Abstract
Many types of glomerulonephritis are initiated by the deposition of immune complexes, which induce tissue injury via either engagement of Fc receptors on effector cells or via complement activation. Four murine Fcγ receptors (FcγRs) have been identified at present. Ligand binding to FcγRI, III, and IV induces cell activation via the immunoreceptor tyrosine-based activation motif on the common γ chain (FcRγ). In this study, FcRγ chain knockout (FcRγ−/−) mice were crossed with thymic stromal lymphopoietin transgenic (TSLPtg) mice, which develop cryoglobulinemic membranoproliferative glomerulonephritis (MPGN). Female mice were studied at 30 and 50 days of age, when MPGN is in early and fully developed stages, respectively. Both TSLPtg and TSLPtg/FcRγ−/− mice developed MPGN with massive glomerular immune deposits, mesangial cell proliferation, extensive mesangial matrix accumulation, and macrophage influx. TSLPtg/FcRγ−/− mice had more glomerular immune complex deposits and higher levels of circulating cryoglobulins, IgG2a, IgG2b, and IgM, compared with TSLPtg mice. TSLPtg and TSLPtg/FcRγ−/− mice developed similar levels of proteinuria. These results demonstrated that deletion of activating FcγRs does not confer protection in this model of immune complex-mediated MPGN. The findings contradict accepted paradigms on the role of activating FcγRs in promoting features of glomerulonephritis as seen in other model systems. We speculate engagement of FcγRs on cells such as monocytes/macrophages may be important for the clearance of deposited immune complexes and extracellular matrix proteins.
Deposition of immune complexes (ICs) is a major initiation step for many types of glomerulonephritis. After deposition in tissues, the Fc portion of immunoglobulins (Ig) in ICs binds to Fc receptors (FcRs) on effector cells. In the mouse, four FcγRs for IgG have been identified.1,2 Among them, FcγRI, III, and IV are polymeric receptors with a common γ adaptor chain (FcRγ) bearing the immunoreceptor tyrosine-based activation motif. The FcRγ chain is necessary for cell surface expression and signal transduction of FcγRI, III, and IV. The FcRγ chain is also a subunit of FcεRI and FcαRI.3,4 Binding of FcγRI, III, and IV by IgG transduces activating signals via phosphorylation of the FcRγ immunoreceptor tyrosine-based activation motif leading to activation of syk tyrosine kinase and downstream signaling pathways such as phospholipase C-γ and phosphatidylinositol-3 kinase. Deletion of the FcRγ subunit leads to functional loss of FcγRI, III, and IV.5 In contrast, the monomeric FcγRIIb has an immunoreceptor tyrosine-based inhibitory motif in its cytoplasmic tail. Ligand binding to FcγRIIb leads to recruitment of inhibitory phosphatases such as src homology domain type-2-containing tyrosine phosphatase 1 and SH2-containing inositol polyphosphate phosphatase that dampen immunoreceptor tyrosine-based activation motif-initiated activation.1,6 An emerging paradigm for the mediation of inflammation is that a balancing of engagement of co-existing activating and inhibitory FcγRs on the surface of effector cells determines the severity of inflammatory response to injury.4,7
Membranoproliferative glomerulonephritis (MPGN) in humans is typically initiated by deposition of immune complexes. The mechanism by which MPGN develops subsequent to immune complex deposition is still not well understood. We have characterized a mouse model of MPGN resulting from deposition of cryoglobulin containing ICs, which result from overexpression of thymic stromal lymphopoietin (TSLP), a cytokine that causes abnormalities in B cell development.8,9 TSLP transgenic (TSLPtg) mice develop mixed cryoglobulinemia and a systemic inflammatory disease that involves the kidney, lung, spleen, liver, and skin. These mice develop renal disease that closely resembles human cryoglobulinemia-associated MPGN.8 The glomerular injury in these mice is characterized by extensive subendothelial and mesangial immune deposits, marked macrophage influx, and mesangial cell proliferation and matrix expansion. Immunofluorescence microscopy shows massive glomerular deposition of immunoglobulins and complement.
We have studied the effect of the inhibitory FcγRIIb on this MPGN model previously and showed that FcγRIIb deficiency in TSLPtg mice resulted in more severe kidney disease with more infiltrating macrophages and increased cell proliferation in glomeruli.10 Here we present complementary studies in which the effect of deletion of the activating FcγRs on the disease in this model was tested, and present the surprising result that this intervention produced no improvement in renal and systemic disease in TSLPtg mice and indeed lead to higher concentration of circulating cryoglobulins and more deposition of ICs in the kidney in these mice.
Materials and Methods
Animals
The experimental protocol of this study was reviewed and approved by the Animal Care Committee of the University of Washington. Mice were housed under standard conditions and received normal chow and water ad libitum in a specific pathogen free facility. TSLPtg mice on C57BL/6 background have been reported previously.8 FcRγ chain knockout (FcRγ−/−) mice on C57BL/6 background were provided by J. V. Ravetch (Rockefeller University, New York, NY) and have been described previously.11 TSLPtg mice were crossed to FcRγ−/− mice. Cohorts of FcRγ+/+ (wild-type), FcRγ−/−, TSLPtg, and TSLPtg/FcRγ−/− mice (n = 6 in each group), all females, were sacrificed and analyzed at age 30 days and 50 days when kidney disease is early in its evolution (30 days) and when it is fully developed (50 days) in female TSLPtg mice, as previously documented.8,10
Immune Thrombocytopenia Induced by 6A6 Antibody
To establish the intact function of activating FcγRs in TSLPtg mice, and its absence in TSLPtg/FcRγ−/− mice, a platelet depletion assay that requires the presence of intact FcγRs was used, as previously described.12,13 Blood samples were collected from TSLPtg and TSLPtg/FcRγ−/− mice and placed into EDTA tubes to prevent clotting. The assay utilizes the monoclonal anti-platelet antibody 6A6 (IgG1).14 Five μg 6A6 antibody in 200 μl of saline was injected into the tail vein of each mouse. After 4 hours, a second blood sample was collected and placed into an EDTA tube. The blood samples underwent manual count of platelets (Phoenix Central Laboratory, Everett, WA).
Tissue Collection and Histological Staining
Mice were anesthetized and blood was collected before sacrifice by retro-orbital bleeding. Kidney, spleen, liver, and lung were collected at sacrifice. Portions of each organ were fixed in 10% neutral-buffered formalin and methyl Carnoy’s solution (60% methanol, 30% chloroform, and 10% acetic acid). These tissues were processed and embedded in paraffin. Four μm sections were cut and used in H&E, periodic acid-Schiff, Masson’s trichrome, and immunohistochemistry staining. Paraffin-embedded kidney tissue was also cut at 2 μm and stained with silver methenamine reagents (silver staining). Portions of kidney in OCT were snap-frozen and sectioned at 5 μm, fixed in ice-cold acetone for 10 minutes, and then used for immunofluorescence and immunohistochemistry studies.
Analysis of Kidney Function
Before sacrifice, mouse spot urine was collected. Urine albumin was measured by enzyme-linked immunosorbent assay (ELISA) using the Albuwell kit (Exocell, Philadephia, PA) and urine creatinine was measured using the Creatinine Companion kit (Exocell) according to protocols of the manufacturer. Proteinuria was evaluated by urine albumin/creatinine ratio.
Measurements of Serum Cryoglobulins, Immunoglobulins, and TSLP
Blood was allowed to clot at 37°C and serum was collected by centrifugation at 3000 rpm for 10 minutes. Serum was stored at 4°C for a minimum of 72 hours and the formation of cryoprecipitate was identified and serum cryoglobulin concentration (cryocrit) was measured as previous described.8,15 Serum IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA were measured by ELISA. Briefly, 96-well ELISA plates were coated overnight with anti-mouse IgM (Southern Biotechnology, Birmingham, AL) or anti-mouse IgG (Southern Biotechnology), blocked with 1% bovine serum albumen in PBS, and incubated for 2 hours with serial dilutions of IgG1, IgG2a, and IgM standards (Southern Biotechnology) and diluted serum samples. Serum samples were diluted 1:20,000 for TSLPtg mice and 1:500 for wild-type and FcRγ−/− mice. After washing, alkaline phosphatase conjugated anti-mouse-IgG1, -IgG2a, or -IgM (Southern Biotechnology) were added and incubated for 1 hour. Then plates were washed and developed with ready-to-use p-nitrophenyl phosphate substrate (Zymed, San Francisco, CA) and absorbance was measured at 405 nm on a microplate reader (Beckman Coulter AD340). IgG2b, IgG3 and IgA were measured using mouse immunoglobulin ELISA kits (Bethyl, Montgomery, TX) according to the manufacturer’s protocol. Serum TSLP levels were measured by ELISA (Duoset Economy Pack for mouse TSLP, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Immunofluorescence
Acetone-fixed frozen kidney sections were air-dried and washed in PBS. Sections were then incubated with fluorescein-conjugated antibodies against mouse IgG, IgM, IgA, and complement C3 (all from Cappel Pharmaceuticals, Aurora, OH). After washing, slides were mounted in Vectashield mounting media (Vector, Burlingame, CA), coverslipped, and viewed under a Zeiss fluorescence microscope. In a blinded manner, the glomerular fluorescence intensity was described semiquantitatively (0, negative; 1, weak; 2, moderate; 3, strong). For every sample, 15 glomeruli were evaluated and mean score was calculated.
Kidney Immunohistochemistry
The general immunohistochemistry protocol used has been described previously.8,16 Type IV collagen was detected by a goat polyclonal anti-human type IV collagen antibody that cross-reacts with mouse type IV collagen (Southern Biotechnology, Birmingham, AL). Glomerular mesangial cell activation was evaluated by α-smooth muscle actin (α-SMA) expression. Tissues were first blocked by Rodent Block M, (Biocare Medical, Concord, CA). After incubation with a mouse monoclonal anti-human α-SMA antibody (Dako, Carpinteria, CA), MM-HRP Polymer (Biocare Medical) was added and then detected with diaminobenzidine substrate. Macrophages were detected using a rat anti-mouse Mac-2 antibody (Cedarlane, Ontario, Canada) in paraffin-embedded tissue or a rat anti-mouse CD68 antibody (Serotech, Raleigh, NC) for frozen tissue. T cells were detected using a rat anti-mouse CD3 antibody (Dako). FcγRI and FcγRIII expression in glomeruli was detected using goat anti-mouse FcγRI antibody (R&D Systems, Minneapolis, MN) in frozen tissues and goat anti-mouse FcγRIII antibody (R&D Systems) in paraffin-embedded tissue, respectively. Double immunolabeling was used to identify the cell types expressing FcγRI and FcγRIII receptors. For FcγRI receptor, frozen kidney tissues were stained with rat anti-mouse CD68 antibody, followed by detection with Alexa Fluor 488-labeled (green color fluorescence) rabbit anti-rat secondary antibody (Invitrogen, Carlsbad, California). Tissues were then incubated with goat anti-mouse FcγRI antibody followed by Alexa Fluor 593-labeled (red color fluorescence) donkey anti-goat secondary antibody (Invitrogen). For FcγRIII receptor, methyl Carnoy’s solution-fixed and paraffin-embedded kidney tissues were first stained with rat anti-mouse Mac-2 antibody followed by biotinylated secondary antibody. Positive staining was visualized by Vector VIP substrate kit (purple-blue color in positive areas). Tissues were than incubated with goat anti-mouse FcγRIII antibody. Biotinylated anti-goat secondary antibody was added and then visualized by diaminobenzidine substrate (brown color in positive area). Tissues were then counterstained with methyl green. Double immunohistochemical staining for FcγRI or FcγRIII and the activated mesangial cell marker α-SMA was performed similarly with FcγRI and FcγRIII stained with diaminobenzidine substrate (brown), α-SMA stained with Vector VIP substrate (purple-blue), and counterstaining with methyl green.
Analytical Methods and Statistical Analysis
Fifteen random glomerular cross sections were photographed using an Olympus DP11 digital camera (Olympus, Melville, NY). The photography and subsequent morphological analyses were performed by an examiner blinded to the origin of the samples. The photographs were imported and analyzed using the ImagePro Plus software (Media Cybernetics, Silver Spring, MD). Glomerular tuft area and the proportion of the glomerular area occupied by black silver methenamine-stained matrix or stained by antibodies to type IV collagen, or the proportion occupied by cells expressing α-SMA, Mac-2, or CD3 were quantified as previously described.10,15,16 In Masson’s trichrome-stained kidney tissue, ICs are stained brown-pink and can be differentiated from cells and extracellular matrix. Accumulations of stained ICs in glomeruli were morphometrically quantified as the proportion of IC-stained area in glomerular tuft area in TSLPtg and TSLPtg/FcRγ−/− mice. In interstitial areas of kidney, the numbers of Mac-2 or CD3 positive cells were counted on 15 high power fields. Liver and lung tissue sections stained with H&E were assessed for evidence of systemic injury. As previously described, the liver and lung inflammation was scored semiquantitatively on a scale of 0 to 3: 0, no inflammation; 1, mild; 2, moderate; 3, severe, with regard to the extent and density of leukocytic infiltration.15,17
All data are expressed as mean ± SEM. Statistical analysis of the data for multiple groups was performed by analysis of variance with Tukey-Kramer multiple comparisons test using the InStat program (Version 3.0, Intuitive Software for science, San Diego, CA). P < 0.05 was considered significant.
Results
TSLPtg Mice Deficient in FcRγ Chain Develop Similar Renal Lesions as TSLPtg Mice
TSLPtg mice have higher serum TSLP levels than wild-type mice (7.2 ± 0.6 vs. 0.7 ± 0.3 ng/ml); TSLPtg/FcRγ−/− mice have higher TSLP levels than FcRγ−/− mice (6.7 ± 0.5 vs. 0.9 ± 0.5 ng/ml). The TSLP levels between TSLPtg and TSLPtg/FcRγ−/− mice are not significantly different. This result demonstrates the crossing of FcRγ−/− with TSLPtg did not change the expression of the TSLP transgene.
The 6A6 antibody induced platelet reduction in wild-type and TSLPtg mice in 4 hours after i.v. injection, whereas in FcRγ−/− and TSLPtg/FcRγ−/− mice, the platelet reduction was mild (% of reduction rate of platelet: wild-type, 78 ± 5%; TSLPtg, 77 ± 3%; FcRγ−/−, 12 ± 2%; TSLPtg/FcRγ−/−, 8 ± 4%). This demonstrates wild-type and TSLPtg mice have intact activating Fcγ receptors, and that the activating Fcγ receptors have been effectively deleted in FcRγ−/− and TSLPtg/FcRγ−/− mice.12,13
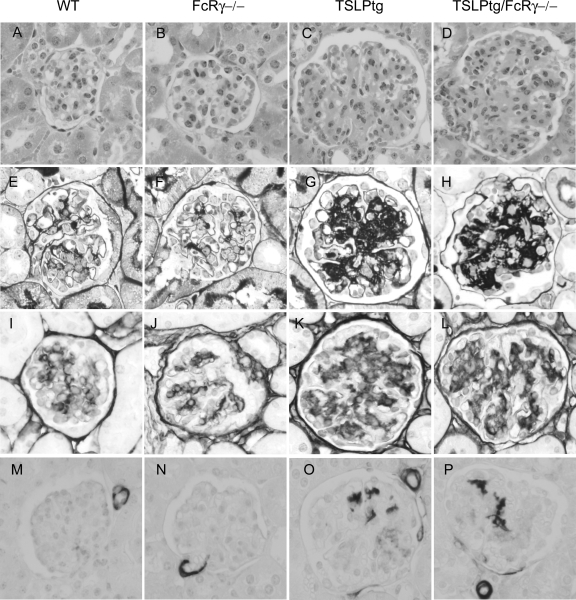
TSLPtg mice developed typical features of cryoglobulin-associated MPGN as described previously.8,10 Mice showed progressive kidney injury from age 30 days to age 50 days, with extensive mesangial cell proliferation and mesangial matrix expansion as demonstrated by glomerular hypercellularity and increase in silver methanamine-stained extracellular matrix (Figure 1, A–P and Table 1). Mesangial cell activation, as assessed by α-SMA expression, was also markedly increased. FcRγ−/− mice have no renal pathology, comparable with wild-type mice. In contrast, TSLPtg/FcRγ−/− mice developed kidney disease comparable with TSLPtg mice, with glomerular hypercellularity, mesangial matrix expansion (% silver-stained extracellular matrix area/glomerular tuft area (age 30 → 50 days): TSLPtg, 9.8 ± 0.9 →14.5 ± 1.3; TSLPtg/FcRγ−/−, 9.0 ± 0.5 → 15.5 ± 1.6; FcRγ−/−, 5.3 ± 0.4 → 7.2 ± 0.5; wild-type, 5.7 ± 0.5 → 6.6 ± 0.8, P < 0.05 TSLPtg and TSLPtg/FcRγ−/− versus wild-type mice of the same age, n = 6 each group) and mesangial cell activation (% α-SMA staining area/glomerular tuft area [age 50 days]: TSLPtg, 0.9 ± 0.2; TSLPtg/FcRγ−/−, 0.9 ± 0.1; FcRγ−/−, 0.3 ± 0.2; wild-type, 0.4 ± 0.1, P < 0.05 TSLPtg and TSLPtg/FcRγ−/− versus wild-type mice, n = 6 each group). Type IV collagen, a major component of extracellular matrix, also increased significantly and progressively in both TSLPtg and TSLPtg/FcRγ−/− mice (% type IV collagen staining area/glomerular tuft area [age 30 → 50 days]: TSLPtg, 6.3 ± 0.3 → 7.6 ± 1.0; TSLPtg/FcRγ−/−, 6.5 ± 0.4 → 8.3 ± 0.3; FcRγ−/−, 4.3 ± 0.2 → 4.0 ± 0.4; wild-type, 4.2 ± 0.5 → 4.5 ± 1.0, P < 0.05 TSLPtg and TSLPtg/FcRγ−/− versus wild-type mice of the same age, n = 6 each group).
Figure 1.
Histological appearance of glomeruli with H&E (A–D), silver methenamine (E–H) staining and immunohistochemical staining for type IV collagen (I–L) and α-SMA (M–P) in wild-type (WT), FcRγ−/−, TSLP transgenic (TSLPtg) and TSLPtg/FcRγ−/− mice of age 50 days. TSLP transgenic and TSLPtg/FcRγ−/− mice show similarly increased cellularity, mesangial matrix accumulation (black silver staining area increase and type IV collagen expression increase) and mesangial α-SMA expression as compared with wild-type and FcRγ−/− mice. Magnification = original ×400.
Table 1.
Morphometric Analysis of Extracellular Matrix, Mesangial Cell Activation in Glomeruli, Macrophage, and T Cell Infiltration in Glomerular and Interstitial Area, and Proteinuria*
| WT–30 days | FcRγ−/−–30 days | TSLPtg 30–days | TSLPtg/FcRγ−/−–30 days | WT–50 days | FcRγ−/−–50 days | TSLPtg–50 days | TSLPtg/FcRγ−/−–50 days | |
|---|---|---|---|---|---|---|---|---|
| Cellularity/glomerulus | 29 ± 4 | 27 ± 5 | 38 ± 5 | 35 ± 5 | 28 ± 4 | 30 ± 3 | 45 ± 5† | 41 ± 4† |
| % Extracellular matrix area/GTA | 5.7 ± 0.5 | 5.3 ± 0.4 | 9.8 ± 0.9† | 9.0 ± 0.5† | 6.6 ± 0.8 | 7.2 ± 0.5 | 14.5 ± 1.3‡ | 15.5 ± 1.6§ |
| % Collagen IV staining area/GTA | 4.2 ± 0.5 | 4.3 ± 0.2 | 6.3 ± 0.3† | 6.5 ± 0.4† | 4.5 ± 1.0 | 4.0 ± 0.4 | 7.6 ± 1.0† | 8.3 ± 0.3‡ |
| % SMA staining area/GTA | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.3† | 0.4 ± 0.1 | 0.3 ± 0.2 | 0.9 ± 0.2† | 0.9 ± 0.1† |
| Macrophage infiltration/glomerulus | 0.3 ± 0.1 | 0.2 ± 0.1 | 2.0 ± 0.3† | 1.8 ± 0.2† | 0.5 ± 0.2 | 0.4 ± 0.1 | 3.1 ± 0.6§ | 2.6 ± 0.6‡ |
| Macrophage infiltration/interstitial HPF | 1.8 ± 0.3 | 2.5 ± 0.8 | 4.0 ± 0.6 | 4.6 ± 0.5† | 1.9 ± 0.3 | 2.1 ± 0.5 | 4.6 ± 0.8† | 4.5 ± 0.5 |
| CD3 T cell infiltration/glomerulus | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| CD3 T cell infiltration/interstitial HPF | 1.2 ± 0.5 | 1.4 ± 0.3 | 3.8 ± 0.3† | 3.5 ± 0.5† | 2.4 ± 0.5 | 2.0 ± 0.7 | 7.0 ± 0.9d | 6.6 ± 1.1§ |
| Proteinuria (albumin/creatinine, μg/mg) | 13.3 ± 4.9 | 15.9 ± 6.0 | 42.2 ± 8.5† | 39.0 ± 9.3† | 22.7 ± 8.0 | 19.2 ± 7.3 | 128.5 ± 22.5§ | 139.9 ± 29.6§ |
GTA, glomerular tuft area; HPF, high power field; WT, wild-type mice; TSLPtg, thymic stromal lymphopoietin transgenic mice; FcRγ−/−, FcR γ chain knock out mice; TSLPtg/FcRγ−/−, TSLP transgenic with FcR γ chain knockout mice.
Data are means ± SEM (n = 6 in each group);
P < 0.05 versus WT mice;
P < 0.01 versus WT mice;
P < 0.001 versus WT mice.
Functionally, TSLPtg and TSLPtg/FcRγ−/− mice had similar levels of proteinuria as assessed by urine albumin/creatinine (ug/mg) ratio (age 30 → 50 days) (TSLPtg: 42.2 ± 8.5 → 128.5 ± 22.5; TSLPtg/FcRγ−/−: 39.0 ± 9.3→ 139.9 ± 29.6; FcRγ−/−: 15.9 ± 6.0→ 19.2 ± 7.3; wild-type: 13.3 ± 4.9 → 22.7 ± 8.0, P < 0.05 TSLPtg and TSLPtg/FcRγ−/− versus wild-type mice of the same age, n = 6 each group) (Table 1).
Leukocyte Infiltration and FcγRI and FcγRIII Receptor Expression in Kidneys in TSLPtg and TSLPtg/FcRγ−/− Mice
Macrophage and T cell infiltration in glomeruli and interstitium were assessed immunohistochemically by Mac-2 expression and CD3 expression, respectively (Figure 2, A–H and Table 1). Both TSLPtg and TSLPtg/FcRγ−/− mice had a marked macrophage influx in glomeruli. There were fewer glomerular macrophages in TSLPtg/FcRγ−/− mice compared with TSLPtg mice, but the difference was not statistically significant. There was no difference for T cell infiltration in glomeruli between the two groups. In interstitium, both groups had similarly increased numbers of macrophages and T cells compared with wild-type and FcRγ−/− mice.
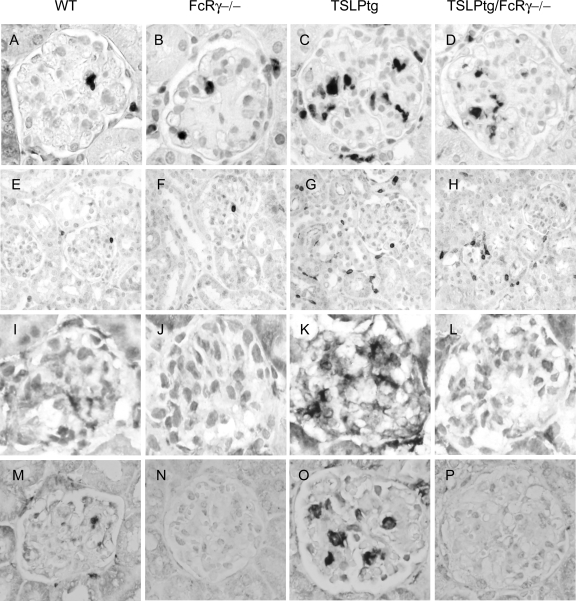
Figure 2.
Representative photographs of expression of Mac-2 (marker of monocytes/macrophages) (A–D), CD3 (marker of T cells) (E–H), FcγRI (I–L), and FcγRIII (M–P) in the kidney of wild-type (WT), FcRγ−/−, TSLPtg, and TSLPtg/FcRγ−/− mice of age 50 days. TSLPtg and TSLPtg/FcRγ−/− mice show markedly increased monocyte/macrophage infiltration in glomeruli and they have increased T cell infiltration in interstitium. TSLPtg mice have markedly increased FcγRI and FcγRIII expressing cells in glomeruli than wild-type mice whereas FcRγ−/− and TSLPtg/FcRγ−/− mice lack expression of FcγRI and FcγRIII. Magnification = original ×400 (Mac-2, FcγRI, and FcγRIII); ×100 (CD3).
Immunohistochemistry showed remarkable FcγRI and FcγRIII staining in glomeruli of TSLPtg mice (Figure 2, I–P). In wild-type mice, there were a few cells expressing FcγRI or FcγRIII. In contrast, there was no FcγRI and FcγRIII staining in glomeruli of FcRγ−/− and TSLPtg/FcRγ−/− mice, as expected, demonstrating the fidelity of the knockout of the common γ chain in eliminating the expression of the whole FcγRI and FcγRIII receptors.
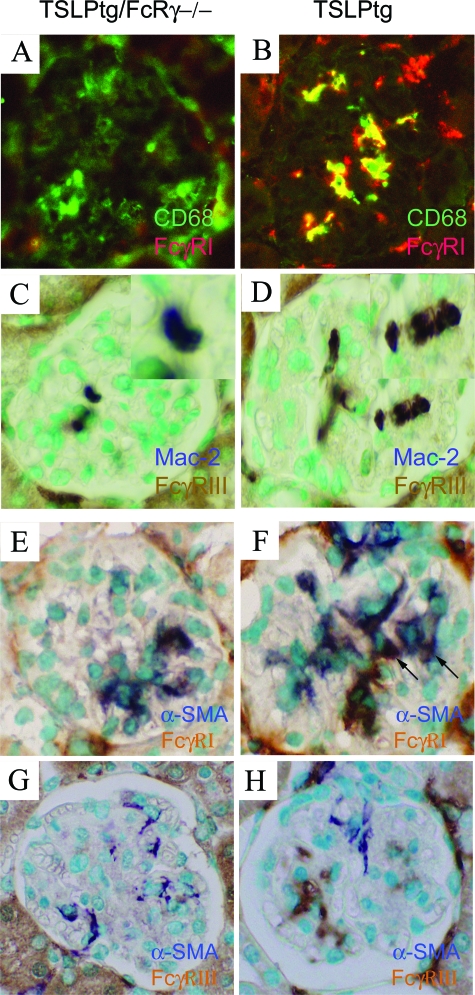
Double immunolabeling of TSLPtg/FcRγ−/− mice showed the presence of glomerular Mac-2 or CD68 positive monocyte/macrophages with absent expression of both FcγRI and FcγRIII receptors (Figure 3, A and C). In contrast, most of the infiltrating glomerular Mac-2- or CD68-expressing monocyte/macrophages in TSLPtg mice expressed both FcγRI and FcγRIII (Figure 3, B and D). Double immunolabeling for expression of the activated mesangial cell marker α-SMA and FcγRI and FcγRIII showed that FcγRIII-expressing cells were distinct from α-SMA-expressing mesangial cells (Figure 3, G and H); and most FcγRI-expressing cells were also distinct from α-SMA-expressing mesangial cells (Figure 3, E and F), although a few α-SMA-expressing mesangial cells also expressed FcγRI (arrows). These results demonstrate that most of glomerular FcγRI- and FcγRIII-expressing cells in TSLPtg mice are infiltrating monocyte/macrophages instead of intrinsic mesangial cells.
Figure 3.
Double immunolabeling for macrophage marker and FcγR receptor expression in glomeruli of 50-day-old TSLPtg and TSLPtg/FcRγ−/− mice. A–B: Immunofluorescence staining of the macrophage marker CD68 (green) and FcγRI (red) on frozen kidney tissue. Colocalization CD68 and FcγRI yields a yellow color in merged picture. C–D: Double immunolabeling of macrophage marker Mac-2 (purple-blue) and and FcγRIII (brown) on methyl Carnoy’s solution fixed kidney tissue. TSLP/FcRγ−/− mice (A, C) show that macrophages infiltrating the glomerulus do not express FcγRI (A) and FcγRIII (C) receptors. TSLPtg mice (B, D) show many macrophages infiltrating the glomeruli express FcγRI (B) and FcγRIII (D) receptors. E–H: Double labeling of the activated mesangial cell markers α- SMA and FcγR receptors expression in glomeruli of TSLPtg and TSLPtg/FcRγ−/− mice of age 50 days. E–F: Immunohistochemical double staining of α-SMA (blue) and FcγRI (brown) on acetone fixed frozen tissue. G–H: Immunohistochemical double staining of α-SMA (blue) and FcγRIII (brown) on methyl Carnoy’s solution fixed tissue. TSLP/FcRγ−/− mice (E, G) show α-SMA expression in mesangial areas of the glomerulus; these areas lack FcγRI (E) and FcγRIII (G) expression. TSLPtg mice (F, H) show α-SMA expression in mesangium and a separate population of cells that express FcγRI and FcγRIII. Most of the cells expressing α-SMA or FcγRI receptors are distinct and non-overlapping. There are a few cells expressing both α-SMA and FcγRI (arrows, F). The cells expressing α-SMA and FcγRIII receptors are completely distinct (H). Magnification = original ×400.
TSLPtg/FcRγ−/− Mice Had More Glomerular Immune Complexes Deposits than TSLPtg Mice
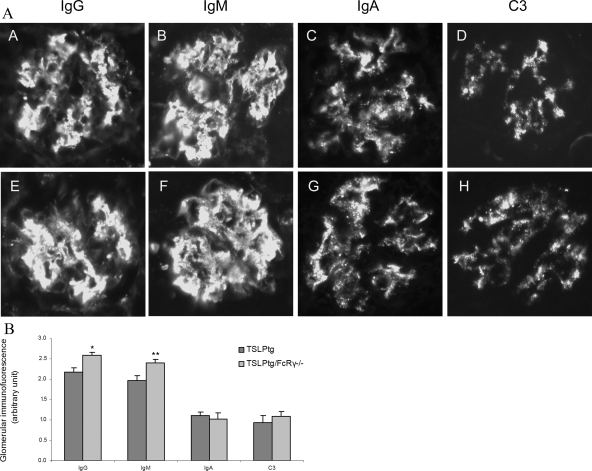
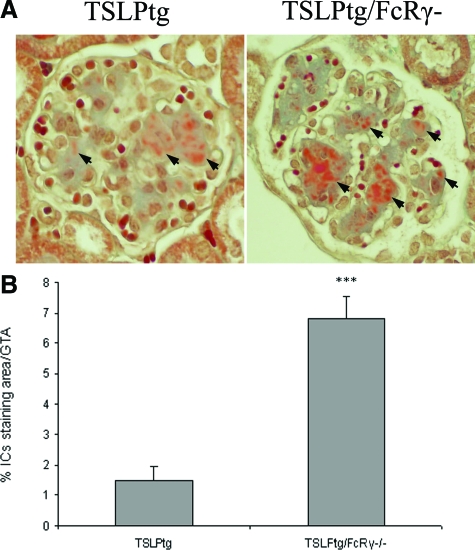
Kidney immunofluorescence studies showed that TSLPtg mice of both age 30 days and age 50 days had prominent deposition of IgG, IgM (semiquantitative scale [30 → 50 days] IgG, 1.5 ± 0.3 → 2.2 ± 0.1, and IgM, 1.8 ± 0.3 → 2.0 ± 0.2, on a scale of 0 to 3), and to lesser extent IgA and complement C3 in glomeruli, whereas wild-type and FcRγ−/− mice had only background immunofluorescence staining. TSLPtg/FcRγ−/− mice had a trend toward greater IgG and IgM deposits (semiquantitative scale [30 → 50 days]: IgG, 2.2 ± 0.4 → 2.6 ± 0.1, and IgM, 2.0 ± 0.3 → 2.4 ± 0.1, on a scale of 0 to 3) than TSLPtg mice (Figure 4, A and B), although only differences at age 50 days reached statistical significance. In Masson’s trichrome-stained kidney tissues, the areas of brown-pink stained IC deposits were measured by morphometry, and TSLPtg/FcRγ−/− mice had strikingly more IC deposit in glomeruli than TSLPtg mice (% IC staining area/glomerular tuft area: TSLPtg 1.5 ± 0.4 versus TSLPtg/FcR γ−/− 6.8 ± 0.7, P < 0.001) (Figure 5, A and B), complementing the immunofluorescence findings.
Figure 4.
Representative photographs of glomerular immunofluorescence in TSLPtg (A–D) and TSLPtg/FcRγ−/− mice (E–H) showing remarkable glomerular deposits of IgG and IgM and to a lesser extent, deposits of IgA and complement C3 in both TSLPtg and TSLPtg/FcRγ−/− mice of age 50 days. Magnification = original ×400. B: Semiquantitative grading of glomerular immunofluorescence staining of glomerular IgG, IgM, IgA and complement C3 in TSLPtg and TSLPtg/FcRγ−/− mice of age 50 days (n = 6 per group). *P < 0.01. **P < 0.05 versus TSLPtg mice.
Figure 5.
A: Representative photographs of Masson’s trichrome staining of kidney in TSLPtg and TSLPtg/FcRγ−/− mice of age 50 days. Arrows point to the brown-pink immune complexes deposits in glomeruli. Magnification = original ×400. B: Morphometric quantification of glomerular ICs deposits in Masson’s trichrome stained glomeruli from TSLPtg and TSLPtg/FcRγ−/− mice of age 50 days (n = 6 per group). ***P < 0.001 versus TSLPtg mice.
Serum Cryoglobulins and Immunoglobulins in TSLPtg and TSLPtg/FcRγ−/− Mice
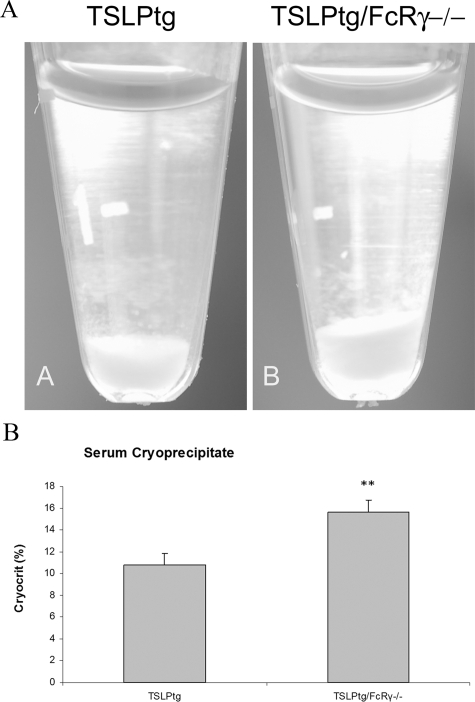
All TSLPtg and TSLPtg/FcRγ−/− mice developed cryoglobulinemia whereas FcRγ−/− and wild-type mice showed no circulating cryoglobulins. TSLPtg/FcRγ−/− mice had increased levels of circulating cryoglobulins, as compared with TSLPtg mice (Figure 6, A and B) (cryocrits [30 → 50 days]: TSLPtg 6.5 ± 0.8% → 10.8 ± 1.0% versus TSLPtg/FcRγ−/− 8.7 ± 1.5% → 15.6 ± 1.1%, P < 0.05 TSLPtg versus TSLPtg/FcRγ−/−, mice of age 50 days).
Figure 6.
A: Representative photographs of serum cryoglobulins precipitated at 4°C. TSLPtg/FcRγ−/− mice had higher levels of circulating cryoglobulins than TSLPtg mice. B: Serum cryoglobulin precipitates measured as cryocrits in TSLPtg/FcRγ−/− and TSLPtg mice of age 50 days (n = 6 per group). **P < 0.05 versus TSLPtg mice.
As quantified by ELISA, serum immunoglobulins including IgG1, IgG2a, IgG2b, IgG3, and IgM were dramatically increased in both TSLPtg and TSLPtg/FcRγ−/− mice (Table 2). Levels of IgG1 and IgG3 were similar between the two groups. TSLPtg/FcRγ−/− mice had a trend toward higher IgG2a, IgG2b, and IgM levels though these differences between TSLPtg and TSLPtg/FcRγ−/− mice were not statistically significant. FcRγ−/− and wild-type mice had comparable serum immunoglobulins levels (Table 2).
Table 2.
Serum Immunoglobulins Profile (μg/ml)*
| IgG1 | IgG2a | IgG2b | IgG3 | IgM | IgA | |
|---|---|---|---|---|---|---|
| WT–50 days | 209.5 ± 27.0 | 48.4 ± 1.6 | 86.1 ± 6.5 | 6.1 ± 1.3 | 132.2 ± 6.3 | 144.0 ± 9.5 |
| FcRγ−/−–50 days | 197.1 ± 17.1 | 45.1 ± 4.9 | 78.2 ± 10.2 | 7.4 ± 2.2 | 139.3 ± 10.3 | 136.8 ± 11.8 |
| TSLPtg–50 days | 2528.8 ± 403.0§ | 793.8 ± 198.5‡ | 936.2 ± 145.0‡ | 331.0 ± 71.7§ | 7803.7 ± 1203.6§ | 1456.7 ± 349.5† |
| TSLPtg/FcRγ−/−–50 days | 2478.3 ± 424.2§ | 1075.6 ± 196.4§ | 1124.2 ± 221.8§ | 325.8 ± 29.4§ | 10487.6 ± 597.0§ | 1367.7 ± 402.3† |
WT, wild-type.
Data are means ± SEM (n = 4 in each group).
P < 0.05 versus WT mice;
P < 0.01 versus WT mice;
P < 0.001 versus WT mice.
TSLPtg and TSLPtg/FcRγ−/− Mice Had Similar Systemic Lesions
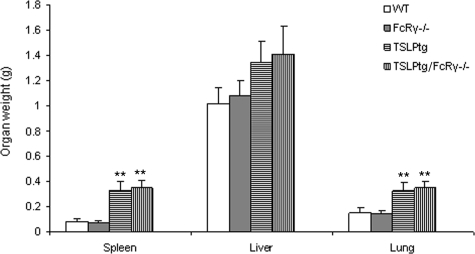
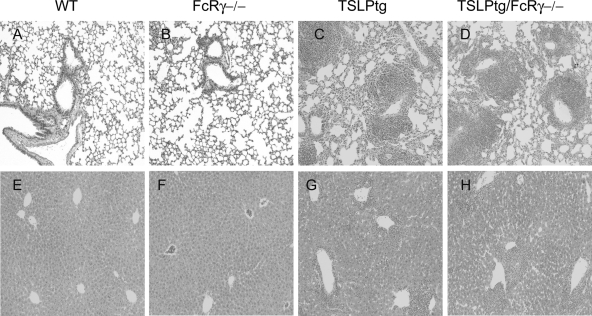
TSLPtg/FcRγ−/− mice demonstrated cryoglobulinemia-associated systemic lesions identical to TSLPtg mice as described previously.8 They had massive splenic enlargement and had significantly increased weights of lungs, with a trend toward increased liver weights, as compared with wild-type and FcRγ−/− mice (Figure 7). The liver showed extensive portal inflammation with extension to hepatic parenchyma. The lung showed perivascular and peribronchiolar inflammation (Figure 8, A–H), with inflammatory infiltrates composed of both lymphoid cells and monocytes/macrophages labeled immunohistochemically by the Mac-2 antibody (data not shown). Semiquantitative assessment showed similar degrees of lung and liver inflammation in TSLPtg and TSLPtg/FcRγ−/− mice (Table 3).
Figure 7.
Weights (g) of the spleen, liver and lung (n = 6 per group) of age 50 days mice. Both TSLPtg and TSLPtg/FcRγ−/− mice have similarly marked increased weights of lung, liver, and spleen, especially the lung and spleen, compared with wild-type (WT) and FcRγ−/− mice. **P < 0.05 versus WT mice.
Figure 8.
Representative photographs of lung (A–D) and liver (E–H) tissues of H&E staining in wild-type (WT), FcRγ−/−, TSLPtg, and TSLPtg/FcRγ−/− mice of age 50 days. Hepatic and lung inflammation is similar in distribution and extent in TSLPtg and TSLPtg/FcRγ−/− mice. Magnification = original ×100.
Table 3.
Semi-Quantitative Grading of Lung and Liver Inflammation
| TSLPtg–30 days | TSLPtg/FcRγ−/−–30 days | TSLPtg–50 days | TSLPtg/FcRγ−/−–50 days | |
|---|---|---|---|---|
| Lung inflammation | 2.0 ± 0.4 | 2.2 ± 0.3 | 2.5 ± 0.6 | 2.8 ± 0.5 |
| Liver inflammation | 1.3 ± 0.5 | 1.5 ± 0.4 | 1.9 ± 0.5 | 1.6 ± 0.4 |
Data are means ± SEM (n = 6 in each group).
Discussion
There is a widely accepted paradigm that the two classes of FcγRs exist in mice—one class of activating FcγRI, III and the recently identified FcγRIV, which have a common FcRγ chain, and one class consisting of the inhibitory FcγRIIb.4,7 The paradigm holds that the balance of their activities determines the extent of local inflammatory responses to tissue injuries initiated by deposition of immune complexes. Binding of the activating FcγRs leads to activation of signaling molecules and subsequent activation of phagocytosis, antibody-dependent cellular cytotoxicity, and release of inflammatory mediators.4,6 Activating FcγRs are important for the amplification of immune complex-mediated diseases. FcRγ deficiency protects NZB/NZW F1 mice from developing pathological features of lupus glomerulonephritis even though IC deposition in glomeruli is unaffected.11 FcRγ−/− mice are also protected from nephrotoxic nephritis.18 In contrast, the inhibitory receptor FcγRIIb has been found to be an important negative regulator of immune responses and B cell activation.5 The previous demonstration that FcγRIIb deficiency aggravates kidney disease in TSLPtg mice indicates the important inhibitory role of FcγRIIb in this model of glomerulonephritis and the overall paradigm that inflammation is regulated by balanced Fc receptor engagement.
However, there is evidence that the applicability of this paradigm is not universal. It is somewhat paradoxical that although the FcRγ-deficient mice were protected from nephrotoxic nephritis, FcγRIII knockout and FcγRI and III double knockout mice developed similar nephritis as wild-type mice.18 Another study of nephrotoxic nephritis showed both FcRγ−/− and FcγRI/III double knockout mice were protected, while FcγRIII knockout mice were only partially protected from the disease.19 In sharp contrast to the studies in the lupus model of NZB/NZW F1 mice, FcRγ deficiency afforded no protection from development of lupus glomerulonephritis in the MRL/lpr mouse model of lupus.20 The present study provides new evidence that the sum of activities of the activating FcγRs may result in little or no significant effects of these receptors in mediating progression of glomerulonephritis in some settings, such as cryoglobulinemic glomerulonephritis, and indeed, as discussed below, may even act to inhibit the initiating event of IC deposition in this setting.
Leukocyte accumulation, including monocyte/macrophage influx after deposition of ICs is an important feature of glomerulonephritis, especially in MPGN. Macrophages express all four FcγRs.21,22 FcγR-mediated endocytosis and phagocytosis by macrophages is important for clearance of IgG containing ICs, including soluble ICs and cells coated by IgG.21,23 Macrophages from FcRγ deficient mice cannot phagocytose ICs, analogous to ligation of FcγRIIb, which inhibits phagocytosis.22,24 In TSLPtg mice, ICs containing cryoglobulins are deposited extensively in glomeruli. Lack of activating FcγRs in TSLPtg/FcRγ−/− mice results in greater accumulation of IC deposits in glomeruli. Clearance of circulating ICs also relies on phagocytic cells, mainly macrophages, in the spleen and the liver. Deficiency of activating FcγRs on macrophages in these organs presumably results in diminished phagocytosis by these cells, leading to the increased IC deposits in the kidney and increased circulating cryoglobulins observed in TSLPtg/FcRγ−/− mice.
This study builds on a body of evidence that indicates Fc receptors on infiltrating monocyte/macrophages are principle determinants of amplification and progression of glomerulonephritis. There is some evidence that cultured human mesangial cells normally do not express FcγRs, but can express FcγRI and FcγRIII after IFN-γ stimulation in vitro.25,26 One study identified low constitutive expression of FcγRIIb, but not FcγRI and FcγRIII in normal glomeruli of C57BL/6 mouse.27 In anti-GBM nephritis of the mice it was found that glomerular expression of FcγRIII mRNA was induced. But whether this glomerular FcγRIII resulted from either infiltrating FcγRIII-positive cells and/or induction of FcγRIII expression on normally FcγRIII-negative resident kidney cells was not established.27 We used double immunostaining of kidney tissue for FcγRI and FcγRIII with the macrophage markers CD68 or Mac-2, and the activated mesangial cell marker α-SMA, to demonstrate that most of the FcγRI- and FcγRIII-bearing cells in glomeruli in this MPGN model are infiltrating monocyte/macrophages, and that intrinsic glomerular cells, such as mesangial cells, show little if any FcγRI and FcγRIII expression. This corresponds with previous studies showing that FcγRs on circulating leukocytes and not intrinsic renal cells mediate nephrotoxic nephritis28 and that FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis.29 We cannot exclude the possibility that α-SMA-negative mesangial cells may express FcγRI and FcγRIII, for α-SMA expression is generally limited to only the activated mesangial cells in the mouse.
In the glomeruli of TSLPtg mice, there are also significant IgM and IgA deposits. Much less is known about Fc receptors for IgM and IgA. FcαRI (CD89), which binds IgA, has been identified in human, but not in mouse.30 A human FcμR has been identified on B cells but not on macrophages. An Fc receptor for both IgA and IgM, named Fcα/μR, has been identified in both human and mouse tissues. Fcα/μR was found to be expressed by B cells and macrophages and also by kidney tubular epithelial cells, but was not expressed by glomerular cells.31 A specific role for IgM, IgA, and Fc receptors that specifically bind to these Ig classes therefore remains uncertain in the development of kidney lesion in IC-mediated glomerulonephritis.
Besides acting on Fc receptors on effector cells, immune complexes can also activate complement pathways to induce injury. However, in TSLPtg mice, C1q staining is absent and C3 deposition is much less than IgG and IgM deposition. Our previous studies showed that overexpression of complement receptor-1 related protein Y, which blocks the classic and alternative pathways of complement activation through inhibition of C3 convertase, did not protect the kidney injury in TSLPtg mice.32 Our studies also showed factor B deficiency with inhibition of the alternate pathway of complement activation, either alone or in addition to complement receptor-1 related protein Y overexpression, did not alleviate, but instead aggravated the renal lesion in TSLPtg mice.33,34 These results demonstrate complement activation may not play a substantially detrimental role in this IC-mediated inflammation, consistent with some other animal studies.12
A critical question remains as to the key determinants of leukocyte recruitment in cryoglobulinemic MPGN if they are not dependent on complement or activating FcγRs. We have shown previously, in contrast to the current findings with activating FcγRs, that the inhibitory receptor FcγRIIb is a critical mediator of glomerulonephritis and the recruitment of leukocytes.10 We have not yet tested the role of specific chemokines in recruitment of leukocytes to sites of glomerular injury in this model, but believe they are important mediators of this process.
Relevant to our findings is a recent study by Hida et al,35 showing FcRγ chain constitutively associates with the transmembrane domain of the β chain of the interleukin-3 receptor, thereby mediating interleukin-3 signal transduction, which in turn regulates production of interleukin-4 in basophils. It was not determined in their study to what extent this finding might be generalized to other classes of leukocytes, but a generalized scenario could have broad consequences for regulation of immune injury. For example, FcRγ deficiency would then be expected to impair the ability of the host to mount a Th2 type immune response, based on impaired production of interleukin-4. Testing of this possibility will be important in future studies of glomerulonephritis.
In conclusion, we demonstrated deficiency of activating FcγRs does not confer protection in the TSLPtg model of immune complex-mediated MPGN. TSLPtg mice deficient in activating FcγRs develop kidney disease comparable with that of TSLPtg mice and in some ways show exacerbation of disease parameters with higher levels of circulating cryoglobulins and more immune complex deposition in glomeruli. This study provides suggestive evidence that activating FcγRs may be important for the clearance of immune complexes both from the circulation and from deposits in tissues. Substantiation of this suggestive evidence may be achieved by generation of chimeric TSLPtg/FcRγ−/− mice by transplantation of bone marrow from FcRγ+/+ mice. These chimeric mice would allow more definitive testing of the importance of FcRγ+/+ monocytes/macrophages for removal of circulating and deposited immune complexes. Given the evidence that activating FcγRs may also have protective effects in diseases associated with immune complex deposition, caution should be taken when considering new therapeutic strategies that target activating FcγRs for diseases such as immune complex-mediated glomerulonephritis.
Acknowledgments
This work was supported by National Institutes of Health grant DK68802 to CEA. We thank Dr. Jeffrey V. Ravetch (Rockefeller University, New York, NY) for the kind provision of FcR γ knockout mice.
Footnotes
Address reprint requests to Charles E. Alpers, Department of Pathology, University of Washington, 1959 NE Pacific Avenue, Box 357470, Seattle, WA 98195. E-mail: calp@u.washington.edu.
Supported in part by NIH grant DK68802 to C.E.A.
References
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- Tarzi RM, Cook HT. Role of Fcgamma receptors in glomerulonephritis. Nephron Exp Nephrol. 2003;95:e7–12. doi: 10.1159/000073018. [DOI] [PubMed] [Google Scholar]
- Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol. 2001;159:2355–2369. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, Hudkins K, Dooley J, Farr A, Alpers CE, Ziegler SF, Rawlings DJ. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. 2007;8:522–531. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- Muhlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE. Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol. 2003;163:1127–1136. doi: 10.1016/s0002-9440(10)63472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- Sylvestre D, Clynes R, Ma M, Warren H, Carroll MC, Ravetch JV. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prislovsky A, Marathe B, Hosni A, Bolen AL, Nimmerjahn F, Jackson CW, Weiman D, Strom TS. Rapid platelet turnover in WASP(−) mice correlates with increased ex vivo phagocytosis of opsonized WASP(−) platelets. Exp Hematol. 2008;36:609–623. doi: 10.1016/j.exphem.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H, Engelman RW, Kurata Y, Ikehara S, Good RA. Development and characterization of monoclonal antiplatelet autoantibodies from autoimmune thrombocytopenic purpura-prone (NZW × BXSB)F1 mice. Blood. 1993;82:837–844. [PubMed] [Google Scholar]
- Guo S, Kowalewska J, Wietecha TA, Iyoda M, Wang L, Yi K, Spencer M, Banas M, Alexandrescu S, Hudkins KL, Alpers CE. Renin-angiotensin system blockade is renoprotective in immune complex-mediated glomerulonephritis. J Am Soc Nephrol. 2008;19:1168–1176. doi: 10.1681/ASN.2007050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneda S, Hudkins KL, Cui Y, Farr AG, Alpers CE, Segerer S. Growth factor expression in a murine model of cryoglobulinemia. Kidney Int. 2003;63:576–590. doi: 10.1046/j.1523-1755.2003.00778.x. [DOI] [PubMed] [Google Scholar]
- Iyoda M, Hudkins KL, Becker-Herman S, Wietecha TA, Banas MC, Guo S, Meyer-Bahlburg A, Kowalewska J, Liu G, Ziegler SF, Rawlings DJ, Alpers CE. Imatinib suppresses cryoglobulinemia and secondary membranoproliferative glomerulonephritis. J Am Soc Nephrol. 2008;20:68–77. doi: 10.1681/ASN.2008010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama H, Hasegawa Y, Kawabe T, Hara T, Matsuo S, Mizuno M, Takai T, Kikutani H, Shimokata K. Abolition of anti-glomerular basement membrane antibody-mediated glomerulonephritis in FcRgamma-deficient mice. Eur J Immunol. 2000;30:1182–1190. doi: 10.1002/(SICI)1521-4141(200004)30:4<1182::AID-IMMU1182>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tarzi RM, Davies KA, Claassens JW, Verbeek JS, Walport MJ, Cook HT. Both Fcgamma receptor I and Fcgamma receptor III mediate disease in accelerated nephrotoxic nephritis. Am J Pathol. 2003;162:1677–1683. doi: 10.1016/s0002-9440(10)64302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Watanabe N, Akikusa B, Kurasawa K, Matsumura R, Saito Y, Iwamoto I, Saito T. Fc receptor-independent development of autoimmune glomerulonephritis in lupus-prone MRL/lpr mice. Arthritis Rheum. 2003;48:486–494. doi: 10.1002/art.10813. [DOI] [PubMed] [Google Scholar]
- McKenzie SE, Schreiber AD. Fc gamma receptors in phagocytes. Curr Opin Hematol. 1998;5:16–21. doi: 10.1097/00062752-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Hirano M, Davis RS, Fine WD, Nakamura S, Shimizu K, Yagi H, Kato K, Stephan RP, Cooper MD. IgE(b) immune complexes activate macrophages through FcgammaRIV binding. Nat Immunol. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- Gresham HD, Dale BM, Potter JW, Chang PW, Vines CM, Lowell CA, Lagenaur CF, Willman CL. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member. Fgr J Exp Med. 2000;191:515–528. doi: 10.1084/jem.191.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke HH, Gessner JE, Uciechowski P, Magert HJ, Schmidt RE, Resch K. Intrinsic human glomerular mesangial cells can express receptors for IgG complexes (hFc gamma RIII-A) and the associated Fc epsilon RI gamma-chain. J Immunol. 1994;153:1281–1292. [PubMed] [Google Scholar]
- Uciechowski P, Schwarz M, Gessner JE, Schmidt RE, Resch K, Radeke HH. IFN-gamma induces the high-affinity Fc receptor I for IgG (CD64) on human glomerular mesangial cells. Eur J Immunol. 1998;28:2928–2935. doi: 10.1002/(SICI)1521-4141(199809)28:09<2928::AID-IMMU2928>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Radeke HH, Janssen-Graalfs I, Sowa EN, Chouchakova N, Skokowa J, Loscher F, Schmidt RE, Heeringa P, Gessner JE. Opposite regulation of type II and III receptors for immunoglobulin G in mouse glomerular mesangial cells and in the induction of anti-glomerular basement membrane (GBM) nephritis. J Biol Chem. 2002;277:27535–27544. doi: 10.1074/jbc.M200419200. [DOI] [PubMed] [Google Scholar]
- Tarzi RM, Davies KA, Robson MG, Fossati-Jimack L, Saito T, Walport MJ, Cook HT. Nephrotoxic nephritis is mediated by Fcgamma receptors on circulating leukocytes and not intrinsic renal cells. Kidney Int. 2002;62:2087–2096. doi: 10.1046/j.1523-1755.2002.00687.x. [DOI] [PubMed] [Google Scholar]
- Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita N, Honda SI, Usui K, Shimizu Y, Miyamoto A, Tahara-Hanaoka S, Shibuya K, Shibuya A. Identification of the Fcalpha/muR isoform specifically expressed in the kidney tubules. Mol Immunol. 2008;46:749–753. doi: 10.1016/j.molimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Muhlfeld AS, Segerer S, Hudkins K, Farr AG, Bao L, Kraus D, Holers VM, Quigg RJ, Alpers CE. Overexpression of complement inhibitor Crry does not prevent cryoglobulin-associated membranoproliferative glomerulonephritis. Kidney Int. 2004;65:1214–1223. doi: 10.1111/j.1523-1755.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- Wietecha TA, Hudkins KL, Iyoda M, Banas MC, Guo S, Wang L, Thurman J, Holers VM, Kowalewska J, Alpers CE. Deletion of murine factor B in thymic stromal lymphopoietin mice aggravates cryoglobulin-associated membranoproliferative glomerulonephritis. J Am Soc Nephrol. 2006;17:F-PO820. [Google Scholar]
- Wietecha TA, Hudkins KL, Iyoda M, Banas MC, Guo S, Wang L, Yi K, Alexandrescu S, Thurman J, Holers VM, Alpers CE. Inhibition of complement pathways of the murine protein crry and deletion of factor B in thymic stromal lymphopoietin mice aggravates cryoglobulin-associated membranoproliferative glomerulonephritis. J Am Soc Nephrol. 2007;18:SA-PO317. [Google Scholar]
- Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, Karasuyama H, Sugane K, Saito T, Taki S. Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10:214–222. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]