Abstract
The urokinase receptor (uPAR) promotes metastasis of human malignancies; however, its mechanism of action remains incompletely understood. Established models focus on the ability of uPAR to bind urokinase-type plasminogen activator (uPA) and promote protease activation in the tumor cell microenvironment; however, uPAR also regulates cell signaling and migration by both uPA-dependent and -independent mechanisms in vitro. The significance of uPAR as a cell-signaling receptor in vivo remains unclear. In this study, we expressed either human or mouse uPAR in human embryonic kidney (HEK-293) cells. We selected HEK-293 cells because, unlike most cancer cells, they do not express uPA or uPAR endogenously. Both mouse and human uPAR increased cell adhesion and migration on vitronectin. Rac1 was activated and responsible for the increase in cell migration. HEK-293 cells that did not express uPAR formed palpable tumors in severe combined immunodeficient mice; however, metastases were exceedingly rare. The xenografts contained abundant mouse uPA, produced by infiltrating mouse cells, but no human uPA. Mouse uPA bound only to mouse uPAR and not human uPAR and, thus, could not interact with human uPAR-expressing HEK-293 cells in xenografts. Nevertheless, both mouse and human uPAR significantly increased HEK-293 cell metastasis into the lungs. The activity of human uPAR suggests that uPAR may promote cancer metastasis independent of uPA. Candidate mechanisms include its effects on adhesion, migration, and Rac1 activation.
The urokinase receptor (uPAR) is a glycosyl phosphatidylinositol-anchored membrane protein that functions as a biomarker for cancer progression and metastasis in many forms of human malignancy.1 In the prostate, malignant epithelial cells are uPAR-positive, whereas benign epithelia are uPAR-negative.2 The degree of uPAR-positivity in malignant prostate epithelial cells correlates with the Gleason grade of the tumor.3 Similarly, in endometrial cancer, uPAR expression in tumor cells correlates with tumor grade, recurrence, and mortality.4 Breast cancer may be more complex because although uPAR expression correlates with disease severity, in many cases, uPAR is expressed by both malignant epithelial cells and nonmalignant stromal cells in the tumor microenvironment.5,6,7,8 uPAR expression by normal cells in the adult is limited5; however, this receptor may play an important role in diseases in addition to cancer, such as rheumatoid arthritis9 and infectious lung diseases.10
Binding of urokinase-type plasminogen activator (uPA) to uPAR substantially increases the catalytic efficiency for plasminogen activation.11 Plasmin, which is generated near the cell surface, may participate in the activation of metalloproteases.12 Based on these findings, the first models developed to explain the cancer-promoting activity of uPAR focused on its ability to support protease activation and remodeling of the extracellular matrix (ECM).13,14 Activated proteases may facilitate cellular penetration of tissue boundaries such as basement membranes.15 In migrating cells, uPAR localizes to leading lamellipodia,16 supporting the hypothesis that remodeling of ECM is an important uPAR activity in cell migration and invasion.
uPAR regulates cell adhesion by binding directly to vitronectin and by lateral association with integrins in the plasma membrane.17,18 Although the effects of uPAR on cell adhesion may be regulated by uPA, there is no strict requirement for uPA. uPAR activates cell-signaling to Rac1 by a pathway that is dependent on vitronectin but not uPA.19,20,21 Cell-signaling factors that are activated downstream of uPAR but typically in response to uPA-binding include H-Ras, ERK/MAP kinase, and Akt.22,23,24 uPAR-initiated cell-signaling controls cell survival, proliferation, and cell migration in vitro.25,26 uPAR also may regulate cell-signaling in response to ligands for receptor tyrosine kinases, such as epidermal growth factor.27
In animal model studies, uPAR expression has been correlated with cancer progression and metastasis28,29,30; however, the mechanisms by which uPAR functions in vivo remain elusive. Recently identified uPAR activities in cell signaling and adhesion remain largely untested in in vivo model systems. To address this problem, we took advantage of the strict species specificity in uPA-binding to uPAR; mouse uPA does not bind with significant affinity to human uPAR.31 Xenografts were developed in severe combined immunodeficient (SCID) mice using human embryonic kidney (HEK-293) cells, which do not express uPA or uPAR endogenously,32 but form tumors because of integrated adenovirus genes.33 We show that nonmalignant mouse cells, which infiltrate HEK-293 cell xenografts, generate abundant mouse uPA. When HEK-293 cells were transfected for stable expression of mouse uPAR, metastasis to the lungs was significantly increased, as anticipated because of the availability of mouse uPA in the tumors. Surprisingly, HEK-293 cells that express human uPAR also demonstrated significantly increased metastasis despite the absence of human uPA. The ability of human uPAR to promote metastasis in mice rules out mechanisms involving activation of proteases, and instead, supports the hypothesis that uPAR may promote metastasis independently of uPA. Candidate mechanisms identified here include regulation of cell adhesion, migration, and Rac1 activation.
Materials and Methods
Reagents
Val-Leu-Lys-p-nitroanilide (VLK-pNA) was from Bachem (Torrance, CA). Mouse uPA was from Molecular Innovations (Novi, MI). [Glu1]plasminogen was purified from human plasma as previously described.34 The MEK inhibitor, PD098059, and the Rac1-GEF inhibitor, NSC23766, were from EMD Biosciences (Gibbstown, NJ). Expression constructs encoding human uPAR and mouse uPAR are previously described.27 An expression construct encoding humanized green fluorescent protein (phr-GFP) was from Stratagene (La Jolla, CA). Rac/Cdc42 assay reagent (PAK1-PBD), which includes residues 67 to 150 of p21-activated kinase fused to glutathione-S-transferase and coupled to glutathione-agarose was from Millipore (Billerica, MA). Expression constructs encoding dominant negative Rac1 (DN-Rac1/N17Rac1) tagged with green fluorescent protein (GFP) and the corresponding empty vector (pCDNA-EGFP) were from Addgene (Cambridge, MA). Polyclonal antibody that recognizes total ERK/MAP kinase was from Millipore. Rac1-specific monoclonal antibody was from BD Biosciences (San Jose, CA). Polyclonal human uPAR-specific antibody 3932, which was used for immunoblot analysis, was from American Diagnostica (Stamford, CT). Monoclonal human uPAR-specific antibody ATN-658, which was used for immunohistochemistry, mouse uPAR-specific antiserum, which was used for immunoblot analysis and immunohistochemistry, and mouse uPA-specific antiserum, which was used for immunofluorescence microscopy, were kindly provided by Dr. Andrew Mazar (Attenuon, San Diego, CA). Horseradish peroxidase-conjugated antibodies specific for mouse or rabbit IgG were from GE Health care (Piscataway, NJ). Vitronectin was purified from outdated human plasma as previously described.35 Vectastatin ABC Elite kit for immunohistochemistry was from Vector (Burlingame, CA). Phalloidin conjugated with Alexa Fluor 568 was from Invitrogen (Carlsbad, CA). Quantitative (q)PCR reagents, including primers and probes, were from Applied Biosystems (Foster City, CA).
Cell Lines
HEK-293 cells (ATCC) were transfected to express full-length human uPAR or mouse uPAR or with empty vector using lipofectamine (Invitrogen). Transfected cells were selected in hygromycin (0.5 mg/ml) and subjected to single-cell cloning. These cells were further transfected with phr-GFP using lipofectamine and selected in puromycin (1.5 μg/ml) for another 2 weeks. Cell lines were maintained in Dulbecco’s Modified Eagle Medium (Hyclone) supplemented with 10% fetal bovine serum (Hyclone), penicillin (100 units/ml), streptomycin (100 μg/ml), hygromycin (0.2 mg/ml), and puromycin (1.5 μg/ml). MDA-MB-468 cells (ATCC) were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml).
Immunoblot Analysis
Immunoblot analysis was performed to detect human uPAR and mouse uPAR in HEK-293 cells and transfected clones. Cell extracts were prepared in RIPA buffer (20 mmol/L sodium phosphate, 150 mmol/L NaCl, pH 7.4, 1% NP40, 0.1% SDS, 0.5% sodium deoxycholate) containing complete protease inhibitor cocktail (Roche). Protein concentrations were determined by bicinchoninic acid assay (Sigma-Aldrich). Equal amounts of cell extract were resolved on an SDS-polyacrylamide gel, electrotransferred to nitrocellulose membrane, and probed with primary antibodies.
uPA-Coupled Enzyme Assay
Plasminogen activation by uPAR-associated uPA was determined as previously described.27 In brief, cells were incubated with mouse uPA (10 nmol/L) for 1 hour at 4°C. The cells then were washed and reconstituted in EHB buffer (Earle’s balanced salt solution, 25 mmol/L HEPES, 1 mg/ml bovine serum albumin, pH 7.4) with [Glu1]plasminogen (1 μmol/L) and VLK-pNA (0.5 mmol/L) for 2 hours at 37°C. VLK-pNA hydrolysis was detected by measuring the absorbance at 406 nm.
qPCR
Total RNA was isolated from xenografts and from cells in culture using the RNeasy kit (Qiagen). cDNA was synthesized using the iScript cDNA synthesis kit (BioRad). qPCR was performed using a System 7300 instrument (Applied Biosystems) and a one-step program: 95°C, 10 minutes; 95°C, 30 s and 60°C, 1 minute for 40 cycles. HPRT-1 gene expression was measured as a normalizer. Results were analyzed by the relative quantification (ΔΔCt) method. Experiments were performed in triplicate with internal duplicate determinations. The human uPA-specific primer/probe set amplified mouse uPA with a relative efficiency of 3 × 10−6. The total uPA primer/probe set amplified mouse and human uPA with essentially identical efficiency.
Immunofluorescence Microscopy
Cells were plated on glass coverslips and then fixed in 4% formaldehyde. Fixed cells were permeabilized in 0.2% Triton X-100 and incubated with phalloidin conjugated with Alexa Fluor 568 for 30 minutes. Preparations were mounted on slides using Pro-long Gold with 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and examined using a Leica DMIRE2 fluorescence microscope. Images were obtained using a ×63 oil-immersion objective and a Hamamatsu digital camera with SimplePCI software. Deconvolution was performed using Simple PCI software. To study mouse uPA-binding to HEK-293 cells, cells plated on coverslips were incubated with mouse uPA (10 nmol/L) for 30 minutes at 4°C and then fixed in 4% formaldehyde. Fixed cells were incubated with polyclonal mouse uPA-specific antibody, and then with secondary antibody conjugated with Alexa Fluor 488. Preparations were mounted on slides using Pro-long Gold with DAPI and examined by fluorescence microscopy.
Cell Adhesion
High-binding 96-well plates (Corning) were coated with purified vitronectin (2 μg/ml). Cells (1 × 104) were suspended in 100 μl of serum-free medium containing 0.1% bovine serum albumin and seeded into vitronectin-coated plates. Cells were allowed to adhere for 1 hour at 37°C. The wells then were washed with phosphate-buffered solution. Adherent cells were fixed in 4% formaldehyde and stained with crystal violet. The dye was eluted with 10% acetic acid, and the absorbance at 570 nm was determined.
Cell Migration
Migration of uPAR-expressing and control HEK-293 cells was studied using 6.5-mm Transwell chambers with 8 μm pores (Costar). The Transwell membranes were precoated with purified vitronectin (1 μg/ml) on the underside only. In some studies, cells (105 in 100 μl) were pre-treated with PD098059 (50 μmol/L) or NSC23766 (100 μmol/L) for 15 minutes in suspension. The cells then were added to the upper chamber of each Transwell unit in the presence of the same pharmacological antagonists. The lower chamber was supplemented with 10% fetal bovine serum to create a chemotactic gradient. Migration was allowed to occur for 24 hours at 37°C. Cell migration was determined by crystal violet-staining of cells on the lower membrane surface, as previously described.19 To study migration of DN-Rac1-expressing cells, HEK-293 cells were transiently transfected with DN-Rac1 tagged with GFP or with pCDNA-EGFP, as a control. Migration studies were performed for 24 hours. Non-migrating cells were removed with a cotton swab and the membranes were fixed in 4% formaldehyde. Cells expressing GFP on the lower surfaces of the membranes were counted by fluorescence microscopy.
Affinity Precipitation of GTP-Rac1
Active GTP-loaded Rac1 was affinity-precipitated using the fusion protein, PAK1-PBD, which specifically recognizes the GTP-bound forms of Rac1 and Cdc42, as previously described.20 uPAR-expressing and control HEK-293 cells (8 × 105) were cultured in 10 cm plates for 18 hours. Cultures then were washed with ice-cold PBS and extracted in 1% (v/v) Triton X-100, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 50 mmol/L Tris-HCl, 0.5 M/L NaCl, 10 mmol/L MgCl2, pH 7.2, supplemented with protease inhibitor cocktail and 1 mmol/L sodium orthovanadate. The extracts were incubated with 15 μg of PAK1-PBD coupled to glutathione-sepharose for 45 minutes at 4°C. The glutathione-sepharose was washed four times and then treated with SDS sample buffer to dissociate the PAK1-PBD and associated proteins. Immunoblot analysis then was performed to detect Rac1. Samples of each cell extract also were subjected to immunoblot analysis before incubation with PAK1-PBD to determine total Rac1.
Xenograft Studies in Mice
Animal experimentation was done in accordance with protocols approved by the University of California San Diego Animal Care Program. Anesthetized 8-week-old C.B-17/lcrCrl-scid-BR mice (Charles River Laboratories) were inoculated in the fourth mammary fat pad with 1 × 106 HEK-293 cells or with MDA-MB 468 cells suspended in 50 μl of Matrigel (Sigma). Primary tumor growth was monitored every 2 to 3 days, using external measurement by calipers, until the tumors were 2.0 cm in maximum diameter. The mice then were euthanized and the xenografts recovered for analysis. Xenograft tumor volumes were calculated using the formula: (4/3)π × largest radius × (smaller radius)2. For the xenografts described in this study, maximum tumor size was achieved without outward evidence of morbidity. In some studies, the HEK-293 cells expressed GFP. This allowed us to screen for metastases using a Leica fluorescence stereomicroscope (MZ FLIII), first in the whole animal at necropsy and then, in each lung. Pulmonary metastatic load was determined by counting the number of fluorescent tumor foci visible on the ventral surface of each lobe at ×8 magnification. We counted only fluorescent foci that were larger than 100 μm. Green-fluorescing metastases were confirmed by histology.
Human tumor cell burden in the lungs also was determined by real-time qPCR to detect human Alu repeats. This technique is modification of the method that was originally developed by Kim et al.36 Genomic DNA was extracted from the cardiac lobe of each mouse lung using the minigenomic DNA extraction kit (Quiagen). The primers were: 5′-ACGCCTGTAATCCCAGCACTT-3′ (sense) and 5′-TCGCCCAGGCTGGAGTGCA-3′ (antisense). Genomic DNA (50 ng) was mixed with SYBR green master mix (Applied Biosystems) and the specific primers. Amplification was performed using an ABI 7300 instrument and the following conditions: 95°C for 2 minutes followed by 30 cycles at 95°C for 30 s, 63°C for 30 s, and 72°C for 30 seconds. Because the amount of mouse DNA in each sample was substantially higher than the amount of human DNA, we standardized results by comparison with the signal obtained for the mouse glyceraldehyde-3-phosphate dehydrogenase gene. To analyze the actual number of human cells in mouse lung, a standard curve was generated through quantitative amplification of genomic DNA extracted from a serial dilution of HEK-293 cells mixed with extract of mouse cardiac lung lobe. The actual number of tumor cells/cardiac lobe was estimated by comparison with the standard curve.
Analysis of Mouse Tissues
Formalin-fixed tissue specimens were paraffin-embedded. Serial 4-μm sections were stained with H&E. These sections were examined by light microscopy. Tissue sections also were analyzed by fluorescence microscopy to detect green-fluorescing tumor cells after freezing in OCT compound (Tissue-Tek). Four-micron frozen sections were fixed in acetone and rinsed with PBS. Preparations were mounted on slides using Pro-long Gold with DAPI (Invitrogen) and examined using a Leica DMIRE2 fluorescent microscope. Images were obtained using a ×20 objective. Immunohistochemistry studies to detect uPAR were performed using 4-μm sections of primary tumor frozen in OCT compound and processed as described above. Incubation with 1% hydrogen peroxide was used to remove endogenous peroxidase activity. Sections were incubated with blocking buffer (goat or horse serum in phosphate-buffered saline) for 2 hours and then with primary antibodies in blocking buffer. Specific binding of primary antibodies was detected by the addition of biotin-conjugated goat anti-rabbit or horse anti-mouse antibody, followed by avidin:biotinylated enzyme complex and 3,3′-diaminobenzidine (Vector Laboratories). Each sample was counterstained using hematoxylin (Sigma). Light microscopy images were obtained using ×10 or ×40 objectives and a Leica DFC 420 digital camera with Leica Application Suite software. When necessary, histological sections were examined by a pathologist that was blinded to their identity.
Data Analysis/Statistics
Data processing and statistical analysis were performed using GraphPad Prism (GraphPad Software Inc.), Microsoft Excel (Microsoft Corporation), and MATLAB (The MathWorks). Depending on the distribution, the Student’s t-test, one-way analysis of variance, or Mann-Whitney rank sum test was performed.
Results
Non-Cancer Cells Produce uPA in Xenografts Established in Mice
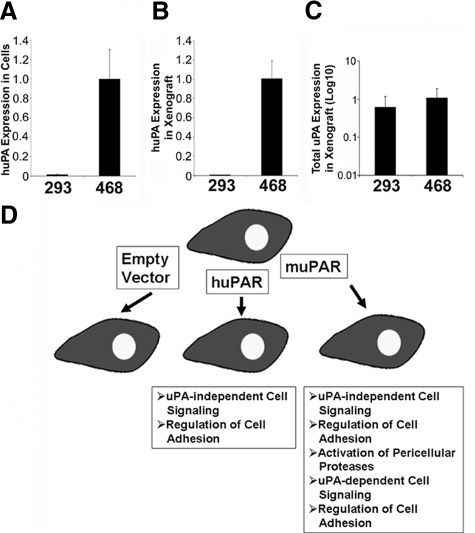
To examine uPA expression in the tumor microenvironment, xenografts were established in SCID mice using MDA-MB 468 human breast cancer cells and HEK-293 cells. The same cell lines also were maintained in cell culture. Total RNA was extracted from the xenografts and from the cell cultures. qPCR was performed to compare uPA mRNA expression. First, we used primers and probes that are specific for human uPA and do not cross-react with mouse uPA. Figure 1A shows that in cell culture, HEK-293 cells fail to express detectable levels of human uPA, compared with MDA-MB 468 cells, which express abundant uPA (the positive control). Equivalent results were obtained when we examined xenografts (Figure 1B); human uPA mRNA expression was essentially undetectable in tumors formed with HEK-293 cells.
Figure 1.
uPA expression in xenografts. A: Human uPA (huPA) mRNA level in HEK-293(293) and MDA-MB 468(468) cells in vitro. B: huPA mRNA in xenografts prepared with HEK-293 and MDA-MB 468 cells. C: Total uPA mRNA level in xenografts prepared with HEK-293 and MDA-MB 468 cells. Relative levels of uPA mRNA were determined by real-time qPCR and normalized to HPRT mRNA. The amount of uPA mRNA in HEK-293 was expressed relative to that present in MDA-MB 468 cells (mean ± SEM). D: Model showing uPAR-associated activities that are possible in HEK-293 cells that express human uPAR or mouse uPAR.
Next, we probed the identical xenograft RNA extracts using primers and probes that are equally efficient in amplifying mRNA for human uPA and mouse uPA. Figure 1C shows that with these primers and probes, uPA was detected in tumors formed with both cell types; the level of uPA mRNA present in HEK-293 cell xenografts was not significantly different (at the P < 0.05 level) from that present in tumors formed with MDA-MB 468 cells. We concluded that in primary tumors formed with HEK-293 cells, uPA is generated exclusively by infiltrating mouse cells, which may include inflammatory cells and other stromal cells. These cells are known to interact with malignant cells in the tumor microenvironment and influence the aggressiveness of the malignancy.37
Characterization of uPAR-Expressing HEK-293 Cells
To determine uPAR activities that may be important for cancer progression in vivo, HEK-293 cells were transfected to express human uPAR or mouse uPAR. We chose HEK-293 cells because it was previously reported that these cells do not express uPA or uPAR endogenously.32 Although HEK-293 cells are not true cancer cells, due to expression of adenoviral early region 1A, these cells are capable of forming tumors in immunodeficient mice.33 Others have shown that binding of uPA to uPAR is species-specific.31 Thus, mouse uPA that is produced by nonmalignant cells in the HEK-293 cell xenograft microenvironment may interact only with HEK-293 cells that express mouse uPAR. By binding uPA, mouse uPAR-expressing cells may mobilize the full continuum of uPAR-associated activities, including activation of cell-surface proteases.25,26 HEK-293 cells that express human uPAR may take advantage only of uPAR activities that do not require uPA. Representative uPAR activities that fall into either category are shown in the model presented in Figure 1D.
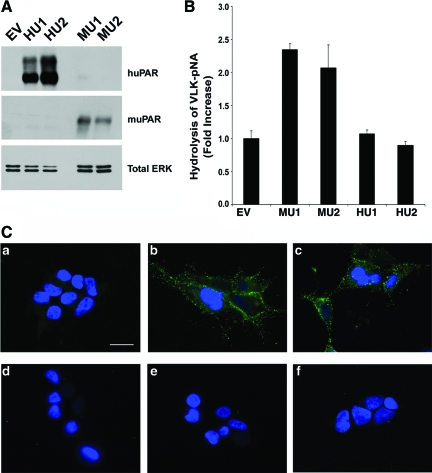
Two single-cell clones that express human uPAR (HU1 and HU2) and two that express mouse uPAR (MU1 and MU2) were selected for further study. As a control, we studied cells that were transfected with empty vector (EV). Immunoblot analysis was performed to detect human or mouse uPAR using antibodies that do not cross-react. Figure 2A shows that the HU1 and HU2 cells expressed comparable levels of uPAR. The presence of more than one band has been previously attributed to heterogeneity in uPAR glycosylation.38 Human uPAR was not detected in the EV cells or in the mouse uPAR-expressing cells, as anticipated. The MU1 and MU2 cells expressed similar levels of mouse uPAR. We confirmed that both human uPAR and mouse uPAR are expressed on the cell surface by flow cytometry (results not shown). Cell-surface uPAR was comparable in the MU1 and MU2 cells and in the HU1 and HU2 cells.
Figure 2.
uPAR expression and activity in transfected HEK 293 cells. A: Extracts from cells transfected with empty vector (EV), human uPAR-expressing clones (HU1, HU2), and mouse uPAR-expressing clones (MU1, MU2) were subjected to immunoblot analysis to detect mouse or human uPAR, and total ERK/MAP kinase as a loading control. B: The ability of mouse uPAR and human uPAR to support plasminogen activation in the presence of mouse uPA was determined by incubating cells with mouse uPA, followed by purified plasminogen and VLK-pNA. Hydrolysis of VLK-pNA was determined by measuring the absorbance at 406 nm (mean ± SEM, n = 4). C: Cells were incubated with mouse uPA (10 nmol/L), washed, fixed and then incubated with mouse uPA-specific antibody (green) and DAPI (blue). Panel (a) shows EV cells that were incubated with mouse uPA; b: MU1 cells that were incubated with mouse uPA; c: MU2 cells that were incubated with mouse uPA; d: HU1 cells that were incubated with mouse uPA; e: HU2 cells that were incubated with mouse uPA; f: MU1 cells were treated with mouse uPA-specific antibody without prior incubation with mouse uPA. Scale bar = 15 μm.
Because HEK-293 cells are exposed exclusively to mouse uPA in a xenograft microenvironment, studies were performed to compare the ability of mouse uPAR and human uPAR to promote plasminogen activation in the presence of mouse uPA. In MU1 and MU2 cells, mouse uPAR promoted plasminogen activation, as determined by a coupled enzyme assay in which the cells were loaded with purified mouse uPA, washed, and then incubated with purified plasminogen and the plasmin-specific substrate, VLK-pNA. As compared with control EV cells, VLK-pNA hydrolysis was increased by about 2.5-fold (Figure 2B). When HU1 and HU2 cells were pre-incubated with mouse uPA and then with plasminogen and VLK-pNA, no increase in plasminogen activation was observed, confirming the anticipated result that human uPAR does not bind mouse uPA. We also examined the ability of HEK-293 cells that express human uPAR or mouse uPAR to bind mouse uPA by immunofluorescence microscopy. All five cell lines (HU1, HU2, MU1, MU2, EV) were incubated with mouse uPA (10 nmol/L), washed, fixed, and then incubated with mouse uPA-specific antibody. Bound uPA was detected only in MU1 and MU2 cells (Figure 2C). In control experiments, no immunofluorescence was detected when MU1 or MU2 cells were treated with mouse uPA-specific antibody, without prior treatment with mouse uPA.
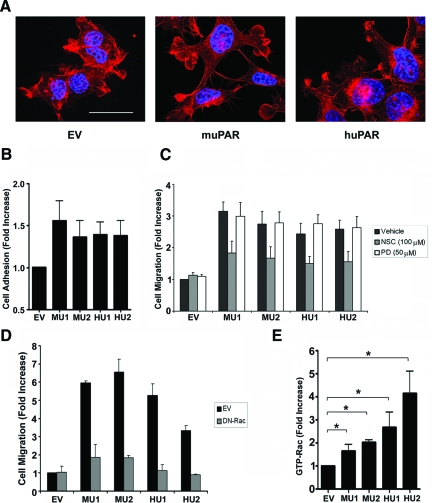
Mouse uPAR and Human uPAR Alter Cell Morphology and Promote Cell Migration Similarly
Madsen et al32 reported that HEK-293 cells, which express human uPAR, demonstrate altered morphology. We confirmed that human uPAR alters HEK-293 cell morphology and demonstrated equivalent changes in the morphology of mouse uPAR-expressing cells. Cells expressing both forms of the receptor developed highly elongated protrusions when cultured on vitronectin (Figure 3A). Although Madsen et al32 attributed these changes to activation of ERK/MAP kinase, others have suggested that this morphology may indicate activation of Rac1 in the absence of substantial Rho-A activation.39
Figure 3.
uPAR expression alters cell morphology, adhesion, and migration. A: Mouse uPAR-expressing MU1 cells, human uPAR-expressing HU2 cells, and control cells that were transfected with empty vector (EV) were stained with phalloidin (red) and DAPI (blue). Scale bar = 15 μm. B: HEK-293 cells transfected with empty vector (EV), human uPAR-expressing cells (HU1, HU2) and mouse uPAR-expressing cells (MU1, MU2) were seeded in vitronectin-coated 96-well plates. Cells were allowed to adhere for 1 hour. Cell adhesion was expressed as the fold-increase relative to EV cells (mean ± SEM, n = 4). C: EV, HU1, HU2, MU1, and MU2 cells were treated with the Rac1-GEF inhibitor, NSC23766 (NSC, 100 μmol/L), the MEK inhibitor, PD098059 (PD, 50 μmol/L), or vehicle and allowed to migrate in Transwells for 24 hours. Cell migration was expressed as the fold-increase relative to vehicle-treated EV cells (mean ± SEM, n = 4). D: EV, HU1, HU2, MU1, and MU2 cells were transiently transfected with DN-Rac1 tagged with EGFP or with empty vector (pCDNA-EGFP) as a control. Cells were allowed to migrate in Transwells for 24 hours. Cell migration was expressed as the fold-increase relative to EV cells (mean ± SEM, n = 4). E: Cells extracts were affinity-precipitated with PAK1-PBD and subjected to immunoblot analysis to determine GTP-bound Rac1. Results were standardized by separate determinations of total Rac1. Immunoblots were analyzed by densitometry. The results of four separate experiments were averaged to generate the bar graph. *P < 0.05 (mean ± SEM, n = 4).
uPAR regulates the activity of vitronectin-binding integrins and, in the absence of these integrins, also may function as an independent adhesion receptor for vitronectin.17,18 HEK-293 cells are known to adhere weakly to vitronectin, due mainly to the function of αvβ1.40 Figure 3B shows that murine uPAR and human uPAR increased adhesion of HEK-293 cells to vitronectin. The increase was statistically significant for all four uPAR-expressing cell lines (compared with EV cells, P < 0.05). The magnitude of the increase in cell adhesion was equivalent for the MU and HU cells. This result confirms that both human uPAR and mouse uPAR were present on the cell surface and biologically active when expressed in HEK-293 cells.
All four uPAR-expressing cell lines demonstrated increased cell migration. The change in migration rate was statistically significant (P < 0.01, one-way analysis of variance) when fetal bovine serum was added to the lower chamber as a chemoattractant (Figure 3C) and when fetal bovine serum was omitted in control experiments (results not shown). NSC23766, which is a pharmacological inhibitor of the Rac1-GEFs, Trio and Tiam1, inhibited the increase in cell migration associated with uPAR expression in HEK-293 cells by about 60%. NSC23766 had no effect on migration of control EV cells. PD098059, which inhibits activation of ERK/MAP kinase, did not affect migration of the uPAR-expressing cells. This result was anticipated because, in our hands, activation of ERK/MAP kinase downstream of uPAR requires uPA.24 Based on these results, we hypothesized that uPAR stimulates migration of HEK-293 cells by activating Rac1. The incomplete effects of NSC23766 may reflect the fact that this reagent does not inhibit all Rac1 GEFs.
To further test the role of Rac1 in promoting cell migration in uPAR-expressing HEK-293 cells, we transfected the cells with DN-Rac1. This construct had no effect on migration of EV cells but nearly entirely blocked the increase in cell migration associated with expression of mouse uPAR or human uPAR (Figure 3D). Figure 3E shows that the level of activated Rac1 was significantly increased in the four uPAR-expressing cell lines (P < 0.01, one-way analysis of variance), as compared with control EV cells. Although human uPAR-expressing cells showed a tendency toward greater Rac1 activation than mouse uPAR-expressing cells, this observation should be interpreted with caution because Rac1 is activated locally at the subcellular level in the migrating cell41 and our pulldown assay examines the overall extent of Rac1 activation. In studies that are not shown, we demonstrated that the basal level of ERK/MAP kinase phosphorylation was not changed by uPAR expression. This result was anticipated because of the inability of PD098059 to inhibit migration of uPAR-expressing HEK-293 cells.
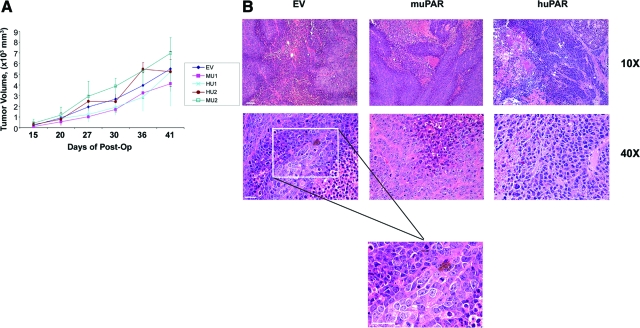
HEK-293 Cells Form Tumors in SCID Mice
The five cell lines (EV, MU1, MU2, HU1, HU2) were transfected to express GFP. The GFP-expressing cells expressed unchanged levels of uPAR in vitro, as determined by immunoblot analysis (data not shown). All five cell lines were inoculated into mammary fat pads in SCID mice and each formed tumors. uPAR expression was not associated with a significant change in the growth rate of the primary tumor, as determined by external measurement with calipers (Figure 4A). At necropsy, sections of the tumors were stained with H&E and analyzed by light microscopy. At low magnification (×10), each tumor showed large areas of necrosis and extensive infiltration with inflammatory cells (Figure 4B). This result is consistent with our observation that mouse uPA mRNA is abundant in HEK-293 cell xenografts and that the source of mouse uPA is nonmalignant infiltrating cells. uPAR expression in HEK-293 cells did not have an obvious effect on the extent of necrosis, inflammation or extracellular matrix deposition. At high power (×40), malignant cells within the xenografts were highly pleomorphic. Mitotic figures were abundant; however, uPAR expression did not clearly affect the range of cellular morphologies observed by microscopy.
Figure 4.
Xenograft growth in SCID mice A: Primary tumor growth and size were monitored using calipers in live mice until the size of tumor reached a maximum diameter of 2 cm. Tumor volume was determined (mean ± SEM). B: H&E-stained paraffin sections of primary tumors established with EV cells, mouse uPAR-expressing cells (MU2) and human uPAR-expressing cells (HU1) are shown. Scale bar = 300 μm in top panel; 100 μm in middle and bottom panel).
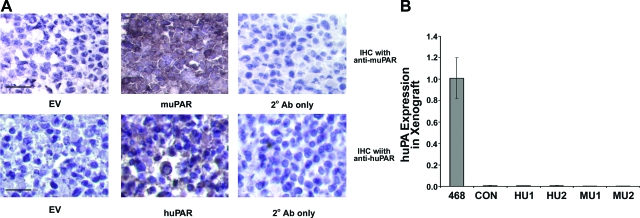
To confirm that transfected HEK-293 cells continue to express uPAR in xenografts, immunohistochemistry studies were performed. Separate antibodies were used to detect mouse uPAR and human uPAR because the available antibodies did not cross-react with both forms of the receptor. Figure 5A shows that both mouse uPAR and human uPAR were detected in tumor cells in vivo. The representative field shown in the top-middle panel demonstrates mouse uPAR expression by MU2 cells. The bottom-middle panel shows human uPAR expression by HU1 cells. uPAR immunopositivity was not detected in HEK-293 cells that were transfected with empty vector (EV), providing a negative control for both antibodies.
Figure 5.
Expression of uPA and uPAR in xenografts of uPAR-expressing HEK-293 cells. A: Immunohistochemistry was performed using mouse uPAR-specific antibody in the top row and human uPAR-specific antibody in the bottom row. EV cells were immunostained with both antibodies (left). MU2 were cells immunostained to detect mouse uPAR and HU1 cells, to detect human uPAR (middle). A control section was treated exclusively with secondary antibody (right). All of the sections were counterstained with hematoxylin (bars, 100 μm). B: Total RNA was extracted from xenografts prepared with EV, MU1, MU2, HU1, HU2, and MDA-MB 468 cells. Relative expression of human uPA mRNA was determined by real-time qPCR, normalized to HPRT mRNA. Results are standardized against the level of uPA mRNA in MDA-MB 468 cells (mean ± SEM, n = 4).
To confirm that uPAR expression in HEK-293 cells does not induce expression of human uPA, RNA was isolated from xenografts formed with all four uPAR-expressing cell lines and probed by qPCR to detect human uPA. As shown in Figure 5B, human uPA mRNA was not detected in any of the primary tumors, whereas abundant uPA mRNA was detected in xenografts of MDA-MB 468 cells (a positive control).
Mouse uPAR and Human uPAR Promote Metastasis in Mice
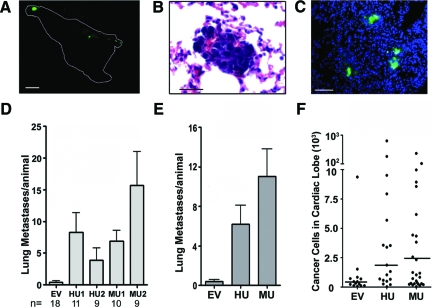
Mice were euthanized when the external diameter of the primary tumor reached 2 cm in maximum diameter. In no case did we observe changes in animal behavior to indicate discomfort or morbidity. The lungs were recovered and examined initially without dissection using a fluorescent stereomicroscope. Metastases were identified by the presence of green-fluorescent foci on the surfaces of the lungs at ×8 magnification. Examples of fluorescent tumors within an intact cardiac lobe (left lung) are shown in Figure 6A. We confirmed that fluorescent foci contained tumor cells by H&E staining and light microscopy (Figure 6B). Fluorescent imaging at higher power revealed green tumor cells interspersed in lung parenchyma, stained with DAPI to identify nuclei (Figure 6C).
Figure 6.
uPAR promotes HEK-293 cell metastasis in mice. A: Xenografts were established in SCID mice with uPAR-expressing HEK-293 cells or control EV cells. The image shows the cardiac lobe of the left lung from an animal inoculated with MU2 cells. Four fluorescent metastases were counted. Scale bar = 1 mm. B: H&E stained paraffin-section of lung with a focus of metastatic cells. Scale bar = 100 μm. C: Cryosections of the diaphragmatic lobe were stained with DAPI and imaged for green fluorescent tumor cells. Scale bar = 200 μm. D: The number of discernable metastases to the lungs is plotted for mice injected with empty vector (EV), human uPAR-expressing cells (HU1, HU2) or mouse uPAR-expressing cells (MU1, MU2). Lung metastases were determined by counting green fluorescent foci that are at least 100 μm in maximum diameter on the surfaces of each lobe (mean ± SEM, P < 0.01, P value determined by one-way analysis of variance test). E: Results obtained with HU1 and HU2 cells or MU1 and MU2 cells were pooled. F: Genomic DNA qPCR was performed to quantitate human-specific Alu repeats in the cardiac lobes of mouse lungs. These results were used to estimate the number of human cells in each cardiac lobe by comparison with a standard curve generated using increasing numbers of HEK-293 cells from cell culture (median, P < 0.05, P values determined by Mann-Whitney rank sum test).
As shown in Figure 6D, metastases to the lungs, which could be detected by low-power fluorescence microscopy, were extremely rare when primary tumors were formed with control EV cells, which lacked uPAR. By contrast, all four cell lines that expressed human uPAR or mouse uPAR demonstrated a significant increase in metastasis, compared with EV cells (P < 0.01, one-way analysis of variance). Results obtained with the two mouse uPAR-expressing clones and with the two human uPAR-expressing clones are pooled for comparison in Figure 6E. Although the pooled data show that the number of metastases was modestly higher with mouse uPAR-expressing cells, compared with human uPAR-expressing cells, the difference was not statistically significant.
As a second method to compare metastasis, we isolated genomic DNA from the cardiac lobe of the left lung of each animal and performed qPCR to detect human-specific Alu repeats.36 The number of cancer cells in the cardiac lobe was determined by comparison with a standard curve in which we plotted the qPCR signal for Alu repeats against known numbers of HEK-293 cells. In control experiments, we demonstrated that the number of GFP foci in the cardiac lobe correlated with the number of tumor cells in the same lobe, as determined Alu qPCR (r2 = 0.87). Figure 6F shows that analysis of Alu qPCR confirmed our major conclusion, that expression of either human uPAR or mouse uPAR in HEK-293 cells increases dissemination of cells to the lungs. The results were statistically significant, as determined by the Mann-Whitney rank sum test (P < 0.05 compared with EV cells).
Discussion
Established models regarding the function of uPAR implicate this receptor in protease activation and ECM remodeling, which may be important for cell migration in vivo.13,42 However, it is now clear that cancer cells migrate individually or collectively, in sheets.43 Migration of individual cells may follow patterns referred to as mesenchymal or amoeboid.44 Each of these patterns of cell migration may have different dependencies on extracellular proteases. Furthermore, the continuum of proteases known to support ECM remodeling in the migrating cell is now quite large, including transmembrane proteases like membrane type 1-matrix metalloprotease.45 Thus, it can no longer be assumed that the activity of uPAR in cancer progression reflects exclusively its activity in promoting protease activation and ECM remodeling.
uPAR associates with integrins in the plasma membrane and may thereby regulate cell adhesion.17 In the absence of integrins, uPAR still may function in cell adhesion by binding directly to vitronectin.18 The function of uPAR in cell adhesion is coupled to its ability to activate cell-signaling to factors such as Rac1.20 Other uPAR-initiated cell-signaling events occur in response to uPA.25,26 In this case, uPA probably modifies the structure of uPAR so that its interaction with signaling co-receptors is altered.46 Co-receptors that have been implicated in uPA-initiated cell-signaling include FPR-like receptor-1/lipoxin A4 receptor47 and receptor tyrosine kinases.19,48,49 Co-receptors may physically associate with uPAR or participate in cell-signaling by classic transactivation pathways.50 Although in vitro studies describing uPA-dependent and -independent mechanisms of uPAR function are abundant, evidence that the effects of uPAR on cell adhesion and cell-signaling are important in vivo is still lacking.
In this study, we demonstrated that both human uPAR and mouse uPAR promote metastasis of HEK-293 cells in mice. Even in the absence of uPAR, HEK-293 cells formed primary tumors in SCID mice. This result is explained by integrated adenovirus genes, which allow HEK-293 cells to behave like cancer cells.33 Although we anticipated that mouse uPAR would promote metastasis of HEK-293 cells, our results with human uPAR were unanticipated. HEK-293 cells are different from most cancer cells in that they do not express uPA (Figure 1), and thus do not provide an endogenous source of ligand for autocrine activation of uPAR. Because mouse uPA cannot bind to human uPAR in a paracrine manner in vivo, the ability of human uPAR to promote HEK-293 cell metastasis in mice implicates an uPA-independent mechanism or mechanisms of action. Most importantly, the activity of human uPAR excludes activation of proteases in the cellular microenvironment as an explanation for the increase in metastasis.
The uPA-independent mechanism by which uPAR promotes HEK-293 cell metastasis in mice remains to be determined; however, the results presented in Figure 3 provide candidate pathways. As reported by Kjoller et al20 and Madsen et al,32 uPAR expression induced substantial changes in the morphology of HEK-293 cells and these changes occurred independently of the form of uPAR introduced (human or mouse). All four uPAR-expressing cell lines showed a comparable increase of cell adhesion to vitronectin. Both human uPAR and mouse uPAR increased HEK-293 cell migration independently of uPA. In all four uPAR-expressing cell lines, the changes in morphology, cell adhesion to vitronectin, and cell migration were associated with a significant increase in Rac1 activation (Figure 3, A–C). Although Rac1 controls multiple downstream targets and diverse aspects of cell physiology, many of its targets regulate dynamic remodeling of the actin cytoskeleton, which is essential for cell migration.51 Our results suggesting that activation of ERK/MAP kinase did not contribute to the increase in cell migration associated with uPAR expression in HEK-293 cells is consistent with our previous work indicating that ERK/MAP kinase activation requires uPA-binding to uPAR.24
Our study is the first to directly test whether uPAR promotes cancer metastasis by mechanisms that are uPA-independent in vivo. However, the function of uPAR in promoting cancer invasion and metastasis in animal model systems is well documented.29,30 A role for uPAR in cancer progression was suggested by the study by Van der Pluijm et al.52 These investigators demonstrated that MDA-MB 231 cells, which express a synthetic peptide known to disrupt uPAR-integrin interactions, form smaller tumors in mouse bones after intraosseus injection. The ability of uPAR to promote metastasis independently of uPA does not rule out important functions for uPA in cancer metastasis. When uPA gene knock-out mice were crossed with MMTV-PymT mice, primary breast cancers developed as expected for MMTV-PymT mice; however, metastasis to the lungs was decreased.53 Furthermore, uPA gene knock-out mice are resistant to chemical induction of melanoma.54 Although our data did not conclusively demonstrate a greater increase in the metastatic potential of mouse uPAR-expressing cells, compared with human uPAR-expressing cells, a trend was observed, which might support a role for uPA-uPAR complex. If this trend can be verified in future studies, this may suggest two layers in the function of uPAR contributing to its activity in cancer metastasis. The first involves uPA-independent mechanisms, proven here by the activity of human uPAR, and the second involving uPA-dependent pathways, suggested by the greater activity of mouse uPAR.
Although we chose to execute the experiments presented here using a cancer model system in mice, we feel these results may be extended to other forms of pathophysiology in which uPAR plays a regulatory role independently of uPA. In this regard, our results support further work to understand the diverse mechanisms by which uPAR regulates cell physiology, in addition to activation of proteases.
Footnotes
Address reprint requests to Steven L. Gonias, UCSD School of Medicine, Department of Pathology, 9500 Gilman Drive, La Jolla, CA, 92093-0612. E-mail: sgonias@ucsd.edu.
Supported by NIH R01 CA-94900.
References
- Charles Edo, de Bock YW. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- Usher P, Thomsen OF, Iversen P, Johnsen M, Brünner N, Høyer-Hansen G, Andreasen P, Danø K, Nielsen BS. Expression of urokinase plasminogen activator, its receptor and type-1 inhibitor in malignant and benign prostate tissue. Int J Cancer. 2005;113:870–880. doi: 10.1002/ijc.20665. [DOI] [PubMed] [Google Scholar]
- Cozzi PJ, Wang J, Delprado W, Madigan MC, Fairy S, Russell PJ, Li Y. Evaluation of urokinase plasminogen activator and its receptor in different grades of human prostate cancer. Hum Pathol. 2006;37:1442–1451. doi: 10.1016/j.humpath.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Memarzadeh S, Kozak KR, Chang L, Natarajan S, Shintaku P, Reddy ST, Farias-Eisner R. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc Natl Acad Sci USA. 2002;99:10647–10652. doi: 10.1073/pnas.152127499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Cohen RL, Thor AT, Todd RF, 3rd, Mizukami IF, Lawrence DA, Ljung BM, Shuman MA, Smith HS. The urokinase receptor is expressed in invasive breast cancer but not in normal breast tissue. Cancer Res. 1994;54:861–866. [PubMed] [Google Scholar]
- Carriero MV, Franco P, Del Vecchio S, Massa O, Botti G, D'Aiuto G, Stoppelli MP, Salvatore M. Tissue distribution of soluble and receptor-bound urokinase in human breast cancer using a panel of monoclonal antibodies. Cancer Res. 1994;54:5445–5454. [PubMed] [Google Scholar]
- Jankun J, Merrick HW, Goldblatt PJ. Expression and localization of elements of the plasminogen activation system in benign breast disease and breast cancers. J Cell Biochem. 1993;53:135–144. doi: 10.1002/jcb.240530206. [DOI] [PubMed] [Google Scholar]
- Pyke C, Graem N, Ralfkiaer E, Ronne E, Hoyer-Hansen G, Brunner N, Dano K. Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res. 1993;53:1911–1915. [PubMed] [Google Scholar]
- Belcher C, Fawthrop F, Bunning R, Doherty M. Plasminogen activators and their inhibitors in synovial fluids from normal, osteoarthritis, and rheumatoid arthritis knees. Ann Rheum Dis. 1996;55:230–236. doi: 10.1136/ard.55.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- Ellis V, Behrendt N, Dano K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266:12752–12758. [PubMed] [Google Scholar]
- Collen D. The plasminogen (fibrinolytic) system. Thromb Haemost. 1999;82:259–270. [PubMed] [Google Scholar]
- Blasi F, Vassalli JD, Dano K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987;104:801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levinson AD. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA. 1993;90:5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Estreicher A, Muhlhauser J, Carpentier JL, Orci L, Vassalli JD. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111:783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380–32388. [PubMed] [Google Scholar]
- Jo M, Thomas KS, O'Donnell DM, Gonias SL. Epidermal growth factor receptor-dependent and -independent cell-signaling pathways originating from the urokinase receptor. J Biol Chem. 2003;278:1642–1646. doi: 10.1074/jbc.M210877200. [DOI] [PubMed] [Google Scholar]
- Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol. 2001;152:1145–1157. doi: 10.1083/jcb.152.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol. 2002;159:1061–1070. doi: 10.1083/jcb.200207070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22:392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem. 1998;273:8502–8507. doi: 10.1074/jbc.273.14.8502. [DOI] [PubMed] [Google Scholar]
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol. 2000;12:613–620. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- Jo M, Thomas KS, Takimoto S, Gaultier A, Hsieh EH, Lester RD, Gonias SL. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene. 2007;26:2585–2594. doi: 10.1038/sj.onc.1210066. [DOI] [PubMed] [Google Scholar]
- Dass CR, Nadesapillai AP, Robin D, Howard ML, Fisher JL, Zhou H, Choong PF. Downregulation of uPAR confirms link in growth and metastasis of osteosarcoma. Clin Exp Metastasis. 2005;22:643–652. doi: 10.1007/s10585-006-9004-3. [DOI] [PubMed] [Google Scholar]
- Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
- Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005;280:36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Estreicher A, Wohlwend A, Belin D, Schleuning WD, Vassalli JD. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem. 1989;264:1180–1189. [PubMed] [Google Scholar]
- Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Mertz ET. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell. 1998;94:353–362. doi: 10.1016/s0092-8674(00)81478-6. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Behrendt N, Ronne E, Ploug M, Petri T, Lober D, Nielsen LS, Schleuning WD, Blasi F, Appella E, Dano K. The human receptor for urokinase plasminogen activator. NH2-terminal amino acid sequence and glycosylation variants. J Biol Chem. 1990;265:6453–6460. [PubMed] [Google Scholar]
- Webb DJ, Horwitz AF. New dimensions in cell migration. Nat Cell Biol. 2003;5:690–692. doi: 10.1038/ncb0803-690. [DOI] [PubMed] [Google Scholar]
- Bodary SC, McLean JW. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Mabilat C, Yeh P, Guitton JD, Li H, Pouchelet M, Shoevaert D, Legrand Y, Soria J, Soria C. Blockage of urokinase receptor reduces in vitro the motility and the deformability of endothelial cells. FEBS Lett. 1996;380:21–24. doi: 10.1016/0014-5793(95)01540-x. [DOI] [PubMed] [Google Scholar]
- Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br J Dermatol. 2006;154 Suppl 1:11–15. doi: 10.1111/j.1365-2133.2006.07231.x. [DOI] [PubMed] [Google Scholar]
- Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi AA, Upson RH, Cohen RL, Manuppello J, Bognacki J, Henkin J, McCrae KR, Kounnas MZ, Strickland DK, Preissner KT, Lawler J, Cines DB. Interaction of single-chain urokinase with its receptor induces the appearance and disappearance of binding epitopes within the resultant complex for other cell surface proteins. Blood. 1996;88:542–551. [PubMed] [Google Scholar]
- Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99:1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase-induced signaling in human vascular smooth muscle cells is mediated by PDGFR-beta. EMBO J. 2005;24:1787–1797. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Jo M, Thomas KS, Marozkina N, Amin TJ, Silva CM, Parsons SJ, Gonias SL. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280:17449–17457. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- van der Pluijm G, Sijmons B, Vloedgraven H, van der Bent C, Drijfhout J-W, Verheijen J, Quax P, Karperien M, Papapoulos S, Lowik C. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol. 2001;159:971–982. doi: 10.1016/S0002-9440(10)61773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almholt K, Lund LR, Rygaard J, Nielsen BS, Dano K, Romer J, Johnsen M. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer. 2005;113:525–532. doi: 10.1002/ijc.20631. [DOI] [PubMed] [Google Scholar]
- Shapiro RL, Duquette JG, Roses DF, Nunes I, Harris MN, Kamino H, Wilson EL, Rifkin DB. Induction of primary cutaneous melanocytic neoplasms in urokinase-type plasminogen activator (uPA)-deficient and wild-type mice: cellular blue nevi invade but do not progress to malignant melanoma in uPA-deficient animals. Cancer Res. 1996;56:3597–3604. [PubMed] [Google Scholar]