Abstract
Increased expression levels of tumor necrosis factor-α (TNFα) is involved in tubulointerstitial cell proliferation and apoptosis in obstructive renal injury. Two TNFα receptors (TNFRs), TNFR1 and TNFR2, are known to exist. On TNFα binding, TNFR1 recruits TNFR-associated death domain (TRADD), an assembly platform to mediate TNFR1 signaling. We investigated postreceptor TRADD regulation in rat kidneys with unilateral ureteral obstruction (UUO). Whereas UUO was associated with increased expression levels of TNFα, TNFR1, TNFR2, and TRADD mRNAs, it resulted in the marked decrease of TRADD protein levels (which appeared at day 1 and persisted thereafter) and a slight decrease in TNFR1 protein levels at days 7 and 14. Both ubiquitination and degradation of TRADD were increased in UUO kidneys, degradation of TRADD was stimulated by TNFα in HK-2 cells, and TRADD degradation was suppressed by proteasome inhibitor. Inhibition of TNFα by soluble TNFR2, etanercept, reduced significantly, although transiently, tubular and interstitial cell proliferation, fibronectin expression, and apoptosis in UUO kidneys, and also suppressed TRADD degradation. These data suggest that the decrease in TRADD resulting from enhanced ubiquitin-dependent degradation is involved in obstructive renal injury. Since TRADD is not incorporated into TNFR2-mediated TNFα signaling, the persistent decrease in TRADD, associated with a mild decrease in TNFR1 levels, may function, at least in part, to divert TNFα signals toward a TNFR2-mediated pathway in UUO kidneys.
Unilateral ureteral obstruction (UUO) is a well-established model of experimental renal injury characterized by significant renal tubular dilatation, proliferation, apoptotic cell death, and followed by tubulointerstitial fibrosis.1,2 In the kidney, cell proliferation is believed to be a central response to injury and culminates in the development of fibrotic renal damage.3 An imbalance between cell proliferation and apoptosis leads to unchecked apoptosis, resulting in progressive cell loss, renal tubular atrophy, and interstitial fibrosis.4 Tumor necrosis factor-α (TNFα) is a highly pleiotropic cytokine that induces diverse cellular responses ranging from proliferation and differentiation to activation of apoptosis.5 Overexpression of TNFα is reported to be involved in proliferation and apoptosis of renal tubular and interstitial cells in obstructive renal injury.6,7,8 However, little is known about the postreceptor regulation of TNFα signaling in renal lesions.
TNFα binds to TNF receptors (TNFR) to elicit its biological functions. There are two different cell-surface TNFRs; TNFR1 and TNFR2, which originate from separate gene products.9 On binding of TNFα, TNFR1 recruits the adaptor protein, TNFR associated death domain (TRADD), directly to its cytoplasmic death domain. In turn, TRADD serves as an assembly platform to diverge TNFR1 signaling. Interaction of TRADD with receptor interacting protein and TNF receptor associated factor 2 (TRAF2) leads to the activation of nuclear factor κB (NFκB).10 Furthermore, TRADD is also involved in the recruitment of Fas-associated protein with death domain, resulting in the initiation of apoptosis through activation of the caspase-8/3 cascade.11
On the other hand, the precise mechanism of TNFR2-mediated signaling is not fully elucidated. One report demonstrated that the binding of TNFα to TNFR2 recruits TRAF2 and induces NFκB activation.12 However, it was also shown that the binding of TNFα to TNFR2 causes ubiquitin-dependent degradation of TRAF2, resulting in the suppression of NFκB activation through the inhibition of TRADD, receptor interacting protein, and TRAF2 complex formation, and finally leading to TNFR1-mediated TNF-α signaling toward the pro-apoptotic direction.13
At present, the differential contribution of TNFR1- and TNFR2-mediated TNFα signaling is not fully elucidated in renal lesions. Ramesh et al9 reported that renal injury induced by cisplatin was less severe in TNFR2-deficient mice than TNFR1-deficient mice. In contrast, Guo et al1 reported that the renal lesions in UUO mice were less severe in TNFR1 knockout mice compared with TNFR2 knockout mice. There is no report on the involvement and regulation of TRADD, an assembly platform to diverge TNFR1 signaling, in the development of renal lesions. In the present study, we investigated the postreceptor regulation of TRADD in the UUO rat kidneys. The effect of TNFα inhibition by etanercept, a soluble TNFR2, was also studied in UUO rat kidneys.
Materials and Methods
Experimental Animals and Design
Male Wistar rats, weighing 200 g at the start of the experiment, were prepared. UUO was achieved by ligating the left ureter with 3-0 silk through a left lateral incision. Sham-operated rats (n = 8) were used as a control. Rats were sacrificed 1, 3, 7, or 14 days after surgery (n = 8 for each group), and obstructed kidneys were harvested and subjected to the studies described below.
To investigate the effects of etanercept, a soluble TNFR2 that inhibits TNFα binding to TNFR, on UUO kidney lesions and postreceptor regulation of TRADD, additional rats were allocated to the following four groups: 1) six rats with UUO treated with subcutaneous injections of etanercept at 1.25 mg/kg/day, 24 hours before UUO and every 24 hours thereafter; 2) six rats with UUO treated with the same amount of human IgG instead of etanercept; 3) six sham-operated rats treated with etanercept using the above dose; and 4) six sham-operated rats treated with human IgG in a manner similar to that described above. The UUO kidney tissues were harvested at day 3 and prepared as described below. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Hamamatsu University School of Medicine.
Histopathological and Immunohistochemical Analysis
Kidney tissues were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and embedded in paraffin. Tissue sections (3 μm thick) were stained with Masson’s trichrome for histopathological analysis. Immunoreactivity for TRADD and Ki-67 proteins was determined by using a standard biotin-streptavidin-peroxidase method, as described previously.14,15 The primary antibodies were mouse anti-human TRADD (BD Bioscience, San Jose, CA) and mouse anti-human Ki-67 (Novocastra, Newcastle, UK). The secondary antibodies were affinity-purified biotinylated goat anti-mouse IgG (Nichirei, Tokyo, Japan). Nuclei were counterstained lightly with hematoxylin. The numbers of Ki-67-positive renal tubular epithelial and interstitial cells were counted separately in 10 randomly selected nonoverlapping renal cortical fields observed at ×400 magnification in each sample.
Evaluation of Apoptosis
Deoxynucleotidyl transferase enzyme for terminal deoxynucleotidyl transferase dUTP nick-end labeling assay was performed to detect apoptotic cell death using the ApopTag plus peroxidase in situ apoptosis detection kit (Chemicon, Temecula, CA) as described previously.16 In each kidney, the numbers of tubular and interstitial apoptotic cells were counted separately in 20 nonoverlapping fields observed at ×400 magnification.
Verification of Specificity of Anti-TRADD Antibody
To verify the specificity of monoclonal anti-human TRADD antibody (BD Bioscience), which was used to detect the levels of rat TRADD protein in the present study, we examined its reactivity against the recombinant FLAG-tagged rat TRADD and human TRADD proteins, which were generated by human embryonic kidney cells (HEK293 cells). The full-length FLAG-tagged rat TRADD (FLAG rat TRADD) plasmid was constructed as follows: rat TRADD cDNA was prepared from NRK cells, a rat kidney cell line, using polymerase chain reaction (PCR) and inserted into pCMV (Stratagene, La Jolla, Ca). The sequence of PCR primers was as follow: rat TRADD (sense: 5′-TATCGAATTCAATGGCAGCTGATCAGAA-3′ and antisense: 5′-AGTACTCGAGTTAAGCCTGGCCGCCTT-3′). The insert was subjected to DNA sequencing and confirmed to have no error. pcDNA3 FLAG-tagged full-length human TRADD (FLAG-TRADD) was kindly provided by N. Fujita (Japanese Foundation for Cancer Research, Tokyo, Japan). HEK293 cells were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum at 37°C under 95% air–5% CO2 atmosphere. HEK293 cells were transfected with FLAG rat TRADD vector and FLAG-TRADD vector by using FuGene 6 (Roche, Mannheim, Germany), respectively. As a control, pCMV lacking TRADD cDNA insert was used. The cells were lysed with Triton X-100 lysis buffer after incubation for 48 hours. Because HEK293 cells carry endogenous TRADD, which also reacts with anti-TRADD antibody, the recombinant FLAG rat TRADD and FLAG-TRADD proteins were purified and collected by immunoprecipitation using anti-FLAG M2 beads (Sigma, St. Louis, MO), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblot analysis with monoclonal anti-human TRADD antibody.
Immunoblot Analysis
Kidney tissues were extracted with Triton X-100 lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 300 mmol/L NaCl, 0.5% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 0.1 mmol/L sodium orthovanadate,1 mmol/L sodium fluoride, and 10 mmol/L β-glycerophosphate. The lysates were homogenized and incubated for 30 minutes at 4°C, and centrifuged for 10 minutes at 9000 × g. The resulting supernatants were subjected to immunoblot analysis as described previously.14,15 The primary antibodies were rabbit anti-TNFR1 (Gene Tex, Irvine, CA), rabbit anti-TNFR2 (Cell Signaling Technology, Danvers, MA), mouse anti-human TRADD (BD Bioscience), rabbit anti-IκB-α (Cell Signaling Technology), and mouse anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies were horseradish peroxidase-conjugated anti-mouse and horseradish peroxidase-conjugated anti-rabbit IgG (Promega, Madison, WI). Immunoblotted protein bands were visualized using an enhanced chemiluminescence system (PerkinElmer, Waltham, MA). We used GAPDH as an internal control. The band intensity was quantified using the National Institute of Health Image analysis software (Bethesda, MD).
RNA Isolation and Quantitative Analysis of mRNA by Real-Time Reverse Transcription-PCR
Total RNA was extracted from renal tissue using ISOGEN (Nippon Gene Inc., Tokyo) according to the instructions provided by the manufacturer. Reverse transcription of the RNA was performed using the first-strand cDNA synthesis kit (Roche). For real-time PCR, the Light Cycler PCR and detection systems (Roche) were used for amplification and online quantification. All PCR experiments were performed using the QuantiTect SYBR Green PCR kit (Qiagen, Tokyo). The sequences of PCR primers were as follow: rat TNFα (sense: 5′-GCTCCCTCTCATCAGTTCC-3′ and antisense: 5′-CTCCTCTGCTTGGTGGTTTG-3′); rat TNFR1 (sense: 5′-TGACCCTCTCCTCTACGGA-3′ and antisense: 5′-CCATCCACCACAGCATACA-3′); rat TNFR2 (sense: 5′-TAGGACTGGCGAACTGCTT-3′ and antisense: 5′-AACTGGGTGCTGTGGTCAAT-3′); rat TRADD (sense: 5′-TGGCAATCTACAAGGCTCTG-3′ and antisense: 5′-GAAACGCAACTGAACGATGA-3′); rat fibronectin (sense: 5′-GTGTCTCCAGCGTGTACGAA-3′ and antisense: 5′-GGCGGTGACATCAGAAGAAT-3′) and rat GAPDH (sense: 5′-ATGACTCTACCCACGGCAAG-3′ and antisense: 5′-TACTCAGCACCAGCATCACC-3′). Data analysis was performed by using Light Cycler software (version 3.3.9; Roche). The mRNA level relative to that of GAPDH was calculated in each sample.
Degradation Assay for TRADD in Renal Extracts
To investigate the degradation activity directed against TRADD in the kidneys, we conducted in vitro degradation assay for endogenous TRADD using renal extracts collected from UUO kidneys at day 1 and those from sham-operated kidneys, as described previously.14,17 Renal extracts (15 μg) were mixed with 15 μl of reaction buffer containing 50 mmol/L Tris-HCl (pH 8.3), 5 mmol/L MgCl2, 2 mmol/L dithiothreitol, 5 mmol/L adenosine-5′-triphosphate, and 2 mg/ml ubiquitin, and incubated for 0, 1, 2, and 4 hours at 37°C. After incubation, each sample was subjected to SDS-PAGE, followed by immunoblot analysis with anti-TRADD antibody. The effect of proteasome inhibitor, 0.25 mmol/L MG132, on the degradation of TRADD was also examined.
In Vitro TRADD Ubiquitination Assay
To investigate whether the ubiquitin-dependent degradation of TRADD is involved in UUO kidneys, we performed in vitro assay of ubiquitination activity directed against exogenous TRADD, as described previously.14,17,18 FLAG-TRADD protein was prepared from HEK293 cells transfected with pcDNA3 FLAG-tagged full-length human TRADD (FLAG-TRADD) using FuGene 6 (Roche). As a control, pcDNA3 lacking FLAG-TRADD was used. Renal extracts that were obtained from UUO and sham-operated kidneys, were lysed in Triton X-100 lysis buffer, and then centrifuged for 8 hours at 10,000 × g. The resulting supernatants were used for the ubiquitination assay. The polyubiquitinated FLAG-TRADD was detected by immunoblotting with anti-ubiquitin antibody (FK2; Nippon Biotest Labo, Tokyo) or anti-FLAG M2 antibody (Sigma).
In Vivo TRADD-Ubiquitination Assay
pcDNA3- FLAG-TRADD or pcDNA3 plasmid lacking FLAG-TRADD plasmid was transfected with or without HA-Ub plasmid into HEK293 cells in 10-cm dishes using FuGene6 (Roche). To induce accumulation of polyubiquitinated FLAG-TRADD, cells were treated with a proteasome inhibitor, 10 μmol/L MG132, for 3 hours starting at 45 hours after transfection. The cells were lysed with Triton X-100 lysis buffer 48 hours after transfection and harvested. The cell lysates were incubated with anti-FLAG M2 affinity gel 20 μl (Sigma) at 4°C for 2 hours and polyubiquitinated FLAG-TRADD were purified and collected by double immunoprecipitation. The first complexes that were immunoprecipitated using anti-FLAG M2 beads were denatured by treatment with SDS sample buffer at 100°C for 7 minutes. Then, ubiquitinated FLAG-TRADD were immunoprecipitated again with anti-FLAG M2 beads, separated by SDS-PAGE (9%), and detected by immunoblot with anti-HA antibody (Roche) or anti-FLAG M2 antibody (Sigma).
Degradation Assay for TRADD in HK-2 Cells Stimulated by TNFα
HK-2 cells, an immortalized proximal tubule cell line derived from normal adult human kidney,19 were obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium/F-12 medium supplemented with 10% fetal calf serum at 37°C in a 95% air–5% CO2 atmosphere and passaged every 7 days.20 To investigate the effect of TNFα on the degradation of endogenous TRADD and IκB, HK-2 cells were incubated with 100 ng/ml of recombinant TNFα (Sigma) for 0, 2, 4 and 8 hours, and then harvested, homogenized, separated by SDS-PAGE (9%), and detected by immunoblot with anti-TRADD or anti- IκB antibody. The effect of proteasome inhibitor, 10 μmol/L MG132, on their degradation was also examined.
Statistical Analysis
All values were expressed as mean ± SEM Differences between groups were examined for statistical significance using analysis of variance. When a significant difference was found, further statistical analysis was performed using the Scheffé F-test between the two groups. P < 0.05 was considered statistically significant.
Results
Renal Lesions, Expression of TNFα, TNFR1, and TNFR2 mRNAs, and Proliferating and Apoptotic Cells in UUO Kidneys
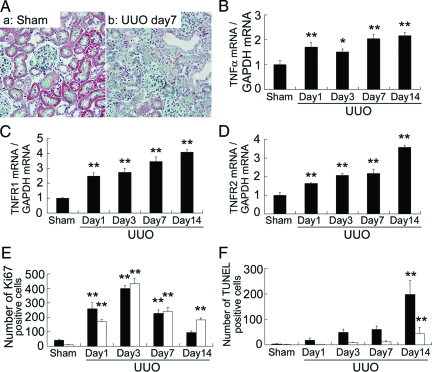
Progression of tubulointerstitial fibrosis, tubular atrophy and dilatation, and interstitial mononuclear cell infiltration were noted in the obstructed kidneys of UUO rats (Figure 1A). These changes were associated with marked and time-dependent overexpression of TNF-α, TNFR1 and TNFR2 mRNAs (Figure 1, B–D). The numbers of Ki-67-positive proliferating tubular epithelial and interstitial mononuclear cells were increased 1 day after UUO, reached peak values at day 3, and then decreased gradually thereafter (Figure 1E). The numbers of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive apoptotic tubular epithelial and interstitial cells were also increased at day 14 after UUO (Figure 1F).
Figure 1.
Renal lesions (A), expression of TNFα (B), TNFR1 (C), and TNFR2 (D) mRNAs, Ki-67-positive proliferating (E), and terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive apoptotic (F) cells in UUO kidneys. A: Representative pictures of sham-operated (Sham) and UUO kidneys at day 7. Note the progression of tubulointerstitial fibrosis, tubular atrophy, and dilatation, and interstitial mononuclear cell infiltration in UUO kidneys. Masson’s trichrome stain; original magnification, ×400. The closed and open bars in E–F represent the number of tubular epithelial and interstitial mononuclear cells, respectively. Data are mean ± SEM, *P < 0.05, **P < 0.01 compared with sham-operated kidneys.
Specificity of Anti-TRADD Antibody
Significant immunoreactivity directed against recombinant rat and human TRADD proteins was noted (Figure 2). No significant nonspecific bands were noted by Western blot analysis in rat kidney lysates.
Figure 2.
Specificity of anti-TRADD antibody. Western blot analysis of monoclonal anti-human TRADD antibody demonstrated significant immunoreactivity directed against recombinant rat and human TRADD proteins. Monospecificity of the antibody was also confirmed in rat kidney lysate. IgG bands are indicated by asterisks.
Low Levels of TRADD and TNFR1 Proteins, but Not mRNA, in UUO Kidneys
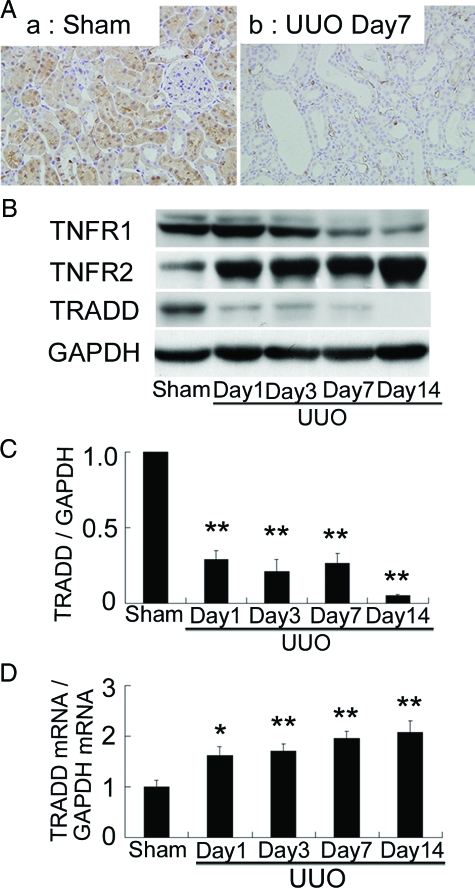
In sham-operated kidneys, significant immunoreactivity for TRADD protein was observed in proximal tubular cells and weak immunoreactivity was also found in the distal tubular cells (Figure 3A). In contrast, immunoreactivity for TRADD was markedly low in UUO kidneys (Figure 3A). No significant immunoreactivity was observed in the control serial sections of sham-operated kidneys incubated with nonimmune mouse IgG instead of mouse anti-TRADD antibody (data not shown). These findings were further confirmed by Western blot analysis. When compared with sham-operated kidneys, the level of TRADD protein was low at day 1 after UUO, and remained at that level thereafter in UUO kidneys (Figure 3, B and C). To investigate whether the reduction in TRADD protein was the result of down-regulation of TRADD mRNA, we evaluated the expression of TRADD mRNA by real-time RT-PCR. In contrast to the marked decrease in TRADD protein, TRADD mRNA expression was significantly augmented in UUO kidneys (Figure 3D). These results suggest that the reduction of TRADD protein in UUO kidneys could be due to enhanced protein degradation.
Figure 3.
Immunostaining and Western blot analysis of TNFR1, TNFR2, and TRADD proteins and expression of TRADD mRNA. A: In sham-operated kidneys, note the significant immunoreactivity for TRADD protein in the proximal tubular cells and the weak immunoreactivity in the distal tubular cells. Note also the markedly decreased immunoreactivity for TRADD in UUO kidneys. Original magnification, ×400. B: Western blot analysis showed mild decreases in TNFR1 protein at days 7 and 14, progressive increases in TNFR2 protein, and marked decreases in TRADD protein in UUO kidneys. The decrease in TRADD protein was noted at day 1 after UUO and remained low thereafter. C: Densitometric evaluation of TRADD protein in UUO kidneys. D: In contrast, expression of TRADD mRNA detected by real-time RT-PCR was significantly augmented in UUO kidneys. Data are mean ± SEM; *P < 0.05, **P < 0.01 compared with sham-operated kidneys.
Mild decreases in TNFR1 protein, despite increased TNFR1 mRNA expression as shown in Figure 1C, were noted at days 7 and 14 after UUO (Figure 3B). In contrast, a marked increase in TNFR2 protein, which was associated with increased TNFR2 mRNA expression (Figure 1D), was observed in UUO kidneys (Figure 3B).
Enhanced Proteasomal Degradation of Endogenous TRADD Protein in UUO Kidneys
To confirm the enhanced degradation of TRADD protein in UUO kidneys, we subsequently subjected the renal extracts to in vitro degradation assay. Degradation of endogenous TRADD was increased in renal extracts collected from UUO kidneys at day 1, when TRADD protein level was low (Figure 4, A and B). In contrast, no significant reduction in TRADD protein was noted in renal extracts collected from sham-operated kidneys (Figure 4, A and B). The degradation of endogenous TRADD protein was abrogated by the addition of proteasome inhibitor, MG132 (Figure 4C). These results indicate enhanced proteasomal degradation of TRADD protein in UUO kidneys.
Figure 4.
Enhanced degradation of endogenous TRADD protein in UUO kidneys. A: Renal extracts collected from sham-operated kidneys and UUO kidneys at day 1 were mixed with the ubiquitination mixture, incubated for 0 to 4 hours at 37°C, and the reaction mixtures were subjected to immunoblot analysis to detect degradation of endogenous TRADD protein. B: Densitometric intensity of TRADD protein relative to the level at time 0 in each group (closed circles, Sham; open circles, UUO) Degradation of endogenous TRADD was significantly increased in UUO kidney extracts, whereas no significant reduction was noted in sham-operated kidney extracts. Data are mean ± SEM *P < 0.01 compared with 0 hours of UUO kidney extracts, #P < 0.01 compared with 4 hours of sham-operated kidney extracts. C: Degradation of TRADD protein in UUO kidney extracts was prevented by the addition of proteasome inhibitor, 0.25 mmol/L MG132.
Enhanced Ubiquitination Activity Directed Against Exogenous TRADD Protein in UUO Kidneys
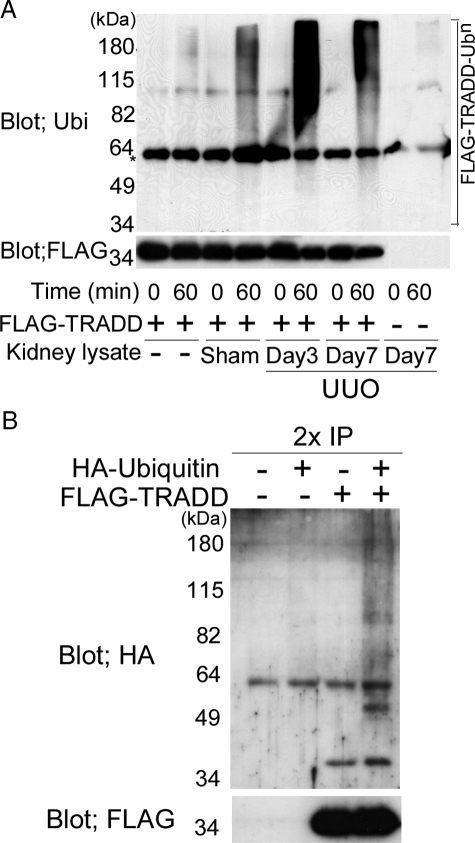
To examine whether the ubiquitin-proteasome system is involved in the increased degradation of TRADD protein in UUO kidneys, we investigated the in vitro ubiquitination activity directed against exogenous TRADD protein, which was derived from HEK293 cells transfected with pcDNA3-FLAG-TRADD expression plasmid, and was immunoprecipitated by anti-FLAG M2 affinity gels as a substrate. Significant ladders were observed when the UUO kidney extracts were incubated with FLAG-TRADD protein and immunoblotted with anti-ubiquitin antibody (Figure 5A). Only a few weak ladders were noted in the sham-operated kidney extracts. Ladders were scarcely observed when FLAG-TRADD protein was incubated with no renal extracts. Furthermore, no significant bands were observed when anti-FLAG immunoprecipitated protein, which was obtained from HEK293 cells transfected with pcDNA3 lacking FLAG-TRADD as a negative control, was incubated with renal extracts collected from UUO rats at day 7 (Figure 5A). These results suggest that the ladders noted in the UUO kidney extracts were polyubiquitinated TRADD proteins.
Figure 5.
In vitro ubiquitination assay of TRADD in UUO kidney extracts (A) and in vivo TRADD-ubiquitination in HEK 292 cells (B). A: Renal extracts collected from sham-operated and UUO kidneys at day 7 were incubated with immunopurified FLAG-tagged TRADD (FLAG-TRADD) protein for 60 minutes at 37°C. The resulting immunoprecipitates were washed thoroughly four times with cold lysis buffer, then FLAG-TRADD was eluted by FLAG peptide, and the assay products were subjected to immunoblot analysis using anti-ubiquitin antibody. Significant ladders were observed when FLAG-TRADD protein was incubated with renal extracts collected from UUO kidneys, but only a few weak ladders were noted when FLAG-TRADD protein was incubated with the extracts from sham-operated kidneys. Scant ladders were observed when FLAG-TRADD protein was incubated without renal extracts. Furthermore, no significant bands were observed when anti-FLAG immunoprecipitated protein, which was derived from HEK293 cells transfected with pcDNA3 lacking FLAG-TRADD as a negative control, was incubated with renal extracts collected from UUO rat at day 7. IgG bands are indicated by an asterisk. B: pcDNA3-FLAG-TRADD plasmid was transfected together with or without pCGN-HA-tagged ubiquitin (HA-Ub) plasmid into HEK293 cells. Forty-five hours after transfection, the cells were incubated with or without 10 μmol/L MG132 for 3 hours. The immunoprecipitates obtained from the cell lysates using anti-FLAG M2 beads were washed four times with lysis buffer and subjected to double immunoprecipitation. The first complexes immunoprecipitated using anti-FLAG M2 beads were denatured by treatment with SDS sample buffer at 100°C for 7 minutes. Then, ubiquitinated FLAG-TRADD was reimmunoprecipitated with anti-FLAG M2 affinity beads, separated by SDS-PAGE (9%), and detected by immunoblot with anti-HA antibody or anti-FLAG M2 antibody. Significant ladders of polyubiquitinated TRADD were observed when FLAG-TRADD was transfected with HA-Ub, but no ladders were noted without HA-Ub transfection.
TRADD Ubiquitination in HEK293 Cells Transfected with TRADD and Ubiquitin
Next, we investigated whether TRADD is ubiquitinated in HEK 293 cells. pcDNA3-FLAG-TRADD plasmid was transfected with or without HA-Ub plasmid into HEK293 cells and polyubiquitinated FLAG-TRADD proteins were purified and collected by double immunoprecipitation. Significant ladders of polyubiquitinated TRADD were observed when FLAG-TRADD was transfected with HA-Ub, but no ladders were noted without HA-Ub transfection (Figure 5B).
TNFα Stimulates Degradation of TRADD and IκB in HK-2 Cells
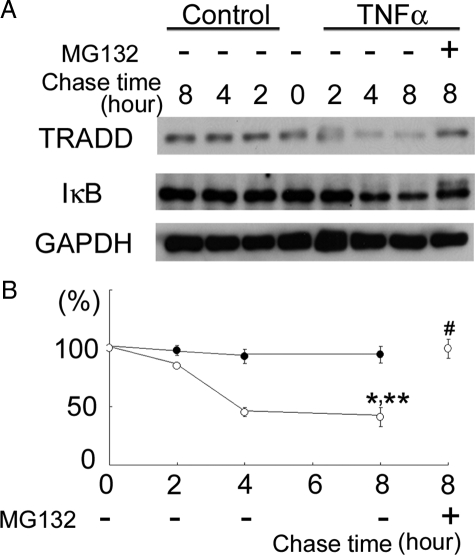
We investigated the degradation activity directed against TRADD in HK-2 cells stimulated by TNFα. The degradation of NFκB inhibitor (IκB) was also examined as a positive control for TNFα-induced degradation, based on a previous study that demonstrated TNFα-induced activation of the IκB kinase (IKK) complex, which is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NEMO (also known as IKKχ), followed by phosphorylation of IκB by IKKβ, resulting in polyubiquitination and subsequent proteasomal degradation of IκB.21 TNFα caused TRADD and IκB degradation in a time-dependent manner, and the degradation was blocked by proteasome inhibitor, MG132 (Figure 6, A and B). These results indicate that TNFα stimulates proteasomal degradation of TRADD and IκB.
Figure 6.
Degradation of TRADD and IκB induced by TNFα in HK-2 cells. A: TNFα, 100 ng/ml, was added to HK-2 cell cultures and incubated for the indicated time periods. TRADD and IκB levels in cell lysates were investigated by immunoblot analysis. B: The densitometric intensity of TRADD protein relative to the level at time 0 in each group [closed circles, TRADD (control HK-2 cells); open circles, TRADD (TNFα-treated HK-2 cells)]. TNFα caused significant degradation of TRADD in a time-dependent manner, and the degradation was blocked by 10 μmol/L MG132. Data are mean ± SEM. *P < 0.01, compared with TRADD level in TNFα-treated HK-2 cells at 0 hours, **P < 0.01, compared with TRADD level in control HK-2 cells at 8 hours, #P < 0.01, compared with TRADD level in TNFα-treated HK-2 cells [MG132 (−)] at 8 hours.
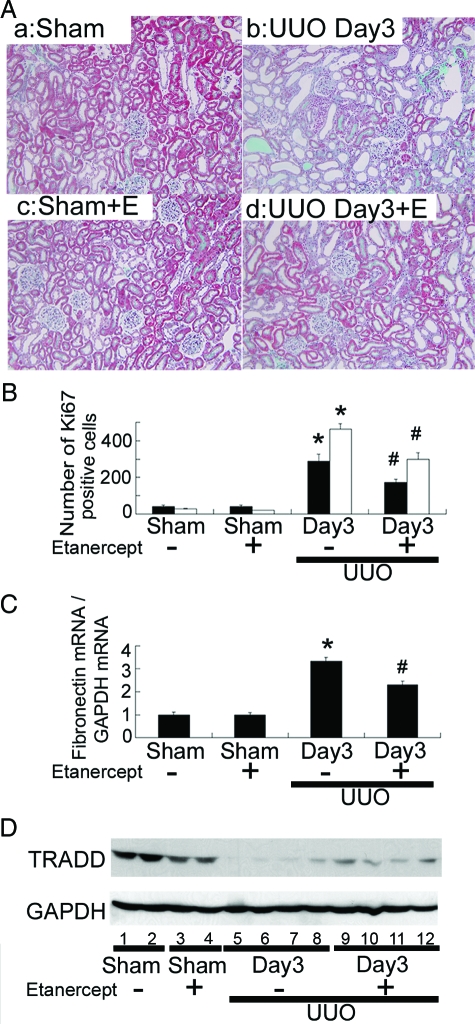
Soluble TNFR2, Etanercept, Reduces Tubulointerstitial Cell Proliferation, Fibronectin mRNA Expression, and TRADD Degradation in UUO Kidneys
Finally, we investigated the effects of inhibition of TNFα binding to TNFR by administration of soluble TNFR2, etanercept, on UUO kidney lesions. Treatment with etanercept significantly reduced tubulointerstitial fibrosis, tubular atrophy and dilatation, and interstitial mononuclear cell infiltration in UUO kidneys at day 3 (Figure 7A). Etanercept also significantly reduced the numbers of Ki-67-positive proliferating tubular and interstitial cells (Figure 7B) and terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive apoptotic tubular and interstitial cells (data not shown), as well as the expression of fibronectin mRNA (Figure 7C) and degradation of TRADD protein (Figure 7D) in UUO kidneys at day 3. However, these ameliorative effects of etanercept were transient and no significant differences were noted between etanercept-treated and -untreated UUO rats at days 7 and 14 (data not shown). No significant renal lesions were noted in sham-operated rats treated with etanercept (Figure 7A).
Figure 7.
Effects of TNFα inhibition by administration of soluble TNFR2, etanercept, on UUO kidney lesions (A), tubulointerstitial cell proliferation (B), fibronectin mRNA expression (C), and TRADD degradation (D) in UUO kidneys. A: Representative photographs of kidneys from sham-operated rats treated with human IgG (a) and etanercept (+E) (c), and from UUO rats treated with human IgG (b) and etanercept (+E) (d) at day 3. A: Treatment with etanercept significantly reduced tubulointerstitial fibrosis, tubular atrophy and dilatation, and interstitial mononuclear cell infiltration in UUO kidneys at day 3. Masson’s trichrome stain; original magnification, ×200. The number of Ki-67-positive proliferating tubular epithelial (closed bars) and interstitial mononuclear cells (open bars) (B), the ratios of fibronectin mRNA/GAPDH mRNA (C), and the degradation of TRADD protein demonstrated by Western blot (D) were also significantly suppressed by etanercept treatment in UUO kidneys at day 3. Data in B and C are mean ± SEM *P < 0.01 compared with sham-operated kidneys, #P < 0.01 compared with UUO kidneys at day 3.
Discussion
Tubulointerstitial fibrosis is the final common feature of end-stage renal damage in various types of kidney diseases, and its severity correlates with renal prognosis.22 Overexpression of TNFα has been shown to be involved in cell proliferation and apoptosis in renal tubular and interstitial cells in obstructive renal injury.1,6,7,8
TRADD serves as an assembly platform to diverge TNFR1 signaling, including activation of NFκB through interaction with receptor interacting protein andTRAF2,10 and induction of apoptosis through activation Fas-associated protein with death domain/caspase-8/3 cascade.11 However, the pathogenic implication of the intracellular TRADD regulatory mechanism in obstructive renal injury remains to be elucidated. Our study was designed to investigate the postreceptor regulation of TRADD-mediated TNFα signaling in rat kidneys with UUO.
In sham-operated kidneys, significant immunoreactivity for TRADD protein was observed in the proximal tubular cells and weak immunoreactivity was also found in the distal tubular cells. Increased expression of TNFα, TNFR1, TNFR2, and TRADD mRNAs was observed in UUO rats. However, TRADD protein was decreased markedly at day 1 after UUO and its level remained low thereafter. Both degradation and ubiquitination of TRADD were increased in UUO kidneys and its degradation was suppressed by proteasome inhibitor. Enhanced polyubiquitination of TRADD was noted in HEK293 cells transfected with TRADD and ubiquitin. Inhibition of TNFα by administration of soluble TNFR2, etanercept, reduced significantly, though temporarily, tubular and interstitial cell proliferation, fibronectin mRNA expression, and apoptosis in UUO kidneys. Inhibition of TNFα by etanercept also suppressed the degradation of TRADD protein in UUO kidneys. The ameliorative effect of etanercept has also been documented in DOCA salt hypertensive renal injury.23 Inhibition of TNFα by the administration of pegylated form of soluble TNFR1 has also been shown to ameliorate obstructive renal injury.6,7,8 It was also demonstrated in the present study that degradation of TRADD was stimulated by the action of TNFα in HK-2 cells and was suppressed by proteasome inhibitor. These data suggest that the decrease in TRADD resulting from enhanced ubiquitin-dependent proteasomal degradation, a process triggered, at least in part, by the action of TNFα, is involved in obstructive renal injury.
In contrast to the present data indicating a decrease in TRADD protein in rat kidneys with UUO, Choi et al24 used a rabbit polyclonal anti-human TRADD antibody purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and reported tubular immunostaining of TRADD in UUO mouse kidneys. We therefore verified the specificity of the anti-TRADD antibody used in the present study using recombinant FLAG-tagged rat TRADD and human TRADD proteins, and confirmed its monospecificity by Western blot analysis. Lotocki et al25 also used the same antibody to investigate the levels of TRADD protein in rat cerebral cortex. Although the exact reason for the different TRADD protein level in UUO kidneys in the two studies is not clear, we suspect that the different results could reflect animal species studied and/or the antibodies used to detect TRADD.
Protein ubiquitination and subsequent proteasomal degradation is a common postreceptor regulatory mechanism for signal transduction.26,27,28 In TNFα signaling, TRAF2 and receptor interacting protein are ubiquitinated by E3 ligases, inhibitor of apoptosis protein 1 (c-IAP1) and A20, respectively, and degraded by the ubiquitin proteasomal system.13,29 Phosphorylation of IκB mediated by IKKβ also activates ubiquitin-dependent proteasomal degradation of IκB.21 However, to our knowledge, no report has determined the intracellular regulation of TRADD. This is the first report that demonstrates the involvement of postreceptor regulation of TRADD, mediated by ubiquitin-dependent proteasomal degradation, in UUO kidneys. However, in the present study, we did not identify the E3 ligase for TRADD ubiquitination, which might be stimulated through the action of TNFα.
The cascade of TNFR2-mediated TNFα signaling has not been fully elucidated. Since TRADD is not incorporated in the TNFR2-mediated TNFα signaling,13 the decrease in TRADD resulting from enhanced ubiquitin-proteasomal degradation may work, at least partly, to tilt TNFα signals toward TNFR2-mediated pathway in UUO kidney lesions. The marked increase in TNFR2 protein, which was associated with mild decrease in TNFR1 protein, may also amplify the importance of TNFR2-mediated TNFα signaling in UUO kidney lesions. In support of this concept, Ramesh et al9 reported that renal injury induced by cisplatin was less severe in TNFR2-deficient mice than TNFR1-deficient mice.
In the present study, we cannot completely rule out possible degradation of TRADD after transmitting TNFα signals mediated through TNFR1 in UUO kidney lesions. However, it seems unlikely that TNFR1-mediated TNFα signal that incorporates TRADD is far predominant through the entire course of the development of obstructive renal injury, because TRADD decreased at day 1 after UUO and continued at low level thereafter. The possibility that TRADD degradation might be a compensatory phenomenon to limit TNFα induced fibrosis in UUO kidneys also remained to be clarified. Although we did not investigate the mechanism of low TNFR1 protein in UUO kidneys in this study, the low level of protein, coupled with increased mRNA expression, suggested the involvement of ubiquitin-dependent degradation of TNFR1 in UUO kidneys. In this regard, Legler et al30 reported enhanced ubiquitin-dependant proteasomal degradation of TNFR1 through the action of TNFα. Further studies using TNFR1 as well as TNFR2 knockout mice are needed to elucidate the precise role of TNFR1 and TNFR2-mediated TNFα signaling in the development of obstructive renal injury. However, our preliminary data of Western blot and immunostaining in the kidneys of wild-type C57BL-6 mice with UUO and those with sham operation indicated that the monospecific mouse monoclonal anti-human TRADD antibody, which we used in the present study, did not react to mouse TRADD protein (data not shown). In addition, although the study using the TRADD knockout mouse is useful to elucidate the pathogenic role in the decrease in TRADD in UUO kidneys, it is embryonic lethal.
In conclusion, the present study showed a decrease in TRADD in rat UUO kidneys, which was due to enhanced ubiquitin-dependent proteasomal degradation. Since TRADD is not incorporated in TNFR2-mediated TNFα signaling, the sustained decrease in TRADD, in association with mild decrease in TNFR1and marked increase in TNFR2, may work, at least in some parts, to tilt TNFα signals toward TNFR2-mediated pathway in UUO kidneys.
Acknowledgments
We thank Dr. Naoya Fujita from the Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, Tokyo, Japan, for providing pcDNA3 FLAG-TRADD plasmid, and Dr. Tomoyasu Isobe for assistance in preparation of pCMV FLAG rat TRADD plasmid.
Footnotes
Address reprint requests to Tatsuo Yamamoto, M.D., Ph.D., Faculty of Health Promotional Sciences, Department of Health and Nutritional Sciences, Hamamatsu University, 1230 Miyakoda-cho, Kita-ku, Hamamatsu, 431-2102, Japan. E-mail: tyama@hamamatsu-u.ac.jp.
Supported by KAKENHI, a grant-in-aid for young scientists (B) 20790591 (to T.M.) and in part by a grant-in-aid for scientific research (C) 20590967 (to T.Y.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol. 1999;277:F766–F772. doi: 10.1152/ajprenal.1999.277.5.F766. [DOI] [PubMed] [Google Scholar]
- Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ. Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis. 1994;23:193–198. doi: 10.1016/s0272-6386(12)80971-1. [DOI] [PubMed] [Google Scholar]
- Gobe GC, Axelsen RA. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat: role of apoptosis. Lab Invest. 1987;56:273–281. [PubMed] [Google Scholar]
- He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol. 2005;288:F406–F411. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- Meldrum KK, Metcalfe P, Leslie JA, Misseri R, Hile KL, Meldrum DR. TNF-alpha neutralization decreases nuclear factor-kappaB activation and apoptosis during renal obstruction. J Surg Res. 2006;131:182–188. doi: 10.1016/j.jss.2005.11.581. [DOI] [PubMed] [Google Scholar]
- Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis. Am J Physiol Regulatory Integrative Comp Physiol. 2007;292:1456–1464. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–618. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- Fukasawa H, Yamamoto T, Togawa A, Ohashi N, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Suzuki S, Kitagawa M, Hishida A. Down-regulation of Smad7 expression by ubiquitin-dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proc Natl Acad Sci USA. 2004;101:8687–8692. doi: 10.1073/pnas.0400035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto T, Ikegaya N, Fujigaki Y, Suzuki H, Togawa A, Fukasawa H, Nagase M, Hishida A. Transforming growth factor-beta receptors in self-limited vs. chronic progressive nephritis in rats. J Pathol. 2002;198:397–406. doi: 10.1002/path.1213. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Fukasawa H, Kitagawa K, Uchida C, Hattori T, Isobe T, Oda T, Misaki T, Ohashi N, Nakayama K, Nakayama KI, Hishida A, Yamamoto T, Kitagawa M. Renal damage in obstructive nephropathy is decreased in Skp2-deficient mice. Am J Pathol. 2007;171:473–483. doi: 10.2353/ajpath.2007.070279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa A, Yamamoto T, Suzuki H, Fukasawa H, Ohashi N, Fujigaki Y, Kitagawa K, Hattori T, Kitagawa M, Hishida A. Ubiquitin-dependent degradation of Smad2 is increased in the glomeruli of rats with anti-thymocyte serum nephritis. Am J Pathol. 2003;163:1645–1652. doi: 10.1016/S0002-9440(10)63521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K. An F-box protein. FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Haussler U, von Wichert G, Schmid RM, Keller F, Schneider G. Epidermal growth factor activates nuclear factor-kappaB in human proximal tubule cells. Am J Physiol Renal Physiol. 2005;289:F808–F815. doi: 10.1152/ajprenal.00434.2003. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Baranowska-Daca E, Nguyen V, Koji T, Ballantyne CM, Sheikh-Hamad D, Suki WN, Truong LD. Mechanism of chronic obstructive uropathy: increased expression of apoptosis-promoting molecules. Kidney Int. 2000;58:1481–1491. doi: 10.1046/j.1523-1755.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- Lotocki G, Alonso OF, Dietrich WD, Keane RW. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci. 2004;24:11010–11016. doi: 10.1523/JNEUROSCI.3823-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Regulating protein degradation by ubiquitination. Immunol Today. 1997;18:189–198. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNF alpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]