Abstract
Protein S-glutathionylation (PSSG) is a posttranslational modification that involves the conjugation of the small antioxidant molecule glutathione to cysteine residues and is emerging as a critical mechanism of redox-based signaling. PSSG levels increase under conditions of oxidative stress and are controlled by glutaredoxins (Grx) that, under physiological conditions, preferentially deglutathionylate cysteines and restore sulfhydryls. Both the occurrence and distribution of PSSG in tissues is unknown because of the labile nature of this oxidative event and the lack of specific reagents. The goal of this study was to establish and validate a protocol that enables detection of PSSG in situ, using the property of Grx to deglutathionylate cysteines. Using Grx1-catalyzed cysteine derivatization, we evaluated PSSG content in mice subjected to various models of lung injury and fibrosis. In control mice, PSSG was detectable primarily in the airway epithelium and alveolar macrophages. Exposure of mice to NO2 resulted in enhanced PSSG levels in parenchymal regions, while exposure to O2 resulted in minor detectable changes. Finally, bleomycin exposure resulted in marked increases in PSSG reactivity both in the bronchial epithelium as well as in parenchymal regions. Taken together, these findings demonstrate that Grx1-based cysteine derivatization is a powerful technique to specifically detect patterns of PSSG expression in lungs, and will enable investigations into regional changes in PSSG content in a variety of diseases.
Oxidants have long been implicated in the pathophysiology of diverse diseases, and highly reactive oxidants have been demonstrated to cause damage to macromolecules. However, a wealth of recent data has demonstrated that oxidants, such as nitric oxide, and hydrogen peroxide are also produced and used in physiological settings as signaling molecules. This signaling function of oxidants is performed via reversible oxidations of targeted cysteine residues within proteins.1 Specifically, cysteines with low-pKa sulfhydryl groups (known as thiolates, or reactive cysteines) are targets for reversible oxidations by hydrogen peroxide or S-nitrosothiols (functional derivatives of nitric oxide), and virtually every major class of proteins contain reactive cysteines that are highly conserved across species. Oxidation of these reactive cysteine groups can cause a change in protein structure and/or function.1 The low-molecular-weight ubiquitous antioxidant molecule glutathione acts primarily to maintain the reduced state of cellular protein thiol groups. Under various conditions of oxidative stress or redox-based signaling, glutathione (GSH) will become covalently bound to reactive cysteines within proteins, a scenario known as protein S-glutathionylation (also referred to as S-glutathiolation, mixed disulfides, or PSSG), and can confer protection against further irreversible oxidations.2,3,4,5,6 The exact biochemical events that lead to S-glutathionylation remain unclear, and have been speculated to involve the formation of sulfenic acid, S-nitrosothiol, sulfenylamide, or thiyl radical intermediates, among other events.4,5
The formation of PSSG has come to the forefront of redox-based signaling, as it has been shown that several signaling proteins that include kinases, phosphatases, proteases, chaperones, transcription factors, etc are impacted both in positive and negative fashions by S-glutathionylation of specific cysteine residues.1,7,8,9 S-glutathionylation has the potential to alter both enzymatic activity and tertiary structure, and as such it has been likened to O-phosphorylation. This comparison is further bolstered by the fact that PSSG formation is both highly specific within a cell and its reversibility is tightly regulated.4 Given that GSH is present within cells at millimolar concentrations, the likelihood that S-glutathionylation may play a critical role in redox-based signaling or pathophysiological events is high.1
The relevance of S-glutathionylation to cell biology is furthermore bolstered by the existence of mammalian glutaredoxins (Grx), which under physiological conditions act to preferentially reverse S-glutathionylated proteins restoring the protein cysteine to the sulfhydryl group, and restoring protein function.4,10 Although the major enzymatic action of Grx under physiological settings has been linked to de-glutathionylation of protein targets,4,11 it should be stressed that under conditions of oxidative stress or damage where oxidized glutathione (GSSG) are increased, Grx causes increases in S-glutathionylation, instead of decreases.12 Nonetheless, S-glutathionylation and glutaredoxin have now emerged as a new module controlling redox-based biological processes.1,4 Grx enzymes contain within their structure the thioredoxin fold common to members of the oxidoreductase class.12 In mammals, three isoforms of Grx have been identified, the cytoplasm/nuclear Grx1, the mitochondrial/nuclear Grx2, and Grx5, whose name is derived from its yeast homolog and whose function remains elusive.13 It has been shown in vitro that up or down-regulation of Grx results in decreases and increases in levels of PSSG, respectively.9,14
Grx protein levels are known to be altered in a number of human diseases, and in various animal models of disease,15,16,17 and increases in overall content of PSSG have been reported in tissue homogenates in various pathological settings, and models of oxidative stress, including models of oxidant-induced acute lung injury.18,19,20 To date very little data exist with regard to the identity of the target proteins of S-glutathionylation in vivo, and about regional patterns of PSSG in tissues. Identification of proteins that are targeted by PSSG thus far has been limited primarily to evaluations of protein in vitro via mass spectrometry, studies using 35S-labeled GSH, or anti-GSH antibodies and subsequent identification of protein targets.9,21 Detection of PSSG in situ using paraffin preserved tissue has been previously reported using peroxidase-conjugated glutathione S-transferase overlays,22 or by using an anti-GSH antibody,23 but these techniques does not absolutely exclude the detection of free GSH within tissues.
In this study, we sought to determine whether the highly specific catalytic activity of mammalian Grx1 toward reduction of PSSG could be used to visualize PSSG in paraffin-embedded tissues in situ using microscopy approaches. For this purpose we adapted a procedure, previously described for cells,11 for use in lung tissue. Using a series of reagent controls we demonstrate that Grx1-catalyzed cysteine derivatization is robust and highly specific for the detection of PSSG. Additionally we demonstrate that this technique allows for the detection of regional changes in PSSG in various models of lung disease, highlighting the usefulness of in situ detection of PSSG, as a new marker of redox-dependent post-translation modification of proteins.
Materials and Methods
In Situ Detection of S-Glutathionylated Proteins in Tissue Following Grx1 Catalyzed Cysteine Derivatization
After dewaxing tissue samples in three changes of xylene, tissue was rehydrated in 100%, 95%, and 75% ethanol. Free thiol groups were then blocked using a buffer that contained 25 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 0.1 mmol/L EDTA, pH 8.0, 0.01 mmol/L neocuproine, 40 mmol/L N-ethylmaleimide (Sigma) and 1% Triton (Sigma) for 30 minutes. After three washes with phosphate-buffered saline (PBS), S-glutathionylated cysteine groups were reduced by incubation with 13.5 μg/ml human Grx1 (Lab Frontiers), 35 μg/ml GSSG reductase (Roche), 1 mmol/L GSH (Sigma), 1 mmol/L NADPH (Sigma), 18 μmol EDTA and 137 mmol/L Tris · HCl, pH 8.0, for 20 minutes. After three washes with PBS, newly reduced cysteine residues were labeled with 1 mmol/L N-(3-maleimidylpropionyl) biocytin (MPB) (Roche) for 1 hour. Excess MPB was removed by three washes with PBS. Next, tissue samples were incubated with 0.5 μg/ml streptavidin-conjugated Alexa Fluor 568 for 30 minutes. Nuclei were stained using 50 nmol/L SYTOX Green (Molecular Probes). All steps were conducted at room temperature. Tissues were then mounted and coverslipped and slides analyzed by confocal microscopy using an Olympus BX50 microscope coupled to a Bio-Rad MRC 1024 confocal scanning laser microscope system. All sections were scanned using identical instrument settings that did not result in saturation of pixel intensities. As negative controls Grx1, GSH, streptavidin, or Grx1, GSH, GSSG reductase, and NADPH were omitted from the reaction mix. As positive controls tissues were incubated with 400 μmol/L diamide (Sigma) and 1 mmol/L GSH for 10 minutes before application of N-ethylmaleimide. Autofluorescence of the tissues was determined for each experimental condition by evaluating tissues that were not subjected to Grx1-based cysteine derivatization, labeling with MPB, or incubation with streptavidin-conjugated Alexa Fluor 568, via confocal laser scanning microscopy, using identical instrument settings. In all cases this revealed no detectable fluorescence signal in either channel. Semiquantitative assessment of the intensity of PSSG reactivity in bronchial epithelium, parenchymal regions, or alveolar macrophages was conducted by evaluating mean red fluorescence intensities (PSSG) in each region of interest and dividing this by the mean green fluorescence intensity (DNA content) present in the same region, and multiplication of this value by 100, to obtain a measure of the relative fluorescence intensity (RFI) of PSSG. Note that bronchial epithelium and parenchymal regions were selected based on their architectural appearance and examination of sections that were stained with hematoxylin and eosin. PSSG staining patterns in macrophages were confirmed following bronchoalveolar lavage of control mice. Normalization to DNA content was done to account for regional differences in tissue densities that characterize lung tissue. Mean RFI values ± SEM were obtained from evaluating images obtained from multiple animals per groups, as is detailed in the figure legends. To evaluate PSSG in bronchial epithelium, images were cropped to remove submucosal regions, before determining RFI values. All semiquantitative analysis was done using .tif images generated from proprietary Bio-Rad .pict images and the Metamorph 7.5 software package (Molecular Devices), according to the manufacturers’ instructions. Different absolute RFI values presented in various figures are reflective of subtle differences in staining intensities and instrument settings between individual experiments.
Grx1 Activity Assay
Grx1 activity was assayed as previously described.24 Briefly, recombinant Grx1 was incubated in buffer containing 137 mmol/L Tris-HCl (pH 8.0), 0.5 mmol/L GSH, 35 μg/ml GSSG reductase, 0.35 mmol/L NADPH, 1.5 mmol/L EDTA (pH 8.0), and 2.5 mmol/L Cys-SO3(Sigma). As a control GSH was omitted from the reaction mix. The reaction was allowed to proceed at room temperature and NADPH consumption was followed spectrophotometrically at 340 nm. Data are expressed as units, where 1 unit equals the oxidation of 1 μmol NADPH/min/mg protein.
Mouse Models of Pulmonary Inflammation and Fibrosis
For NO2 exposure C57BL/6 mice were exposed to 25 ppm NO2 for 6 hours a day for 3 consecutive days, and tissue sections were used from previously published studies.25 Alternatively, C57BL/6 mice were exposed to >95% O2 continuously for 3 consecutive days.26 Pulmonary fibrosis was induced following administration of bleomycin, 7.5 U/kg (Gensia Sicor Pharmaceuticals, Irvine, CA) in sterile PBS, by oropharyngeal aspiration (40 μl),27 and evaluation of lung tissues on day 21, a time point where a fibrotic response is readily apparent. The presence of fibrosis was confirmed in replicate sections from the same study.28 Lastly, mice were subjected to administration of lipopolysaccharide (LPS, 10 μg, List Biological Laboratories) in sterile saline by oropharyngeal aspiration, and lung tissue evaluated 16 hours later, a time frame when neutrophilic infiltration is apparent (see below) and levels of inflammatory cytokines are increased.29 Mice were sacrificed and lungs were lavaged with 1 ml of PBS. The left lung lobe was subsequently instilled with 4% paraformaldehyde in PBS for 10 minutes at a pressure of 25 cm H2O, and placed into 4% paraformaldehyde in PBS at 4°C overnight for further fixation of the tissue. Tissues were embedded in paraffin, and 5-μm sections we used in all staining procedures.25 All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
In Situ Detection of S-Glutathionylated Proteins Using an Anti-GSH Antibody
Tissue samples were dewaxed and permeabilized by incubation with 1% Triton in PBS for 30 minutes at room temperature. After permeabilization tissue samples were blocked using 1% normal goat serum (Jackson ImmunoResearch Laboratories, Inc.). Samples were then incubated with an anti-GSH (10 μg/ml) (Invitrogen) overnight at 4°C. Tissue samples were then incubated with Alexa Fluor 568-conjugated secondary antibody (Molecular Probes), and nuclei were counterstained using SYTOX green. Slides were analyzed using confocal microscopy. As negative controls, either primary or secondary antibody was omitted from the protocol or tissue sections were incubated with 2 mmol/L β-mercaptoethanol (BME) (Sigma) for 10 minutes to decompose S-glutathionylated proteins before staining.
In Situ Detection of Grx1
Tissue samples were dewaxed and permeabilized by incubation with 1% Triton in PBS for 30 minutes at room temperature. After permeabilization tissue samples were blocked using 1% bovine serum albumin (Fischer). Samples were then incubated with (10 μg/ml) anti-Grx1 antibody (Lab Frontier) overnight at 4°C. Tissue samples were subsequently incubated with Alexa Fluor 568-conjugated secondary antibody (Molecular Probes), and nuclei were counter stained using SYTOX green. Slides were analyzed by confocal microscopy. As a negative control primary antibody was omitted from the protocol.
Statistical Analyses
Data were analyzed by one-way analysis of variance, using the Tukey test to adjust for multiple comparisons (Microsoft Excel, Redmond, WA).
Results
Endogenous Levels of PSSG Are Detectable in Paraffin-Embedded Lung Tissues and Can Be Manipulated through Direct Exposure to Oxidants
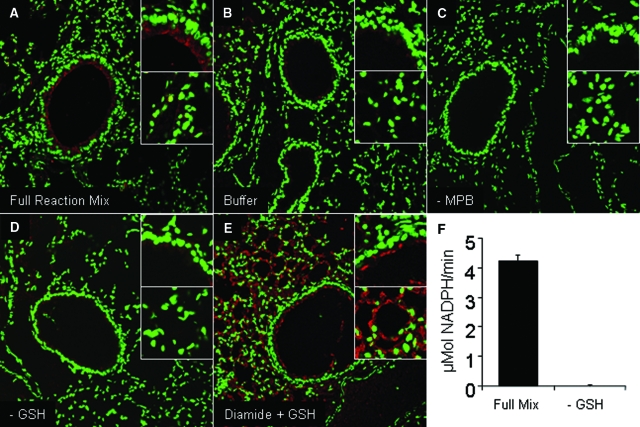
Because of the newly appreciated significance of PSSG in signal transduction and redox homeostasis,1 we sought to establish a method to detect this posttranslational modification in paraffin-embedded lungs sections using a method of Grx1-catalyzed derivatization. This protocol involves the sequential permeabilization of the lung tissue, blocking of reduced thiols with an alkylating agent, Grx1-catalyzed reduction of PSSG, labeling of newly generated thiols with biotin, and subsequent detection of label via confocal laser scanning microscopy. Application of this protocol resulted in detectable levels of endogenous PSSG (red signal) in the lungs of control mice (Figure 1A). Signal was detectable primarily within the epithelium of the conducting airways, with less reactivity detectable in the lung parenchyma. Note that these regions were identified based on their architectural appearance and examination of sections that were stained with hematoxylin and eosin. As a control, omission of all components from the enzymatic reaction mix resulted in almost a complete loss of detectable signal (Figure 1B). In addition, omission of the biotin-conjugated detection agent, MPB, abolished the fluorescence signal (Figure 1C). To test the dependence of the reaction on the presence of functionally active Grx1, GSH was omitted from the reaction mix. Results in Figure 1D demonstrate that omission of GSH from the reaction mix almost completely abolished detectable reactivity in the lung tissue, consistent with a complete loss of detectable Grx1 catalytic activity in vitro (Figure 1F). For all subsequent experiments, the omission of GSH from the reaction mix was therefore used as a negative control. Last, as a positive control, tissue sections were treated with diamide in the presence of 1 mmol/L GSH, to increase the PSSG content in tissues, before subjecting the slides to the protocol of Grx1-based cysteine derivatization. This regimen causes oxidation of thiols, which in the presence of GSH will lead to increases in PSSG content. As is demonstrated in Figure 1E, incubation of the tissue with diamide plus GSH resulted in a marked increase in detectable PSSG throughout the parenchyma, as expected.
Figure 1.
In situ analysis of PSSG in mouse lung tissue using Grx1-based cysteine derivatization. A: Pattern of PSSG reactivity in control mouse lung. Red, PSSG reactivity; green, DNA content. Magnification, ×200. B: Omission of derivatization buffer as a negative control. C: Omission of MPB labeling agent as a negative control. D: Selective omission of GSH from the reaction mix as a negative control. E: Evaluation of PSSG reactivity using Grx1-based cysteine derivatization following pretreatment of tissue with diamide and GSH as a positive control. Following dewaxing and rehydration, lung tissue was incubated with 400 μmol/L diamide plus 1 mmol/L GSH for 10 minutes to cause PSSG formation. Lungs were then processed and evaluated as described in A. Insets: Top, PSSG reactivity in bronchial epithelium; bottom, PSSG reactivity in parenchymal regions (zoom = 4×). F: Assessment of Grx1 activity in vitro using a full enzymatic mix or omitting GSH as a negative control. Semiquantitative RFI values for PSSG were derived from the mean red fluorescence intensities (PSSG) in each region of interest divided by the mean green fluorescence intensity (DNA content). RFI values, mean (SEM) for bronchial epithelium and parenchymal regions based on scanning of four optical images per panel were as follows. A: Full reaction mix: bronchial epithelium, 39 (10); parenchyma, 24 (4). B: Buffer: bronchial epithelium, 9 (5); parenchyma, 10 (8). C: −MPB: bronchial epithelium, 0 (0); parenchyma, 0 (0). D: −GSH; bronchial epithelium, 6 (2); parenchyma, 3 (1). E: Diamide + GSH: bronchial epithelium, 18 (1); parenchyma, 46 (1); *P < 0.05 (analysis of variance) compared with A.
Comparative Evaluation of Immunofluorescence Patterns of PSSG Using Grx1-Based Cysteine Derivatization and Anti-GSH Antibody
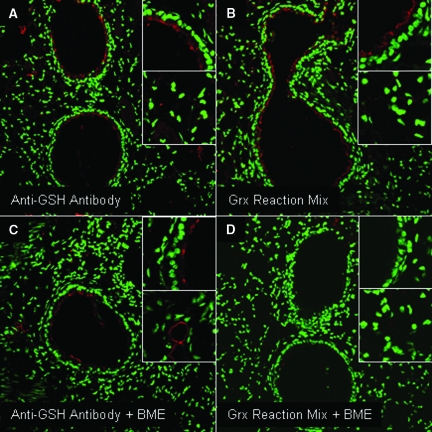
Antibodies have been developed against GSH, and are being considered for the detection of PSSG in tissues.23 We therefore comparatively evaluated staining patterns using Grx1-based derivatization or anti-GSH antibody. In these studies, tissues were pretreated with the reducing agent BME, to decompose PSSG, as a reagent control. Evaluation of paraffin-embedded lung tissue with anti-GSH antibody resulted in a fluorescence profile similar to that observed using the Grx1-based derivatization protocol. Fluorescence was detected primarily in the epithelium of the conducting airways, with lower reactivity apparent in the parenchyma (Figure 2, A and B). As expected, pretreatment of tissues with BME resulted in a complete loss of fluorescence, when subsequently evaluated using the Grx1-based derivatization protocol (Figure 2D). However, despite pre-incubation with BME, detectable immunoreactivity remained when using the anti-GSH antibody (Figure 2C). These findings suggest that while Grx1-based cysteine derivatization is dependent on the presence of oxidized (S-glutathionylated) cysteines, the anti-GSH antibody might detect other oxidative events, or free GSH, in addition to PSSG.
Figure 2.
Comparative evaluation of PSSG reactivity in lung tissue using Grx1-based cysteine derivatization, and an anti-GSH antibody. A: Evaluation of PSSG in control mouse lung using anti-GSH antibody (red). B: Evaluation of PSSG in control mouse lung using Grx1-catalyzed cysteine derivatization (red). Green reflects DNA content. C and D: Assessment of PSSG reactivity in control mouse lung following reduction of PSSG with the thiol reducing agent, BME, before analysis of the tissue with anti-GSH antibody (C) or Grx1-based cysteine derivatization (D). Insets: Top, PSSG reactivity in bronchial epithelium; bottom. PSSG reactivity in parenchymal regions. Mean RFI values for PSSG or anti-GSH antibody reactivity based on scanning of four optical images per panel were as follows. A: Anti-GSH antibody; bronchial epithelium, 31 (2); parenchyma, 21 (4). B: Grx reaction mix; bronchial epithelium, 23 (2); parenchyma, 16 (2). C: Anti-GSH antibody plus BME; bronchial epithelium, 19 (4); parenchyma, 11(2). D: Grx reaction mix plus BME; bronchial epithelium, 2 (1); parenchyma, 2 (2).
Detection of PSSG in Alveolar Macrophages
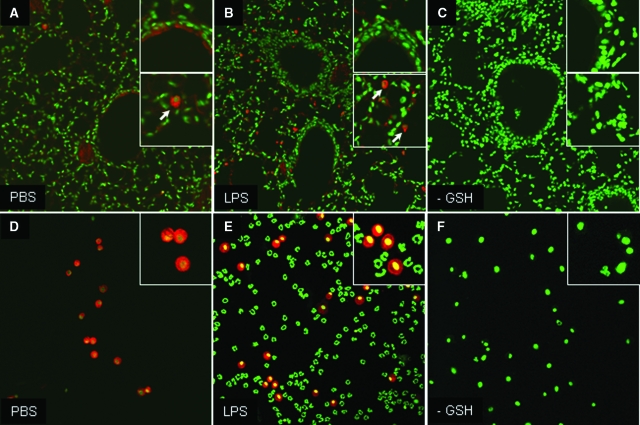
We next evaluated whether presence of PSSG could be detected in specific cell types, and whether this was modulated following acute exposure to the proinflammatory agent, LPS. Scanning of sections from control mice revealed distinct cells, with the appearance of alveolar macrophages, which contained high fluorescence following Grx1-catalyzed derivatization, and increases in numbers of cells with high PSSG content were apparent in mice exposed to LPS (Figure 3, A and B), which likely represent infiltrating macrophages.29 Bronchoalveolar lavage (BAL) analysis of control mice confirmed that macrophages were highly reactive when subjected to Grx1-based cysteine derivatization. However, only minor further increases in PSSG reactivity occurred in BAL samples obtained from LPS treated mice that were non-statistically significant (Figure 3, D and E). In contrast, infiltrating neutrophils recognized based on their characteristic nuclear shape did not have detectable PSSG, when evaluated using the Grx1-based cysteine derivatization strategy (Figure 3E). Detectable PSSG in the epithelium appeared to decrease slightly, but not statistically significantly, following LPS challenge, compared with PBS controls (Figure 3, A and B). As was demonstrated earlier in Figure 1, omission of GSH from the reaction mix did not result in detectable fluorescence signal in lung tissues (Figure 3C) or BAL samples (Figure 3F). It is possible that the BAL procedure may have affected the patterns of PSSG subsequently observed in the lung tissue. However, comparative evaluation of PSSG in situ in lavaged and non-lavaged lungs demonstrated no differences in the extent or regional patterns of PSSG (data not shown).
Figure 3.
In situ analysis of PSSG reactivity in lung tissue (A–C) or cells obtained via bronchoalveolar lavage (D–F) from mice 16 hours following oropharyngeal aspiration of PBS or LPS. PSSG was evaluated using Grx1-catalyzed cysteine derivatization as described in Figure 1. Magnification, ×200. Red, PSSG; green, DNA content. Insets in A–C: Top, PSSG reactivity in bronchial epithelium; bottom: PSSG reactivity in parenchymal regions. Insets in D–F reflect PSSG reactivity in BAL cells, as described in Figure 1. C and F: Negative control in which GSH was omitted from the derivatization reaction in lung tissue (C), or BAL cells (F). RFI values, mean (SEM), obtained from analyzing four optical images per panel were as follows. A: PBS; bronchial epithelium, 52 (13); parenchyma, 40 (12); B: LPS; bronchial epithelium, 36 (10); parenchyma, 31 (17). C: −GSH; bronchial epithelium, 5 (2); parenchyma, 2 (1). D–F: RFI values for macrophages obtained via bronchoalveolar lavage. D: PBS; 89 (14); E: LPS; 104 (10); F: −GSH, 0 (0). Note that in B, PSSG reactive cells within airspaces, which resembled macrophages were omitted from the analyses. Note that in E, neutrophils were omitted from the semiquantitative analyses. Arrows in A and B represent cells with the morphological appearance of alveolar macrophages.
Modulation of PSSG Reactivity in Lung Tissue in Mice with Acute Lung Injury or Fibrosis
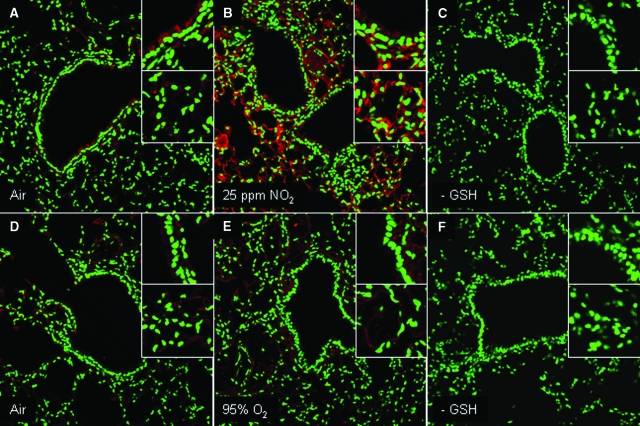
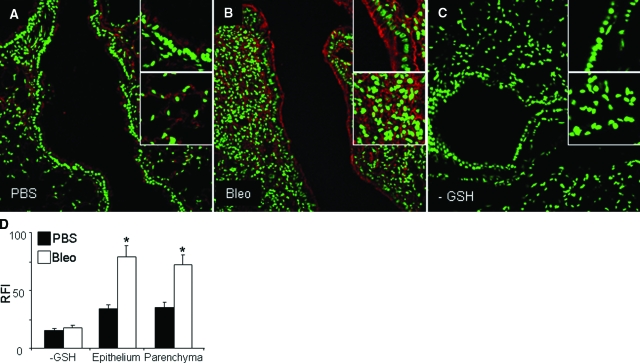
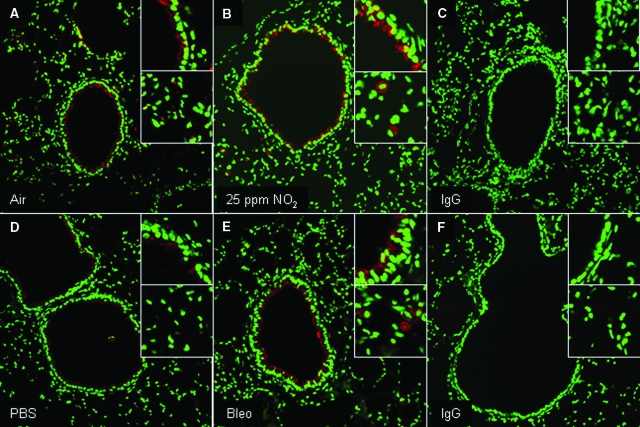
We next evaluated whether modulation of PSSG occurred in models of acute lung injury and fibrosis. As is demonstrated in Figure 4B, exposure of mice to 25 ppm NO2 for 6 hours/day for 3 days resulted in a marked increase in detectable PSSG (Figure 4, A–C). Increases in PSSG were most apparent and statistically significant in the lung parenchymal and vascular regions, while less appreciable changes that were not statistically significant occurred in the epithelium of the conducting airways. Conversely, exposure of mice to >95% O2 for 3 days’ exposure resulted in no clear increases in PSSG (Figure 4, D–F), suggesting that modulation of PSSG depends on the nature of the oxidative insult. Changes in the oxidative environment have been described in lungs from patients with pulmonary fibrosis or in mouse models of fibrosis, and changes in homeostasis of glutathione herein have been reported.30,31 We therefore determined next whether the PSSG patterns were affected in the bleomycin model of fibrosis, using the Grx1-based cysteine derivatization protocol. In mice exposed to bleomycin, statistically significant increases in PSSG reactivity were apparent within the bronchial epithelium and parenchymal regions, as compared with PBS controls (Figure 5, A–D).
Figure 4.
Evaluation of PSSG in lungs of mice following inhalation of NO2 (A–C) or >95% oxygen (D–F). Red, PSSG reactivity; green, DNA content. Magnification, ×200. Insets: Top, PSSG reactivity in bronchial epithelium; bottom, PSSG reactivity in parenchymal regions. A and D: Respective sham air exposures for the individual experiments. C and F: Negative control in which GSH was omitted from the derivatization reaction in lung tissue. RFI values; mean (SEM) values for bronchial epithelium and parenchymal regions in each inset, obtained from evaluating four to seven optical images per group, were as follows. A: Air; bronchial epithelium, 42 (5); parenchyma, 31 (7). B: NO2; bronchial epithelium, 69 (6); parenchyma, 77 (8)*. C: –GSH control; bronchial epithelium, 10 (2); parenchyma, 3 (1). D: Air; bronchial epithelium, 33 (3); parenchyma, 40 (5). E: 95% oxygen; bronchial epithelium, 53 (13); parenchyma, 30 (5). F: −GSH control; bronchial epithelium, 7 (1); parenchyma, 12 (1)*. *P < 0.05; analysis of variance compared with parenchymal region in A.
Figure 5.
Evaluation of PSSG in lungs of mice with bleomycin-induced pulmonary fibrosis using Grx1-catalyzed cysteine derivatization. A and B: Lungs were evaluated 21 days after administration of bleomycin (Bleo) or PBS as a vehicle control. Red, PSSG reactivity; green, DNA content. Magnification, ×200. C: Negative control in which GSH was omitted from the derivatization reaction in lung tissue. Insets: Top, PSSG reactivity in bronchial epithelium; bottom, PSSG reactivity in parenchymal regions. D: Semiquantitative assessment of increases in PSSG in lung tissues of mice exposed to bleomycin as compared with controls. RFI values were determined in bronchial epithelial and parenchymal regions of seven mice/group. Three optical fields per mouse were evaluated for each region. *P < 0.05 (analysis of variance) compared with PBS controls.
Modulation of Grx1 Expression in Lung Tissue of Mice in Situ
The observed changes in PSSG in response to NO2 or bleomycin could be due to alterations in endogenous expression of Grx1, the enzyme responsible for de-glutathionylation. We therefore examined whether expression of Grx1 was affected in response to these agents. Results in Figure 6 demonstrate that in response to NO2 exposure, increases in Grx1 immunofluorescence occurred within the bronchial epithelium, while Grx1 expression was lower in parenchymal regions (Figure 6, A–C). Overall patterns of expression of Grx1 inversely correlated with PSSG staining patterns in mice exposed to NO2 (compare Figures 6B and 4B). However, in mice exposed to bleomycin, increases in Grx1 expression occurred within the bronchial epithelium (Figure 6, D–F), which also showed marked increases in PSSG (Figure 5B). It is noteworthy to highlight that isolated cells within parenchymal regions, which morphologically resembled alveolar macrophages, showed apparent increases in Grx1 expression, both in response to NO2 or bleomycin (Figure 6, B and D, insets), consistent with previous observations demonstrating Grx1 expression in human lung macrophages.17
Figure 6.
In situ analysis of Grx1 expression in the lungs of mice following inhalation of 25 ppm NO2 (A–C) or administration of bleomycin (D–F) or vehicle controls. Red, Grx1 expression; green, DNA content. Magnification, ×200. Insets: Zoom = 4×. C and F: Negative controls in which the primary antibody was omitted.
Discussion
While oxidants have been implicated in a multitude of lung diseases, their exact role in the disease process has remained enigmatic. This is partially related to the occurrence of multiple redox changes, in association with the production of diverse oxidants with unique reactivities. Despite the recent development of new a redox biosensor probe that enables the imaging of changes in GSH/GSSG status in real time with great specificity and special/temporal resolution,32 specific tools to probe oxidative events with relevance for cell signaling in biological settings, such as S-glutathionylation, in situ were unavailable, contributing to the lack of understanding of the mechanism whereby redox changes affect lung homeostasis. The recently required ability to trap and label proteins that are targeted by reversible cysteine oxidations enables investigators to determine, for the first time, which proteins are regulated via cysteine oxidations in intact tissues.
In the present study we describe and validate a new method to detect PSSG in tissues in situ, taking advantage of the highly specific enzymatic activity of Grx1 toward reduction of PSSG back to free thiols in this setting.4,5 This procedure is somewhat analogous to the TUNEL assay, which employs the enzymatic properties of terminal deoxytransferase to nick translate and subsequently label fragmented DNA, generated in cells undergoing apoptosis.33 Our results demonstrate that Grx1-based cysteine derivatization can be applied to paraffin embedded lung tissues, is highly specific, and allows the demonstration of regional and stimulus-specific changes in PSSG. It also does not require the use of antibodies, avoiding the inherent problems with specificity of antibodies. Indeed comparative evaluation of Grx1-based cysteine derivatization, and an antibody directed against GSH demonstrated that, although in control lung tissue overall staining patterns were somewhat similar, chemical reduction of PSSG with β-mercaptoethanol failed to abolish the reactivity of the anti-GSH antibody (Figure 2, compare C with A), suggesting that this antibody might detect other oxidative events, or free GSH in lung tissues, in addition to PSSG.
The primary locations of PSSG reactivity within lung observed here were the bronchial epithelium, and alveolar macrophages. Although the PSSG staining pattern in macrophages in the intact lung tissue awaits further confirmation using a macrophage specific marker, evaluation of BAL samples from control mice, which consists of >99% alveolar macrophages (data not shown) confirms marked PSSG reactivity in alveolar macrophages. Although PSSG reactivity has not been evaluated in lung tissue in situ previously, we recently demonstrated that Grx1 expression was increased in bronchial epithelium in mice with allergic airways disease.16 Grx1 immunoreactivity also has been detected in bronchial epithelium from humans.17 The most striking reactivity of PSSG was observed in alveolar macrophages, which also have been demonstrated to express Grx1.17 In contrast to our expectations, we did not observe detectable PSSG reactivity in neutrophils obtained via BAL following administration of LPS. This is surprising given the high oxidant producing capacity of these cells, and the decreases in GSH content that have been observed following their activation34 in association with increases in PSSG.35 It is plausible that under the current experimental conditions, insufficient neutrophil activation occurred to induce increases in PSSG, although this possibility remains to be formally tested.
This study also demonstrates that PSSG reactivity changes in lungs of mice subjected to oxidant-induced acute lung injury and the bleomycin model of pulmonary fibrosis. PSSG reactivity was affected in a stimulus-specific manner. While the oxidant gas, NO2, caused widespread increases in PSSG in lung tissue, which were most apparent in parenchymal regions, virtually no increases in PSSG were observed in lungs of mice exposed to >95% oxygen for similar times (Figure 4), suggesting that changes in PSSG do not occur as a result to all forms of oxidant stress, but appear to be stimulus-specific. These results are in agreement with previous observations demonstrating that the sites and mechanisms of injury induced by NO2 and oxygen are distinct.36,37 It is, however, possible that increases in PSSG could have occurred to a lesser extent, or with different kinetics in response to hyperoxia. Evaluation of these possibilities would require additional investigation, which was beyond the scope of the present manuscript. Nonetheless, the lack of overt changes in PSSG reactivity in response to hyperoxia observed in the present study, are in agreement with a recent study demonstrating that mice lacking Grx1 are not more susceptible to hyperoxia-induced lung injury,14 findings that collectively suggest that the Grx1/PSSG redox module may not be a major determinant of acute lung injury in response to hyperoxia.
Increases in oxidative stress have been observed in humans with pulmonary fibrosis as well as in mouse models of fibrosis, and a role of glutathione herein is apparent.30,31,38 The outcome of a recent clinical study that involved the glutathione precursor, N-acetyl-l-cysteine has suggested a potential therapeutic effect,39 warranting additional studies into the role of changes in the glutathione homeostasis in pulmonary fibrosis. Our present study demonstrates for the first time that increases in PSSG content occur in the bleomycin model of fibrosis, and suggests that changes in PSSG may play a role in the process of fibrogenesis. A recent study also demonstrated that PSSG content increased in patients with non-alcoholic fatty liver disease in correlation with steatohepatitis and liver fibrosis, based on evaluation of tissues using an antibody directed against GSH,23 and supports a putative link between PSSG and fibrogenesis. However, additional studies that specifically modulate of PSSG content in vivo, and evaluate sections from patients with fibrosis, will be required to solidify those speculations, and implementation of the Grx1-based derivatization assay described herein will be instrumental in those endeavors.
Steady-state levels of PSSG in cells are controlled by the activity of Grx. Thus, the extent of PSSG reactivity observed in tissue in situ is likely dependent on the amount of expression of Grx. We have demonstrated previously that mRNA expression en enzymatic activity of Grx1 is affected by a wide array of cytokines, and also that its expression is increased bronchial epithelium of mice with allergic inflammation.16 Similarly we demonstrate in the present study that immunoreactivity of Grx1 is increased in mice with NO2-induced acute lung injury, or bleomycin-induced fibrosis, and that increases in Grx1 expression predominantly occurred in the bronchial epithelial cells. These increases in Grx1 expression might have dampened the extent to which increases in PSSG content increased in these regions, and absence or low levels of apparent Grx1 expression in parenchymal regions might have permitted the larger increases in PSSG reactivity observed in those locations. Evaluation of regional differences in PSSG content, and expression of Grx1 will require additional studies based on strict stereological criteria to solidify conclusions about the extent to which these parameters change in different compartments of the lung. Such studies, which were beyond the scope of the present study, can also include the use of antibodies to label specific cells, to determine the extent to which PSSG reactivity is affected in individual cell types.
In conclusion, this study describes and validates a novel approach that allows investigators for the first time to evaluate PSSG in lung tissue in situ. The methodology described herein relies on sequential steps of cysteine blocking, reduction and labeling, and takes advantage of the highly specific catalytic activity of Grx1 toward reduction of PSSG, in this setting, and can be used in paraffin embedded tissues. Grx1-catalyzed cysteine derivatization allows detection of reversible cysteine oxidations that are only beginning to be evaluated in intact tissues, and may represent a highly sensitive marker for subtle changes in the redox environment, for which currently no other assays exist. Application of the presently described method will open the door toward the unraveling of the functional significance of PSSG in pulmonary as well as other human diseases where changes in glutathione homeostasis have been documented.
Acknowledgments
We thank Dr. Lin Mantell, Department of Pharmaceutical Sciences, St. John’s University College of Pharmacy, Queens, NY, for providing expertise with the hyperoxia exposures.
Footnotes
Address reprint requests to Yvonne M.W. Janssen-Heininger, Ph.D., University of Vermont College of Medicine, Department of Pathology, 89 Beaumont Avenue, Burlington, VT 05405. E-mail: yvonne.janssen@uvm.edu.
Supported by National Institutes of Health grants R01 HL60014, R01 HL079331 and R03 HL 095404.
Y.M.W.J.-H. has a pending patent that involves the detection of S-glutathionylated proteins in situ.
Current address of K.C.: SUNY Plattsburgh, Department of Chemistry, Hudson Hall 318, 101 Broad Street, Plattsburgh, NY 12901.
References
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford RE, Heinloth AN, Heard SC, Paules RS. Cellular and molecular targets of protein S-glutathiolation. Antioxid Redox Signal. 2005;7:940–950. doi: 10.1089/ars.2005.7.940. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Xiao R, Lundstrom-Ljung J, Holmgren A, Gilbert HF. Catalysis of thiol/disulfide exchange: glutaredoxin 1 and protein-disulfide isomerase use different mechanisms to enhance oxidase and reductase activities, J Biol Chem. 2005;280:21099–21106. doi: 10.1074/jbc.M411476200. [DOI] [PubMed] [Google Scholar]
- Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, Cohen RA. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta, Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, Ckless K, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta. 2006;1760:380–387. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- Sagemark J, Elgan TH, Burglin TR, Johansson C, Holmgren A, Berndt KD. Redox properties and evolution of human glutaredoxins. Proteins. 2007;68:879–892. doi: 10.1002/prot.21416. [DOI] [PubMed] [Google Scholar]
- Ho YS, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi MJ, Rytila PH, Harju TH, Soini YM, Salmenkivi KM, Ruddock LW, Kinnula VL. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res. 2006;7:133. doi: 10.1186/1465-9921-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Akerboom TP, Bilzer M, Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982;257:4248–4252. [PubMed] [Google Scholar]
- DeLucia AJ, Mustafa MG, Hussain MZ, Cross CE. Ozone interaction with rodent lung: III. Oxidation of reduced glutathione and formation of mixed disulfides between protein and nonprotein sulfhydryls, J Clin Invest. 1975;55:794–802. doi: 10.1172/JCI107990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini I, Vandekerckhove J, Gianazza E, Ghezzi P. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics. 2003;3:1154–1161. doi: 10.1002/pmic.200300436. [DOI] [PubMed] [Google Scholar]
- Cheng G, Ikeda Y, Iuchi Y, Fujii J. Detection of S-glutathionylated proteins by glutathione S-transferase overlay. Arch Biochem Biophys. 2005;435:42–49. doi: 10.1016/j.abb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Piemonte F, Petrini S, Gaeta LM, Tozzi G, Bertini E, Devito R, Boldrini R, Marcellini M, Ciacco E, Nobili V. Protein glutathionylation increases in the liver of patients with non-alcoholic fatty liver disease, J Gastroenterol Hepatol. 2008;23:e457–e464. doi: 10.1111/j.1440-1746.2007.05070.x. [DOI] [PubMed] [Google Scholar]
- Gan ZR, Wells WW. Purification and properties of thioltransferase. J Biol Chem. 1986;261:996–1001. [PubMed] [Google Scholar]
- Poynter ME, Persinger RL, Irvin CG, Butnor KJ, van Hirtum H, Blay W, Heintz NH, Robbins J, Hemenway D, Taatjes DJ, Janssen-Heininger Y. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L144–L152. doi: 10.1152/ajplung.00131.2005. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;40:422–432. doi: 10.1165/rcmb.2008-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- Day BJ. Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal. 2008;10:355–370. doi: 10.1089/ars.2007.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Packer L, Maguire JJ. Neutrophil antioxidant capacity during the respiratory burst: loss of glutathione induced by chloramines. Free Radic Biol Med. 1997;23:445–452. doi: 10.1016/s0891-5849(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Carr AC, Winterbourn CC. Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid. Biochem J. 1997;327:275–281. doi: 10.1042/bj3270275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo JD, Barry BE, Chang LY, Mercer RR. Alterations in lung structure caused by inhalation of oxidants. J Toxicol Environ Health. 1984;13:301–321. doi: 10.1080/15287398409530500. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Marsh-Salin J, Ingram P, Pratt PC. Tolerance and cross-tolerance using NO2 and O2 II. Pulmonary morphology and morphometry. J Appl Physiol. 1978;44:370–379. doi: 10.1152/jappl.1978.44.3.370. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]