Abstract
Impairment of the colonic epithelial barrier and neutrophil infiltration are common features of inflammatory bowel disease. Luminal proteases affect colonic permeability through protease-activated receptors (PARs). We evaluated: (i) whether fecal supernatants from patients with ulcerative colitis (UC) trigger alterations of colonic paracellular permeability and inflammation, and (ii) the roles of cathepsin G (Cat-G), a neutrophil serine protease, and its selective receptor, PAR4, in these processes. Expression levels of both PAR4 and Cat-G were determined in colonic biopsies from UC and healthy subjects. The effects of UC fecal supernatants on colonic paracellular permeability were measured in murine colonic strips. Involvement of Cat-G and PAR4 was evaluated using pepducin P4pal-10 and specific Cat-G inhibitor (SCGI), respectively. In addition, the effect of PAR4-activating peptide was assessed. UC fecal supernatants, either untreated or pretreated with SCGI, were infused into mice, and myeloperoxidase activity was determined. PAR4 was found to be overexpressed in UC colonic biopsies. Increased colonic paracellular permeability that was triggered by UC fecal supernatants was blocked by both SCGI (77%) and P4pal-10 (85%). Intracolonic infusion of UC fecal supernatants into mice increased myeloperoxidase activity. This effect was abolished by SCGI. These observations support that both Cat-G and PAR4 play key roles in generating and/or amplifying relapses in UC and provide a rationale for the development of new therapeutic agents in the treatment of this disease.
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are organic diseases resulting from chronic dysregulation of the gut mucosal immune system.1 Although these diseases are often differentiated clinically on the basis of disease distribution and morphology, they share many common characteristics.1 IBD profoundly increases patient morbidity, decreases quality of life, and increases patients’ susceptibility to colorectal cancer. However, the etiology of these diseases is still not fully understood. Current models of disease identify as principal determinants of IBD pathogenesis: (i) an increased permeability of the intestinal epithelial barrier occurring in CD and UC,2 which leads to penetration of luminal products, including bacteria, into the mucosa, and (ii) an abnormal immune response to these products leading to neutrophil infiltration and cytokine-driven inflammation.3
It was shown that the acute phase of UC is characterized by colonic mucosal injury and the presence of leukocytes, including large numbers of neutrophils in the area of tissue damage.4 Neutrophils contain two major granule populations, primary (azurophil) and secondary (specific) granules, formed at different stages of neutrophil maturation. Primary granules mainly contain several proteolytic enzymes and a wide range of bactericidal proteins including cathepsin G (Cat-G), elastase, myeloperoxidase (MPO), and lysozyme.5 Cat-G is a serine protease that makes up approximately 20% of the neutrophil azurophilic granule proteins. Cat-G plays important roles in neutrophil function during inflammatory processes, including degradation of extracellular matrix components and cytokines, modulation of integrin clustering on neutrophils, and direct chemoattraction of T cells and other leukocytes.6
Recent studies have highlighted the Cat-G-dependent cleavage of seven transmembrane spanning domain G protein-coupled protease-activated receptors (PARs).7 PARs can be cleaved by different proteases from the circulation, inflammatory cells, digestive glands and microorganisms. These signaling proteases are generated or released during injury and disease, and regulate the critically important processes in hemostasis, inflammation, neurotransmission, cell migration, and division.8 Although protease signaling has been described in all organ systems, it is of particular relevance in the gastrointestinal tract, which, more than any other organ system, is a rich source of proteases that derive from digestive secretions, inflammatory cells, and resident microorganisms.8 Four members (PAR1, 2, 3, and 4) of the PAR family have been cloned so far; PAR4 is a recently discovered member of the PAR family.9 It is highly expressed throughout the gastrointestinal tract and can be activated by trypsin, thrombin, and Cat-G.10 It was shown that in the gastrointestinal tract, luminal proteases are able to increase paracellular permeability through PAR activation.11
Based on the recent literature showing a high activity of serine-proteases11 in fecal supernatants of patients with UC we conducted this study to: (i) evaluate whether this high colonic luminal serine-protease activity may be responsible for increasing colonic paracellular permeability (CPP) and to determine which factor is involved in this process, (ii) investigate whether such protease activity can trigger inflammation, and (iii) examine the possible involvement of Cat-G in developing inflammation.
Materials and Methods
Human Samples
Biopsies of human colon and fecal samples were collected at Rangueil Hospital, Toulouse, France and University of Szeged, Szeged, Hungary from patients during routine colonoscopy related to colorectal cancer or polyp screening for healthy subjects (n = 25, range 30 to 65, years old) and routine follow-up for active patients with UC (n = 18, range 18 to 40, years old, Mayo scores 1 to 2, all patients were under immunosuppressant treatment). The study protocol was approved by the Ethical Committee of the University of Szeged and Rangueil Hospital, Toulouse. All subjects provided written informed consent to participate.
To prepare fecal supernatants, samples were thawed at 4°C and 1 g of each sample was dissolved and homogenized in 7 ml of physiological saline, then centrifuged (4500 rpm, 10 minutes, 4°C). Coarse particles and bacteria were removed from this solution by filtration on 0.8 μm size syringe filter (Nalgene, Rochester, NY) on ice.
Animals
Six- to eight-week-old male C57Bl.6/J and BALB/c wild-type mice (Janvier, Le Genest St-Isle, France) were used in this study. Mice were housed in polypropylene cages in a light- and temperature-controlled room (12 hours/12 hours cycles, 20 ± 2°C) and fed with standard pellets (Harlan Teklad, Oxon, UK), and water was provided ad libitum. The experimental protocols described in the study were approved by the local Institutional Animal Care and Use Committee.
Measurement of Fecal Enzymatic Activities
To measure total fecal proteolytic activity supernatants of fecal homogenates (25 μl) were incubated with 1 ml of reaction buffer (0.15 M/L NaCl and 20 mmol/L Tris-HCl, pH 8.3) and 1 ml of 0.5% (w/v) azocasein (Sigma, St. Quentin Fallavier, France) at 37°C. The reaction was stopped after 20 minutes with 1 ml of 10% (v/v) trichloroacetic acid (Sigma). Following centrifugation (4500 rpm, 10 minutes, 4°C), absorption of the clear supernatant was measured at 366 nm. Enzyme activities of the supernatants were normalized to protein content. The effect of protease inhibitors on proteolytic activity was tested by the measuring the effect of pre-incubation (30 minutes at 37°C) of the fecal supernatants with either the serine-protease inhibitor 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (Sigma, 2 mmol/L) or specific Cat-G inhibitor (SCGI, Calbiochem, Pessac, France, 2 mmol/L). MPO activity in mice distal colon (3 cm from anus) was measured as described earlier.12 Cat-G activity was measured in fecal supernatants from patients with UC and healthy subjects, using as a substrate N-succinyl-Ala-Ala-Pro-Phe p-Nitroanilide (Sigma). Enzymatic activity was measured at 410 nm for 5 minutes at 37°C.
Immunohistochemistry of PAR4
PAR4 labeling was performed in human colon biopsies from patients with UC and healthy subjects. Samples were fixed in buffered paraformaldehyde (4%), incubated in 30% sucrose (24 hours, 4°C), embedded (Tissue Tek medium) and frozen in isopentane at −45°C. Cryostat section (7 μm) were fixed with acetone (10 minutes, −20°C), hydrated with PBS and permeabilized with PBS-0.5% Triton X100. After incubation in blocking solution (PBS containing 5% bovine serum albumin), sections were incubated overnight (4°C) with rabbit anti-human PAR4 polyclonal antibody (1:200, Abcys, Paris, France), then 1 hour at room temperature with fluorescein isothiocyanate (FITC)-labeled donkey anti-rabbit antibody (1:2000, Uptima, Montluçon, France). Sections were rinsed in PBS, mounted in Vectashield HardSet Mounting Medium (Abcys) and examined under a Nikon 90i fluorescent microscope.
Western Blotting of Cat-G
Proteins contained in fecal supernatants from UC patients and healthy subjects were extracted with RIPA buffer (100 ml Tris-buffered saline, 1 ml Igepal CA-630, 0.5 g sodium deoxycholate, and 0.1 g SDS) and quantified. Equal amounts of protein extracts were electrophoresed in 12% SDS-polyacrylamide gel and then electrotransferred onto Hybond-P polyvinylidene difluoride membrane (GE Health care). The membrane was blocked with Tris-buffered saline-milk 4%, then incubated for 2 hours at room temperature with the primary antibody 1:1000 (rabbit anti-human Cat-G antibody, Abcam, Paris, France). After washing, goat anti-rabbit IgG (Upstate Biotechnology, Molsheim, France) was used as a secondary antibody (1:20000) with 1 hour incubation. The membrane was developed with SuperSignal West Femto Substrate (Pierce, Brebieres, France).
RT-PCR Detection of PAR4 and Cat-G
Total RNA from colon biopsy samples (∼20 μg), from UC patients, healthy subjects, and wild-type C57Bl.6/J was extracted using RNeasy mini Kit (Qiagen, Courtaboeuf, France). Total RNA was then reverse-transcribed into complementary (c)DNA using Omniscript Reverse Transcriptase (Qiagen). PCR amplification of 1 μl of template cDNA was performed using standard PCR protocol (HotStarTaq Plus PCR, Qiagen). After an initial step for enzyme activation (95°C for 15 minutes), 32 cycles were performed as followed: denaturation at 94°C for 30 seconds; annealing for 30 seconds at 55°C (Cat-G, human and mouse glyceraldehyde-3-phosphate dehydrogenase [GAPDH], mouse PAR4), or 57°C (human PAR4); extension at 72°C for 1 minute; and a final extension step at 72°C for 10 minutes. Aliquots of 10 μl of PCR products were analyzed on a 2% agarose gel run using Tris-Acetate-EDTA buffer, and stained with SYBRGold (Molecular probes, Cergy-Pontoise, France).
The primers sequence (Eurogentec, Seraing, Belgium) were, for Cat-G: 5′-ATAATCAGCGGACCATCCAG-3′ sense, and 5′-CTCTGCA-CTCTCAGCTGCAC-3′ antisense; human PAR4: 5′-GATGACAGCACGCCCTCAATC-3′ sense, and 5′-CATCAGCAGCAT-GGTGGAGG-3′ antisense; human GAPDH: 5′-GAAGGTC-GGAGTCAACGGATTTGGT-3′ sense, and 5′-ATGTGGGCCATGAGGTCCACCAC-3′ antisense; mouse PAR4: 5′-GCAGACCTTCCGATTAGCTG- 3′ sense, and 5′-cagtctgagtgcatggctgt-3′ antisense; and mouse GAPDH: 5′-CACCATCTTCCAGGAGCGAG-3′ sense, and 5′-GCCTTCTCCATGGTGGTGAA-3′ antisense.
In Vitro Permeability Studies
C57Bl.6/J mice were sacrificed by cervical dislocation and the distal part of colon was removed. Colonic strips were mounted in Ussing-type chambers (Physiological Instruments, San Diego, CA) with an exposed area of 0.3 cm2. Both sides of each colonic layer were bathed in Krebs-Henseleit buffer (Sigma) and oxygenated at a maintained temperature of 37°C. After 15 minutes for equilibrium, 1 ml of buffer solution was replaced with 500 μl of supernatant from UC, healthy subjects or physiological saline, and 500 μl of FITC-dextran (4000 MW, 0.022 g/ml, Sigma) on the mucosal side of each chamber. After 60 minutes, fluorescence intensity was measured. To assess the respective role of PAR1, PAR2, and PAR4 in the influence of UC fecal supernatants on CPP: (i) P4-pal10 pepducin (final concentration 1 μmol/L) (NeoMPS, Strasbourg, France), (ii) PAR1 antagonist FLLRN (Phe-Leu-Leu-Arg-Asn, 10 μmol/L, Peptides International, Louisville, KY),13,14 (iii) PAR2 antagonist FSLLRY (Phe- Ser-Leu-Arg-Tyr, 10 μmol/L, Bachem, Weil am Rhein, Germany),15 or (iv) physiological saline, as a control, were added to the mucosal side of each chamber before administration at the mucosal side of either fecal supernatants or physiological saline. In another series of experiments, PAR4 agonist peptide (Ala-Tyr-Pro-Gly-Lys-Phe-NH2, 50 μmol/L, Sigma) was added into the mucosal compartment, and CPP was measured after 1 hour. Finally the effect of a Cat-G inhibitor was assessed. UC fecal supernatants were pre-incubated 30 minutes on ice with inhibitor (0.2 mmol/L), then added to the mucosal side of the colonic strip to determine the permeability to FITC-dextran.
Mice Infusion of Fecal Supernatants
BALB/c mice were fasted for 24 hours and then placed in plastic tubes (3 cm in diameter, 9.5 cm in length) for 60 minutes to empty their bowel contents, thus reducing the risk of perforation during catheter insertion. Mice were anesthetized with sodium pentobarbital (Ceva, Libourne, France) (1:10; 0.2 ml per mice) and a polyethylene perfusion catheter was inserted into the distal colon ending 3.5 cm from the anus. After recovery from anesthesia, animals received either 0.3 ml of fecal supernatants of patients with UC, healthy subjects, or supernatants of patients with UC previously incubated with SCGI (0.2 mmol/L) for 30 minutes on ice, or physiological saline, intracolonically with a perfusion rate of 170 μl/h.
One day after infusion mice were sacrificed by cervical dislocation and we collected: distal colon (3 cm from anus) for MPO activity analyze and feces (0.3 g) for serine-protease activity measurement.
Statistical Analysis
All data are expressed as means ± SEM. Densitometry analysis was conducted using Image J software (NIH, Bethesda, MD). For statistical analysis Graph Pad Prism 4.0 (GraphPad, San Diego, CA) was used. Between-group comparisons within groups were performed by Student’s paired t-test. Multiple comparisons within groups were performed by repeated measures one-way analysis of variance followed by Tukey’s posthoc test. Statistical significance was accepted at P < 0.05.
Results
Cat-G and PAR4 Expression in Biopsies and Fecal Supernatants
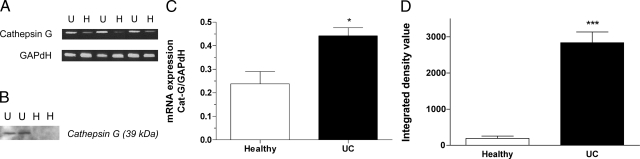
Cat-G mRNA expression was higher in biopsies obtained from patients with UC than in those from healthy subjects (0.44 ± 0.08 vs. 0.24 ± 0.03, P < 0.05, Figure 1, A and C). Moreover, Western blotting experiments indicated that fecal supernatants from patients with UC had much higher Cat-G content when compared with supernatants from healthy subjects (Figure 1, B and D).
Figure 1.
A: Expression of Cat-G in biopsy samples from healthy (H) (n = 6) and UC (U) (n = 7) subjects. B: Western blotting of Cat-G in fecal supernatants from UC patients (n = 8) and healthy subjects (n = 7). C: Densitometry analysis of Cat-G expression in biopsy samples normalized to GAPDH. D: The integrated density values of Cat-G in fecal supernatants. Data are expressed as means ± SEM, *P < 0.05, ***P < 0.001 compared with healthy subjects.
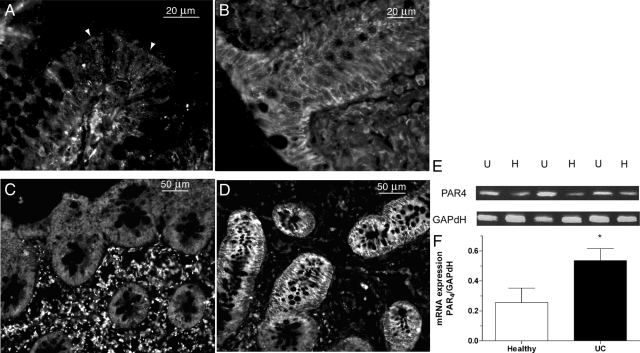
Immunolocalization of PAR4 in biopsies from healthy subjects showed expression of PAR4 in cytoplasm of non epithelial cells, but no expression or only very little in crypts (Figure 2, A and C). In contrast, in biopsies from patients with UC we noticed very high PAR4 labeling in crypts and rather weak expression of PAR4 in non epithelial cells (Figure 2, B and D). Indeed, a PCR product was amplified from RNA prepared from colonic biopsies of healthy subjects and patients with UC, showing the presence of PAR4 in those tissues. Compared with the level of expression of PAR4 in healthy colon, the expression of PAR4 was 107.7% higher in patients with UC (0.26 ± 0.1 vs. 0.54 ± 0.08, P < 0.05, Figure 2, E and F).
Figure 2.
Immunohistochemical staining of longitudinal (A, B) and transverse serial section (C, D) for PAR4 expression (arrowheads) in human colonic biopsies from healthy subjects (n = 7) (A, C) and UC patients (n = 6) (B, D). RT-PCR was performed on similar samples (E), and expression of PAR4 was normalized to GAPDH and analyzed by densitometry by using ImageJ software (F). Data are expressed as means ± SEM (n = 6/7), *P < 0.05 compared with healthy subjects.
Colonic Paracellular Permeability
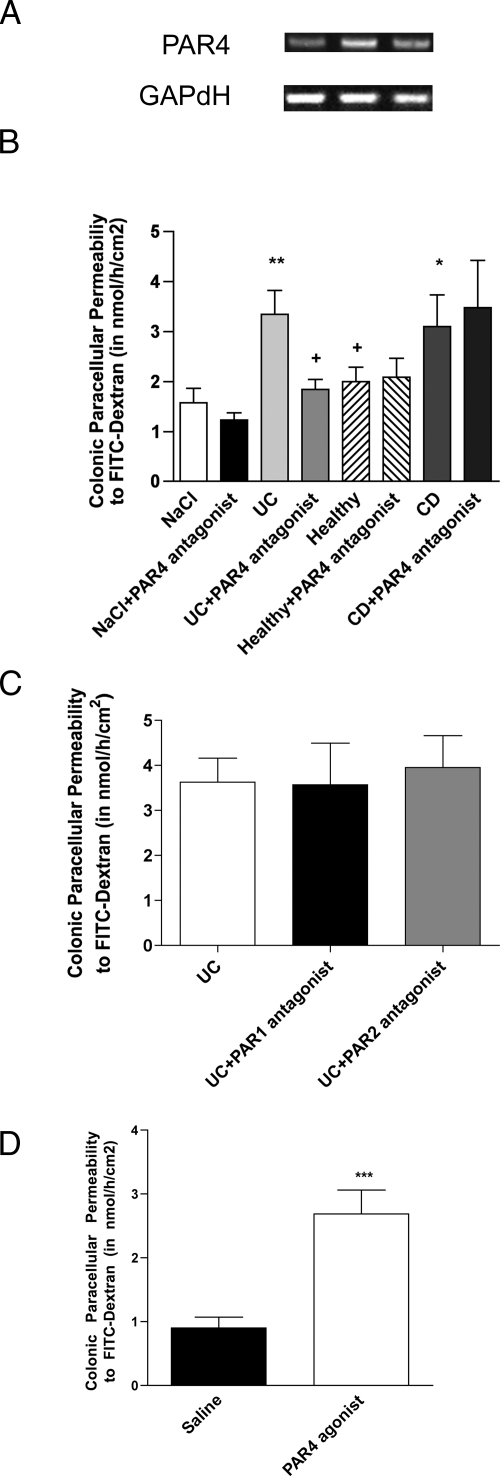
PAR4 was constitutively expressed in mouse colon (Figure 3A). Administration of fecal supernatants from healthy subjects to the mucosal side of mouse colon mounted in Ussing chambers did not significantly alter CPP, as compared with saline (1.58 ± 0.29 vs. 2.00 ± 0.28, NS, Figure 3B). In contrast, addition of fecal supernatants from UC and CD samples significantly increased (3.34 ± 0.47, 3.1 ± 0.63) the FITC-dextran flux compared with supernatants from healthy subjects (P < 0.05) and vehicle (P < 0.01) (Figure 3B). The addition of FLLRN (10 μmol/L) and FSLLRY (10 μmol/L) did not affect significantly (P < 0.05) the increase in CPP triggered by UC fecal supernatants (Figure 3C). In contrast, at the concentration tested (1 μmol/L) pepducin P4pal-10 reduced by 85% (1.84 ± 0.20, P < 0.05 vs. UC; NS versus NaCl) the effect of UC fecal supernatants on permeability to dextran but not the effect of CD fecal supernatants (Figure 3B). Finally, the activation of PAR4 by its activating peptide (AYPGKF-NH2; 50 μmol/L) increased by 199% the dextran flux across mice colonic epithelium in comparison with vehicle (2.69 ± 0.17 vs. 0.90 ± 0.17, P < 0.001, Figure 3D).
Figure 3.
A: Expression of PAR4 in colonic mucosa from wild-type C57Bl.6/J mice (n = 6). GAPDH served as control. B: Colonic paracellular permeability (CPP) to FITC-Dextran in Ussing chambers 60 minutes after the administration of fecal supernatants of UC patients (n = 7), CD patients (n = 6), healthy controls (n = 6), and saline (n = 10) on colonic strips of wild-type mice, and CPP after prior incubation of fecal supernatant of UC patients (n = 15), CD patients (n = 7), healthy controls (n = 12), and saline (n = 12) with pepducin P4pal-10 targeting PAR4 (N-palmitodyl-SGRRYGHALR-NH2, 1 μmol/L). +P < 0.05 compared with fecal supernatants of UC and CD patients, *P < 0.05 compared with saline and **P < 0.01 compared with saline. C: CPP after prior incubation of fecal supernatant of UC patients (n = 6) with the PAR1 antagonist, FLLRN (10 μmol/L) and the PAR2 antagonist, FSLLRY-amide (10 μmol/L). No significant differences were observed. D: CPP 60 minutes after the administration of PAR4 agonist (AY-NH2, 50 μmol/L) (n = 10) and saline (n = 7) controls on colonic strips of wild-type mice. Data are expressed as means ± SEM, ***P < 0.001.
In fecal supernatants from healthy subjects, the total proteolytic activity was 73.10 ± 6.12. This proteolytic activity was significantly greater in fecal supernatants of patients with UC (127.50 ± 27.51, P < 0.05) as compared with healthy controls (Table 1). Pre-incubation of fecal supernatants from healthy subjects and patients with UC with 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, a broad spectrum serine-protease inhibitor, revealed that 31.8% and 45.4%, respectively, of total proteolytic activity were linked to serine-proteases (Table 1). Furthermore, pre-incubation of UC fecal supernatants with SCGI showed that, among luminal serine-proteases found in healthy subjects and UC supernatants, 7% and 15%, respectively, belong to Cat-G (Table 1).
Table 1.
Proteolytic Activity in Fecal Supernatants from Healthy Subjects (n = 7) and UC Patients (n = 12)
| Healthy | UC | P value | |
|---|---|---|---|
| Proteolytic activity (U/mg protein) | 73.10 ± 6.12 | 127.50 ± 27.51 | P < 0.05 |
| Serine protease activity among total proteases (inhibition with AEBSF) | |||
| U/mg protein | 23.25 | 57.89 | P < 0.05 |
| Percentage | 31.8% | 45.4% | P < 0.05 |
| Cathepsin G activity among serine proteases (inhibition with SCGI) | |||
| U/mg protein | 1.62 | 8.68 | P < 0.05 |
| Percentage | 7% | 15% | P < 0.05 |
Serine proteases activity among total proteolytic activity of fecal supernatants from healthy subjects and UC patients, was calculated after incubation of fecal supernatants with wide range serine protease inhibitor AEBSF (4-(2-aminoethyl)benzenesulphonyl fluoride hydrochloride). Cathepsin G activity among serine proteases in fecal supernatants from healthy subjects and UC patients, was calculated after incubation with specific Cathepsin G inhibitor (SCGI).
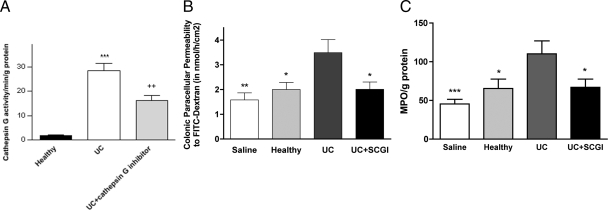
Fecal supernatant from patients with UC showed a high activity, whereas those from healthy subjects presented a very weak ability to cleave the substrate (N-succinyl-Ala-Ala-Pro-Phe p-Nitroanilide), widely used to assess Cat-G activity (28.34 ± 3.05 vs. 2.02 ± 0.22, P < 0.001, Figure 4A). Moreover, SCGI decreased enzymatic activity in UC supernatants by 43% (16.14 ± 2.02, P < 0.01, Figure 4A), showing that Cat-G is present in significant amounts within the lumen in UC, but not in healthy subjects.
Figure 4.
A: Cat-G activity assessed with the substrate N-Suc-Ala-Ala-Pro-Phe-pNA, in fecal supernatant from healthy subjects (n = 6) and UC patients (n = 9), pretreated or not with a specific Cat-G inhibitor (SCGI) (1 μmol/L). Data are expressed as means ± SEM, ***P < 0.001 compared with fecal supernatants from healthy subjects, and ++P < 0.01 compared with fecal supernatants from UC patients. B: Colonic paracellular permeability to FITC-Dextran after prior incubation of fecal supernatants of UC patients with SCGI (0.2 μmol/L). Data are expressed as means ± SEM (n = 10), *P < 0.05, **P < 0.01 compared with fecal supernatants from UC patients. C: Fecal MPO activity in mice colon 1 day after intracolonic infusion of fecal supernatants of UC patients, healthy controls, saline and UC supernatants previously incubated with SCGI. Data are expressed as means ± SEM (n = 7), *P < 0.05, ***P < 0.001 compared with UC supernatants infusion.
Pre-incubation of UC fecal supernatants with SCGI abolished by 77% the effect of UC fecal supernatants alone on paracellular permeability to dextran (3.49 ± 0.53 vs. 2.01 ± 0.30, P < 0.05; NaCl versus UC + SCGI, NS, Figure 4B).
Induction of Colitis
In mice, intracolonic infusion of 0.3 ml of fecal supernatants from patients with UC (170 μl/h) resulted in elevation of the level of serine-protease activity in feces from 51 U/mg/ml (for vehicle) to 177 U/mg/ml (for UC supernatants, data not shown). This was associated with increased activity of inflammatory marker MPO, when compared with administration of fecal supernatants from healthy subjects (P < 0.05) and vehicle (P < 0.001) (Figure 4C). Moreover, SCGI was able to abolish the increase in MPO activity (P < 0.05), observed after UC fecal supernatants infusion (Figure 4C).
Discussion
The present study shows that Cat-G and PAR4 are expressed in higher levels in patients with UC than in healthy subjects. Cat-G and PAR4 are involved in increasing CPP, after UC fecal supernatants infusion to mice. Our data also revealed that UC fecal supernatants can be responsible for triggering a colonic inflammation, which can be decreased by SCGI, a specific cathepsin G inhibitor.
It is well known that neutrophil transmigration across mucosal epithelium is a hallmark of inflammatory conditions, such as IBD. It was shown that the predominant histopathological feature of IBD is the infiltration of immune cells including neutrophils in the affected intestine.16 Moreover, neutrophil accumulation within epithelial crypts and in the intestinal lumen directly correlates with clinical disease activity and epithelial injury.17,18 We have described earlier that activity of MPO and elastase, two components of neutrophils, is markedly increased in fecal supernatants from patients with UC, in comparison with supernatants from healthy subjects.11 However, even though Cat-G is one of the most abundant proteins found in human and mouse neutrophils,19 no clinical studies have evaluated so far its involvement in the pathogenesis of IBD. It has been shown earlier that expression of tissue-degrading cathepsins B, L, and D may play an important role in the pathophysiology of IBD and that they can provide a new target for IBD therapy.20 We can speculate that large amounts of Cat-G may be released from neutrophils into tissues but also into the colonic lumen consecutively to epithelial barrier disruption. Interestingly, anti-neutrophil cytoplasmic antibodies targeting Cat-G are found in the sera of more than half of UC patients.21 We have shown by RT-PCR and Western blotting that Cat-G is overexpressed in patients with UC, suggesting that Cat-G is specifically released from colonic wall into the lumen in patients with UC, but not in healthy subjects. Therefore, we speculate that Cat-G may be an important factor in pathogenesis of IBD.
PAR4 receptor can be activated by Cat-G, which is also referred as the “Cat-G receptor,”10 despite cleavage by other serine-proteases being suspected. Earlier, it was shown that mRNA encoding PAR4 is present in human small and large intestine,22 as well as in rat colon.23 PAR4 was detected at the surface of leukocytes, endothelial cells, and smooth muscle cells.24 In the present study, we investigated the PAR4 expression in human biopsies and showed that it was markedly higher in biopsies taken from patients with UC than these from healthy subjects. Moreover, we observed internalization of PAR4 in UC biopsies, depicting the activation of this receptor on epithelial cells. Regarding PAR4 expression in biopsies from patients with UC, we clearly identified that its expression was mainly located in crypts. By contrast, in biopsies from healthy subjects PAR4 expression was mainly located in cytoplasm of non epithelial cells. Thus, overexpresion of Cat-G in UC patients is associated with high expression of PAR4. We can presume here that Cat-G may be an important factor involved in UC pathogenesis, acting through PAR4 activation.
Increased epithelial permeability plays a central role in inflammatory processes. Indeed, permeability changes are involved in perpetuating the chronic mucosal damage observed in IBD.25 This impaired permeability reflects a primary defect of the tight junctions between epithelial cells. For instance, the expression of a dominant negative N-cadherin mutant in mouse epithelium leads to leaky tight junctions and severe inflammation in the lamina propria subjected to epithelial cells expressing the transgene.26 In addition, decreased expression of tight junction proteins is observed in IBD patients, particularly with changes in expression and distribution of claudins 2, 5, and 8.27 Recent studies have highlighted that direct activation of PAR128 and PAR229 on enterocytes is responsible for increased CPP in IBD and a significant increase of proteolytic activity has been observed in feces of patients with UC.11,30 Our group has shown previously that fecal supernatants from patients with IBS (irritable bowel syndrome), with high serine-proteases activity, can trigger paracellular permeability in mice colon.11 We hypothesize that the level of serine-protease in the lumen plays a key role in the development of inflammation in UC by affecting the mucosal barrier integrity, through PAR4 activation. Our present findings show that UC and CD fecal supernatants increased paracellular permeability of mouse distal colon. Pepducin P4pal-10, a lipopeptide that specifically blocks PAR4 signaling,31 abolished the increased paracellular permeability caused by UC fecal supernatants, but not by CD fecal supernatants. Moreover, PAR1 and PAR2 antagonists had no effect on increased paracellular permeability triggered by UC fecal supernatants. In addition, the synthetic peptide AYPGKF-NH2, which can activate PAR4 but no other PARs,32 was able to reproduce the effect of UC fecal supernatants on CPP in mice. Accordingly, we concluded that fecal supernatants from patients with UC contained an amount of serine-proteases able to activate PAR4 located on epithelial cells, to initiate an increase in CPP. In addition, CD fecal supernatants also increase CPP in mice, but though a mechanism independent of PAR4 activation. We then speculated that Cat-G represents a significant part of serine-proteases present into the lumen that are able to contribute to the PAR4-mediated increase in permeability found in vitro. Indeed, we showed in experiments with a more selective substrate (N-succinyl-Ala-Ala-Pro-Phe p-Nitroanilide),33 that Cat-G is present within the lumen of UC patients in greater amount than in healthy subjects. These results showed that 15% of serine-protease activity, in fecal supernatants from UC patients, belongs to Cat-G. Nevertheless, the remaining enzymatic activity (85%) can be related to other serine-proteases, either from endogenous, as chymotrypsin, or exogenous, ie, bacterial, origin34 or to proteases such as metalloproteinases.35 Indeed, it was shown that mast cell-derived metalloproteinase take part in inflammation during IBD.36 However, it is unlikely that these enzymes are able to activate PAR4, since we showed that pre-incubation of fecal supernatants with SCGI abolished the increased CPP induced by fecal supernatants of UC patients. Thus, Cat-G appears to be the major colonic luminal factor present in UC, but not in CD, able to stimulate PAR4 to trigger epithelial barrier alternations.
MPO is a neutrophil enzyme and its activity in the colon is linearly related to infiltration of neutrophils. The assessment of MPO activity is well established for quantification of intestinal inflammation.37 Intracolonic infusion of mice with supernatants from patients with UC, rich in serine-proteases, increased MPO activity. Pre-incubation of UC fecal supernatants with SCGI abolished this MPO increase. Thus, Cat-G present in UC fecal supernatants is able to trigger an inflammatory reaction, likely through epithelial barrier impairment.
Finally, we have characterized that one neutrophil mediator, Cat-G, secreted in the colonic lumen, is able to trigger inflammation through epithelial PAR4 activation. This duet, Cat-G and PAR4, is responsible for the primary event of epithelial barrier disruption, that is, the increase in paracellular permeability that contributes to the onset of the disease. In addition to being novel, these data also highlight new targets for a non-immune treatment of UC particularly intended for the prevention of relapses. Indeed, by using P4-pal10, a pepducin that blocks the signal transduction induced by PAR4 activation,31 we were able to abolish the dramatic increase in colonic paracellular permeability triggered by UC fecal supernatants. Moreover, recent studies have shown that pepducins targeting PARs may be of interest in other inflammatory situations38 and arterial thrombosis.39 We propose that, in a context of inflammatory bowel diseases, pepducins and/or Cat-G inhibitors could represent a new, promising therapeutic approach.
Acknowledgments
We thank Andras Rosztoczy, Ferenc Nagy, Tamas Molnar, and Jean-Pierre Vinel for their contribution in patients selection and screening, and Raphael Garcia-Villar and Hervé Guillou for critical reading and linguistic improvement of the manuscript.
Footnotes
Address reprint requests to Dr. Lionel Bueno, Neuro-Gastroenterology & Nutrition Unit, Institut National de la Recherch Agronomique, 180 Chemin de Tournefeuille, BP 3, 31931 Toulouse Cedex 9, France. E-mail: lbueno@toulouse.inra.fr.
Supported by grants from INRA, BREMICI, and Association François Aupetit. M.D. was supported by a post-doc fellowship grant from INRA.
M.D. and L.F. contributed equally to this work.
References
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Robinson CE, Kottapalli V, D'Astice M, Fields JZ, Winship D, Keshavarzian A. Regulation of neutrophils in ulcerative colitis by colonic factors: a possible mechanism of neutrophil activation and tissue damage. J Lab Clin Med. 1997;130:590–602. doi: 10.1016/s0022-2143(97)90109-8. [DOI] [PubMed] [Google Scholar]
- Carlson M, Raab Y, Seveus L, Xu S, Hallgren R, Venge P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut. 2002;50:501–506. doi: 10.1136/gut.50.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda N, Fukazawa N, Nonomura K, Fairchild RL. Cathepsin g is required for sustained inflammation and tissue injury after reperfusion of ischemic kidneys. Am J Pathol. 2007;170:930–940. doi: 10.2353/ajpath.2007.060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri A, Alcott SG, Elouardighi H, Pak E, Derian C, Andrade-Gordon P, Kinnally K, Steinberg SF. Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J Biol Chem. 2003;278:23944–23954. doi: 10.1074/jbc.M302718200. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Bunnett N. Protease-activated receptors: protease signaling in the gastrointestinal tract. Curr Opin Pharmacol. 2004;4:551–556. doi: 10.1016/j.coph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. International Union of Pharmacology XXVIII proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Day JR, Haskard DO, Taylor KM, Landis RC. Effect of aprotinin and recombinant variants on platelet protease-activated receptor 1 activation. Ann Thorac Surg. 2006;81:619–624. doi: 10.1016/j.athoracsur.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Poullis M, Manning R, Laffan M, Haskard DO, Taylor KM, Landis RC. The antithrombotic effect of aprotinin: actions mediated via the protease activated receptor 1. J Thorac Cardiovasc Surg. 2000;120:370–378. doi: 10.1067/mtc.2000.108531. [DOI] [PubMed] [Google Scholar]
- Sethi AS, Lees DM, Douthwaite JA, Corder R. Factor VIIa stimulates endothelin-1 synthesis in TNF-primed endothelial cells by activation of protease-activated receptor 2. Clin Sci (Lond) 2005;108:255–263. doi: 10.1042/CS20040237. [DOI] [PubMed] [Google Scholar]
- Ando T, Nobata K, Watanabe O, Ina K, Kusugami K, Maeda O, Ishiguro K, Ohmiya N, Niwa Y, Goto H. Abnormalities in the upper gastrointestinal tract in inflammatory bowel disease. Inflammopharmacology. 2007;15:101–104. doi: 10.1007/s10787-006-0735-1. [DOI] [PubMed] [Google Scholar]
- Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann NY Acad Sci. 2006;1072:276–287. doi: 10.1196/annals.1326.018. [DOI] [PubMed] [Google Scholar]
- Hanai H, Takeuchi K, Iida T, Kashiwagi N, Saniabadi AR, Matsushita I, Sato Y, Kasuga N, Nakamura T. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004;49:1438–1443. doi: 10.1023/b:ddas.0000042243.47279.87. [DOI] [PubMed] [Google Scholar]
- MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- Menzel K, Hausmann M, Obermeier F, Schreiter K, Dunger N, Bataille F, Falk W, Scholmerich J, Herfarth H, Rogler G. Cathepsins B, L, and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin Exp Immunol. 2006;146:169–180. doi: 10.1111/j.1365-2249.2006.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Sato Y, Saka M, Kondo Y, Miyata M, Obara K, Nishimaki T, Kasukawa R. Anti-cathepsin G antibodies in the sera of patients with ulcerative colitis. J Gastroenterol. 2000;35:682–689. doi: 10.1007/s005350070047. [DOI] [PubMed] [Google Scholar]
- Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA: 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf WA, Hellmich HL, Micci MA, Winston JH, Zou L, Pasricha PJ. Molecular cloning of the rat proteinase-activated receptor 4 (PAR4). BMC Mol Biol. 2002;3:2. doi: 10.1186/1471-2199-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Derian CK, D'Andrea MR, Steinhoff M, Andrade-Gordon P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential proinflammatory role for proteinase-activated receptor-4. J Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AC, Vergnolle N, MacNaughton WK, Wallace JL, Hollenberg MD, Buret AG. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci USA: 2003;100:11104–11109. doi: 10.1073/pnas.1831452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roka R, Rosztoczy A, Leveque M, Izbeki F, Nagy F, Molnar T, Lonovics J, Garcia-Villar R, Fioramonti J, Wittmann T, Bueno L. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:550–555. doi: 10.1016/j.cgh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- Houle S, Papez MD, Ferazzini M, Hollenberg MD, Vergnolle N. Neutrophils and the kallikrein-kinin system in proteinase-activated receptor 4-mediated inflammation in rodents. Br J Pharmacol. 2005;146:670–678. doi: 10.1038/sj.bjp.0706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Barillec K, Pidard D, Balloy V, Chignard M. Human neutrophil cathepsin G down-regulates LPS-mediated monocyte activation through CD14 proteolysis. J Leukoc Biol. 2000;68:209–215. [PubMed] [Google Scholar]
- de Leeuw E, Li X, Lu W. Binding characteristics of the Lactobacillus brevis ATCC 8287 surface layer to extracellular matrix proteins. FEMS Microbiol Lett. 2006;260:210–215. doi: 10.1111/j.1574-6968.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Reimann J, Claesson MH, Bland PW. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol. 2000;157:1927–1935. doi: 10.1016/S0002-9440(10)64831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai LA, Mohammadirad A, Yasa N, Minaie B, Nikfar S, Ghazanfari G, Zamani MJ, Dehghan G, Jamshidi H, Boushehri VS, Khorasani R, Abdollahi M. Benefits of zataria multiflora boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med. 2007;4:43–50. doi: 10.1093/ecam/nel051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slofstra SH, Bijlsma MF, Groot AP, Reitsma PH, Lindhout T, ten Cate H, Spek CA. Protease-activated receptor-4 inhibition protects from multiorgan failure in a murine model of systemic inflammation. Blood. 2007;110:3176–3182. doi: 10.1182/blood-2007-02-075440. [DOI] [PubMed] [Google Scholar]
- Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]