Abstract
The implantation of synthetic biomaterials initiates the foreign body response (FBR), which is characterized by macrophage infiltration, foreign body giant cell formation, and fibrotic encapsulation of the implant. The FBR is orchestrated by a complex network of immune modulators, including diverse cell types, soluble mediators, and unique cell surface interactions. The specific tissue locations, expression patterns, and spatial distribution of these immune modulators around the site of implantation are not clear. This study describes a model for studying the FBR in vivo and specifically evaluates the spatial relationship of immune modulators. We modified a biomaterials implantation in vivo model that allowed for cross-sectional in situ analysis of the FBR. Immunohistochemical techniques were used to determine the localization of soluble mediators, ie, interleukin (IL)-4, IL-13, IL-10, IL-6, transforming growth factor-β, tumor necrosis factor-α, interferon-γ, and MCP-1; specific cell types, ie, macrophages, neutrophils, fibroblasts, and lymphocytes; and cell surface markers, ie, F4/80, CD11b, CD11c, and Ly-6C, at early, middle, and late stages of the FBR in subcutaneous implant sites. The cytokines IL-4, IL-13, IL-10, and transforming growth factor-β were localized to implant-adherent cells that included macrophages and foreign body giant cells. A better understanding of the FBR in vivo will allow the development of novel strategies to enhance biomaterial implant design to achieve better performance and safety of biomedical devices at the site of implant.
It is estimated that at least 20 million people in the United States have a biomaterial device implant.1 Implant device failure or implant-associated infections can have disastrous consequences for the implant device function and the host. Although the risk of implant-associated infections is small (1 to 7%), these infections are associated with considerable morbidity, expensive health care, and prolonged antibiotic therapy.2 The medical and surgical cost of treating certain device failures or implant-associated infections can average up to $50,000 per patient.3,4 These significant burdens and the increasing use of biomaterial implants in a myriad of medical applications warrants a clear understanding of the immune response to these materials.
The foreign body response (FBR) also known as the host response to implanted biomaterials, involves a complex cascade of immune modulators, including various cell types, soluble mediators, and cellular interactions5 Although the FBR has proven detrimental to many implanted devices and dangerous to the patient in many cases, it has not been studied in depth. Two major problems that compromise the function of the implant are associated with the FBR; first is the fibrotic encapsulation of the device (eg, glucose sensor implant) and second is the promotion of enzymes and reactive intermediaries by activated cells in the FBR that over time are capable of degrading the biomaterials. At the present time, we do not have a clear understanding of the FBR and how to control it to avoid failure of the biomaterial implant devices. Thus, a major goal in both clinical medicine and the biomaterial industry is to obtain a better understanding of FBR response to enable further development of novel devices, materials, therapies, and treatments that can improve the safety and function of biomaterials in medicine.
Previous studies have made it clear that the macrophage is the dominant cell in the FBR and both macrophages and multinucleated foreign body giant cells (FBGCs) are commonly observed in the FBR.5,6 It is believed that macrophages adherent on the implant fuse through a complex series of events to form FBGCs.5 FBGCs have been characterized as cells expressing a wide variety of surface markers including CD14, F4/80, CD11b, and others, linking the origin of these cells to the fusion of monocyte-derived macrophages.5 The adherence of FBGCs on biomaterial surfaces is correlated to the release of enzymes (eg, esterases, lipases) and other bio-reactive intermediates that can degrade the biomaterial and cause a loss of function in the implant.5,7
Although several studies in vitro8,9 and in vivo10,11,12,13,14,15,16,17,18 have implicated immunoregulatory cytokines [interleukin (IL)-4, IL-13, IL-10, and transforming growth factor (TGF-β)], inflammatory cytokines [IL-6 and tumor necrosis factor (TNF)-α], and chemokines (MCP-1) in the formation of the FBR, the spatial tissue distribution, localization, specific cell expression and proximity of these cytokines and chemokines around the implant is not clear. We believe this knowledge is essential to the development of new therapies aiming to treat the harmful effects of the FBR or in the development of new biomaterial devices that avoid the deleterious effects of the FBR.
We have used a subcutaneous implantation model and in situ immunohistochemical techniques to assess the spatial relationship and expression patterns between FBR-associated cytokines, chemokines, and specific cell types known to be critical to the progression of the FBR. Our murine subcutaneous biomaterials implantation model reliably induces a characteristic FBR. Analyzing the tissue implantation site using immunohistochemical techniques at early (2 weeks), middle (4 weeks), and late (10 weeks) time points allowed for direct in situ analysis of the FBR. Results provide spatially resolved data correlating cells, cytokines, time, and tissue locations to biomaterials and the FBR. This approach should further the understanding of the FBR and the immune environment around biomaterial implants in vivo to facilitate improvements in materials and methods in biomaterials applications in medicine.
Materials and Methods
Mice
Specific pathogen-free female C57BL/6 mice, 6 to 8 weeks old, were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were maintained at the animal care facilities at Colorado State University, and given sterile water, mouse chow, bedding, and enrichment for the duration of the experiments. The specific pathogen-free nature of the mouse colonies at these facilities was demonstrated by testing sentinel animals. These were shown to be negative for 12 known mouse pathogens. All experimental protocols used in this study were approved by the Animal Care and Use Committee of Colorado State University.
Implant Model
A 4- × 5-mm section of nylon mesh was cut from a sterile cell strainer (BD Biosciences, San Jose, CA) under sterile conditions. The mesh consisted of woven nylon filaments approximately 50 μm in diameter with a regular pore size of 70 μm providing a high intrinsic implanted material surface area for tissue contact. The mesh was then rolled lengthwise and loaded into the tip of a sterile 16-gauge needle. Female mice 6 to 8 weeks old (n = 4) each received two materials implants without incision using only subcutaneous materials injection on the dorsal side at least 3 cm apart. The implantation procedure was performed while the mice were anesthetized using an intraperitoneal injection of 10 mg/kg body weight ketamine and 10 mg/kg xylazine. The sterile needle loaded with the prepared nylon mesh was gently inserted subcutaneously and a sterile syringe plunger was used to push the implant into the subcutaneous pocket in the dorsal tissue formed by the needle insertion. All mice were monitored until they recovered from anesthesia. Inflammation at the implant site, behavioral changes and other adverse reactions to the implant were monitored for the duration of the experiment and no significant abnormalities were observed. Sham control mice had a 16-gauge needle inserted subcutaneously and then removed with no implant.
Tissue Collection
On weeks 2, 4, and 10 postimplantation, selected mice cohorts were euthanized by CO2 asphyxiation. The implant and a 2-mm area of full thickness dermal tissue around the implant were excised together, placed into histology cassettes and fixed in formalin-free zinc fixative (BD PharMingen, San Diego, CA) for 48 hours. Following fixation, the tissue was dehydrated by incubating the cassettes in 70%, 85%, 95%, and 100% ethanol solutions followed by incubation in xylene. The tissue was then embedded in paraffin. Paraffin-embedded tissue was cut into 5- to 7-μm sections and stained with hematoxylin and eosin or Masson’s trichrome stain or left unstained for immunohistochemical analysis.
Histological and Cell Analysis
On weeks 2, 4, and 10 postimplantation, adherent cell densities and the percentage of macrophage fusion in and around the implant material were determined by adapting a previously described method.19 Cell densities on hematoxylin and eosin-stained sections of implant tissue were determined by counting the number of cells surrounding each section of implant in five representative 20× fields per sample. Cell density was expressed as cells/mm2. Macrophage fusion was determined by counting the total number of nuclei in each 20× field and the number of nuclei within FBGCs (defined as cells with three or more nuclei). Percent fusion was the number of nuclei within FBGCs divided by the total number of nuclei. Both the cell densities and percent fusion data were averaged for each sample. These analyses were performed by a researcher blinded to sample information.
Immunohistochemistry
For immunohistochemical analysis, paraffin-embedded blocks were cut in sections 5- to 7 μm thick and the paraffin was removed from the tissue sections using EZ-DeWax solution (Biogenex Lab, San Ramon, CA). The slides were then rehydrated in an ethanol gradient by incubating for 5 minutes in 100%, 90%, 85%, and 70% ethanol solutions. Slides were then washed in deionized water for 3 minutes and endogenous peroxidase was blocked with a solution of methanol containing 0.3% H202 for 20 minutes at room temperature. Subsequently, unspecific antibody binding was blocked by incubating the slides in 3% bovine serum albumin in phosphate-buffered saline for 30 minutes at room temperature and this solution was used to wash between the next steps. Following this step, each section was incubated overnight at 4°C with a primary antibody at the determined dilution (Table 1). The slides were then washed and incubated with the appropriate secondary antibody for 1 hour at room temperature. The slides were washed and thereafter, the specific antibody binding reaction was amplified using the Vectastain ABC system (Vector Laboratories, Burlingame, CA). After the amplification step, the slides were washed and incubated again for 5 minutes with at room temperature with 3-amino-9-ethylcarbazole (AEC; Vector Laboratories). Finally the slides were counterstained with hematoxylin QS (Vector Laboratories) and mounted for microscopic observation. Samples were observed using an Olympus BX41 microscope. Lung tissue from Mycobacterium tuberculosis infected mice was used as a positive control for interferon (IFN)-γ and TNF-α as this tissue has been shown to have high levels of both of these cytokines.20 The extent of staining for the cytokines/chemokines was evaluated by a researcher blinded to sample information and given a score of − for no staining, + for slight staining, and ++ for extensive staining.
Table 1.
Antibodies Used for Immunohistochemistry Studies
| Antibody | Company | Dilution | 2° Antibody |
|---|---|---|---|
| F4/80 | Invitrogen (Carlsbad, CA) | 1/25 | Goat anti-rat |
| CD11c | BD PharMingen (San Diego, CA) | 1/100 | Goat anti-rat |
| CD11b | Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA) | 1/100 | Rabbit anti-goat |
| Ly-6C | BD PharMingen | 1/75 | Goat anti-rat |
| B220 | BD PharMingen | 1/200 | Goat anti-rat |
| CD3 | DAKO (Dako, Denmark) | 1/100 | Goat anti-rabbit |
| IL-4 | Santa Cruz Biotechnologies, Inc. | 1/50 | Rabbit anti-goat |
| IL-13 | Santa Cruz Biotechnologies, Inc. | 1/75 | Rabbit anti-goat |
| IL-10 | Santa Cruz Biotechnologies, Inc. | 1/50 | Rabbit anti-goat |
| IFN-γ | Invitrogen | 1/50 | Rabbit anti-goat |
| TNF-α | Invitrogen | 1/100 | Rabbit anti-goat |
| IL-6 | R&D Systems (Minneapolis, MN) | 1/300 | Rabbit anti-goat |
| MCP-1 | Santa Cruz Biotechnologies, Inc. | 1/50 | Rabbit anti-goat |
| TGF-β1/2/3 | Santa Cruz Biotechnologies, Inc. | 1/100 | Goat anti-rabbit |
Antibody dilutions were determined for optimal staining intensity.
To quantify the average distance from lymphocytes to the implant, five 10× images were taken per sample and analyzed on Adobe Photoshop CS using the measure tool. The distances between the stained cells and closest implant surface were measured for CD3, B220, and FBGCs and at least 50 cells were counted per group.
For fluorescent double immunostaining, paraffin embedded sections were rehydrated and blocked as described above. Each section was incubated overnight at 4°C with the appropriate primary antibody at the determined dilution (Table 1). The slides were then washed and incubated with Alexa Fluor 594 anti-rat IgG (F4/80) and an Alexa Fluor 647 anti-goat IgG (Cytokines) secondary antibodies (Invitrogen) for 1 hour at room temperature. Isotype controls were labeled with either goat IgG isotype (Santa Cruz Biotechnologies) or rat IgG2a isotype (BD PharMingen). Following this, the slides were mounted using Prolong Gold (Invitrogen) mounting media containing the nuclear stain 4′-6-diamidino-2-phenylindole. Images were taken using a Zeiss LSM510 confocal microscope using a 63× objective.
Quantitative Image Analysis of Fibrosis Using Masson’s Trichrome Staining
The extent of fibrosis around the implants was quantified by Masson’s trichrome staining and computer-assisted image analysis as previously reported.21,22 Images were taken using an Olympus BX41 microscope and DP70 camera. Image data were analyzed on Adobe Photoshop CS. All images were obtained using a 20x objective. Camera settings were under manual control and held constant to produce comparable conditions for image collection. For each group (n = 4), 25 randomly selected images of the implant lesion site were captured by a blinded researcher. Positive blue signal, indicating collagen, was selected based on its color ranges and the proportional area in each image was quantified using the histogram tool and expressed as a percentage of the image.
Statistical Analysis
All results presented represent two independent experiments. Data are expressed as the mean ± the SEM (n = 4). Student’s t-test test and analysis of variance were used to assess statistical significance. P values <0.05 (*) were considered significant.
Results
Subcutaneous Implantation of Biomaterial Resulted in Strong FBR and Formation of FBGCs in Vivo
The subcutaneous implantation of the sterile synthetic nylon mesh induced a strong FBR. At 2 weeks postimplantation, there was a prominent cellular response with dispersed granulocyte and macrophage influx. The lesion had noncellular evidence of acute inflammatory changes including necrosis, hemorrhage with erythrophagocytosis, hemosiderin-laden macrophages, and neovascularization of blood vessels (Figure 1, A–D). After 4 and 10 weeks postimplantation, many of the acute inflammatory changes are resolved. The overall cellular response is less when compared with 2 weeks and the granulocyte and macrophage distribution is focal around the implant (Figure 1A). To confirm the presence of neutrophils immunohistochemical staining was performed for Ly-6C and compared with the pathological observations (Figure 1E).
Figure 1.
Histological analysis of a subcutaneous mouse model of biomaterial implantation was performed by analysis of tissue sections of the implant and lesion area at 2, 4, and 10 weeks postimplantation. A–D: H&E-stained sections of the implant and lesion area at 4 weeks. E: Immunohistochemical staining for Ly-6C showing neutrophil infiltration around a section of implant. F: Adhesion cell density. G: Percentage of macrophage fusion. H: The extent of fibrosis was quantified by computer-assisted image analysis as previously reported. Positive blue stain for collagen was selected based on its color ranges and the proportional area in each image was quantified and expressed as a percentage of the image. The statistical significance of week 2 was compared with the control group using Student’s t-test. Week 4 and 10 were compared with control and 2 weeks using analysis of variance. There was no significant difference between week 4 and 10 when analyzed by Student’s t-test. The data represent the mean ± SEM. I–K: Masson’s trichrome stained sections of implant and lesion area at 2, 4, and 10 weeks postimplantation shows areas of fibrosis. Blue stain is for collagen, red/pink is for cellular cytoplasm, and black is for nuclei. Arrows indicate areas of fibrosis. *P < 0.05.
Adherent cell densities decreased significantly from 2 to 4 weeks postimplantation (Figure 1F). FBCGs were present around the implant at 2, 4, and 10 weeks postimplantation. The percentage of macrophage fusion increases through 4 and 10 weeks postimplantation (Figure 1G).
The FBR Develops into Fibrotic Encapsulation of the Implant
Fibrotic encapsulation by the host of the implanted medical devices can be one of the major drawbacks for medical devices such as glucose biosensors.23 The fibrotic encapsulation of the implant was observed as early as 2 weeks and extended through 10 weeks (Figure 1, I–K). Using computer-assisted image analysis, the area of fibrosis around each implant was semiquantified. There was a greater area of fibrosis, defined as blue collagen staining, after 2 weeks when compared with control mice and after 4 and 10 weeks there was a significant increase in the fibrotic area over both control mice and 2 weeks (Figure 1H). There was no significant change between 4 and 10 weeks.
Macrophages Recruited to the Implant Site Express F4/80, CD11b, and Lysozyme
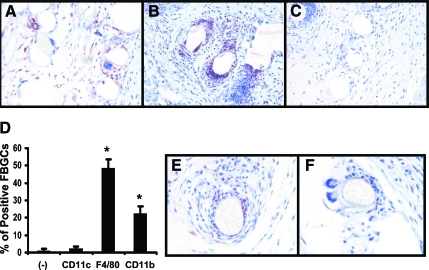
Macrophages around the implant site were positive for F4/80 and CD11b when compared with negative controls (Figure 2A). F4/80+ and CD11b+ macrophages were mainly localized to within the first layer of cells surrounding the implant. FBGCs expressed both F4/80 and CD11b (Figure 2, A–D). No cells around the implant site were positive for CD11c, a marker commonly expressed in dendritic cells.24 Positive staining for lysozyme was found in areas immediately adjacent to the implant. This staining was predominately found in small macrophages and granulocytes surrounding the implant and in direct contact with the implant (Figure 2E). However, no staining for this enzyme was observed in FBGCs at any time postimplantation (Figure 2F).
Figure 2.
Immunohistochemical staining of macrophages and FBGCs in implant tissue sections. Tissue sections at 4 weeks postimplantation were stained for F4/80 (A) CD11b (B), and CD11c (C). D: The number of FBGCs with positive staining for these markers was determined by counting FBGCs in stained sections (n = 4). A total of >50 FBGCs were counted per slide and represented as a percentage of total FBGCs. The data represent the mean ± SEM. E and F: Representative images of macrophages staining positive for lysozyme at 10 weeks postimplantation at 40×. *P < 0.05.
Lymphocytes at the Implant Site
Immunohistochemical staining for CD3 and B220 was used to detect the presence and location of T cells and B cells, respectively. At all time points, the presence of CD3 and B220 positive cells was observed at the implant site (Figure 3A), and both CD3 and B220 positive cells were observed in blood vessels in close proximity to the lesion area (data not shown). The lymphocytes were not present in high numbers with fewer than 25 positively stained lymphocytes per section (data not shown), randomly dispersed around the implant lesion area. Lymphocytes were not found in close proximity to the biomaterial surface when compared with FBGCs (Figure 3B).
Figure 3.
Immunohistochemical staining of B cells and T cells in implant tissue sections. A: Tissue sections at 4 weeks postimplantation were stained for B220 or CD3. Images on right are higher magnification of the same section from the left. Arrows indicate stained lymphocytes. B: The distances between stained CD3, B220 cells or FBGCs and the nearest implant surface.
Localized Expression of Immunosuppressive Cytokines Around the Implant Surface
With the intent of locating immunoregulatory cytokines and chemokines critical to the FBR, immunohistochemical analysis was performed for IL-13, IL-4, IL-10, and TGF-β on samples obtained from 2, 4, and 10 weeks postimplantation (Figure 4, A and B). IL-13, IL-4, IL-10, and TGF-β were present at 2, 4, and 10 weeks (Table 2). IL-4 was seen in small macrophages and FBGCs surrounding the implant but was not found in the periphery of the lesion. IL-13 was seen in FBGCs and macrophages spread throughout the lesion. IL-10 was observed only in close proximity to the implant and found in FBGCs and small macrophages. Positive staining for TGF-β was seen in FBGCs and macrophages around the lesion site. TGF-β also was associated with fibroblasts and the regions of fibrosis (Figure 4A).
Figure 4.
Immunohistochemical staining of implant tissue sections at 4 weeks postimplantation for cytokines and chemokines important in the FBR. A: Representative images of IL-4, IL-13, IL-10, and TGF-β staining at 20×. B: representative images of FBGCs stained for IL-4, IL-13, IL-10, and TGF-β at 40×. Arrows indicate positive staining in FBGCs C: Representative images of IL-6, TNF-α, IFN-γ, and MCP-1 staining at 20×. D: Representative images of FBGCs stained for IL-6, TNF-α, IFN-γ, and MCP-1 at 40×. Arrows indicate positive staining in FBGCs.
Table 2.
Extent of Staining for Cytokines/Chemokines
| Cytokine/chemokine | 2 weeks | 4 weeks | 10 weeks | FBGC |
|---|---|---|---|---|
| IL-4 | ++ | ++ | + | ++ |
| IL-10 | + | ++ | ++ | ++ |
| IL-13 | ++ | ++ | + | ++ |
| TGF-β | ++ | ++ | + | + |
| IL-6 | ++ | ++ | ++ | − |
| TNF-α | − | − | − | − |
| IFNγ | − | − | − | − |
| MCP-1 | + | + | + | ++ |
The extent of staining for the cytokines/chemokines was evaluated by a researcher blinded to sample information and given a score of − for no staining, + for slight staining, and ++ for extensive staining.
Lack of Th1 Type Inflammatory Immune Response
Immunohistochemistry for IL-6, TNF-α, IFN-γ, and the chemokine MCP-1 was performed to assess the extent and location of classical inflammatory cytokines and chemokines associated with a Th1 type of immune response (Figure 4, C and D). Positive staining for IL-6 was seen at all time points (Table 2). IL-6 was located in fibroblasts and macrophages in the periphery of the lesion area. FBGCs did not stain positive for IL-6. Staining for TNF-α was not seen at all time points. However, at very early time points, (3 and 7 days) TNF-α was expressed in the lesion area (data not shown). Positive IFN-γ staining was not observed at any time point. The chemokine MCP-1 was expressed at 2, 4, and 10 weeks postimplantation. This staining was observed in macrophages surrounding the lesion and in FBGCs.
Localization of Cytokines with Macrophage Populations
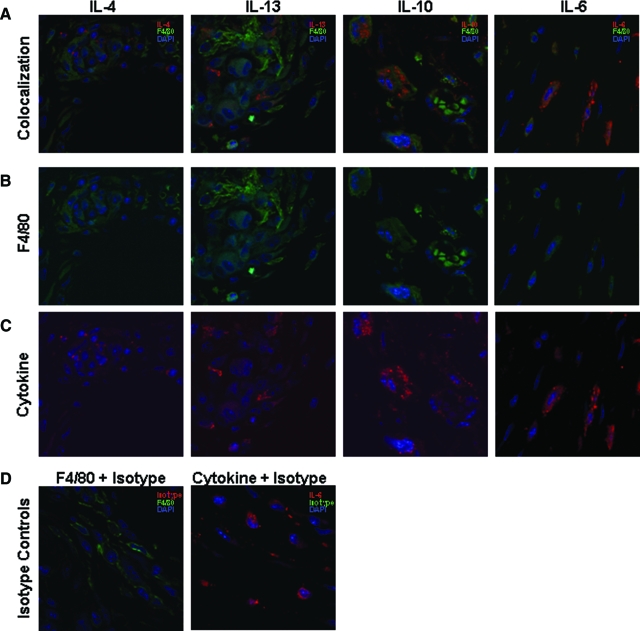
With the intent of determining the spatial relationship of cytokines and macrophage populations, fluorescent double immunostaining was performed for IL-4, IL-13, IL-10, and IL-6 along with the macrophage marker F4/80 on samples obtained from 4 weeks postimplantation (Figure 5, A–D). Control slides were prepared with isotype control antibodies. IL-4, IL-13 and IL-10 were co-localized around cells positive for the F4/80 marker and were located in close proximity to the implant. IL-6 was also co-localized with cells positive for the F4/80 marker but these cells were located in the periphery of the lesion.
Figure 5.
Fluorescent double immunostaining for the cytokines IL-4, IL-13, IL-10, and IL-6 and the macrophage marker F4/80 on implant tissue sections at 4 weeks postimplantation. A–D: Sections were incubated with Alexa 594 (F4/80) and Alexa 647 (IL-4, IL-13, IL-10, and IL-6) fluorescent secondary antibodies and mounted with mounting media containing the nuclear stain 4′-6-diamidino-2-phenylindole. Green stain correlates with F4/80, red correlates with the cytokine and blue staining correlates with the 4′-6-diamidino-2-phenylindole. Negative controls were stained using the appropriate primary antibody isotype control.
Discussion
This study reports the localization and cellular expression of various soluble and cellular immune mediators during the course of the FBR in soft tissue in situ. Although subcutaneous implantation of biomaterials has been used before to study the FBR, relatively few studies have addressed the immune mediators during the FBR in vivo and even fewer have analyzed both the spatial and temporal components of these mediators in situ.15,16,17 The mouse model described in this study allows for in situ observation of cytokines, chemokines, and cell types to better understand the environment around an implanted biomaterial during the FBR.
FBGCs and macrophages around the biomaterial are proposed as the primary cellular mediators in the foreign body response.5 We demonstrate here that the FBGCs around the implant express both F4/80 and CD11b, both markers of the monocyte-macrophage cell lineage, supporting the previously published idea that these multinucleated cells result from fusion of smaller adherent macrophages.25,26,27,28 Although studies have demonstrated that biomaterials can influence the maturation of dendritic cells in vitro,29 the lack of CD11c expression around the lesion suggests that these cells do not exhibit a dendritic cell phenotype and perhaps therefore that dendritic cells do not play a significant role in the FBR in vivo.
Lysozyme cleaves peptidoglycans and is up-regulated on macrophage activation.30 Lysozyme is still expressed at the implant site as late as 10 weeks postimplantation (data not shown). The up-regulation of lysozyme in adherent macrophages at the implant site and its noted lack of expression in adherent FBGCs suggest that macrophages undergo a phenotypic switch after fusion to form the FBGCs. Because polyesters and other biomaterials (eg, celluloses) are susceptible to degradation by lysozyme,31,32,33 further investigation into the in vivo degradation of biomaterials from wound site enzymes is justified.
Although it has been shown that the memory component of the adaptive immune response is not present in the FBR,34 the presence of T and B lymphocytes at the implant site suggests a role for these cells.6 Th2-polarized T cells were originally theorized to be the source of the cytokines IL-10, IL-4, and IL-13 during the FBR.35 However, a recent report demonstrated that T-cell-deficient mice are still able to develop a normal FBR and that the levels of IL-13 and IL-4 were not affected by the T-cell deficiency.36 It has been demonstrated in vitro that lymphocytes can affect FBGC activation and development through both indirect and direct cell-cell interactions.37 However, the data in this study suggest that both B cells and T cells are not in close proximity to the FBGCs and therefore may not have significant direct cell to cell interactive effects. Furthermore, the presence of only a small number of lymphocytes in the lesion area overall suggests that the high levels of T-cell-associated cytokines found in the lesion are from another source. Together these data diminish the role of lymphocytes in the development of the FBR; it is unlikely that the T-cell or B-cell lymphocytes are the source of the observed Th2 cytokines. More likely, the production of these cytokines may be either macrophage- or neutrophil-derived.38,39,40
The immunoregulatory cytokines IL-4 and IL-13 are both thought to induce macrophage fusion to form FBGCs and have been described as inducing alternatively activated macrophages.5,41 Blocking IL-4 in vivo inhibits FBGC development,42 and IL-13 can stimulate macrophage fusion in vitro.43 In our study, the expression of both IL-4 and IL-13 was concentrated to the areas of FBGC development and macrophage fusion. This indicates that a combination of these cytokines may prompt macrophage fusion and not one single cytokine acting alone. Moreover, FBGCs had positive staining for both IL-4 and IL-13 as late as 10 weeks postimplantation, suggesting that the FBGCs are formed by the fusion of alternatively activated macrophages or acquire that phenotype after fusion. Additionally, IL-13 has been implicated as having a role in foreign body fibrotic capsule formation that is independent of TGF-β.18
IL-10 is an immunosuppressive cytokine associated with alternatively activated macrophages that is able to block the generation of classically activated macrophages.44 Low levels of IL-10 have been found at the site of biomaterial implants in vivo.13,14 We show here for the first time that IL-10 is located at the sites of macrophage fusion and FBGC development. Additionally, FBGCs stained positive for IL-10 with a similar staining pattern to IL-14 and IL-13. Therefore IL-10 may have a role in macrophage fusion and in the suppression of inflammatory responses during the FBR.
The cytokine TGF-β has three isoforms, TGF-β 1, 2, and 3, with similar biological properties that have been associated with the development of fibrosis in a number of disorders.45 In this study, TGF-β1/2/3 (referred to as TGF-β) expression was observed throughout the lesion area, specifically correlated with areas of fibrosis. This observation is consistent with another biomaterials implant study where TGF-β1 was found within activated fibroblasts, neutrophils, and mononuclear cells in the implant lesion area.46 Additionally, correlation of TGF-β expression with areas of fibrosis suggests a role for TGF-β in the formation of fibrotic implant encapsulation. Therefore, neutralizing TGF-β species may decrease the formation of the fibrotic capsule. Furthermore, TGF-β staining was located in FBGCs suggesting that this cytokine may have an effect on the development of FBGCs or their function.
MCP-1 and IL-6 are pro-inflammatory cytokines/chemokines that can act as chemoattractants for macrophages.47,48 The presence of IL-6 observed in this study indicates it may have a significant role in recruiting macrophages to the implant site. However, Kyriakides and co-workers49 recently demonstrated that rats lacking MCP-1 still recruit macrophages to the implant site but these rats had significantly less macrophage fusion than control rats. Our study correlates with this and with other previous studies since FBGCs were observed positive for MCP-1.
TNF-α is a proinflammatory cytokine that induces apoptosis of biomaterial adherent macrophages.50 Although TNF-α was not observed in this study at the time points presented, we have observed TNF-α at very early time points in this model of implantation (ie, at 1 and 3 days postimplantation; unpublished data). The sterile nylon mesh biomaterial in this study and the injection technique were specifically used to limit the amount of endotoxins and contaminates (eg, lipopolysaccharide) that could stimulate TNF-α production. Given the abundance of these highly reactive species ubiquitous in the environment, in most water, and on biomaterials, it is essential to address this confounding influence of the procedure and outcomes when studying the FBR in vivo.51 The absence of the proinflammatory cytokine IFN-γ in this study correlates with the lack of Th1 type inflammatory response observed.
Although the expression of the immunosuppressive cytokines IL-10 and TGF-β around the implant may have a role in protecting the host from an over reactive immune response to the implant leading to increased disease pathology, we suggest that the localized concentration of these cytokines may increase susceptibility to certain opportunistic implant-associated infections by inhibiting the antimicrobial capacity of these cells located near the implant. These cytokines down-regulate certain leukocyte functions by inhibiting inflammatory cytokine production (ie, IFN-γ, TNF-α, and IL-6), abrogating activation by IFN-γ, and inhibiting bactericidal mechanisms.52,53,54 The localized loss of both IL-6 and lysozyme expression in FBGCs or adherent macrophages supports the hypothesis that these immunosuppressive cytokines are inhibiting the inflammatory immune response around the implant. Consequently, high expression of IL-4, IL-10, IL-13, and TGF-β may exacerbate certain opportunistic infections where an efficient Th1 and inflammatory response is necessary. Therefore this immunosuppressive environment may need to be considered as a potential obstacle in treating implant-associated infections and developing implants that are less susceptible to these infections.
Many published in vitro models have provided a wealth of basic cellular and cytokine signaling knowledge about FBGC development, biomaterial adherent macrophage activity, and cellular interactions during the FBR.5 However, these models use a variety of different cell lines and culture protocols that may or may not correlate either temporally or spatially with the actual FBR in vivo. The need to study the FBR in vivo to confirm in vitro reports is clear, but few studies have attempted to correlate these models. Additionally, previous in vivo studies of the FBR are complex and have used various, often incomparable implantation models to study the host materials immune response. Fortunately, many of these studies have been quantitative in demonstrating the amount of cellular or soluble mediators around implant sites using exudates, cell counting, quantitative reverse transcription-polymerase chain reaction, or enzyme-linked immunosorbent assay techniques. The current study sought not only to demonstrate the presence or absence of these immune mediators, but also define their spatial relationship within the lesion over time. Results from this study begin to give a picture of the spatial dynamics in the environment during the FBR to biomaterials and illuminate many possible areas of further study to correlate in vitro and in vivo cytokine profiling8,9,10,11,12,13 to tissue sites and cellular components of the FBR surrounding the implant.
Acknowledgments
We acknowledge Lisa Chamberlain for technical advice and discussions.
Footnotes
Address reprint requests to Dr. Mercedes Gonzalez-Juarrero, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, CO 80523-1682. E-mail: malba@mail.colostate.edu.
Supported by National Institutes of Health grant R01 EB000894.
References
- Marwick C. Implant recommendations. JAMA. 2000;283:869. [PubMed] [Google Scholar]
- Anderson JM, Marchant RE. Biomaterials: factors favoring colonization and infection. Waldvogel FA, Bisno AL, editors. Washington DC: ASM Press; Infections associated with biomedical devices. 2000:pp 89–109. [Google Scholar]
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33:1567–1572. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2007:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18:311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Meyerson H, Anderson JM. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J Biomed Mater Res A. 2008;89:152–159. doi: 10.1002/jbm.a.31939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A. 2003;64:320–329. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- Kalltorp M, Oblogina S, Jacobsson S, Karlsson A, Tengvall P, Thomsen P. In vivo cell recruitment, cytokine release and chemiluminescence response at gold, and thiol functionalized surfaces. Biomaterials. 1999;20:2123–2137. doi: 10.1016/s0142-9612(99)00115-5. [DOI] [PubMed] [Google Scholar]
- Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17:669–687. doi: 10.1163/156856206777346340. [DOI] [PubMed] [Google Scholar]
- Wang X, Lennartz MR, Loegering DJ, Stenken JA. Multiplexed cytokine detection of interstitial fluid collected from polymeric hollow tube implants-a feasibility study. Cytokine. 2008;43:15–19. doi: 10.1016/j.cyto.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Lappalainen R, Laine P, Kitti U, Santavirta S, Teronen O. Immunohistochemical evaluation of inflammatory mediators in failing implants. Int J Periodontics Restorative Dent. 2006;26:135–141. [PubMed] [Google Scholar]
- Scheidbach H, Tamme C, Tannapfel A, Lippert H, Kockerling F. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc. 2004;18:211–220. doi: 10.1007/s00464-003-8113-1. [DOI] [PubMed] [Google Scholar]
- Luttikhuizen DT, Harmsen MC, van Luyn MJ. Cytokine and chemokine dynamics differ between rats and mice after collagen implantation. J Tissue Eng Regen Med. 2007;1:398–405. doi: 10.1002/term.50. [DOI] [PubMed] [Google Scholar]
- Ward WK, Li AG, Siddiqui Y, Federiuk IF, Wang XJ. Increased expression of Interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J Biomater Sci Polym Ed. 2008;19:1065–1072. doi: 10.1163/156856208784909408. [DOI] [PubMed] [Google Scholar]
- Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci USA. 2002;99:10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Higgins JR, Lee EJ, Orme IM, Gonzalez-Juarrero M. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J Immunol. 2008;180:4892–4900. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- Lehr HA, van der Loos CM, Teeling P, Gown AM. Complete chromogen separation and analysis in double immunohistochemical stains using Photoshop-based image analysis. J Histochem Cytochem. 1999;47:119–126. doi: 10.1177/002215549904700113. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal Dendritic Cells” Comprise Two Distinct Populations: CD1(+) Dendritic Cells and CD209(+) Macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasou NA, Quinn J. Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol. 1990;43:997–1003. doi: 10.1136/jcp.43.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussis IA, Puddle B, Athanasou NA. Immunophenotype of multinucleated and mononuclear cells in giant cell lesions of bone and soft tissue. J Clin Pathol. 1992;45:398–404. doi: 10.1136/jcp.45.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbondanzo SL, Young VL, Wei MQ, Miller FW. Silicone gel-filled breast and testicular implant capsules: a histologic and immunophenotypic study. Mod Pathol. 1999;12:706–713. [PubMed] [Google Scholar]
- Al-Saffar N, Revell PA, Kobayashi A. Modulation of the phenotypic and functional properties of phagocytic macrophages by wear particles from orthopaedic implants. J Mater Sci Mater Med. 1997;8:641–648. doi: 10.1023/a:1018575504518. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Mata J, Babensee JE. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J Biomed Mater Res A. 2007;80:7–12. doi: 10.1002/jbm.a.30832. [DOI] [PubMed] [Google Scholar]
- Gordon S, Todd J, Cohn ZA. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974;139:1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Jiang H, Chen W. The biodegradability of electrospun Dextran/PLGA scaffold in a fibroblast/macrophage co-culture. Biomaterials. 2008;29:1583–1592. doi: 10.1016/j.biomaterials.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P, Vila-Jato JL, Alonso MJ. Effect of lysozyme on the stability of polyester nanocapsules and nanoparticles: stabilization approaches. Biomaterials. 1997;18:1305–1310. doi: 10.1016/s0142-9612(97)00061-6. [DOI] [PubMed] [Google Scholar]
- Lim SM, Song DK, Oh SH, Lee-Yoon DS, Bae EH, Lee JH. In vitro and in vivo degradation behavior of acetylated chitosan porous beads. J Biomater Sci Polym Ed. 2008;19:453–466. doi: 10.1163/156856208783719482. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Voskerician G, Meyerson H, MacEwan SR, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J Biomed Mater Res A. 2008;85:556–565. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, MacEwan SR, Meyerson H, Kirk JT, Anderson JM. The foreign body reaction in T-cell-deficient mice. J Biomed Mater Res A. 2008;90:106–113. doi: 10.1002/jbm.a.32050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Colton E, Anderson JM. Paracrine and juxtacrine lymphocyte enhancement of adherent macrophage and foreign body giant cell activation. J Biomed Mater Res A. 2008;89:490–498. doi: 10.1002/jbm.a.31981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, Holtzman MJ. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E, Woerly G, Younes AB, Loiseau S, Capron M. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. 2000;68:125–130. [PubMed] [Google Scholar]
- Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29:1267–1275. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–3390. [PubMed] [Google Scholar]
- Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Li AG, Quinn MJ, Siddiqui Y, Wood MD, Federiuk IF, Duman HM, Ward WK. Elevation of transforming growth factor beta (TGFbeta) and its downstream mediators in subcutaneous foreign body capsule tissue. J Biomed Mater Res A. 2007;82:498–508. doi: 10.1002/jbm.a.31168. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, Rollins BJ, Bornstein P. The CC chemokine ligand. CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–2166. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck WG, Shive MS, Colton E, Ziats NP, Anderson JM. Interleukin-4 inhibits tumor necrosis factor-alpha-induced and spontaneous apoptosis of biomaterial-adherent macrophages. J Lab Clin Med. 2002;139:90–100. doi: 10.1067/mlc.2002.121260. [DOI] [PubMed] [Google Scholar]
- Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25:5681–5703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis AR. Interleukin-4. Mire-Sluis AR, Thorpe R, editors. San Diego: Academic Press,; Cytokines. 1998:pp 51–68. [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]