Abstract
Myoglobin is a multifunctional heme protein that is thought to be expressed exclusively in myocytes. Its importance in both oxygen transport and free radical scavenging has been extensively characterized. We hypothesized that solid tumors could take advantage of proteins such as myoglobin to cope with hypoxic conditions and to control the metabolism of reactive oxygen and nitrogen species. We therefore sought to establish whether myoglobin might be expressed and functionally regulated in epithelial tumors that are known to face hypoxia and oxidative stress during disease progression. We analyzed the expression of myoglobin in human epithelial cancers at both transcriptional and protein levels; moreover, we investigated the expression levels of myoglobin in cancer cell lines subjected to different conditions, including hypoxia, oxidative stress, and mitogenic stimuli. We provide evidence that human epithelial tumors, including breast, lung, ovary, and colon carcinomas, express high levels of myoglobin from the earliest stages of disease development. In human cancer cells, myoglobin is induced by a variety of signals associated with tumor progression, including mitogenic stimuli, oxidative stress, and hypoxia. This study provides evidence that myoglobin, previously thought to be restricted to myocytes, is expressed at high levels by human carcinoma cells. We suggest that myoglobin expression is part of a cellular program aimed at coping with changed metabolic and environmental conditions associated with neoplastic growth.
Myoglobin (Mb) is an oxygen-binding heme protein that plays a key role in oxygen transport and free radical scavenging.1,2 Capable of binding oxygen at an affinity intermediate between those of hemoglobin and cytochrome oxidase-c, Mb controls intracellular oxygen concentration by several mechanisms, including O2 storage, pO2 buffering, and facilitated O2 diffusion. Mb belongs to the hemoprotein superfamily, which also includes hemoglobin, neuroglobin, and cytoglobin. While the role of hemoglobin and myoglobin has been extensively studied, the functions of neuroglobin and cytoglobin, which have been discovered only recently, are still largely unknown.3,4,5,6
Mb plays a key role in oxidative myocytes of skeletal and cardiac muscles by working as an oxygen reservoir during myofiber contraction.1 Expression of Mb is highest in the skeletal muscles of diving mammals such as whales and seals that are capable of prolonged breath hold dives. Transgenic mice lacking a functional Mb gene have been generated. Interestingly and to some extent unexpectedly, these animals are viable and fertile and do not show the expected hypoxia sensitive phenotype likely because of compensatory mechanisms.7
In addition to its role in oxygen storage and diffusion Mb has also been shown to act as a powerful scavenger of nitric oxide (NO) and of reactive oxygen species (ROS). The latter are important mediators of cell signals indicating that myoglobin might have a broader functional role in mammalian cells.
Recent findings indicate that in the common carp (Cyprinius carpio), which can adapt to live in differently oxygenated environments, expression of Mb extends to several non-muscle tissues including liver, gills, and brain.8 Interestingly, Mb expression in nonmuscular tissues is increased when the fish is kept in low oxygen, suggesting that Mb could play a functional role in the cellular response to hypoxia.
Several lines of evidence suggest that overcoming hypoxia is a key aspect of tumor progression.9 Accordingly, understanding and targeting the mechanisms by which cancer cells cope with increased hypoxic conditions is a very active and exciting field of research. Current efforts are concentrated on hypoxia inducible factor-1α, the master controller of hypoxia-induced transcription and on its regulatory apparatus.10,11
We hypothesized that solid tumors could take advantage of proteins such as myoglobin to cope with hypoxic conditions and to control the metabolism of ROS/NO. We therefore sought to determine whether Mb might be expressed and functionally regulated in epithelial tumors that are known to face hypoxia and oxidative stress during their progression.
Materials and Methods
Cell Culture
OS-9 osteosarcoma cells and GTL16 gastric carcinoma cells were obtained as described.12,13 All other cell lines were purchased from ATCC (Rockville, Maryland). The following cell lines were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 1% glutamine and 10% fetal bovine serum (Sigma, St. Louis, Missouri): BT-474, MCF-7, MDA-MB-231, HT-29, SW48, MG-63, K-HOS/NP, SJSA-1, SAOS-2, OS-9, MNNG and SUIT-2. The following cell lines were maintained in RPMI supplemented with 1% glutamine and 10% fetal bovine serum: ZR-75-1, T-47D, SK-BR-3, HCT-116, DLD-1, SW480, SW620, GTL16, A549 and RPE. MCF-10A were maintained in a 1:1 mixture of Dulbecco’s Modified Eagle’s Medium and F12 (Sigma) supplemented with 1% glutamine, 10% fetal bovine serum, 20 ng/ml epidermal growth factor, 500 ng/ml hydrocortisone, and 10 μg/ml insulin (all from Sigma).
Myoglobin Gene Expression Analysis
Analysis of myoglobin gene expression in human tumor cell lines was performed by real time-PCR. Total RNA was extracted from the cell lines indicated in Table 1 using Trizol Reagent (Invitrogen, Carlsbad, California). Retro-transcription was performed with RNase-free Moloney murine leukemia virus reverse-transcriptase (Promega, Madison, Wisconsin). Expression analysis was performed by TaqMan assay using the following oligonucleotides as primers: 5′-GTCTGAGGACTTAAAGAAGCATGGT-3′ (Mb forward); 5′-CCTCATGATGCCCCTTCTTCTTAA-3′ (Mb reverse). The myoglobin-specific fluorigenic probe had the following sequence: 5′-CAGGGCGGTGAGCACA-3′.
Table 1.
Analysis of Mb mRNA Expression in a Panel of Human Tumor Cell Lines
| Tumor type | Cell line | 2^−ΔΔCT | SD |
|---|---|---|---|
| Breast carcinoma | ZR-75-1 | 557,7 | 0149 |
| Breast carcinoma | T-47D | 171,5 | 0076 |
| Breast carcinoma | BT-474 | 158,7 | 0067 |
| Breast carcinoma | MCF-7 | 51,1 | 0021 |
| Breast carcinoma | SK-BR-3 | 2,7 | 0138 |
| Breast carcinoma | MDA-MB-231 | 0,5 | 0456 |
| Colon carcinoma | HCT-116 | 9,5 | 0222 |
| Colon carcinoma | DLD-1 | 59,9 | 0111 |
| Colon carcinoma | HT-29 | 0,5 | 0885 |
| Colon carcinoma | SW480 | ND | − |
| Colon carcinoma | SW48 | 6,2 | 0392 |
| Colon carcinoma | SW620 | ND | − |
| Colon carcinoma | LoVo | 0,9 | 1065 |
| Osteosarcoma | MG-63 | 29,3 | 0076 |
| Osteosarcoma | K-HOS/NP | 0,9 | 0062 |
| Osteosarcoma | SJSA-1 | ND | − |
| Osteosarcoma | OS-9 | 1,2 | 0028 |
| Osteosarcoma | SAOS-2 | 1,1 | 0026 |
| Osteosarcoma | MNNG | 2,1 | 0115 |
| Pancreas carcinoma | SUIT-2 | 0,9 | 0299 |
| Gastric carcinoma | GTL16 | 3,4 | 0114 |
| Lung carcinoma | A549 | 0,6 | 0044 |
| Normal breast | MCF-10A | ND | − |
| Normal retina | RPE | ND | − |
The levels of Mb mRNA were determined by TaqMan analysis using a Mb-specific probe as described in Materials and Methods. ND, not detected.
Amplification was performed using an ABI PRISM 7900HT analyzer (Applied Biosystems, Foster City, California). In each sample, myoglobin expression was normalized to that of RNase P using a specific kit (Applied Biosystems) as described.14
Myoglobin Protein Expression Analysis
Mb expression was determined by Western blot analysis and enzyme-linked immunosorbent assay (ELISA). For Western blotting, cells were lysed in extraction buffer as described.15 Equal amounts of cellular proteins (150 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis, electro-transferred to nitrocellulose, and analyzed by Western blotting using an anti-human myoglobin monoclonal antibody (Abcam, Cambridge, United Kingdom; catalogue number ab8343). To normalize protein loading, blots were re-probed with either an anti-actin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, California) or an anti-human β-tubulin polyclonal antibody (Santa Cruz). Protein signal was detected using ECL PLUS (Amersham Biosciences, Piscataway, New Jersey) as recommended by the manufacturer. For ELISA analysis, cells were lysed as above and the absolute amount of myoglobin was determined using a human myoglobin ELISA kit (Life Diagnostics, West Chester, Pennsylvania).
Immunohistochemical Analysis of Tumor Samples
Paraffin-embedded human breast, lung, colon, and ovarian carcinoma samples were obtained from the Unit of Pathology of the Institute for Cancer Research and Treatment (Candiolo, Turin, Italy). Histological sections from the above samples were processed for immunohistochemical analysis as follows. After dewaxing and hydration, sections were treated with 3% H2O2 for 5 minutes at room temperature to block endogenous peroxidase activity and saturated with 5% goat serum (Sigma) for 20 minutes at room temperature. Slides were incubated with a 1:500 dilution of a polyclonal rabbit anti-human myoglobin antibody routinely used in clinical diagnostics (Dako, Glostrup, Denmark; catalog number A0324) for 30 minutes at room temperature, washed three times in TBS buffer containing 0.1% Tween 20, and exposed to horseradish peroxidase-labeled goat anti-rabbit secondary antibody (Dako). Peroxidase activity was detected with diaminobenzidine substrate solution (Dako). All slides were examined by two independent pathologists not informed of sample identity, and photographed.
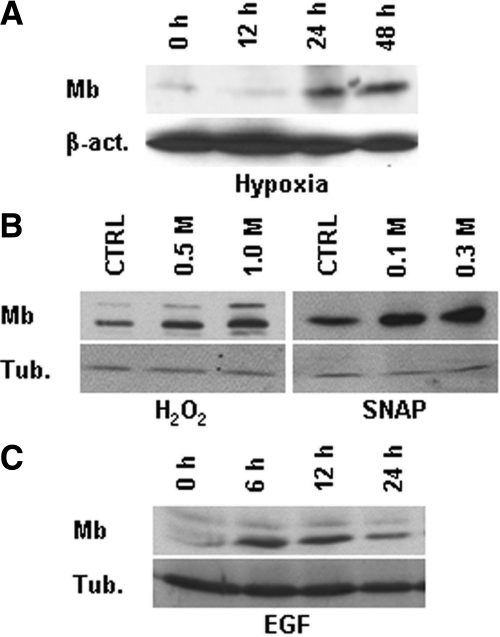
Modulation of Myoglobin Expression
For analysis of hypoxia-induced Mb expression, MCF-7 breast carcinoma cells were incubated in a 1% O2 atmosphere for the indicated time in the presence of 1% serum using a Ruskinn INVIVO2 400 hypoxic station (Biotrace, Bridgend, United Kingdom). For NO-mediated oxidative stress, MCF-7 cells were stimulated with 0.1 mmol/L or 0.3 mmol/L S-nitroso-N-acetylpenicillamine (Sigma) in the presence of 1% serum for 16 hours. For ROS-mediated oxidative stress, MCF-7 cells were stimulated with 0.5 mmol/L or 1.0 mmol/L H2O2 for 1 hour in the absence of serum, and then incubated for 16 hours in the presence of 10% serum. For mitogenic stimulation, MCF-7 cells were incubated for the indicated time in the presence of 100 ng/ml recombinant human epidermal growth factor (Sigma) and 1% serum. Mb expression was determined by Western blot analysis as described above.
Results
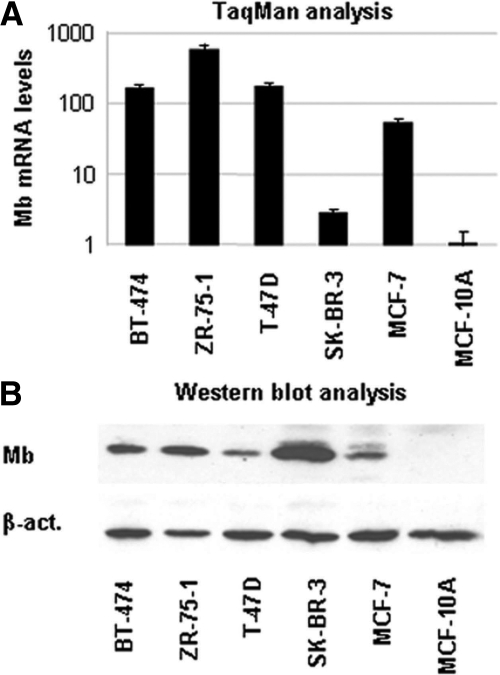
Muscle cells, which face harsh hypoxic conditions during active myofiber contraction, have adapted to their adverse environmental state by expressing high levels of myoglobin. We reasoned that cancer cells could adopt a similar strategy by expressing proteins specialized in oxygen transport and metabolism. To test this hypothesis, we first analyzed Mb gene expression in a panel of human tumor cell lines using an Mb-specific TaqMan assay. Surprisingly, this analysis revealed that Mb is expressed in a number of cancer cell lines of epithelial origin, including colon and breast carcinoma cells (Table 1). The latter displayed the highest Mb expression. On average, Mb mRNA levels were higher in breast carcinomas than in the osteosarcoma cells, which represented our internal positive control, and were undetectable in normal breast epithelial cells (Figure 1A).
Figure 1.
RNA and protein expression analysis of Mb in normal and cancer breast cell lines. The levels of Mb mRNA and protein expression in human breast carcinoma cell lines were analyzed by quantitative PCR (A) and Western blotting (B). MCF-10 is a normal breast epithelial cell line.
Prompted by these results, we determined Mb protein levels in cancer cells by Western blotting. We found that breast carcinoma cells express very high levels of Mb, while no Mb protein could be detected in normal breast epithelial cells (Figure 1B). The absolute amount of Mb was determined by ELISA and found to range between 24 ng/106 cells (MCF-7) to 32 ng/106 cells (SK-BR-3). These results do not closely match those obtained by RNA analysis, thus suggesting that Mb expression may be regulated at a post-transcriptional level by a yet unknown mechanism.
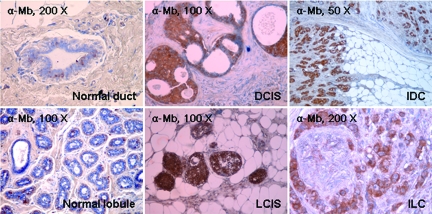
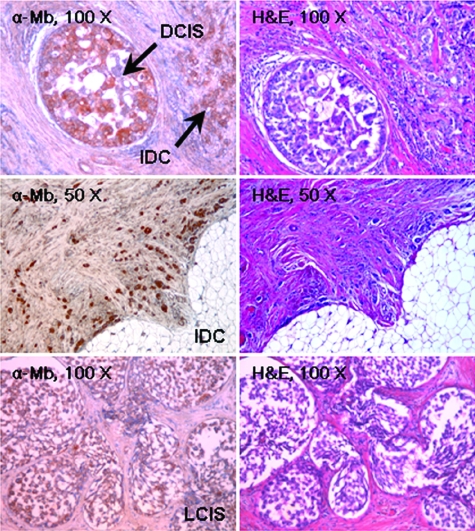
To test whether Mb was expressed not only in cancer cell lines but also in actual tumor tissues, we examined Mb expression in a panel of human primary breast carcinomas by immunohistochemistry (Table 2). Out of 31 tumor samples analyzed, 9 were ductal in situ carcinomas, 2 were lobular in situ carcinomas, 14 were invasive ductal carcinomas, and 6 were invasive lobular carcinomas. Remarkably, in 68% of the tumor samples analyzed, carcinoma cells were strongly positive for Mb staining regardless of their histological classification; 29% of the tumor samples were less strongly positive and only 3% were negative. On average, in all positive samples, more than 40% of carcinoma cells were found to express Mb. In contrast, the corresponding normal epithelial cells and the stroma were invariably negative (Figure 2). Mb staining was present in carcinoma cells of early stage breast cancers (Figures 2 and 3), suggesting that the genetic switch that turns Mb on during tumor progression is a very early event. In normal tissue, in contrast, Mb staining was confined to myoepithelial cells around the ductal and lobular epithelium.
Table 2.
Immunohistochemical Analysis of Myoglobin Expression in Breast Tumor Specimens
| A) Relative Immunoreactive Score | |||||
|---|---|---|---|---|---|
| Immunoreactivity score
|
Invasive ductal carcinoma
|
Invasive lobular carcinoma
|
Ductal carcinoma in situ
|
Lobular carcinoma in situ
|
Total
|
| 0 | 1 | 1 | |||
| 1+ | 1 | 1 | |||
| 2+ | 4 | 3 | 1 | 8 | |
| 3+ | 9 | 3 | 7 | 2 | 21 |
| Total
|
14
|
6
|
9
|
2
|
31
|
|
B) Percentage of Positive Cells | |||||
|
Immunoreactivity score |
Invasive ductal carcinoma |
Invasive lobular carcinoma |
Ductal carcinoma in situ |
Lobular carcinoma in situ |
Total |
| <5% | 2 | 2 | |||
| 5–10% | 1 | 1 | |||
| 10–40% | 1 | 1 | 1 | 3 | |
| >40% | 13 | 5 | 5 | 2 | 25 |
| Total | 14 | 6 | 9 | 2 | 31 |
Figure 2.
Immunohistochemical analysis of Mb expression in breast cancers. Mb expression in normal and cancerous human breast tissues was analyzed by immunohistochemistry using an anti-Mb antibody. The tumor histotype is indicated. DCIS, ductal carcinoma in situ; IDC, infiltrating ductal carcinoma; LCIS, lobular carcinoma in situ; ILC, infiltrating lobular carcinoma.
Figure 3.
Immunohistochemical analysis of Mb expression in breast cancers. Mb expression in normal and cancerous human breast tissues was analyzed by immunohistochemistry using an anti-Mb antibody. The tumor histotype is indicated. DCIS, ductal carcinoma in situ; IDC, infiltrating ductal carcinoma; LCIS, lobular carcinoma in situ.
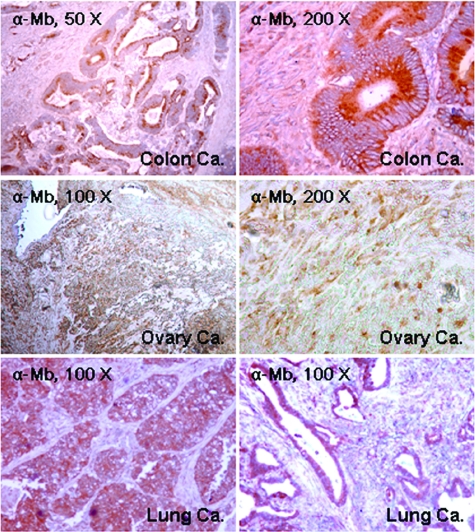
We also analyzed a representative panel of colon, lung, and ovary carcinoma samples, as well as the corresponding normal tissues. In these tumors normal epithelial cells were invariably negative for Mb staining, while, in a subset of the tumor samples, carcinoma cells expressed Mb at variable levels (Figure 4).
Figure 4.
Immunohistochemical analysis of Mb expression in a representative panel of human colon, ovary and lung carcinomas. Mb expression was analyzed by immunohistochemistry using an anti-Mb antibody. The tumor histotype is indicated. Colon Ca: infiltrating adenocarcinoma of the colon. Ovary Ca: clear cell carcinoma of the ovary. Lung Ca: non-small cell lung carcinoma.
Therefore, normal epithelial cells acquire Mb expression at some point during their transition to carcinoma cells, conceivably at a very early stage. We wondered what signals could be responsible for Mb induction. Analysis of Mb gene copy number ruled out that increased Mb expression could be due to gene amplification (data not shown). Next, we subjected breast carcinoma cells to a series of stimuli that are associated with tumor progression, including hypoxia, oxidative stress and mitogenic signals, and then determined Mb protein expression by Western blotting (Figure 5, A–C). Consistent with a role of Mb in O2 transport and ROS/NO scavenging, Mb levels were increased following exposure of cells to hypoxia (1% O2), H2O2 (0.5–1.0 mmol/L), or S-nitroso-N-acetylpenicillamine, a NO donor (0.1−0.3 mmol/L). Furthermore, Mb was also transiently but distinctly induced on cell stimulation with mitogens, including epidermal growth factor.
Figure 5.
Functional regulation of Mb expression in breast carcinoma cells. Human MCF-7 breast carcinoma cells were stimulated as indicated, and Mb expression was determined by Western blotting. Protein loading was normalized using anti-β-actin (β-act.) or tubulin (tub.) antibodies. (A) Hypoxia (1% O2); (B) S-nitroso-N-acetylpenicillamine; (C) epidermal growth factor.
Discussion
So far, the expression of Mb in cancer cells was thought to be restricted to a few tumor types that originate from muscle tissues such as sarcomas and rhabdomyosarcomas or anyway to represent a marker for the so-called ‘myoid differentiation’ of tumors of different origin.16,17,18,19,20,21,22,23,24,25 Our results instead indicate that Mb expression, while confined to muscle cells in normal tissues, is commonly observed in cancerous cells of bona fide epithelial origin in a wide array of human tumors. Importantly, Mb expression in breast cancer cells was confirmed by four independent experimental approaches including quantitative-PCR, Western blotting, ELISA, and immunohistochemistry.
The discovery that Mb is expressed and functionally regulated in epithelial cancer cells opens up new and fascinating avenues in basic and translation cancer research. From a genetic perspective it will be important to determine whether the Mb gene is altered (wild-type or mutated) in human cancer cells. Assessing whether Mb plays a functional role in tumor progression is also an important issue. In particular, our results show that Mb is induced by a variety of signals associated with tumor progression, including mitogenic stimuli, oxidative stress, and hypoxia. Interestingly, all these signals involve the generation of reactive oxygen or nitrogen species as second messengers or as biochemical byproducts. Given the well-documented ability of Mb to scavenge free radicals, we can hypothesize that the ultimate signal that triggers Mb induction is oxidative stress itself, and that Mb expression by cancer cells is part of a cellular program aimed at coping with increased ROS and NO levels.
Alternatively, Mb could help tumor cells to produce energy in hypoxic conditions by supplying mitochondria with oxygen. However, this possibility is less likely because most tumor cells display aerobic glycolysis and are adapted to produce most of their ATP through the glycolytic pathway. A third possibility is that Mb expression in cancer cells affects hypoxia inducible factor-1α induction and stabilization by modulating prolyl-hydroxylase enzymes. RNA interference-mediated down-regulation of Mb expression in tumor cell lines and subsequent analysis of cell metabolism and signaling will help determining which hypothesis is more solid.
The discovery that Mb is expressed in breast and other epithelial cancers also opens up potential avenues for translational research. For example, Mb expression in epithelial cancer tissues could be used as a tumor marker. In this regard, the measurements of Mb levels in the serum of cancer patients could be attempted with the technologies that are routinely used to monitor Mb expression in acute myocardial infarction.
Should myoglobin prove to play a causative role in tumor progression, for example by detoxifying cancer cells of free radicals, modulating NO/ROS signaling or helping tumors to overcome hypoxia, it is tempting to speculate that targeting one or more of its multiple functions by pharmacological agents or more advanced molecular tools could represent a novel therapeutic strategy in oncology. To this end, it will be of interest to cross Mb knock-out mice26 with several transgenic mouse models of cancer to determine whether the lack of Mb expression affects tumor susceptibility or anyway alters tumor progression in experimental systems.
Acknowledgments
We thank Mauro Risio for helpful discussion on tumor pathological samples and Catherine Tighe for manuscript editing.
Footnotes
Address reprint requests to Alberto Bardelli, Institute for Cancer Research and Treatment (IRCC), Laboratory of Molecular Genetics, University of Turin Medical School, Strada Provinciale 142, km 3.95, I-10060 Candiolo (Turin), Italy. E-mail: a.bardelli@ircc.it.
Supported by grants from Italian Association for Cancer Research (AIRC), Italian Ministry of Health, Italian Ministry of University and Research, the Compagnia di S. Paolo Foundation, Regione Piemonte, CRT Progetto Alfieri, EU FP6 MCSCs contract 037297 and EU FP7 Cancer Gene contract 218071 and Association for International Cancer Research (AICR-UK).
References
- Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol. 2004;207:3441–3446. doi: 10.1242/jeb.01172. [DOI] [PubMed] [Google Scholar]
- Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- Fago A, Hundahl C, Malte H, Weber RE. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life. 2004;56:689–696. doi: 10.1080/15216540500037299. [DOI] [PubMed] [Google Scholar]
- Hankeln T, Wystub S, Laufs T, Schmidt M, Gerlach F, Saaler-Reinhardt S, Reuss S, Burmester T. The cellular and subcellular localization of neuroglobin and cytoglobin—a clue to their function? IUBMB Life. 2004;56:671–679. doi: 10.1080/15216540500037794. [DOI] [PubMed] [Google Scholar]
- Pesce A, Bolognesi M, Bocedi A, Ascenzi P, Dewilde S, Moens L, Hankeln T, Burmester T. Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep. 2002;3:1146–1151. doi: 10.1093/embo-reports/kvf248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivapurkar N, Stastny V, Okumura N, Girard L, Xie Y, Prinsen C, Thunnissen FB, Wistuba II, Czerniak B, Frenkel E, Roth JA, Liloglou T, Xinarianos G, Field JK, Minna JD, Gazdar AF. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Cancer Res. 2008;68:7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci USA. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J, de Mello LV, Ward D, Rees HH, Williams DR, Fang Y, Brass A, Gracey AY, Cossins AR. Hypoxia-inducible myoglobin expression in nonmuscle tissues. Proc Natl Acad Sci USA. 2006;103:2977–2981. doi: 10.1073/pnas.0508270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Giordano S, Di Renzo MF, Narsimhan RP, Tamagnone L, Gerbaudo EV, Chiado-Piat L, Comoglio PM. Evidence for autocrine activation of a tyrosine kinase in a human gastric carcinoma cell line. J Cell Biochem. 1988;38:229–236. doi: 10.1002/jcb.240380402. [DOI] [PubMed] [Google Scholar]
- Patane S, Avnet S, Coltella N, Costa B, Sponza S, Olivero M, Vigna E, Naldini L, Baldini N, Ferracini R, Corso S, Giordano S, Comoglio PM, Di Renzo MF. MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res. 2006;66:4750–4757. doi: 10.1158/0008-5472.CAN-05-4422. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ C[T]) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longati P, Bardelli A, Ponzetto C, Naldini L, Comoglio PM. Tyrosines 1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor). Oncogene. 1994;9:49–57. [PubMed] [Google Scholar]
- Carda C, Ferrer J, Vilanova M, Peydro A, Llombart-Bosch A. Anaplastic carcinoma of the thyroid with rhabdomyosarcomatous differentiation: a report of two cases. Virchows Arch. 2005;446:46–51. doi: 10.1007/s00428-004-1123-0. [DOI] [PubMed] [Google Scholar]
- Emoto M, Iwasaki H, Kikuchi M, Shirakawa K. Characteristics of cloned cells of mixed mullerian tumor of the human uterus. Carcinoma cells showing myogenic differentiation in vitro. Cancer. 1993;71:3065–3075. doi: 10.1002/1097-0142(19930515)71:10<3065::aid-cncr2820711029>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Frable WJ. Pathologic classification of soft tissue sarcomas. Semin Surg Oncol. 1994;10:332–339. doi: 10.1002/ssu.2980100505. [DOI] [PubMed] [Google Scholar]
- Kimi K, Onodera K, Kumamoto H, Ichinohasama R, Echigo S, Ooya K. Alveolar soft-part sarcoma of the cheek: report of a case with a review of the literature. Int J Oral Maxillofac Surg. 2000;29:366–369. [PubMed] [Google Scholar]
- Lamovec J, Zidar A, Bracko M, Golouh R. Primary bone sarcoma with rhabdomyosarcomatous component. Pathol Res Pract. 1994;190:51–60. doi: 10.1016/s0344-0338(11)80496-6. [DOI] [PubMed] [Google Scholar]
- Miyagi J, Tsuhako K, Kinjo T, Iwamasa T, Hashimoto H, Ishikawa S. Rhabdoid tumour of the lung is a dedifferentiated phenotype of pulmonary adenocarcinoma. Histopathology. 2000;37:37–44. doi: 10.1046/j.1365-2559.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio S, Malpica A, Schroeder B, Silva EG. Primary ovarian rhabdomyosarcoma associated with clear cell carcinoma of the ovary: a case report and review of the literature. Int J Gynecol Pathol. 2000;19:169–173. doi: 10.1097/00004347-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Nicolas MM, Taylor W. Mediastinal rhabdomyoma: a case report and review of the literature. Int J Surg Pathol. 2002;10:313–318. doi: 10.1177/106689690201000414. [DOI] [PubMed] [Google Scholar]
- Yuri T, Danbara N, Shikata N, Fujimoto S, Nakano T, Sakaida N, Uemura Y, Tsubura A. Malignant rhabdoid tumor of the liver: case report and literature review. Pathol Int. 2004;54:623–629. doi: 10.1111/j.1440-1827.2004.01672.x. [DOI] [PubMed] [Google Scholar]
- Yang GC, Yee HT, Waisman J. Metaplastic carcinoma of the breast with rhabdomyosarcomatous element: aspiration cytology with histological, immunohistochemical, and ultrastructural correlations. Diagn Cytopathol. 2003;28:153–158. doi: 10.1002/dc.10243. [DOI] [PubMed] [Google Scholar]
- Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature. 1998;395:905–908. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]