Development of biomarkers for assessment of activation of cellular and molecular pathways associated with fibrogenesis is an active area of investigation. Identification of common downstream “funneling” factors where signals converge is likely to provide optimal therapeutic targets that will allow treatment of fibrosis regardless of the upstream initiating events.
Summary and Future Directions
Recent studies have added significantly to our understanding of pulmonary fibrogenesis. Emerging concepts include the critical role of the epithelium, particularly type II pneumocytes, in the initiation and perpetuation of fibrosis, in response to either endogenous or exogenous stress; a growing awareness of alternative activation of macrophages in tissue remodeling; growing appreciation of the alternative origins and phenotypic plasticity of fibroblasts; the roles of epigenetic reprogramming and context-dependent signaling in profibrotic phenotype alterations; and recognition of the importance of cross talk and convergence of intracellular signaling pathways in designing antifibrotic interventions. Remarkable overlap exists in molecular and cellular programming among normal development, wound healing, fibrogenesis, and cancer,170 such that careful attention to novel findings in related fields is warranted. Overall, the paradigm that emerges is one of “disordered re-development” of the lung. Only by careful analysis of the developmental context, understanding of the key molecular “players” and mechanisms of context disruption, and by using a coordinated, multidisciplinary approach, can we hope to discover the critical points of convergence and begin to restore order and function.
Acknowledgments
We thank Ms. Elaina Harris and Ms. Cassie Woodley for expert assistance with manuscript preparation.
Footnotes
Address reprint requests to James S. Hagood, M.D., Pediatrics (Pulmonary Division), University of Alabama-Birmingham, 1918 University Blvd., 648 A VH, Birmingham AL 35294-0019. E-mail: jhagood@uab.edu.
Supported in part by NIH grants HL086598 (to W.D.H.), HL50046 and HL061646 (to S.G.), and HL 082818 (to J.S.H.).
References
- Atlanta: American Cancer Society; Cancer Facts & Figures. 2008 [Google Scholar]
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Ferrans VJ, Basset F. Biologic Basis of Pulmonary Fibrosis. Crystal RG, editor. New York: Raven Press,; 1991:pp. 2031–2057. [Google Scholar]
- Mapel DW, Samet JM, Coultas DB. Corticosteroids and the treatment of idiopathic pulmonary fibrosis. Past, present, and future. Chest. 1996;110:1058–1067. doi: 10.1378/chest.110.4.1058. [DOI] [PubMed] [Google Scholar]
- Clark RA. The commonality of cutaneous wound repair and lung injury. Chest. 1991;99:57S–60S. doi: 10.1378/chest.99.3_supplement.57s. [DOI] [PubMed] [Google Scholar]
- Rennard SI. Repair mechanisms in asthma. J Allergy Clin Immunol. 1996;98:S278–S286. doi: 10.1016/s0091-6749(96)70076-3. [DOI] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med. 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- Adamson IYR, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Path. 1990;137:385–392. [PMC free article] [PubMed] [Google Scholar]
- Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol. 2002;283:L510–L517. doi: 10.1152/ajplung.00144.2002. [DOI] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol. 2007;292:L608–L611. doi: 10.1152/ajplung.00379.2006. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol. 2004;66:601–623. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem. 2003;278:52739–52746. doi: 10.1074/jbc.M309599200. [DOI] [PubMed] [Google Scholar]
- Amin RS, Wert SE, Baughman RP, Tomashefski JF, Jr, Nogee LM, Brody AS, Hull WM, Whitsett JA. Surfactant protein deficiency in familial interstitial lung disease. J Pediatr. 2001;139:85–92. doi: 10.1067/mpd.2001.114545. [DOI] [PubMed] [Google Scholar]
- Tredano M, Griese M, Brasch F, Schumacher S, de Blic J, Marque S, Houdayer C, Elion J, Couderc R, Bahuau M. Mutation of SFTPC in infantile pulmonary alveolar proteinosis with or without fibrosing lung disease. Am J Med Genet A. 2004;126A:18–26. doi: 10.1002/ajmg.a.20670. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, Collins RD, Hull WM, Glasser SW, Whitsett JA, Blackwell TS. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol. 2005;167:1267–1277. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Wert SE, Nogee LM. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin Perinatol. 2006;30:327–334. doi: 10.1053/j.semperi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172:1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res. 2007;62:176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, Fisher AB, Savani RC, Shuman H. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax. 1995;50:1234–1239. doi: 10.1136/thx.50.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1999;159:1336–1341. doi: 10.1164/ajrccm.159.4.9807077. [DOI] [PubMed] [Google Scholar]
- Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41:2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Maes R, Del Piero F, Lim A, Wise A, Bolin DC, Caswell J, Jackson C, Robinson NE, Derksen F, Scott MA, Uhal BD, Li X, Youssef SA, Bolin SR. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol. 2007;44:849–862. doi: 10.1354/vp.44-6-849. [DOI] [PubMed] [Google Scholar]
- Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L711–L721. doi: 10.1152/ajplung.00007.2005. [DOI] [PubMed] [Google Scholar]
- McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med. 2008;177:771–780. doi: 10.1164/rccm.200708-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:466–473. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan B, Hoeve MA, Allen JE, Ebrahimi B, Rhind SM, Dutia BM, Nash AA. Murine gammaherpesvirus-induced fibrosis is associated with the development of alternatively activated macrophages. J Leukoc Biol. 2008;84:50–58. doi: 10.1189/jlb.0507270. [DOI] [PubMed] [Google Scholar]
- Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, Bulau P, Eickelberg O. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L34–L45. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
- Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- Bruder E, Hofmeister J, Aslanidis C, Hammer J, Bubendorf L, Schmitz G, Rufle A, Buhrer C. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod Pathol. 2007;20:1009–1018. doi: 10.1038/modpathol.3800928. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Senft AP, Whitsett JA, Maxfield MD, Ross GF, Richardson TR, Prows DR, Xu Y, Korfhagen TR. Macrophage dysfunction and susceptibility to pulmonary Pseudomonas aeruginosa infection in surfactant protein C-deficient mice. J Immunol. 2008;181:621–628. doi: 10.4049/jimmunol.181.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay SE, Kazerooni EA, Toews GB, Lynch JP, 3rd, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, Popovich J, Hyzy R, Martinez FJ. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157:1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Clark RAF, editor. New York: Plenum Press; Wound RepairOverview and General Considerations. 1996:p. pp. 3–50. [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Olman MA. Muscle fatigue: mK2 signaling and myofibroblast differentiation. Am J Respir Cell Mol Biol. 2007;37:503–506. doi: 10.1165/rcmb.2007-0005ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Levrey H, Dahm C, Polunovsky VA, Rubins J, Bitterman PB. Lovastatin induces fibroblast apoptosis in vitro and in vivo. A possible therapy for fibroproliferative disorders. Am J Respir Crit Care Med. 1999;159:220–227. doi: 10.1164/ajrccm.159.1.9802104. [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Dunphy JE. The fibroblast–a ubiquitous ally for the surgeon. N Engl J Med. 1963;268:1367–1377. [Google Scholar]
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, Huang KH, Ko YS, Chung KF, Kuo HP. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees JL, Pociask DA, Sullivan DE, Whitney MJ, Lasky JA, Prockop DJ, Brody AR. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:385–394. doi: 10.1164/rccm.200607-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114:99–108. doi: 10.1042/CS20070073. [DOI] [PubMed] [Google Scholar]
- Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, Xu J. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res. 2007;8:1. doi: 10.1186/1465-9921-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C, Forrest IA, Murphy DM, Johnson GE, Robertson H, Cawston TE, Fisher AJ, Dark JH, Lordan JL, Kirby JA, Corris PA. Phenotype of airway epithelial cells suggests epithelial to mesenchymal cell transition in clinically stable lung transplant recipients. Thorax. 2005;60:865–871. doi: 10.1136/thx.2005.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, Kasper M. Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol. 2008;129:453–462. doi: 10.1007/s00418-008-0388-9. [DOI] [PubMed] [Google Scholar]
- Chilosi M, Zamo A, Doglioni C, Reghellin D, Lestani M, Montagna L, Pedron S, Ennas MG, Cancellieri A, Murer B, Poletti V. Migratory marker expression in fibroblast foci of idiopathic pulmonary fibrosis. Respir Res. 2006;7:95. doi: 10.1186/1465-9921-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps RP. Boca Raton, FL: CRC Press,; Pulmonary Fibroblast Heterogeneity. 1992 [Google Scholar]
- Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S87–S92. [PubMed] [Google Scholar]
- Worrall JG, Whiteside TL, Prince RK, Buckingham RB, Stachura I, Rodnan GP. Persistence of scleroderma-like phenotype in normal fibroblasts after prolonged exposure to soluble mediators from mononuclear cells. Arthritis Rheum. 1986;29:54–64. doi: 10.1002/art.1780290108. [DOI] [PubMed] [Google Scholar]
- Torry DJ, Richards CD, Podor TJ, Gauldie J. Anchorage-independent colony growth of pulmonary fibroblasts derived from fibrotic human lung tissue. J Clin Invest. 1994;93:1525–1532. doi: 10.1172/JCI117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma H, Sato A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax. 1995;50:984–989. doi: 10.1136/thx.50.9.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am. 2003;29:371–390. doi: 10.1016/s0889-857x(03)00025-5. [DOI] [PubMed] [Google Scholar]
- Phipps RP, Penney DP, Keng P, Quill H, Paxhia A, Derdak S, Felch ME. Characterization of two major populations of lung fibroblasts: distinguishing morphology and discordant display of Thy 1 and class II MHC. Am J Respir Cell Mol Biol. 1989;1:65–74. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- Phipps RP, Penney DP, Keng P, Silvera M, Harkins S, Derdak S. Immune functions of subpopulations of lung fibroblasts. Immunol Res. 1990;9:275–286. doi: 10.1007/BF02935527. [DOI] [PubMed] [Google Scholar]
- Penney DP, Keng PC, Derdak S, Phipps RP. Morphologic and functional characteristics of subpopulations of murine lung fibroblasts grown in vitro. Anat Rec. 1992;232:432–443. doi: 10.1002/ar.1092320312. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–3614. [PubMed] [Google Scholar]
- Hagood JS, Mangalwadi A, Guo B, MacEwen MW, Salazar L, Fuller GM. Concordant and discordant interleukin-1-mediated signaling in lung fibroblast thy-1 subpopulations. Am J Respir Cell Mol Biol. 2002;26:702–708. doi: 10.1165/ajrcmb.26.6.4547. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Miller PJ, Lasky JA, Tousson A, Guo B, Fuller GM, McIntosh JC. Differential expression of platelet-derived growth factor-alpha receptor by Thy-1(−) and Thy-1(+) lung fibroblasts. Am J Physiol. 1999;277:L218–L224. doi: 10.1152/ajplung.1999.277.1.L218. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(−) lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36:226–235. doi: 10.1165/rcmb.2006-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-{beta} in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–669. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713–719. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Kaneko A. Voltage-gated sodium currents in isolated retinal ganglion cells of the cat: relation between the inactivation kinetics and the cell type. Neurosci Res. 1991;11:261–275. doi: 10.1016/0168-0102(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Chang S. Modeling aging and cancer in the telomerase knockout mouse. Mutat Res. 2005;576:39–53. doi: 10.1016/j.mrfmmm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- Mason PJ, Wilson DB, Bessler M. Dyskeratosis congenita – a disease of dysfunctional telomere maintenance. Curr Mol Med. 2005;5:159–170. doi: 10.2174/1566524053586581. [DOI] [PubMed] [Google Scholar]
- Utz JP, Ryu JH, Myers JL, Michels VV. Usual interstitial pneumonia complicating dyskeratosis congenita. Mayo Clin Proc. 2005;80:817–821. doi: 10.1016/S0025-6196(11)61538-3. [DOI] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, Hagood JS. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Tsue I, Han RNN, Smith BT. Spatial and temporal differences in fibroblast behavior in fetal rat lung, Am Phys Soc. 1991:424–433. doi: 10.1152/ajplung.1991.261.6.L424. [DOI] [PubMed] [Google Scholar]
- Jarecki J, Johnson E, Krasnow MA. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell. 1999;99:211–220. doi: 10.1016/s0092-8674(00)81652-9. [DOI] [PubMed] [Google Scholar]
- Makale M. Cellular mechanobiology and cancer metastasis. Birth Defects Res C Embryo Today. 2007;81:329–343. doi: 10.1002/bdrc.20110. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293:L417–L428. doi: 10.1152/ajplung.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem. 2008;283:26839–26849. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS ONE. 2008;3:e3220. doi: 10.1371/journal.pone.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- Phan SH, Thrall RS, Ward PA. Bleomycin-induced pulmonary fibrosis in rats: biochemical demonstration of increased rate of collagen synthesis. Am Rev Respir Dis. 1980;121:501–506. doi: 10.1164/arrd.1980.121.3.501. [DOI] [PubMed] [Google Scholar]
- Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SN, Howie SE, Wallace WA, Brown DM, Lamb D, Ramage EA, Donaldson K. A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol. 1995;176:309–318. doi: 10.1002/path.1711760313. [DOI] [PubMed] [Google Scholar]
- Davis GS, Leslie KO, Hemenway DR. Silicosis in mice: effects of dose, time, and genetic strain. J Environ Pathol Toxicol Oncol. 1998;17:81–97. [PubMed] [Google Scholar]
- Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation-induced pulmonary fibrosis: examination of chemokine and chemokine receptor families. Radiat Res. 2002;157:256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lok SS, Haider Y, Howell D, Stewart JP, Hasleton PS, Egan JJ. Murine gammaherpes virus as a cofactor in the development of pulmonary fibrosis in bleomycin resistant mice. Eur Respir J. 2002;20:1228–1232. doi: 10.1183/09031936.02.00272902. [DOI] [PubMed] [Google Scholar]
- Emtage PC, Xing Z, Wan Y, Zlotnik A, Graham FL, Gauldie J. Adenoviral-mediated gene transfer of lymphotactin to the lungs of mice and rats results in infiltration and direct accumulation of CD4+. CD8+, and NK cells. J Interferon Cytokine Res. 2002;22:573–582. doi: 10.1089/10799900252982052. [DOI] [PubMed] [Google Scholar]
- Palmer K, Emtage PC, Strieter RM, Gauldie J. Transient gene transfer of non-ELR chemokines to rodent lung induces mononuclear cell accumulation and activation. J Interferon Cytokine Res. 1999;19:1381–1390. doi: 10.1089/107999099312849. [DOI] [PubMed] [Google Scholar]
- Braciak TA, Bacon K, Xing Z, Torry DJ, Graham FL, Schall TJ, Richards CD, Croitoru K, Gauldie J. Overexpression of RANTES using a recombinant adenovirus vector induces the tissue-directed recruitment of monocytes to the lung. J Immunol. 1996;157:5076–5084. [PubMed] [Google Scholar]
- Foley R, Driscoll K, Wan Y, Braciak T, Howard B, Xing Z, Graham F, Gauldie J. Adenoviral gene transfer of macrophage inflammatory protein-2 in rat lung. Am J Pathol. 1996;149:1395–1403. [PMC free article] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Braciak T, Ohkawara Y, Sallenave JM, Foley R, Sime PJ, Jordana M, Graham FL, Gauldie J. Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation. J Leukoc Biol. 1996;59:481–488. doi: 10.1002/jlb.59.4.481. [DOI] [PubMed] [Google Scholar]
- Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9:39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, Lawler J. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9:421–437. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O. Endless healing: TGF-beta, SMADs, and fibrosis. FEBS Lett. 2001;506:11–14. doi: 10.1016/s0014-5793(01)02875-7. [DOI] [PubMed] [Google Scholar]
- Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- Martinez-Salgado C, Fuentes-Calvo I, Garcia-Cenador B, Santos E, Lopez-Novoa JM. Involvement of H- and N-Ras isoforms in transforming growth factor-beta1-induced proliferation and in collagen and fibronectin synthesis. Exp Cell Res. 2006;312:2093–2106. doi: 10.1016/j.yexcr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Jr, Lonning S, Gauldie J, Jordana M. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med. 2008;177:593–603. doi: 10.1164/rccm.200706-958OC. [DOI] [PubMed] [Google Scholar]
- Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L741–L749. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- Mozaffarian A, Brewer AW, Trueblood ES, Luzina IG, Todd NW, Atamas SP, Arnett HA. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol. 2008;181:7243–7253. doi: 10.4049/jimmunol.181.10.7243. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, Toews GB. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10). Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. Am J Pathol. 1999;155:213–221. doi: 10.1016/S0002-9440(10)65115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, Huber PE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers CL. Will kinase inhibitors have a dark side? N Engl J Med. 2006;355:313–315. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]
- Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, Chen Y, Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol. 2007;27:495–502. doi: 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, Chambers RC, Egan JJ. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002;19:1124–1127. doi: 10.1183/09031936.02.00281602. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, Hardie WD: Rapamycin prevents transforming growth factor-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2009, doi:10.1165/rcmb.2008-0377OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Eferl R, Hasselblatt P, Rath M, Popper H, Zenz R, Komnenovic V, Idarraga MH, Kenner L, Wagner EF. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci USA. 2008;105:10525–10530. doi: 10.1073/pnas.0801414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR: TNF-alpha induces TGF-beta(1) expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med 2009, doi:10/1111/j-1582-4934.2008.00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J, Kolb M. Animal models of pulmonary fibrosis: how far from effective reality? Am J Physiol Lung Cell Mol Physiol. 2008;294:L151. doi: 10.1152/ajplung.00520.2007. [DOI] [PubMed] [Google Scholar]
- Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]