Abstract
Parkinson disease (PD) typically affects the cortical regions during the later stages of disease, with neuronal loss, gliosis, and formation of diffuse cortical Lewy bodies in a significant portion of patients with dementia. To identify novel proteins involved in PD progression, we prepared synaptosomal fractions from the frontal cortices of pathologically verified PD patients at different stages along with age-matched controls. Protein expression profiles were compared using a robust quantitative proteomic technique. Approximately 100 proteins displayed significant differences in their relative abundances between PD patients at various stages and controls; three of these proteins were validated using independent techniques. One of the confirmed proteins, glutathione S-transferase Pi, was further investigated in cellular models of PD, demonstrating that its level was intimately associated with several critical cellular processes that are directly related to neurodegeneration in PD. These results have, for the first time, suggested that the levels of glutathione S-transferase Pi may play an important role in modulating the progression of PD.
Parkinson disease (PD), the second most common neurodegenerative disease after Alzheimer disease (AD), has been recognized recently as a disease that includes more than just nigrostriatal degeneration with motor dysfunction.1,2 More specifically, a significant portion of PD patients suffer from non-motor symptoms, including anosmia, constipation, depression, autonomic failure, and cognitive dysfunction.1,2,3 In fact, PD patients commonly develop reduced cognitive abilities, ranging from mild cognitive impairment to full-blown dementia, as the disease advances.1,2
The prevailing hypothesis for PD development is that the disease likely results from genetic or environmental causes, or their combinations with processes of aging, that culminate in mitochondrial and/or proteasomal dysfunction and increased oxidative stress.1,3 Additionally, pathological observations derived from human PD cases and toxicant-based animal models suggest a dying-back mode of neurodegeneration in PD.1,4 More specifically, loss of presynaptic terminals (for example, the axonal connections from the substantia nigra pars compacta to the striatum) precedes the loss of neuronal cell bodies. Nonetheless, the molecular pathways leading to PD progression are largely unknown. While progressive dopaminergic degeneration can be important, its link to cognitive impairment is not clear. On the other hand, most, if not all, PD patients with dementia demonstrate cortical Lewy bodies (LBs), a pathological hallmark of PD, and/or pathology related to AD.1,2,5 Indeed, though still controversial, it appears that, at least in a subset of PD patients, progression of the disease is accompanied by spreading of LBs from the brainstem to the limbic system and, eventually, to the isocortex, including the frontal cortex.5,6 In this study, we have elected to focus on this particular cohort of PD patients, given our ability to classify them unequivocally.
Herein, using a robust shotgun proteomic approach in conjunction with an isotope labeling technique, isobaric tagging for relative and absolute quantification (iTRAQ), we quantitatively compared the protein profiles of synaptosomal fractions, the subcellular compartment likely involved in the early process of neurodegeneration, from the frontal cortex of patients with PD at different pathological stages of disease, with and without dementia, as well as with age-matched controls. The study revealed many novel proteins with quantitative expression differences as the disease progressed, and provided more detailed molecular mechanisms for one of these proteins, glutathione S-transferase Pi (GSTP1), that may modulate the progression of human PD.
Materials and Methods
Human Brain Tissue
Samples of the middle frontal gyrus from each case were collected at autopsy following the protocols approved by Emory University Medical School, the University of Michigan, and the University of Washington School of Medicine, and characterized as previously described.7,8 Four groups of samples (5/group, male/female ratio = 3/2; 20 cases total) were analyzed: Age-matched controls, PD-brainstem, PD-limbic, and PD-isocortex. Age-matched normal controls were individuals who did not have a diagnosis of neurological disease, were not taking medications prescribed for neurological diseases, and whose neuropathologic examination revealed age-related changes only. All PD patients had been diagnosed with PD (with and without cognitive impairment) according to the National Institute of Neurological Disease criteria and final diagnoses were established by neuropathological examination according to established criteria.9,10,11 All of the PD cases used were free of AD pathologies in the cortex. In addition, all PD cases had been given a clinical diagnosis of PD initially, which meant that dementia with Lewy body disease cases, a disease overlapping with PD with dementia cases pathologically, were excluded from the study. All five cases with cortical LBs (PD-isocortex) demonstrated at least 3 to 5 LBs/section examined, with appreciable increased gliosis but without apparent spongiform changes. However, the extent of gliosis and spongiform was not quantified. All subjects were age-, gender-, and postmortem interval (<12 hours)-matched.
Notably, the investigation was based on anatomical distribution of LBs rather than the cognitive state of each case for two reasons. First, there were no established or generally accepted criteria to diagnose cognitive impairment in PD, including dementia, available until recently,12,13 whereas the cases used in this study were collected and characterized years before. Second, the time lag between last clinical assessment of “mental status” and autopsy in each case varied significantly; thus it is not really possible to perform a precise clinicopathological correlation.
Synaptosome Preparation
Synaptosomes from human frontal cortical tissues were prepared as described by Schratt et al14 with minor modifications. In brief, rapidly thawed samples were dissected in ice-cold homogenization buffer (0.32 mol/L sucrose, 5 mmol/L HEPES, pH7.4, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, and protease inhibitor cocktail from Sigma, St. Louis, MO), and disrupted with a glass-Teflon homogenizer (Wheaton, Millville, NJ) by eight gentle up and down strokes. The protein concentrations of the homogenates were determined using a BCA Protein Assay kit (Pierce, Rockford, IL). For pooled samples, an equal amount of proteins from five individual samples in each group were combined. The pooled homogenate was then centrifuged at 2000 × g for 2 minutes to remove nuclei and cell debris. The supernatant (S1) was collected and centrifuged for 10 minutes at 14,000 × g, and the pellet (P2) was resuspended in 3 ml of homogenization buffer and centrifuged at 14,000 × g for 10 minutes. The resulting pellet (P2′), representing a crude synaptosomal/mitochondrial fraction, was subsequently resuspended and layered over a 6% to 9% to 13% discontinuous Ficoll (Sigma) gradient that had been equilibrated at 4°C for at least 1 hour. The gradient was centrifuged at 86,800 × gmax for 35 minutes on a Beckman (Fullerton, CA) Optima XL-100K ultracentrifuge using a SW 41 Ti swing bucket rotor. Synaptosomes were collected from the 6% to 9% and 9% to 13% gradient interfaces and washed by diluting with five volumes of homogenization buffer and centrifuging at 20,800 × g for 20 minutes. The synaptosomal pellet was then resuspended in homogenization buffer.
Multidimensional Separation and Matrix-Assisted Laser Desorption Ionization Tandem Mass Spectrometry Analysis
To compare the relative abundance of protein levels in PD patents at different stages versus controls, 100 μg of protein from each group was digested with trypsin in parallel and then labeled with iTRAQ reagents (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. The labeled digests from the four groups were combined together, separated using a strong cation exchange column followed by reverse phase nano-capillary liquid chromatography and spotted onto stainless steel matrix-assisted laser desorption ionization (MALDI) plates as previously described.7,8 Quantitative tandem mass spectrometry (MS)/MS analysis was completed using a 4700 Proteomics Analyzer with time-of-flight (TOF)/TOF Optics (Applied Biosystems). The MS/MS spectra were searched against the International Protein Index (IPI) human protein database (version 3.18) using MASCOT algorithm (version 2.0, Matrix Science, Boston, MA) for peptide and protein identification and quantification as described.7,8
Gene Ontology Analysis
Bioconductor package topGO15 was used to determine enhanced gene ontology (GO) categories. The topGO elim method was used to identify categories by evidence of over-representation of significant proteins.
Western Blotting
A detailed experimental protocol for Western blotting has been previously described.8 The following antibodies were used in Western blotting: mouse anti-β-actin monoclonal antibody (1:8000; Abcam, Cambridge, MA); mouse anti-2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) monoclonal antibody [11–5B] (1:2000; Abcam); mouse anti-C/EBP-homologous protein (CHOP) (GADD 153) monoclonal antibody [B-3] (1:300; Santa Cruz Biotech, Santa Cruz, CA); mouse monoclonal [2A5] to glial fibrillary acidic protein (GFAP) (1:4000; Imgenex, San Diego, CA); rabbit polyclonal to GSTP (1:2000; Stressgen/Assay Designs, Ann Arbor, MI); rabbit monoclonal [16F8] to phosphor-protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK) (Thr980) (Cell Signaling, Danvers, MA); goat polyclonal to PSD-95 (1:200; Santa Cruz Biotech); rabbit monoclonal [EP1576Y] to S-100B (1:1000, Abcam); mouse monoclonal [5A6] to SH3-containing GRB2-like protein 2 (SH3GL2) (1:1000; Novus, Littleton, CO); mouse monoclonal [HPC-1] to syntaxin (1:2000; Sigma); and mouse monoclonal to neuron specific class III β-tubulin [TUJ1] (1:2000; Covance, Berkeley, CA).
Immunohistochemistry
This study was performed using a separate set of controls and PD cases with clinical dementia and cortical LBs. Paraffin sections of human frontal cortex tissue were prepared and blocked as previously described.16 The sections were incubated at 4°C overnight with rabbit anti-GSTP antibody (1:300, Stressgen/Assay Designs) plus mouse anti- microtubule-associated protein 2 (MAP2) antibody (1:500, Abcam) or mouse monoclonal [syn211] against α-synuclein (1:400, Biosource/Invitrogen, Carlsbad, CA) antibody, and then incubated with secondary antibodies (Flex Fluor 488 goat anti-rabbit IgG, 1:200; Flex Fluor 568 goat anti-mouse IgG, 1:200; Invitrogen) for 1 hour at room temperature. Tissue sections were then treated with 0.1% Sudan Black B for 30 minutes to block autofluorescence. After brief destaining in 75% ethanol and rinsing, the sections were counterstained with TO-PRO-3 iodide (1:1000, Invitrogen) to label DNA with blue fluorescence and then mounted with Prolong Gold with 4′,6-diamidino-2-phenylindole (Invitrogen). Images were captured using a laser scanning confocal microscope (Bio-Rad LS2000, Hercules, CA) as previously described.16 As a negative control, the primary antibodies were omitted.
Plasmid Construction
Human GSTP1 (hGSTP1*A–D) cDNAs were obtained by PCR of the corresponding pET-17b vectors.17 PCR amplification primers were used that allowed for addition of XhoI-Kozak and EcoRI sites to the 5′ and 3′ ends of the hGSTP1 cDNA, respectively (forward primer: 5′-CTACTCGAGCACCATGCCGCCCTACACCGTGGTC-3′ and reverse primer: 5′-CGATGGATATCTGCAGAATTCCAGCACACTGG-3′). The PCR fragments were then subcloned into the SalI and EcoRI sites of the expression vector pCIG2-IRES-EGFP (gift from Dr. R.F. Hevner, University of Washington), which contains a (cDNA)-internal ribosome entry site-enhanced green fluorescent protein (GFP) expression cassette under the control of a cytomegalovirus-enhancer and a chicken β-actin promoter.18 The C47A + C101A and Y7F mutant GSTP1 constructs were generated using the hGSTP1A construct and the QuikChange (Multi) Site-Directed Mutagenesis Kits (Stratagene, La Jolla, CA) with the primers (5′-CTCACTCAAAGCCTCCGCCCTATACGGGCAGCTC-3′,5′-CGTGGAGGACCTCCGCGCCAAATACATCTCCCTC-3′), (5′-CCTACACCGTGGTCTTTTTCCCAGTTCGAGG-3′,5′-CCTCGAACTGGGAAAAAGACCACGGTGTAGG-3′), respectively. All constructs were confirmed by nucleic acid sequencing.
Cell Culture, Transient Transfection, and Drug Treatment
Mouse Neuro2A neuroblastoma cells (American Type Culture Collection, Rockville, MD) were maintained in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (1:1) supplemented with 10% fetal bovine serum and penicillin/streptomycin at 100 U/ml and 100 μg/ml, respectively. The day before the experiment, cells were seeded at ∼2.5 × 105 cells/cm2 and left to adhere overnight. The cells were then transfected with pCIG2 plasmids using lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The transfection efficiency was 60% to 80% by measuring GFP expression, and 24 to 48 hours after transfection, cells were treated with 20 or 30 nmol/L of rotenone (Sigma) or dimethyl sulfoxide (DMSO; final concentration, 0.1% v/v) as a vehicle control for the indicated periods.
Primary Cortical Culture
Mouse cortical neuron-enriched cultures were prepared as previously described19 with minor modifications. Briefly, cortical tissues were dissected from postnatal mouse pups (C57BL/6J, P0-P1), partially digested with papain (20 U/ml) and DNase I (100 U/ml), and gently disrupted before plating at ∼5 × 105 cells/well in poly-d-lysine/fibronectin coated 4-well Lab-Tek II chamber slides (Nalge Nunc, Rochester, NY). Cytosine arabinoside (Ara-C) was added at 20 to 30 μmol/L on the second day for 3 days to inhibit the growth of glial cells. The cells were then maintained in 5 μmol/L of Ara-C.
Five days after the initial seeding, primary cultures were transfected with pCIG2 plasmids encoding GFP only or hGSTP1A together with GFP. Two days after transfection, cells were treated with rotenone or vehicle for two more days.
Cell Viability
Neuro2A cells in 96-well plates were transfected and treated with rotenone as indicated, and cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay as described by Cookson et al.20 For each experiment, eight wells were used per condition, and the experiment was repeated more than three times.
Neurite Outgrowth Assay
Neuro2A cells were transfected and transferred into poly-d-lysine/fibronectin coated 4-well Lab-Tek II chambered coverglass (Nagle Nunc) at 1 × 104 cells/cm2. After 24 hour incubation, cells were treated with rotenone or vehicle (DMSO) for 48 hours. Images of five randomly selected fields were then captured using a laser scanning confocal microscope (Bio-Rad LS2000), and neurite total length and number of branching points were quantified using the Neurolucida software (version 8.0, MicroBrightField, Williston, VT) by an observer blind to the experiment settings. For primary cultures, after rotenone treatment, cells were fixed in 4% formaldehyde and stained with a rabbit anti-MAP2 antibody (1:1000; Chemicon/Millipore, Temecula, CA) and a goat anti-rabbit IgG Alexa Fluor 598 secondary antibody (Invitrogen). Neurites on GFP+ and MAP2+ neurons were traced and quantified.
Protein Carbonyl Assay
Protein oxidation was measured by quantifying total protein carbonyl content using 2,4-dinitrophenylhydrazine as previously described.21 The results were expressed as the relative absorption at 375 nm (ie, amount of 2,4-dinitrophenylhydrazine incorporated) per mg of protein.
Lipid Peroxidation Assay
Cells cultured in 10-cm dishes were collected and a small fraction was used to determine the protein concentration. The remaining cells were resuspended in Folch solution and lysed by sonication. Isoprostane analysis was then conducted using the procedure as described previously.22 The results were expressed as the amount of isoprostane (ng) per mg of total protein.
JNK Activation Assay
The SuperArray CASE kit was used for cell-based detection of JNK protein phosphorylation (SuperArray, Frederick, MD) as described.23 Cells were treated with rotenone (or H2O2 as a positive control) before fixation in a 4% formaldehyde:PBS solution. The enzyme-linked immunosorbent assay was then performed according to the manufacturer’s instructions. The colorimetric change on cell total and phosphorylated JNK protein expression was measured at 450 nm. Relative cell number was also determined using the absorbance of 595 nm. The relative extent of JNK protein phosphorylation was then determined by normalizing the phosphoprotein-specific antibody A450:A595 ratio to the pan-protein-specific antibody A450:A595 ratio for the same experimental conditions.
Statistical Analysis
Unless otherwise indicated, all data are presented as the mean ± SEM of at least three independent experiments. The statistical significance (*P < 0.05 or **P < 0.01) was determined by one-way analysis of variance and the Tukey test using GraphPad (San Diego, CA) Prism 4.0.
Results
Discovery of Synaptosomal Proteins Potentially Involved in Disease Progression
Synaptosomal fractions were prepared from the pooled frontal cortex homogenate of PD patients at different stages (five subjects/group), ie, PD patients with LBs in the brainstem only (PD-brainstem), LBs in the brainstem and limbic system (PD-limbic), and LBs in the frontal cortex in addition to the brainstem and limbic system (PD-isocortex), respectively, along with normal age-matched controls. Notably, none of the PD-brainstem cases had demonstrable clinical dementia, whereas all PD-isocortex cases had unequivocal clinical dementia. Although no clear dementia was documented in PD-limbic cases, some kind of “cognitive impairment” was noted in the clinical history of all five patients. Also, of note, the pooling strategy was used to minimize the limitation commonly associated with all current MS technology, ie, variation in ionization of a sample and sampling of the ionized peptides. More detailed discussions on this issue can be found in our recent articles.8,24,25 The quality of the synaptosome preparation was assessed by Western blotting. As shown in supplementary Figure S1 (see http://ajp.amjpathol.org), the synaptosome preparation was enriched in both presynaptic (syntaxin) and postsynaptic (PSD-95) proteins relative to the tissue homogenate. Additionally, the levels of GFAP, which constitutes intermediate filaments in the astrocyte processes,26 and S-100B, a glial marker localized in the cytosol and nucleus of astrocytes,27 decreased significantly in the synaptosomal fraction compared with the homogenate, indicating that the glial contamination in the synaptosome preparation was minimal.
Repeated 4-plex comparison (each group labeled with a unique iTRAQ reagent) revealed a total of 1423 non-redundant (unique) proteins from synaptosomal fractions, after combining the results from all experiments. A full list of proteins identified in human cortex by two or more peptides and their characteristics, together with proteins identified with other fractionation and quantification methods, is reported separately.7 Among the total proteins identified, 318 unique proteins displayed significant changes (50% or more) in relative abundance between PD and control groups; 99 of these proteins were identified by two or more unique peptides. A complete list of the proteins identified by two or more peptides as well as by a single peptide can be found in supplementary Tables S1A and S1B (see Table S1 at http://ajp.amjpathol.org), respectively (proteins identified by a single peptide should be considered provisional according to consensus guidelines of most proteomics experts28). Notably, some of the proteins displayed progressive changes as the disease advanced; these include progressively increased proteins such as GSTP1, SH3GL2, and ubiquitin carboxyl-terminal hydrolase isozyme L1. Others, such as CNPase, progressively decreased (Table 1, and supplementary Table S1 at http://ajp.amjpathol.org) with disease progression.
Table 1.
Top Ranked Proteins (Gene Ontology Analysis) with Changes between Parkinson Disease Stages and Controls
| IPI number | Protein name | Peptides found* | PD-B/Ctrl† | PD-L/Ctrl† | PD-I/Ctrl† |
|---|---|---|---|---|---|
| IPI00306667 | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | 23 | 0.92 ± 0.02 | 0.87 ± 0.01 | 0.60 ± 0.02 |
| IPI00021369 | Alpha crystallin B chain | 4 | 0.60 ± 0.05 | 0.74 ± 0.07 | 0.51 ± 0.16 |
| IPI00032904 | Beta-synuclein | 4 | 1.52 ± 0.48 | 1.87 ± 0.90 | 2.33 ± 1.43 |
| IPI00218782 | Capping protein (Actin filament) muscle Z-line, beta | 2 | 1.04 ± 0.33 | 1.39 ± 0.27 | 1.68 ± 0.39 |
| IPI00456925 | Drebrin-like protein, isoform 1 | 2 | 0.72 ± 0.34 | 0.68 ± 0.09 | 0.52 ± 0.04 |
| IPI00027438 | Flotillin-1 | 4 | 1.53 ± 0.40 | 1.76 ± 0.53 | 1.67 ± 0.35 |
| IPI00219757 | Glutathione S-transferase P | 3 | 1.39 ± 0.17 | 1.60 ± 0.12 | 1.72 ± 0.26 |
| IPI00297714 | Gamma-synuclein | 3 | 1.23 ± 0.22 | 1.54 ± 0.27 | 1.66 ± 0.27 |
| IPI00025512 | Heat-shock protein beta-1 | 2 | 1.01 ± 0.02 | 1.57 ± 0.23 | 1.50 ± 0.49 |
| IPI00216475 | Myelin basic protein | 9 | 0.81 ± 0.08 | 0.91 ± 0.03 | 0.59 ± 0.09 |
| IPI00219813 | Reticulon-1, isoform RTN1-C | 3 | 0.99 ± 0.15 | 1.37 ± 0.20 | 1.52 ± 0.26 |
| IPI00021766 | Reticulon-4, isoform 1 | 3 | 0.66 ± 0.14 | 0.77 ± 0.08 | 0.59 ± 0.13 |
| IPI00019171 | SH3-containing GRB2-like protein 2 | 6 | 1.34 ± 0.28 | 1.53 ± 0.42 | 1.62 ± 0.42 |
| IPI00251507 | Synapsin-1, isoform IB | 18 | 1.38 ± 0.22 | 1.40 ± 0.21 | 1.51 ± 0.29 |
| IPI00023302 | Synapsin-2, isoform IIa | 6 | 1.63 ± 0.38 | 1.69 ± 0.49 | 1.72 ± 0.63 |
| IPI00018352 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 9 | 1.35 ± 0.29 | 1.45 ± 0.16 | 1.60 ± 0.13 |
Ctrl, Control; PD-B, PD-brainstem; PD-L, PD-limbic; PD-I, PD-isocortex. Protein names are from the IPI human protein database (version 3.18).
Peptides found denote the sum of all different peptides found and relatively quantified from the proteins shown in two replicate experiments.
The ratio shown is the mean of at least two independent experiments ± SE.
One of the key tasks in proteomic analysis involves reducing the dataset of candidate proteins to a reasonable panel of proteins for further investigation. To achieve this goal, GO analysis was applied for biological category analysis via the cumulative hypergeometric distribution method.15 This approach uses protein lists and identifies GO categories by evidence of over-representation of significant proteins. For the 99 significantly changed proteins identified by two or more peptides (ie, the proteins that were identified with high confidence), the top ranked GO biological process categories or most statistically over-represented biological functions include: (mitochondrial) electron transport, central nervous system (CNS) development, neurofilament/cortical actin cytoskeleton organization and biogenesis, negative regulation of apoptosis, and vesicle-mediated transport.
Table 1 shows proteins from the top ranked GO categories that were also consistently identified in at least two independent experiments. Several of the proteins are known to have potential roles in PD or other neurodegenerative disease pathogenesis, including ubiquitin carboxyl-terminal hydrolase isozyme L13 and GSTP1.29,30,31,32,33,34,35 In addition to these proteins, many other proteins, eg, CNPase36 and SH3GL2,37 are known to be important in the function of the CNS, but have yet to be linked to PD pathogenesis. Thus, we have not only confirmed the presence of significant proteins involved in PD pathophysiology, but also generated a list of proteins that may yield insight into novel mechanisms underlying PD progression.
Validation of Protein Expression by Western Blotting and Immunohistochemistry
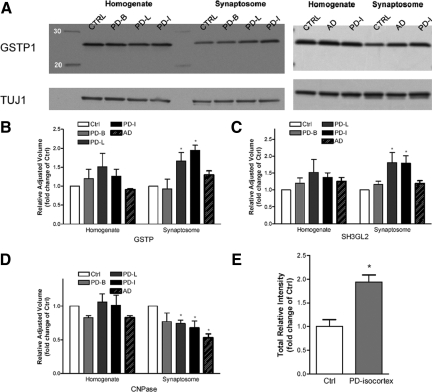
It cannot be overemphasized that any candidate proteins revealed by a high throughput discovery proteomic analysis need to be confirmed in the same samples by alternative means and then validated in different samples or models before their biological roles are investigated extensively, largely because proteins can be identified incorrectly due to current incomplete human protein databases.24,38 From the top-ranked proteins (Table 1), we chose GSTP1, SH3GL2, and CNPase as our first panel of candidates for confirmation. By proteomic analysis GSTP1 and SH3GL2 were significantly increased whereas CNPase was decreased in the late stages of PD cases (Table 1). These findings were all confirmed by Western blot analysis in the synaptosomal fraction (Figure 1, A–D, and supplementary Figure S2 at http://ajp.amjpathol.org). Of note, CNPase is expressed as two isoforms with a molecular mass of around 46 kDa (CNPI) and 48 kDa (CNPII), differing only by a 20-amino acid extension at the N-terminus.36 Only CNPI was significantly decreased under disease conditions (Figure 1D, and supplementary Figure S2 at http://ajp.amjpathol.org).
Figure 1.
Confirmation of selected protein expressions with Western blotting. A: Equal amounts of pooled homogenate and synaptosome samples (five/group, five groups total) were analyzed sequentially with an antibody against GSTP1, and TUJ1 (a neuron-specific β-tubulin) or β-actin (not shown) as a loading control. B–D: Quantitative analysis of Western blots for GSTP1 (B), SH3GL2 (C), and CNPase (D) in pooled samples. Protein expression differences were quantified by measuring the band intensities, and normalized to the level of TUJ1 expression. Similar results were obtained when using β-actin as the loading control (data not shown). E: With an identical approach as in (A), GSTP1 protein expression was analyzed and quantified again in synaptosome samples prepared from individual cases of the control and PD-isocortex groups. Data shown in (B–E) are the average ± SD of at least three independent experiments (*P < 0.05 compared with controls). CTRL: control; PD-B: PD-brainstem; PD-L: PD-limbic; PD-I: PD-isocortex. PD, Parkinson disease; AD: Alzheimer disease.
To determine whether the protein expression changes are specific to PD, we also prepared pooled homogenate and synaptosome samples from five cases of AD, another neurodegenerative disease that primarily affects the hippocampus and regions of cerebral cortex, including the frontal lobe.39 Western blot analyses revealed that the CNPase protein levels significantly decreased in both AD and PD groups (Figure 1D, and supplementary Figure S2 at http://ajp.amjpathol.org), suggesting its change is not PD-specific. In contrast, the levels of GSTP1 and SH3GL2, though also increased in the AD group, were not significant when compared with controls (Figure 1, A–C, and supplementary Figure S2 at http://ajp.amjpathol.org).
Thus far, both proteomic and Western blot analyses were done with pooled samples; this approach, although advantageous in many aspects,24 precludes statistical analysis and cannot assess whether the changes in protein levels resulted from a large change in a single patient or from similar changes in multiple patients. To address this issue, we prepared synaptosomal fractions from individual cases of the control and PD-isocortex groups. Western blot analyses were then conducted in these individual cases from which the samples were pooled. GSTP1 expression was increased in the majority of the synaptosomal fractions from the PD-isocortex cases as compared with the controls and average values in PD-isocortex were significantly greater than controls (P < 0.05) (Figure 1E). SH3GL2 and CNPase were not assessed in individual samples due to the limited amount of remaining human tissues.
GSTP1 has been shown to express in both glia and neurons,35,40,41,42,43 but the precise distribution of GSTP1 in neurons remains unclear. To examine the cellular and subcellular distribution of GSTP1 in human cortex, we performed immunohistochemical and confocal analyses in a separate set of PD patients and controls, asking whether GSTP1 is localized to neuronal processes, the focus of the current investigation. The results, shown in supplementary Figure S3 at http://ajp.amjpathol.org, demonstrated that although GSTP1 expression was relatively high in glia (as expected based on others’ observations35,40,41,42,43), it was indeed present in the neurites of at least a subset of the MAP2+ neurons. Additionally, it appeared that its neuronal expression was higher in PD cases than that in controls, but it was largely absent in LBs visualized by α-synuclein immunostaining (data not shown). Notably, the impression of increased expression of this protein in PD cases is consistent with what we observed by quantitative proteomic analysis and Western blotting (Table 1 and Figure 1). More definitive studies (eg, reciprocal immunoprecipitation of LBs and GSTP1) are needed, however, to confirm the confocal observation of the absence of GSTP1 in LBs.
Effects of Manipulating GSTP1 Expression on Rotenone-Induced Neurotoxicity
Among the two proteins whose expression significantly changed in the synaptosome fractions in PD, but not in AD, GSTP1 previously was suggested to be associated with PD in several case studies.29,30,31,32,33,34 Additionally, it has been demonstrated that dopaminergic neurons in Gstp1− knockout mice have an increased vulnerability to the Parkinsonian toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).35 There are no reports, however, that address whether GSTP1 is involved in PD progression, including development of dementia. Thus, we asked whether the increase in GSTP1 protein levels is a cellular defense response to offset the neurotoxicity as the disease progresses, or the increase contributes/correlates to the PD progression. We first generated plasmid vectors to overexpress human GSTP1A (wild-type) along with enhanced green fluorescent protein (eGFP) in cultured Neuro2A cells (a model for human cortical rather than midbrain dopaminergic neurons). At this point, it should be stressed that one of the major assumptions for clinical dementia in PD is a loss of cortical non-dopaminergic neurons with increased gliosis and formation of diffuse LBs in a significant portion of these cases.1,2,5 Supplementary Figure S4 (see Figure S4 at http://ajp.amjpathol.org) indicates that transient transfection of GSTP1 plasmids led to a moderate increase in expression over the normal endogenous level (two- to threefold), which was sufficient to reduce neurotoxicity-induced by rotenone (Figure 2A), a mitochondrial toxin that increases oxidative stress substantially and is widely used to model PD in animals and cells, given that oxidative stress and associated pathological changes are consistently observed in PD patients.25,44,45,46 More specifically, a 2-day rotenone treatment resulted in ∼50% cell death as measured by MTT assay, but had no significant effect on endogenous GSTP1 protein level when total cell lysate was analyzed (data not shown). Overexpressing GSTP1A, while having no effect on cells treated with vehicle (DMSO), significantly reduced rotenone-induced cell death from about 50% to 30% (Figure 2A).
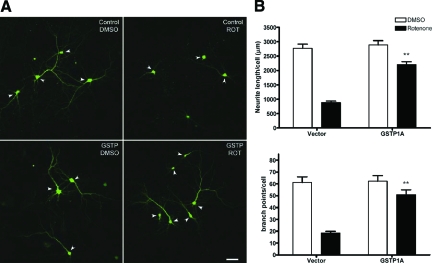
Figure 2.
Overexpression of GSTP1 protected Neuro2A cells from rotenone-induced cell death and neurite loss. A: Neuro2A cells were transfected with empty vector and wild-type GSTP1 before treatment with 20 nmol/L rotenone or DMSO as a control for 48 hours. The extent of cell survival was assessed by the MTT assay. In each experiment, averages of eight replicates were normalized to the average of the DMSO-treated vector control. Data represent mean ± SEM from at least three independent experiments. *P < 0.05 compared with rotenone-treated vector control. B: Images of five randomly selected fields for each condition were captured using a confocal microscope, and neurite processes were traced and quantified using the MicroBrightField Neurolucida software. Images from GSTP1B-D and GSTP1 Y7F mutant-transfected cells are not shown. C–D: Quantification of total neurite process length (C) and neurite branch points (D) per cell as in (B). Bars labeled C47A + C101A and Y7F represent two site directed mutants with reduced GST catalytic activity. Scale bar = 50 μm. *P < 0.05, **P < 0.01 compared with rotenone-treated vector control. For vector control and GSTP1A, n = 215 to 245 cells from eight independent experiments; for GSTP1B, C and D variants and the two mutants, n = 75 to 135 cells from three to five experiments.
A major caveat associated with our cell viability assay is that not all of the counted cells were transfected with GSTP1, meaning that the protective effect of GSTP1 may have been underestimated (Figure 2A). To address these issues and to investigate the effects of GSTP1 overexpression on earlier indices of neuronal health, changes in the number and branches of neurites were measured on transfected (GFP-positive) cells only. While rotenone treatment led to a dramatic decrease in neurite length and branching, overexpression of wild-type GSTP1 (GSTP1A) largely prevented this loss (Figure 2, B–D). It is known that GSTP1 displays polymorphisms in humans: the wild-type GSTP1A (Ile104 + Ala113) and three variant haplotypes, GSTP1B (Val104 + Ala113), GSTP1C (Val104 + Val113), and GSTP1D (Ile104 + Val113).47,48 These variants have reduced transferase activities toward some GSTP1 substrates compared with the wild-type.48 For example, the catalytic activities of GSTP1 variants toward acrolein, a reactive aldehyde that may be generated from lipid peroxidation following oxidative stress, are GSTP1A ≈ GSTP1B > GSTP1D > GSTP1C.49 We found that overexpression of GSTP1B, 1C, or 1D could also protect Neuro2A cells from rotenone-induced neurite loss, though at reduced levels (Figure 2C and D).
To ensure that the observed effect of GSTP1 overexpression was not an artifact associated with immortalized cell lines, we overexpressed GSTP1 using lipid-mediated transfection in primary cultured cortical neurons, which are more similar to human terminally differentiated cortical neurons. As can be seen in Figure 3, A and B, GSTP1A overexpression also prevented the neurite loss induced by rotenone in the primary cultured neurons relative to cultures transfected with vector alone. Of note is also the observation that the concentration of rotenone needed to produce toxicity was less than in cell lines.
Figure 3.
GSTP1 protected primary cortical cultures from rotenone-induced neurotoxicity. A: Representative images of mouse primary cortical neuron-enriched cultures overexpressing GFP only (control) or human GSTP1A together with GFP (GSTP) and treated with 2 nmol/L rotenone or DMSO as control for 2 days. Arrowheads indicate the MAP2+ neurons. Scale bar = 50 μm. B: Neurite length and complexity on GFP+/MAP2+ neurons were measured using the MicroBrightField Neurolucida software (**P < 0.01 compared with rotenone-treated vector control; n = 55 to 70 cells from ∼20 random selected fields and two independent cultures).
Transferase Activity Versus c-jun NH2-Terminal Kinase Signaling in the Protective Action of GSTP1
Two major functions have been proposed for GSTP1: detoxification by conjugation of reduced glutathione to many hydrophobic and electrophilic substrates,47,48 and regulation of c-jun NH2-terminal kinase (JNK) signaling through a direct protein-protein interaction.48,50 To test whether the protective role of GSTP1 is related to the JNK pathway, JNK activity was determined in Neuro2A cells transfected with GSTP1 and treated with rotenone. The results indicated that rotenone did not activate JNK, a result consistent with a previous report that rotenone treatment did not activate JNK or mitogen–activated protein kinase,51 and overexpression of GSTP1 had no effect on JNK activation in cells treated with or without rotenone (see supplementary Figure S5 at http://ajp.amjpathol.org).
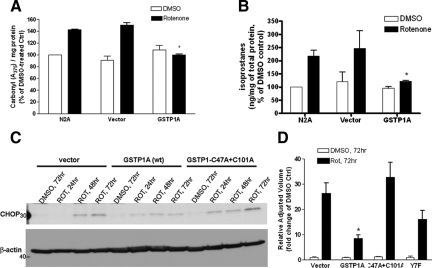
In contrast, when mitochondrial activity, oxidative stress and proteasome function (the major targets of rotenone-mediated neurotoxicity25,44,46,52) were measured, we observed marked protection of GSTP1 against rotenone-induced oxidative stress, ie, protein oxidation as indicated by carbonyl levels (Figure 4A) and lipid peroxidation as indicated by F2-isoprostane levels (Figure 4B). However, no significant difference in mitochondrial complex I or proteasomal activities was observed between rotenone-treated cells with and without GSTP1 overexpression (data not shown).
Figure 4.
GSTP1 protected cultured cells from rotenone-induced oxidative stress and ER stress. Non-transfected Neuro2A cells (N2A) and cells transfected with empty vector or wild-type GSTP1 (GSTP1A) were treated with 20 nmol/L rotenone or DMSO as a control for 72 hours. Extent of oxidative stress, ie, protein oxidation as indicated by carbonyl levels (A) and lipid peroxidation as indicated by isoprostane levels (B), is shown. C: Cells transfected with vector, wild-type and mutant GSTP1 were treated with rotenone or DMSO as a control for 24, 48, or 72 hours. Cell lysates were analyzed by immunoblotting using an antibody against CHOP, and β-actin as a loading control. D: Quantitative analysis of CHOP expression levels after 72 hour treatment obtained in (C). Results are expressed as percentage (A, B) or fold change (D) of DMSO-treated controls and are the mean ± SE from at least three independent experiments (*P < 0.05 compared with rotenone-treated controls).
Finally, to further confirm the role of transferase versus JNK-inhibiting activity of GSTP1 in neuroprotection, we generated a GSTP1 double mutant whose cysteines were mutated at amino acids 47 and 101 (required for GSTP1-1 dimerization) and a single GSTP1 mutant whose tyrosine at position 7 was replaced by phenylalanine (which abrogates catalytic proton transfer activity), respectively. These mutants retain the ability to inhibit JNK activity, but have greatly reduced transferase activity.50 Overexpressing either of the two mutants failed to protect against rotenone-induced neurite loss (Figure 2, B–D).
GSTP1 and ER Stress
While oxidative stress is well known to be important in human PD as well as PD models, including those induced by rotenone, precise downstream mechanisms have not been delineated. Given that proteasome function is apparently not involved in GSTP1-mediated protection, we investigated the potential effects of GSTP1 on ER stress, an adaptive mechanism that responds to the accumulation of unfolded proteins in the ER lumen (induced by exposure to oxidative stress53 or other mechanisms) by activating intracellular signal transduction pathways—cumulatively called the unfolded protein response.54 ER stress has been reported to be linked to neurite loss and neuronal death in neurodegenerative diseases such as AD and PD.55,56,57,58 To investigate whether GSTP1 protected cells from neurite loss and cell death via alleviating the ER stress and ER stress-induced apoptosis, we examined the effects of GSTP1 overexpression on rotenone-induced ER stress in Neuro2A cells. As shown in Figure 4, C and D, and Supplementary Figure S6 (see Figure S6 at http://ajp.amjpathol.org), rotenone treatment induced the phosphorylation of PERK, one of the ER stress sensors, and the expression of CHOP, an ER stress-induced pro-apoptotic protein.54 Overexpression of wild-type GSTP1 before rotenone treatment largely attenuated PERK activation and CHOP induction, but GSTP1 mutants with low catalytic activity had little effect, providing strong evidence that GSTP1 catalytic activity effectively reduced oxidative stress and the associated ER stress that are important components of neurodegeneration in PD progression.
Discussion
In this study, we discovered approximately 100 candidate proteins from synaptosomal fractions in human tissue that displayed significant differences in their relative abundance between PD patients at various stages (ie, clinical and pathological progression as far as cognitive impairment is concerned) and controls. The changes in expression of three of these proteins (GSTP1, SH3GL2, and CNPase) were confirmed using Western blotting. Furthermore, we validated that overexpressing GSTP1 in cultured cortical neuronal cells provided protection from rotenone-induced neurotoxicity and the protection appeared to be secondary to reduced oxidative stress and ER stress.
In the current study, it appeared that there were more increased than decreased proteins when more advanced PD cases were compared with the controls (see supplementary Table S1 at http://ajp.amjpathol.org). To explain this phenomenon, it is important to understand that protein quantification in our proteomic investigation is relative but not absolute. In an extreme case, for example, where there is a significant decrease in expression levels of a highly enriched protein (eg, 90% of total proteins for the sake of argument), it is easily understandable that there will be many more proteins of low abundance with increased values if a constant amount of protein was used. It should also be noted that quite a few proteins listed in Table 1 and supplementary Table S1 are traditionally considered as glial proteins. One possibility is that, despite the fact that only minimal glial contamination was observed in our synaptosomal preparation (see supplementary Figure S1 at http://ajp.amjpathol.org), the mass spectrometric technology used for protein identification is extremely sensitive, ie, even a trivial amount of contamination can be identified by this technology. Alternatively, it is also possible (our favored explanation) that a majority of these proteins are expressed by neurons as well. In fact, there is experimental evidence to suggest that this is indeed the case for some of these proteins. For example, myelin basic protein is an integral component of CNS myelin and a product of a larger gene complex called Golli (Genes of OLigodendrocyte Lineage).59 However, in situ hybridization and immunocytochemical analyses indicated that the golli-mbp products were expressed in selected neuronal populations in postnatal mouse brain, in addition to oligodendrocytes, and a shift in subcellular localization from nuclei and cell soma to the cell processes during development was evident within specific populations of neurons.60
The candidate proteins implicated in PD progression by proteomic analysis, though requiring further validation, are significant because many of them have been suggested to play important roles in PD pathogenesis or are important to the CNS in general. For example, ubiquitin carboxyl-terminal hydrolase isozyme L1, identified as progressively increased as the disease advanced (Table 1), is a neuron-specific ubiquitin hydrolase and ligase and its mutation has been identified in one German family affected by autosomal dominant familial PD.3,61 SH3GL2 (also known as endophilin-1), another progressively increased protein (Table 1, Figure 1C), localizes in brain presynaptic nerve termini and plays important roles in synaptic vesicle formation and recycling as well as intracellular signaling regulation.37 Two proteins that were decreased in the PD groups, CNPase and myelin basic protein, are known to be critically involved in myelination and other signaling pathways in the CNS.36,59 Although α-synuclein was not identified as significantly changed in this study, both β- and γ-synuclein displayed increased protein levels in PD groups compared with controls (Table 1). The biological roles of increased β- and γ-synuclein as a function of disease progression require further investigation. It should also be stressed that in profiling proteomics, the absence of a protein (eg, α-synuclein) simply means that it was not detected, ie, it does not necessarily mean it was absent from the sample or unchanged.8 This is because all current mass spectrometric techniques are associated with significant variations in ionizing and sampling of ionized peptides. Consequently, the overlapping rate of protein identification is rarely beyond 50% to 60% when an identical complicated sample is analyzed multiple times.24,38 Additionally, when a protein/peptide is extensively modified by post-translational modifications, which is likely the case for α-synuclein in PD,62 it will be exceedingly difficult to identify (and thus quantify) unless being targeted at specifically. Indeed, investigating various post-translational modifications of α-synuclein as PD progresses, including the development of dementia, is one of our ongoing projects.
Among the three proteins confirmed by alternative means, the GSTP1 protein levels increased in the synaptosomal fraction from PD patients at advanced stages (Figure 1). Given the role of oxidative stress in PD pathogenesis,3 it is likely that an increase in GSTP1, a major enzyme involved in regulation of oxidative stress by either conjugation of electrophiles or inhibiting JNK signaling,48 is an adaptive mechanism in response to progressive neurodegeneration as PD advances. Using GSTP1 mutants that lack the conjugating transferase activity but have preserved JNK-inhibiting activity, we have unequivocally demonstrated that the protection of GSTP1 against rotenone-induced neurotoxicity depends on its transferase activity (Figure 2, B–D). Additionally, we have provided evidence to explain a marginal or negative association between known GSTP1 polymorphisms and PD development in most epidemiology studies, except when the data are stratified against pesticide exposure and/or smoking.29,30,31,32,33,34 More specifically, our results demonstrated that, unlike the transferase-abolished mutants, overexpression of the three GSTP1 variants (GSTP1B, 1C, or 1D) provided significant protective effects (though less than the “wild-type” GSTP1A) against rotenone-induced neurite loss (Figure 2C and D), suggesting the association of these allelic variants to PD might not be apparent (unless a system is stressed by other conditions like pesticide exposure) and screening for new variants or mutants might be necessary. It needs to be stressed that, although we identified increased protein levels of GSTP1 in the synaptosomal preparations, suggesting that it might play a protective role in synapses, we only intended to reveal its general protective role in the in vitro PD models in this study. Further studies may be necessary to examine its roles in synapses, eg, in cortical cultures after synapses are developed and fully formed.
Our findings again emphasize a critical role of the enzymes involved in metabolism of glutathione, which has been shown to be decreased in PD patients,63,64 in neuronal loss. A recent study showed that transgenic mice with deficient expression of γ-glutamyl cysteine ligase, the rate-limiting enzyme in de novo glutathione synthesis in dopaminergic substantia nigra neurons, exhibited a selective reversible thiol-oxidation-dependent mitochondrial complex I inhibition in substantia nigra synaptosomal preparations, followed by an age-related nigrostriatal neurodegeneration in vivo.65 Another previous investigation demonstrated that knocking down GSTP1 in mice conferred the vulnerability of dopaminergic neurons to a parkinsonian toxicant—MPTP.35 However, our results suggest that an increase of GSTP1 expression in the brain may be an essential compensatory mechanism in non-dopamine neurons in PD progression as well. The fact that increased GSTP1 expression protected non-dopamine neurons from oxidative stress is of potential therapeutic relevance, because the level of GSTP1 expression can be induced, for example, by a variety of antioxidants and the Nrf2/antioxidant response element pathway in many tissues.47,66 The activation of the Nrf2/ antioxidant response element pathway is known to produce pleiotropic changes in gene expression that enhances the ability of cells to protect against oxidative stress.47
Our data also indicated that increased levels of GSTP1 preserved neurites in neurons exposed to rotenone and ER stress, which may be one of the major targets. Neurons contain the ER not only in the soma, but also in axons, dendrites, growth cones, and synaptic terminals.56,67 Recently, Murakami et al reported that ER stress sensors, inositol-requiring protein-1, activating transcription factor-6, and PERK exist in the ER of dendrites in primary mouse neurons, and that under ER stress conditions, GRP78/BiP and phosphorylated eIF2α were induced.68 ER stress has been reported to be linked to neurite loss and neuronal death in neurodegenerative diseases such as AD and PD.55,56,57,58 Analyses of brain tissues from PD patients also revealed increased levels of phosphorylated PERK and eIF2α compared with controls.69 On the other hand, compounds that can produce PD, including rotenone, 6-hydroxydopamine, and MPP+ (the active metabolite of MPTP), which induce oxidative stress and impair mitochondrial respiration and energy metabolism, were also shown to trigger ER stress/unfolded protein response and to induce CHOP and the phosphorylated PERK and inositol-requiring protein-1 in exposed neuronal cells.70,71 Our results suggest that neurodegeneration from rotenone exposure may result, at least in part, from ER stress. In addition, increased GSTP1 protein levels at synaptic termini may protect against prolonged or severe ER stress-induced neurodegeneration. The questions remain, however, how GSTP1 regulates ER stress and how ER stress ultimately causes neurodegeneration in brain regions underlying PD progression.
In conclusion, our report of proteomic profiling of human brain by the iTRAQ method provides a novel approach to study the proteins associated with the mechanisms of PD progression that cannot be investigated readily with current animal models. With pathologically verified human tissues at different disease stages, we identified many novel proteins that likely contribute to the progression of the disease. We confirmed three of these proteins including GSTP1, and validated this finding by demonstrating that increased GSTP1 expression protected cells from rotenone-induced neurotoxicity via attenuating oxidative stress and ER stress. Further elucidation of the mechanisms by which GSTP1 mediates neuroprotection may yield novel therapeutic targets in slowing PD progression.
Supplementary Material
Acknowledgments
We thank Drs. Robert Hevner and Francesco Bedogni for providing pCIG2 plasmids, Dr. Sheng Pan, Natalie Holroyd and Daniel Kashima for their assistance on data analysis, Dr. Richard Beyer for GO analysis, and Ms. Huihua Zhu for image capture and tracing.
Footnotes
Address reprint requests to Jing Zhang, M.D., Ph.D., Department of Pathology, University of Washington School of Medicine, HMC Box 359635, 325 9th Avenue, Seattle, WA 98104. E-mail: zhangj@u.washington.edu.
Supported by the National Institutes of Health (grants R01ES012703, R01AG025327, and R01AG033398) and the UW NIEHS sponsored Center for Ecogenetics and Environmental Health (NIEHS P30ES07033), as well as a Shaw Endowment to J.Z.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D, Jr, Price DL. The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol. 2007;66:251–257. doi: 10.1097/nen.0b013e3180415e42. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci USA. 2007;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults CW. Lewy bodies. Proc Natl Acad Sci USA. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Pan S, Shi M, Jin J, Albin RL, Lieberman A, Gearing M, Lin B, Pan C, Yan X, Kashima DT, Zhang J. Proteomics identification of proteins in human cortex using multi-dimensional separations and MALDI tandem mass spectrometer. Mol Cell Proteomics. 2007;6:1818–1823. doi: 10.1074/mcp.M700158-MCP200. [DOI] [PubMed] [Google Scholar]
- Shi M, Jin J, Wang Y, Beyer RP, Kitsou E, Albin RL, Gearing M, Pan C, Zhang J. Mortalin: a protein associated with progression of Parkinson disease? J Neuropathol Exp Neurol. 2008;67:117–124. doi: 10.1097/nen.0b013e318163354a. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22:1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J. Proteomic identification of novel proteins in cortical Lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Opp SM, Verlinde CL, Bammler TK, Eaton DL. Characterization of atrazine biotransformation by human and murine glutathione S-transferases. Toxicol Sci. 2004;80:230–238. doi: 10.1093/toxsci/kfh152. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Ince PG, Shaw PJ. Peroxynitrite and hydrogen peroxide induced cell death in the NSC34 neuroblastoma x spinal cord cell line: role of poly (ADP-ribose) polymerase. J Neurochem. 1998;70:501–508. doi: 10.1046/j.1471-4159.1998.70020501.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shie FS, Piccardo P, Montine TJ, Zhang J. Proteasomal inhibition induced by manganese ethylene-bis-dithiocarbamate: relevance to Parkinson’s disease. Neuroscience. 2004;128:281–291. doi: 10.1016/j.neuroscience.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Holley SL, Fryer AA, Haycock JW, Grubb SE, Strange RC, Hoban PR. Differential effects of glutathione S-transferase pi (GSTP1) haplotypes on cell proliferation and apoptosis. Carcinogenesis. 2007;28:2268–2273. doi: 10.1093/carcin/bgm135. [DOI] [PubMed] [Google Scholar]
- Abdi F, Quinn JF, Jankovic J, McIntosh M, Leverenz AB, Peskind E, Nixon R, Nutt J, Chung K, Zabetian C, Samii A, Lin M, Hattan S, Pan C, Wang Y, Jin J, Zhu D, Li JY, Liu Y, Waichunas D, Montine TJ, Zhang J. Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J Alzheimer Dis. 2006;9:293–348. doi: 10.3233/jad-2006-9309. [DOI] [PubMed] [Google Scholar]
- Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics. 2006;5:1193–1204. doi: 10.1074/mcp.M500382-MCP200. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: gFAP-thirty-one years (1969–2000). Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976;165:197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Domon B, Aebersold R. Challenges and opportunities in proteomics data analysis. Mol Cell Proteomics. 2006;5:1921–1926. doi: 10.1074/mcp.R600012-MCP200. [DOI] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Kelada SN, Stapleton PL, Farin FM, Bammler TK, Eaton DL, Smith-Weller T, Franklin GM, Swanson PD, Longstreth WT, Jr, Checkoway H. Glutathione S-transferase M1. T1, and P1 polymorphisms and Parkinson’s disease. Neurosci Lett. 2003;337:5–8. doi: 10.1016/s0304-3940(02)01286-7. [DOI] [PubMed] [Google Scholar]
- Deng Y, Newman B, Dunne MP, Silburn PA, Mellick GD. Case-only study of interactions between genetic polymorphisms of GSTM1. P1, T1 and Z1 and smoking in Parkinson’s disease. Neurosci Lett. 2004;366:326–331. doi: 10.1016/j.neulet.2004.05.061. [DOI] [PubMed] [Google Scholar]
- Wilk JB, Tobin JE, Suchowersky O, Shill HA, Klein C, Wooten GF, Lew MF, Mark MH, Guttman M, Watts RL, Singer C, Growdon JH, Latourelle JC, Saint-Hilaire MH, DeStefano AL, Prakash R, Williamson S, Berg CJ, Sun M, Goldwurm S, Pezzoli G, Racette BA, Perlmutter JS, Parsian A, Baker KB, Giroux ML, Litvan I, Pramstaller PP, Nicholson G, Burn DJ, Chinnery PF, Vieregge P, Slevin JT, Cambi F, MacDonald ME, Gusella JF, Myers RH, Golbe LI. Herbicide exposure modifies GSTP1 haplotype association to Parkinson onset age: the GenePD Study. Neurology. 2006;67:2206–2210. doi: 10.1212/01.wnl.0000249149.22407.d1. [DOI] [PubMed] [Google Scholar]
- Vilar R, Coelho H, Rodrigues E, Gama MJ, Rivera I, Taioli E, Lechner MC. Association of A313 G polymorphism (GSTP1*B) in the glutathione-S-transferase P1 gene with sporadic Parkinson’s disease. Eur J Neurol. 2007;14:156–161. doi: 10.1111/j.1468-1331.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Di Iorio G, Markopoulou K, Athanassiadou A, Papapetropoulos S, Watts RL, Vance JM, Bonifati V, Williams TA, Spychala JR, Stenroos ES, Johnson WG. Glutathione S-transferase polymorphisms and onset age in alpha-synuclein A53T mutant Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144:254–258. doi: 10.1002/ajmg.b.30450. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci USA. 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Reutens AT, Begley CG. Endophilin-1: a multifunctional protein. Int J Biochem Cell Biol. 2002;34:1173–1177. doi: 10.1016/s1357-2725(02)00063-8. [DOI] [PubMed] [Google Scholar]
- Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- Rathmann KL, Conner CS. Alzheimer’s disease: clinical features, pathogenesis, and treatment. Drug Intell Clin Pharm. 1984;18:684–691. doi: 10.1177/106002808401800902. [DOI] [PubMed] [Google Scholar]
- Cammer W, Tansey F, Abramovitz M, Ishigaki S, Listowsky I. Differential localization of glutathione-S-transferase Yp and Yb subunits in oligodendrocytes and astrocytes of rat brain. J Neurochem. 1989;52:876–883. doi: 10.1111/j.1471-4159.1989.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Tansey FA, Cammer W. A pi form of glutathione-S-transferase is a myelin- and oligodendrocyte-associated enzyme in mouse brain. J Neurochem. 1991;57:95–102. doi: 10.1111/j.1471-4159.1991.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, el Barbary A, Kornguth SE, Brugge JF, Siegel FL. Glutathione S-transferase isoenzymes in rat brain neurons and glia. J Neurosci. 1993;13:2013–2023. doi: 10.1523/JNEUROSCI.13-05-02013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carder PJ, Hume R, Fryer AA, Strange RC, Lauder J, Bell JE. Glutathione S-transferase in human brain. Neuropathol Appl Neurobiol. 1990;16:293–303. doi: 10.1111/j.1365-2990.1990.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gu G, Goodlett DR, Zhang T, Pan C, Montine TJ, Montine KS, Aebersold RH, Zhang J. Analysis of alpha-synuclein-associated proteins by quantitative proteomics. J Biol Chem. 2004;279:39155–39164. doi: 10.1074/jbc.M405456200. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Tew KD. Redox in redux: emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem Pharmacol. 2007;73:1257–1269. doi: 10.1016/j.bcp.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Pal A, Hu X, Zimniak P, Singh SV. Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of alpha, beta-unsaturated aldehydes. Cancer Lett. 2000;154:39–43. doi: 10.1016/s0304-3835(00)00390-6. [DOI] [PubMed] [Google Scholar]
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat. MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry CF, Ellison JA, Pribyl TM, Campagnoni C, Kampf K, Campagnoni AT. Myelin basic protein gene expression in neurons: developmental and regional changes in protein targeting within neuronal nuclei, cell bodies, and processes. J Neurosci. 1996;16:2452–2462. doi: 10.1523/JNEUROSCI.16-08-02452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Beyer K. Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol. 2006;112:237–251. doi: 10.1007/s00401-006-0104-6. [DOI] [PubMed] [Google Scholar]
- Perry TL, Godin DV, Hansen S. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Perry TL, Yong VW. Idiopathic Parkinson’s disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16:5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Hino SI, Saito A, Imaizumi K. Endoplasmic reticulum stress response in dendrites of cultured primary neurons. Neuroscience. 2007;146:1–8. doi: 10.1016/j.neuroscience.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Holtz WA, O'Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.