Abstract
Presently, little is understood about how endometriosis is established or maintained, or how genetic factors can predispose women to the disease. Because of the crucial role that the progesterone receptor polymorphism PROGINS plays in predisposing women to the development of endometriosis, we hypothesized that this variant may influence critical steps during endometrial cell metabolism that are involved in the pathogenesis of endometriosis. Eutopic endometria were collected from three sources: women with endometriosis who had a single PROGINS allele (from the progesterone receptor gene); women with endometriosis who had the wild-type progesterone receptor allele; and women without endometriosis who had the wild-type allele. Cells prepared from the eutopic endometria of these women were stimulated with both estradiol and progesterone, and then examined for cell proliferation, viability, and apoptosis. The cells from women with endometriosis that carried the PROGINS allele demonstrated increased proliferation, greater viability, and decreased apoptosis following progesterone treatment. In general, these parameters were very different as compared with those of women with endometriosis but without the PROGINS allele and women in the control group. This result indicates there is a reduced level of progesterone responsiveness in women who carry the PROGINS polymorphism. Because progesterone responsiveness is known to be an important characteristic of women with endometriosis, these data support the contention that the PROGINS polymorphism enhances the endometriosis phenotype.
Endometriosis is a chronic inflammatory disease characterized by implantation and growth of endometrial tissue outside of the uterus.1 It affects 10% to 15% of all women of reproductive age, and it is significantly associated with infertility,2 chronic pain,3 and morbidity,4 making endometriosis a significant problem for public health. In 1925, Sampson et al5 suggested that the transtubarian reflux of viable endometrial cells represents the origin of endometriosis. However, several subsequent studies reported that approximately 90% of women have viable endometrial cells in the peritoneal cavity,6,7 disputing the notion that retrograde menstruation theory could explain the cause of the disease. It is also noteworthy that only a small portion of women with retrograde menstruation develops endometriosis.
Environmental, endocrine, immune, and genetic factors have all been related to the pathogenesis of endometriosis. Of note, genetic studies of close relatives suggest that there is a 6% increase in the risk of developing endometriosis.8 Several studies also suggest that women with endometriosis present abnormalities in the eutopic endometrium, raising questions about whether the uterine mucosa is involved in the pathogenesis of the disease.
In that context, modifications of cell cycle control, with increased levels of cell proliferation9 and decreased apoptosis,10 emerge as major mechanisms responsible for endometriosis development. Likewise, enhanced cell adhesion and invasion via increased expression of matrix metalloproteinases and the simultaneous down-regulation of their inhibitors,11,12,13 as well as abnormal sex hormone metabolism,14,15 are additional hallmarks of the disease.
Progesterone plays a major role in the processes mentioned above,16 reinforcing the theory that impaired progesterone function could facilitate the genesis and development of endometriosis.17,18 Interestingly, several studies demonstrated that progesterone is able to induce the expression of a large number of genes in the eutopic endometrium. Microarray analysis reported by Burney et al19 demonstrated that FOX10, MIG6, and CYP26A1 expression was significantly modified in the uterine mucosa. In another study, Wang et al used a baboon model of endometriosis and demonstrated that the expression of progesterone responsive factors is altered during the secretory stage of the menstrual cycle, suggesting that progesterone resistance plays a major role in the genesis of endometriosis.20
Several studies have addressed the question of whether genetic mutations contribute to the development of endometriosis. In these studies, several candidate genes and polymorphisms were associated with the development of endometriosis.21 One particular candidate, the progesterone receptor (PR) gene variant named PROGINS (NCBI Data Bank accession numbers AF016381 and Z49816), has emerged as an important disease component of endometriosis. PROGINS is characterized by a 306-bp insertion in intron G, which exists in linkage disequilibrium with point mutations in exons 4 and 5.22 Epidemiological studies have shown that women carrying the PROGINS polymorphism have an increased risk for the development of hormone-dependent gynecological disorders, such as endometrial and ovarian carcinomas, recurrent abortions, and uterine fibroids,23,24,25,26,27,28 conditions in which progesterone plays a critical role.
Several researchers, including those in our laboratory, have reported that patients carrying a single PROGINS allele have an increased risk for endometriosis development.29,30,31,32 In addition, in vitro data from Romano et al demonstrated that the PROGINS variant of the PR gene is less responsive to progestins, as compared with wild-type PR, resulting in reduced mRNA stability and protein activity, as well as a diminished ability to efficiently inhibit cell proliferation in rodent ovarian cell lines.33 Yet the effects of the PROGINS polymorphism on the phenotype of eutopic endometrial cells have never been described. The goal of the present study was to evaluate the influence of the PR variant PROGINS on cell viability, apoptosis, and proliferation in cell cultures of eutopic endometrium from women with and without endometriosis.
Materials and Methods
Study Participants and Sample Collection
Endometrial tissues were obtained from informed volunteers in the Endometriosis Unit/Department of Gynecology at the Federal University of São Paulo (UNIFESP). The Institutional Ethics Review Board (CEP0571/06) had previously approved the study. Women undergoing laparoscopy for routine evaluation of infertility, pelvic pain, or elective tubal sterilization were recruited. All patients had a history of regular menses and were not taking any sex steroids or steroid-modulating medications 3 months before surgery. After general anesthesia and just before the surgical procedure, endometrial tissues were collected using the Nowak’s curette. During laparoscopy, a systematic observation of the pelvis was conducted and the patients were assigned to either the endometriosis or the control group. The collected endometrial tissue was separated into two parts: one for histological analysis according to the criteria of Noyes et al34 and the other for cell culture processing. Data for the patients’ laparoscopic diagnosis, age, menstrual cycle date, and stage of endometriosis were also recorded (Table 1).
Table 1.
Characteristics of Samples Used in the Study
| Groups | Samples | Age (yr) | Cycle date | Stage of endometriosis* | Laparoscopic diagnose | PROGINS genotype | Sample selected for an experiment [+]/Passage no. used
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IF | qPCR | B/AB | Ct | Tm | CC | V/A | A5 | CM | |||||||
| Endometriosis (E-Alu) | 1 | 41 | Proliferative | Stage I | Endometriosis | T1/1 | +/1, 4–7 | +/5 | +/5 | − | − | +/6 | +/7 | +/7 | − |
| 2 | 34 | Secretory | Stage I | Endometriosis | T1/1 | +/1, 4–7 | − | − | − | − | +/6 | − | +/7 | − | |
| 3 | 23 | Proliferative | Stage II | Endometriosis | T1/1 | +/1, 4–7 | +/5 | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | − | |
| 4 | 26 | Proliferative | Stage IV | Endometriosis | T1/1 | +/1, 4–7 | +/5 | +/5 | − | − | +/6 | +/7 | +/7 | +/5 | |
| 5 | 42 | Secretory | Stage I | Endometriosis | T1/1 | +/1, 4–7 | − | +/5 | − | − | − | +/7 | − | − | |
| 6 | 33 | Proliferative | Stage I | Endometriosis | T1/1 | +/1, 4–7 | − | − | − | − | − | +/7 | − | − | |
| 7 | 33 | Proliferative | Stage III | Endometriosis | T1/1 | +/1, 4–7 | − | +/5 | +/5 | +/5 | − | − | − | +/5 | |
| 8 | 35 | Proliferative | Stage III | Endometriosis | T1/1 | +/1, 4–7 | +/5 | − | +/5 | +/5 | +/6 | +/7 | +/7 | − | |
| 9 | 33 | Secretory | Stage II | Endometriosis | T1/1 | +/1, 4–7 | +/5 | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 | |
| 10 | 22 | Secretory | Stage I | Endometriosis | T1/1 | +/1, 4–7 | +/5 | − | +/5 | +/5 | +/6 | − | +/7 | +/5 | |
| Endometriosis (E+Alu) | 11 | 24 | Proliferative | Stage I | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 |
| 12 | 36 | Secretory | Stage IV | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 | |
| 13 | 35 | Secretory | Stage I | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 | |
| 14 | 37 | Proliferative | Stage I | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 | |
| 15 | 28 | Secretory | Stage I | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | +/5 | |
| 16 | 29 | Proliferative | Stage IV | Endometriosis | T1/2 | +/1, 4–7 | − | +/5 | − | − | − | − | − | − | |
| Control (CP-Alu) | 17 | 31 | Secretory | N/A | Normal pelvis | T1/1 | +/1, 4–7 | − | − | +/5 | +/5 | − | +/7 | − | +/5 |
| 18 | 38 | Secretory | N/A | Ovarian cyst | T1/1 | +/1, 4–7 | − | +/5 | +/5 | +/5 | − | − | − | +/5 | |
| 19 | 30 | Secretory | N/A | Pelvic adhesions | T1/1 | +/1, 4–7 | +/5 | +/5 | +/5 | +/5 | +/6 | +/7 | +/7 | − | |
| 20 | 26 | Proliferative | N/A | Adnexal cyst | T1/1 | +/1, 4–7 | +/5 | +/5 | +/5 | +/5 | +/6 | − | +/7 | − | |
| 21 | 36 | Proliferative | N/A | Normal pelvis | T1/1 | +/1, 4–7 | − | − | +/5 | − | − | +/7 | − | − | |
| 22 | 33 | Proliferative | N/A | Adnexal cyst | T1/1 | +/1, 4–7 | +/5 | +/5 | − | − | +/6 | +/7 | +/7 | − | |
| 23 | 31 | Proliferative | N/A | Pelvic adhesions | T1/1 | +/1, 4–7 | +/5 | − | − | − | +/6 | +/7 | +/7 | − | |
| 24 | 25 | Secretory | N/A | Ovarian cyst | T1/1 | +/1, 4–7 | − | − | − | − | − | +/7 | − | +/5 | |
| 25 | 28 | Proliferative | N/A | Normal pelvis | T1/1 | +/1, 4–7 | − | − | − | − | − | +/7 | − | +/5 | |
| 26 | 34 | Proliferative | N/A | Normal pelvis | T1/1 | +/1, 4–7 | +/5 | +/5 | − | − | +/6 | +/7 | +/7 | − | |
Eutopic endometrium was obtained from control patients (CP-Alu) without the PROGINS allele, and from women with endometriosis with (E+Alu) and without (E-Alu) the single PROGINS allele.
N/A = Not applicable.
PROGINS genotype: T1/1 = homozygous major allele, T1/2 = PROGINS heterozygous.
[+] = sample selected in an experiment, [−] = sample not used.
IF = immunofluorescence, qPCR = time and dose response quantitative real-time PCR for PR; B/AB = PRB/AB mRNA expression ratio; Ct = Cell Counting; Tm = thymidine incorporation assay; CC = cell cycle analysis; V/A = viability and apoptosis (Viacount assay); A5 = Annexin-V labeling; and CM = chromatin morphology analysis. Experiments were performed between passages 4 and 7.
Revised American Society for Reproductive Medicine classification of endometriosis.
PROGINS Genotyping
Before the endometrial biopsy procedure, peripheral blood samples were obtained from the patients. Genomic DNA (gDNA) was extracted and purified using the GFX DNA extraction kit (GE Healthcare, Little Chalfont Buckinghamshire, UK). Genotyping of the PROGINS polymorphism was performed by PCR as described previously.29 Endometriosis and control groups were subdivided based on detection of PROGINS: control (CP-Alu), endometriosis wild-type homozygous (E-Alu), and endometriosis PROGINS heterozygous (E+Alu).
Tissue Isolation and Culture
Specimens were transported to the laboratory in culture medium supplemented with antibiotics and antimycotics. Tissues were minced into small pieces and treated with collagenase IA (Life Technologies, Inc., Grand Island, NY), as previously described.35 No procedures were performed to isolate epithelial or stromal cells. Cells from each individual specimen were plated in 100-mm diameter culture dishes (TPP, Trasadingen, Switzerland). After 16 hours of incubation, non-adherent cells were washed away. The typical yield of stromal cells using this technique is 90%.36 Cells were grown in phenol-free Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO) containing 10% heat-inactivated fetal bovine serum (Life Technologies, Inc., Rockville, MD), 100 IU/ml penicillin, 100 mg/ml streptomycin (Sigma-Aldrich), and 250 μg/ml amphotericin-B (Cultilab, Campinas, Brazil) in a humidified incubator at 37°C with 5% CO2. Adherent cells were characterized by immunofluorescence using specific monoclonal antibodies against cytokeratin (Santa Cruz Biotechnology, Santa Cruz, CA) and vimentin (Dako Corp., CA). The proportions of cytokeratin-positive cells (endometrial glandular cells) and vimentin-positive cells (endometrial stromal cells) were assessed in each cell culture, as described previously.37 Normal goat serum (Sigma-Aldrich) was used instead of the primary antibody as a negative control. Because the cultures presented high percentages of vimentin-positive cells, human breast ductal carcinoma cells (T47D cell line), which constitutively express cytokeratin in the form of intermediate filaments, were used as positive controls for cytokeratin to ensure the specificity of the method.
Quantitative Real-Time Reverse Transcription PCR
A time-course and dose-response study was conducted with 17β-estradiol (Sigma-Aldrich) to induce optimal progesterone receptor mRNA expression for the cytometric experiments with progesterone.38 Total RNA was extracted from the endometrial cells (RNeasy kit, Qiagen Inc, Hilden, Germany), and the reverse transcriptase reaction (Superscript-III, Invitrogen) was performed with 1 μg of DNase I (G&E Healthcare Biosciences, Upsala, Sweden)-treated RNA. qPCR was performed using the ABI 7700 thermocycler (Applied Biosystems, Foster City, CA) and the Power SYBr Green Mastermix PCR kit (Applied Biosystems), according to the manufacturer’s instructions. PCR primers (IDT, Coralville, IA) were synthesized with the following sequences: total progesterone receptor PR (A+B): forward primer 5′-ACACAAAACCTGACACCTCC-3′ and reverse primer 5′-TACAGCATCTGCCCACTGAC-3′; progesterone receptor subunit B (PR-B): forward primer 5′-GCCAGAGAAAAAGTCGGGAG-3′ and reverse primer 5′-TGGGGAGCGCAAGAAAAAG-3′39; and glyceraldehyde- 3-phosphate dehydrogenase (GAPDH): forward primer 5′-ACCACAGTCCATGCCATCAC-3′ and reverse primer 5′-TCCACCACCCTGTTGCTGTA-3′.40 The polymerase chain reaction was performed with the following conditions: one cycle at 95°C for 15 minutes and 35 cycles at 94°C for 15 seconds, 60°C for PR (A+B) and PR-B or 58°C for GAPDH for 30 seconds, and 72°C for 30 seconds. Cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted control samples. The expression of target PR (A+B) and PR-B genes was normalized to GAPDH mRNA levels, which were measured concurrently.41 The specificity of each reaction was confirmed by melting curve analysis and agarose gel electrophoresis.
Viability and Apoptosis Analyses by the Viacount Assay
Endometrial cells (2 × 104) seeded in 6-well plates at 50% to 70% confluence were treated with 100 nmol/L 17β-estradiol for 24 hours, followed by 100 nmol/L progesterone for 24 hours. Cells were mixed with Guava Viacount reagent and allowed to stain for 10 minutes (Guava Technologies, Hayward, CA). The Guava System differentiates viable from non-viable cells by detecting fluorescence signals from two fluorescent DNA-binding dyes: one membrane-permeable dye stains all nucleated cells while the second dye enters cells when membrane integrity has been compromised, ie, non-viable cells.42 Viable cells were quantified using a Guava Personal Analyzer (PCA) flow cytometer (Guava Technologies), according to the manufacturer’s specifications.
Quantification of Apoptotic Cells by Annexin V Labeling
Endometrial cells (2 × 104) seeded in 6-well plates at 50% to 70% confluence were treated with 100 nmol/L 17β-estradiol for 24 hours, followed by 100 nmol/L progesterone for an additional 24 hours. To detect apoptosis, the cells were double stained with annexin V and nexin 7-AAD, according to the manufacturer’s recommendations (Guava Nexin Method; Guava Technologies). Cell-associated fluorescence was analyzed using a Guava PCA flow cytometer (Guava Technologies). Results were expressed as the percentage of apoptotic-positive cells. Both early apoptotic (annexin V-positive) and late apoptotic (annexin V and 7-AAD-positive) cells were included in the analysis.43
Quantification of Apoptotic Cells by Chromatin Morphology Analysis
Endometrial cells (3 × 104) were seeded in 6-well plates and cultured until subconfluence (50% to 70%), at which time the cells were serum-deprived for 24 hours. After treating the cells with 100 nmol/L 17β-estradiol for 24 hours followed by 100 nmol/L progesterone stimulation for 24 hours, 0.5 μg/ml Hoescht 33342 (Sigma-Aldrich) was added to the medium for 30 minutes at 37°C. Attached and non-attached cells were collected and analyzed by UV fluorescence microscopy (×400). Apoptosis was evaluated based on chromatin morphology.44 Two hundred cells per sample were counted. Results are expressed as the percentage of apoptotic cells in the solution.
DNA Synthesis by the [3H]-Thymidine Incorporation Assay
Endometrial cells (2 × 104) were grown in 24-well plates until 50% to 70% confluence was attained, and then quiescence of the cells was achieved by addition of phenol/serum-free Dulbecco’s Modified Eagle’s Medium for 24 hours. Cells were treated with 100 nmol/L 17β-estradiol for 24 hours, followed by 100 nmol/L progesterone for 20 hours. After 14 hours of progesterone exposure, cells were labeled with 1 μCi/ml [3H]-methyl-thymidine (Amersham Biosciences, Piscataway, NJ) for 6 hours. At the end of this period, the cells were washed in saline solution and methanol, and precipitated with trichloroacetic acid. Samples were dissolved with NaOH and diluted in scintillation buffer. Radioactivity was measured in a Packard Tri-Carb LS β-counter (PerkinElmer, Wellesley, MA).
Cell Number Measurement
Changes in the number of cells were determined by flow cytometry using the Guava Viacount kit (Guava Technologies). Briefly, 1.5 × 104 endometrial cells were plated and grown until a confluence of 50% to 70% was attained. Synchronization of the cell cycle was then induced by serum-starvation for 24 hours. Furthermore, the cells were stimulated with 100 nmol/L 17β-estradiol for 24 hours, followed by another 24 hours of 100 nmol/L progesterone treatment, during which the cells were counted at specified time points (three times per well). Results are expressed as the number of cells/ml.
Cell Cycle Analysis
Endometrial cells (2 × 105) were seeded in 100-mm dishes and grown until a confluence of 50% to 70% was reached. After synchronization of the cell cycle by serum starvation for 24 hours, cells were stimulated with 100 nmol/L 17β-estradiol for 24 hours, followed by treatment with 100 nmol/L progesterone in phenol-free Dulbecco’s Modified Eagle’s Medium for an additional 24 hours. Cells were fixed with 70% ethanol for 1 hour, stained with propidium iodine, and cell cycle populations were determined using the Guava EasyCyt system, according to the manufacturer’s recommendations (Guava Technologies). The Guava Cell Cycle software was used to determine cell populations in the different cell cycle phases, while the amount of propidium iodine was quantified in the G0/G1, S, and G2/M phases.45
Statistical Analyses
Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS v14.0). Comparisons between two groups were made using the Student’s t-test (paired). Analysis of variance was applied whenever differences among three or more groups were analyzed. When a positive global difference between groups was found, post hoc Tukey’s Honestly Significant Difference test (when variances were homogeneous) or Games Howell (when variances were considered unequal) tests were performed to identify which coupled groups presented significant differences. qPCR values were log2-transformed before the analysis. At least four biological replicates were analyzed in each experimental group. Data are expressed as the ratio between hormonal treatment and basal conditions in the majority of experiments, and P < 0.05 was considered as significant.
Results
Experimental Subjects
Endometrial biopsies from 16 patients with endometriosis, age 31.2 ± 4 years (mean ± SEM), were staged according to the guidelines from the American Society for Reproductive Medicine46 and were selected for the study. For comparison, endometrial biopsies were obtained from 10 women (age 31.9 ± 6) submitted for laparoscopy for benign ovarian cysts without signs of endometriosis in the pelvis. Tissues were collected at various times during the menstrual cycle and were dated according to Noyes’ criteria.34 No homozygous cases of PROGINS were included in the study, and all of the listed endometrial biopsies yielded viable cell cultures (Table 1).
Endometrial Cell Culture
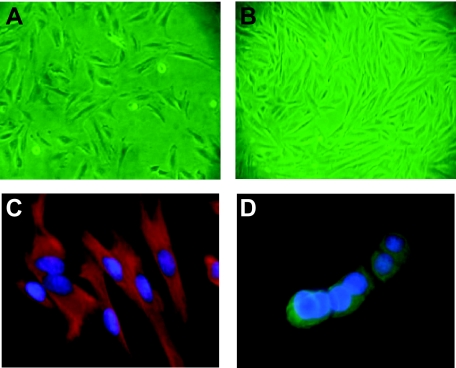
Cell cultures were established from human endometrial tissues as described by Piva et al.36 Cultures reached 90% to 95% confluence within 3 ± 1 day (mean ± SEM) of the initial isolation (Figure 1, A and B). Cells were used from passages 3 until 7, and cell characterization was performed for each passage used in the experiments. The results from each passage showed approximately 90% vimentin-positive cells in all samples, as indicated by immunofluorescence microscopy (Figure 1, C and D). Cells were frozen at the third passage, and experiments were performed using cells from subsequent passages to ensure that a homogeneous population of cells was used for each phenotypic analysis (Table 1).
Figure 1.
Figures A and B show nonconfluent and subconfluent endometrial cells from eutopic endometrium used in all experiments. Triple immunofluorescence for vimentin (red), cytokeratin (green), and 4,6-diamidino-2-phenylindole for the nuclei, was performed to characterize percentages of endometrial mesenchymal (C) and epithelial constituents in each sample. Figure (D) shows T47D cells used as positive controls for cytokeratin staining. Magnification = original ×200 (A), ×100 (B), and ×400 (C and D).
Induction of PR mRNA Expression by 17β-Estradiol and PRB/PRAB Ratio Analysis
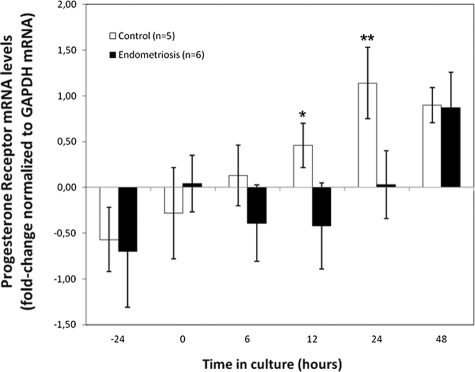
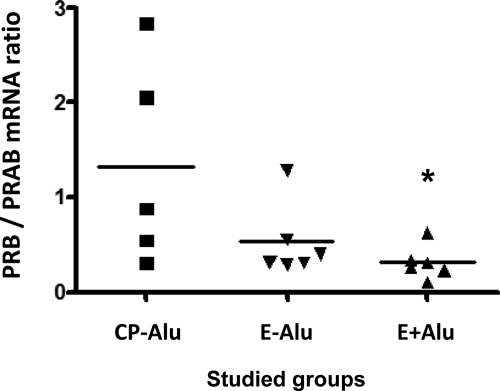
Endometrial cell cultures were treated with 100 nmol/L 17β-estradiol (E2) for different time periods, and total PR mRNA levels were analyzed by real-time PCR. PR showed a time-dependent modulation for both groups (Figure 2), but demonstrated more significant results for the controls (6 hours to 12 hours, P = 0.44; 6 hours to 24 hours, P = 0.04; 6 hours to 48 hours, P = 0.007) when compared with the endometriosis group (6 hours to 12 hours, P = 0.91; 6 hours to 24 hours, P = 0.23; 6 hours to 48 hours, P = 0.04). This pattern was also noticed 24 hours before the time course study, when the cells were maintained in phenol-free Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (−24 hours) immediately before serum-deprivation for 24 hours (0 hours) and E2 treatment (6 to 48 hours). Administration of 1 nmol/L or 10 nmol/L E2 did not modulate PR expression levels (data not shown). Significant differences among groups began to appear only after 12 hours of treatment (P = 0.05), and increased after 24 hours (P = 0.047). Interestingly, up-regulation of PR in the endometriosis group was noticed only 48 hours after E2 treatment, at which time it reached the same levels displayed by the control cells (P = 0.94). This delayed PR gene transcription could suggest a resistance mechanism in endometrial cells from women with endometriosis (Figure 2). Analysis of cells carrying the PROGINS polymorphism and consideration of the individual expression levels of PR subunits during the 24 hours treatment (Figure 3) revealed higher PRB mRNA expression levels in the control group (0.82 ± 0.410) compared with the endometriosis E-Alu (0.31 ± 0.047) and E+Alu (0.16 ± 0.049) groups. The mean ratio of PRB to PRAB (PRA+PRB) mRNA expression was decreased in the endometriosis group consisting of PROGINS carriers (0.31 ± 0.070) as compared with the endometriosis group of non-carriers (0.53 ± 0.158) and the controls (1.32 ± 0.483). However, the mean ratio was also significantly lower when the E+Alu and CP-Alu groups were compared (P = 0.048), whereas the CP-Alu versus E-Alu (P = 0.12) and E-Alu versus E+Alu (P = 0.24) comparisons were not significant (Figure 3).
Figure 2.
Quantitative real-time PCR analysis for progesterone receptor (PR) was performed from samples of eutopic endometrium with cDNA from controls and endometriosis without the PROGINS allele. Time course induction of PR by 100 nmol/L 17β-estradiol at 6 hours, 12 hours, 24 hours, and 48 hours of exposure was applied to determine optimum concentration of progesterone used as treatment for cytometric analyses. PR was also detected in basal conditions (Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum and serum-free medium) before estradiol exposure (indicated as −24 hours and 0). Control (n = 5) vs. endometriosis (n = 6). Differences among studied groups appeared after 12 hours (*P = 0.05), and reached highest significance at 24 hours (**P = 0.047). Expression levels were normalized by GAPDH and compared with baseline. The 100 nmol/L E2 treatment for 24 hours followed by the same regimen with progesterone was the chosen procedure for all cytometric studies.
Figure 3.
Analysis of the PRB/PRAB balance at transcriptional levels by quantitative real-time PCR using total RNA from samples of endometrial cells from CP-Alu (n = 5), E-Alu (n = 6), and E+Alu (n = 6) groups, after treatment with 100 nmol/L 17β-estradiol for 24 hours. *P = 0.0485 (CP-Alu versus E+Alu). GAPDH was used to normalize the values. Experiments were performed in triplicate. The statistical significance was determined using Student’s t-test.
Effects of Progesterone on Cell Viability and Apoptosis
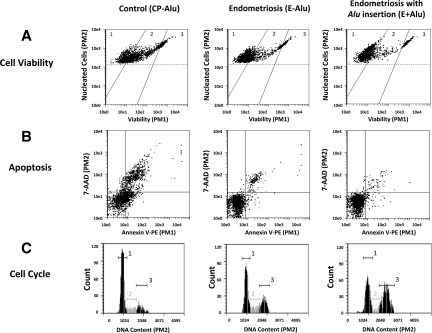
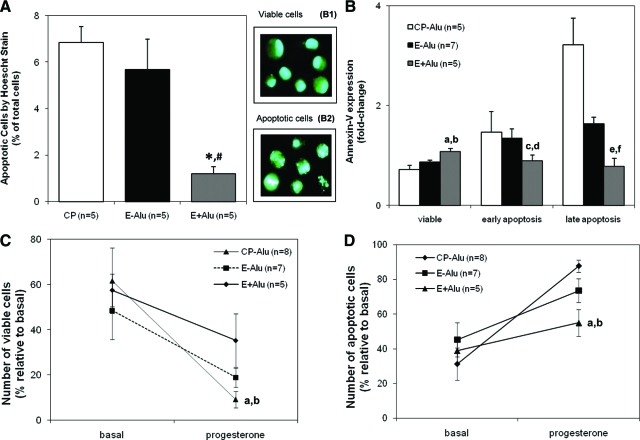
Analysis of cell culture phenotypes revealed sustained viability and decreased apoptosis in E+Alu cells compared with the CP-Alu (P < 0.05) and E-Alu (P < 0.05) groups (Figure 4, A and B). Lower percentages of apoptotic cells were observed in the E+Alu group compared with CP-Alu and E-Alu based on chromatin morphology analysis after progesterone administration (Figure 5A, CP-Alu 6.8% ± 0.7, E-Alu 5.7% ± 1.3, E+AluI. 2% ± 0.3, E+Alu versus CP-Alu, P = 0.001; E+Alu versus E-Alu, P = 0.006). Annexin-V labeling (Figures 4B and 5B) permitted identification of viable (CP-Alu 7.2% ± 0.9, E-Alu 8.7% ± 0.3, E+Alu 10.8% ± 0.6), early apoptotic (CP-Alu 14.7% ± 4.1, E-Alu 13.5% ± 1.8, E+Alu 8.9% ± 1.2), and late apoptotic cells (CP-Alu 32.2% ± 5.3, E-Alu 16.5% ± 1.2, E+Alu 7.8% ± 1.7). The decrease in cell viability and induction of apoptosis were monitored by flow cytometry with the Viacount kit under basal conditions and after a pulse of 100 nmol/L progesterone for 24 hours (Figure 5, C and D).
Figure 4.
Flow cytometry diagrams of representative experiments with progesterone acquired and analyzed by the Guava CytoSoft (v2.0) Software. Line (A) represents the cell viability analysis representing the distinction of viable (1), apoptotic (2) and dead (3), cells dependent on the incorporation of the Viacount dyes. Line (B) shows percentages of apoptotic cells determined by Annexin-V labeling with standard quadrant gating: annexin-V negative/7-AAD negative (alive), annexin-V positive/7-AAD negative (early apoptotic), annexin-V positive/7-AAD positive (late apoptotic) and annexin-V negative/7-AAD positive (necrotic) cells. In line (C) the cell cycle analysis was applied to determine percentages of cell populations in different phases of the cell cycle, and propidium iodide was quantified from the G0/G1 (1), S (2), and G2/M (3) stages.
Figure 5.
Progesterone fails to promote death by apoptosis in endometrial cells with PROGINS. Endometrial cells were serum-deprived and treated with 100 nmol/L progesterone for 24 hours. Apoptosis was measured by flow cytometry with the annexin-V labeling kit (A) which allows the detection of viable (aE+Alu versus CP-Alu and bE+Alu versus E-Alu: aP = 0.002, bP = 0.05), early apoptotic (cE+Alu versus CP-Alu and dE+Alu versus E-Alu: cP = 0.42, dP = 0.13); and late apoptotic cells (eE+Alu versus CP-Alu and fE+Alu versus E-Alu: eP = 0.02, fP = 0.01). Apoptosis was detected morphologically with Hoescht 3342 staining (B), where and viable (B1) and apoptotic nuclei (B2) were distinguished: *E+Alu versus CP-Alu (P = 0.001), and #E+Alu versus E-Alu #(P = 0.006); and the Viacount assay (C): which monitors apoptosis (aE+Alu versus CP-Alu, and bE+Alu versus E-Alu: aP < 0.0001, bP = 0.003); and cell viability (D): (aE+Alu versus CP-Alu, and bE+Alu versus E-Alu: aP = 0.04, bP = 0.15).
Effects of Progesterone on Cell Proliferation
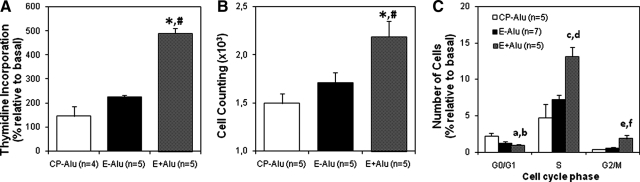
DNA synthesis analysis by [3H]-thymidine incorporation revealed that both the CP-Alu and E-Alu groups significantly differed from the E+Alu group (Figure 6A; CP-Alu 146 ± 40%, E-Alu 224 ± 9%, E+Alu 490 ± 21%, CP-Alu versus E-Alu, P = 0.27; CP-Alu versus E+Alu, P = 0.002), suggesting that the presence of PROGINS can increase the proliferation of cells treated with 17β-estradiol and progesterone. This data were confirmed by cell proliferation analysis, in which the number of cells relative to the corresponding number under basal conditions was counted (Figure 6B; CP-AluI.5 ± 0.10-fold-change, E-AluI.71 ± 0.11-fold-change, E+Alu 2.19 ± 0.16-fold-change; CP-Alu versus E-Alu, P = 0.47; CP-Alu versus E+Alu, P < 0.01). Both methods demonstrated that cells carrying the PROGINS polymorphism exhibit higher proliferation rates than CP-Alu and E-Alu cells (Spearman Correlation of 0.554, P = 0.04). A cell cycle analysis (Figure 4C) also confirmed this tendency, revealing exuberant progression to S and G2/M phases in the E+Alu group compared with the CP-Alu (P = 0.001) and E-Alu (P = 0.01) groups (Figure 6C).
Figure 6.
Cell proliferation was monitored by the [3H]-thymidine incorporation assay (A), after synchronization with serum starvation, followed by consecutive treatments with 100 nmol/L 17β-estradiol and progesterone both for 24 hours.*E+Alu versus CP-Alu (P = 0.002), and #E+Alu versus E-Alu #(P = 0.0001). Similar hormonal stimuli were used to monitor cell counting (B): 6-well plates were seeded with 5 × 103 cells at day 0 and counted again after treatment. Electronic cell counts were performed in triplicate. Cell cycle analysis (C) showed progression of E+Alu (in relation to CP-Alu and E-Alu) throughout the cell cycle into S and G2/M phases. *E+Alu versus CP-Alu (P = 0.01), and #E+Alu versus E-Alu (P = 0.04). G0/G1 (aP = 0.07, bP = 0.15), S (cP = 0.001, dP = 0.01), and G2/M (eP = 0.05, fP = 0.14).
Discussion
The etiology of endometriosis has been exhaustively studied over recent decades. Several studies have focused at the endometrial cellular and molecular levels to understand the pathogenesis of ectopic endometriosis.47 Some of the proposed mechanisms point to progesterone as the main effecter in the process. An increasing number of studies describe the differential PR gene expression and signaling that occur in patients with endometriosis, suggesting that progesterone resistance in the endometrium48 may cause this differential response, purported to be one of the mechanisms responsible for the development of endometriosis.
Progesterone signaling depends on interactions between the hormone and its receptors.49 It was recently shown that progesterone signaling is blocked in cells carrying the PROGINS allele and the V660L mutation on exon 4 of the PR gene. Indeed, comparisons with rodent ovarian (CHO) cells carrying common polymorphic variants for PR gene demonstrated that cells with the PROGINS allele display diminished PR mRNA stability, altered post-translational modifications (such as phosphorylation), and modified protein degradation patterns, which may account for the disrupted response to progesterone.33
The present study demonstrates that there are significant alterations in the cellular phenotype of women with endometriosis carrying the PROGINS polymorphism using an in vitro model consisting of endometrial cell cultures exposed to progesterone. It suggests that the PROGINS polymorphism promotes changes in the cellular phenotype that lead to disrupted cell cycle control in the endometrium, reinforcing the hypothesis that endometrial resistance to progesterone could have deleterious effects and contribute to the genesis of endometriosis.50
Analysis of the cell culture phenotype of eutopic endometrium from women diagnosed with endometriosis allowed us to perform a comprehensive study of cell proliferation, viability, and apoptosis. This approach provided more reliable results, illustrating that the in vitro model is appropriate for such a comprehensive study. Because the cells were not manipulated to establish pure cultures of stromal or epithelial cells, possible sources of stress that could trigger cell differentiation, signaling mechanisms, or other kinds of perturbations were avoided.
One limitation of the study is the absence of any homozygous PROGINS samples, mainly because of their low frequency in our population.29 Another issue is the absence of disease-free women with a single PROGINS allele. Such a group would be helpful in proving the relationship between PROGINS and the cell cycle deregulation observed in endometriosis.
Progesterone signaling is mediated by two functionally distinct receptor isoforms (PRA and PRB) that are expressed from a single gene at which transcription can occur from two alternative promoters.51 Some studies have revealed that the mRNA ratio of PRA to PRB is critical for the cellular response to progesterone. In addition, the relative expression of PRA and PRB is species-dependent and differs in each cellular context.52,53,54,55 In human gynecological cancers, especially ovarian cancers, the dominant expression of PRB mRNA is a marker for a malignant phenotype.56 With respect to endometriosis, Misao et al57 showed that PRB mRNA is highly expressed in endometrioma. A recent study from Wu et al58 demonstrated how knockdown of PRB can promote cell proliferation in immortalized endometrial stromal cells. In the present study, we observed that endometrial cells carrying the PROGINS polymorphism exhibit a decreased expression ratio of PRB to PRAB mRNA when compared with E-Alu and CP-Alu samples. This evidence suggests that PRA and PRB mRNA expression in endometriosis might be altered in the presence of PROGINS, which could lead to a disruption in the control of progesterone effects and responses to sex steroid-mediated growth regulation.
Progesterone is described as an inhibitor of endometrial cell proliferation.59 In cultured endometrial cells from women with endometriosis carrying the PROGINS allele, a significant increase in cell proliferation after treatment with progesterone was observed compared with controls and cells from non-carrier women with endometriosis. This finding could indicate an impaired ability of progesterone to inhibit cell proliferation, an expected effect of this hormone.60
Cell proliferation is, in fact, a balance between cells that progress through the cell cycle and those that undergo cell death. For this reason, we also evaluated the effect of PROGINS on cell apoptosis and cell cycle progression. Interestingly, apoptosis is less pronounced in the eutopic and ectopic endometrium of patients with endometriosis.61 Our results show impaired induction of cell death by apoptosis after progesterone stimulation of cells from the endometriosis group with PROGINS, in contrast to the non-carriers and controls. An analogous pattern was observed for cell viability, as evidenced by endometriosis cells presenting sustained viability after progesterone stimulation; especially those with PROGINS. This information clearly shows that progesterone was unable to induce death by apoptosis in cells carrying the PROGINS allele. Additionally, the disparity between both endometriosis groups may explain why some women with endometriosis respond differently than others to progesterone-based therapies and why such therapies fail to treat the disease. Nonetheless, studies examining larger numbers of samples from different stages will be necessary to test the role of this polymorphism in the progression of the disease.
Our results show that the presence of PROGINS significantly influenced the behavior of cultured endometrial cells. Our results suggest that this polymorphism may be involved in the pathogenesis of the disease, and that further studies are necessary to clarify the role of PROGINS in different aspects of endometriosis.
The identification of genetic markers related to the genesis of endometriosis is critical for developing preventive strategies. Considering our results, further in vivo and epidemiological studies are required to better understand the relevance of PROGINS to the cellular response to progesterone, and its role in the development of endometriosis. Such information would be vital to developing novel therapeutic approaches or to using traditional therapies in a more patient-specific manner.
Acknowledgments
We thank the physicians from the Pelvic Pain and Endometriosis Unit, Gynecology Department (UNIFESP-EPM) for providing endometrial tissue and detailed clinical information about the patients. We are grateful to Mr. Marcelo Anéas (Product Specialist from G&E Healthcare Bio-Sciences, Brazilian Branch) for his assistance with Flow Cytometry, and to Dr. Camila S.C. Guindalini for her advice regarding Statistics. We are also thankful to Mr. Ivan H. Cordeiro for precious technical help at the Cell Culture Facility (Biophysics Dept., UNIFESP-EPM). We were impressed with his enthusiasm and dedication to cell culture. We also thank Ms. Rita C.S. Figueria, MSc., and Professor Mari C. Sogayar (Chemistry Institute, University of São Paulo, IQ-USP) for their valuable help with quantitative PCR analysis of PRB.
Footnotes
Address reprint requests to Paulo D’Amora, Ph.D., Molecular Gynecology and Proteomics Laboratory, and Pelvic Pain and Endometriosis Unit, Gynecology Department, Universidade Federal de São Paulo - Escola Paulista de Medicina (UNIFESP-EPM). Rua Pedro de Toledo, 781, 4° andar/frente-CEP 04039-032, São Paulo, Brasil. E-mail: paulo.toco@epm.br.
Supported by grants (03/04533-1 and 04/09810-6) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Dr. Paulo D’Amora received doctoral fellowship from Coordenação e Aperfeiçoamento de Pessoal em Nível Superior (CAPES).
Some preliminary results of this paper were presented at the 10th World Congress on Endometriosis, held in Melbourne, Australia (in March 2008), and it was honored with the “Best Scientific Poster Award” by the World Endometriosis Society (WES) and the Australian Gynaecological Endoscopy Society (AGES).
References
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–257. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Murakami T, Terada Y, Yaegashi N, Okamura K, Kuriyama S, Tsuji I. Management of the pain associated with endometriosis: an update of the painful problems. Tohoku J Exp Med. 2006;210:175–188. doi: 10.1620/tjem.210.175. [DOI] [PubMed] [Google Scholar]
- Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–1572. doi: 10.1016/j.fertnstert.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of the endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Liu DT, Hitchcock A. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br J Obstet Gynaecol. 1986;93:859–862. doi: 10.1111/j.1471-0528.1986.tb07995.x. [DOI] [PubMed] [Google Scholar]
- Kennedy S. The genetics of endometriosis. Eur J Obstet Gynecol Reprod Biol. 1999;82:129–133. doi: 10.1016/s0301-2115(98)00213-9. [DOI] [PubMed] [Google Scholar]
- Schor E, Silva IDCG, Sato H, Baracat EC, Girão MJBC, Freitas V. P27Kip1 is down-regulated in the endometrium of women with endometriosis. Fertil Steril. 2008;90:2086–2090. doi: 10.1016/j.fertnstert.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10:29–38. doi: 10.1093/humupd/dmh007. [DOI] [PubMed] [Google Scholar]
- Collete T, Maheux R, Mailloux J, Akoum A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum Reprod. 2006;21:3059–3067. doi: 10.1093/humrep/del297. [DOI] [PubMed] [Google Scholar]
- Collete T, Bellehumecr C, Kats R, Maheux R, Mailloux J, Villeneuve M, Akoum A. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum Reprod. 2004;19:1257–1964. doi: 10.1093/humrep/deh290. [DOI] [PubMed] [Google Scholar]
- Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril. 2001;75:152–159. doi: 10.1016/s0015-0282(00)01670-8. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Fushiki S, Honjo H. Detection of aromatase cytochrome P-450 in endometrial biopsy specimens as a diagnostic test for endometriosis. Fertil Steril. 1999;72:1100–1106. doi: 10.1016/s0015-0282(99)00424-0. [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE. Deficient 17ß-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17ß-estradiol. J Clin Endocrinol Metab. 1999;83:4474–4480. doi: 10.1210/jcem.83.12.5301. [DOI] [PubMed] [Google Scholar]
- Fang Z, Yang S, Lydon JP, DeMayo F, Tamura M, Gurates B, Bulun SE. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil Steril. 2004;82:673–678. doi: 10.1016/j.fertnstert.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117–126. doi: 10.1016/s0020-7292(01)00577-x. [DOI] [PubMed] [Google Scholar]
- Vinatier D, Cosson M, Dufour P. Is endometriosis an endometrial disease? Eur J Obstet Gynecol Reprod Biol. 2000;91:113–125. doi: 10.1016/s0301-2115(99)00263-8. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Wang C, Mavrogianis PA, Fazleabas AT. Endometriosis is associated with progesterone resistance in the baboon (Papio anubis) oviduct: evidence based on the localization of oviductal glycoprotein 1 (OVGP1). Biol Reprod. 2009;80:272–278. doi: 10.1095/biolreprod.108.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempfer CB, Simoni M, Destenaves B, Fauser BCJM. Functional genetic polymorphisms and female reproductive disorders: part II – endometriosis. Hum Reprod Update. 2009;15:97–118. doi: 10.1093/humupd/dmn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Coughlan SJ, McKenna NJ, Garrett E, Kieback DG, Carney DN, Headon DR. Ovarian carcinoma-associated TaqI restriction fragment length polymorphism in intron G of the progesterone receptor gene is due to an Alu sequence insertion. Cancer Res. 1995;55:2743–2745. [PubMed] [Google Scholar]
- Junqueira MG, da Silva ID, Nogueira-de-Souza NC, Carvalho CV, Leite DB, Gomes MT, Baracat EC, Lopes LA, Nicolau SM, Goncalves WJ. Progesterone receptor (PROGINS) polymorphism and the risk of endometrial cancer development. Int J Gynecol Cancer. 2007;17:229–232. doi: 10.1111/j.1525-1438.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Pijnenborg JM, Romano A, Dam-de Veen GC, Dunselman GA, Fischer DC, Groothuis PG, Kieback DG. Aberrations in the progesterone receptor gene and the risk of recurrent endometrial carcinoma. J Pathol. 2005;205:597–605. doi: 10.1002/path.1738. [DOI] [PubMed] [Google Scholar]
- Leite DB, Junqueira MG, de Carvalho CV, Massad-Costa AM, Gonçalves WJ, Nicolau SM, Lopes LA, Baracat EC, da Silva ID. Progesterone receptor (PROGINS) polymorphism and the risk of ovarian cancer. Steroids. 2008;73:676–680. doi: 10.1016/j.steroids.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Romano A, Lindsey PJ, Fischer DC, Delvoux B, Paulussen AD, Janssen RG, Kieback DG. Two functionally relevant polymorphisms in the human progesterone receptor gene (C331G/A and progins) and the predisposition for breast and/or ovarian cancer. Gynecol Oncol. 2006;101:287–295. doi: 10.1016/j.ygyno.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Schweikert A, Rau T, Berkholz A, Allera A, Daufeldt S, Wildt L. Association of progesterone receptor polymorphism with recurrent abortions. Eur J Obstet Gynecol Reprod Biol. 2004;113:67–72. doi: 10.1016/j.ejogrb.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Gomes MT, Castro Rde A, Villanova FE, da Silva ID, Baracat EC, de Lima GR, Girao MJ. The progesterone receptor gene polymorphism. PROGINS, may be a factor related to the development of uterine fibroids. Fertil Steril. 2007;87:1116–1121. doi: 10.1016/j.fertnstert.2006.08.099. [DOI] [PubMed] [Google Scholar]
- De Carvalho CV, Nogueira-De-Souza NC, Massad-Costa AM, Baracat EC, Girão MJBC, D'Amora P, Schor E, Silva IDCG. Genetic polymorphisms of cytochrome P450c17a (CYP17) and progesterone receptor genes (PROGINS) in the assessment of endometriosis risk. Gynecol Endocrinol. 2007;23:29–33. doi: 10.1080/09513590601024707. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Somigliana E, Viganò P, Candiani M, Pardi G, Di Blasio AM. Genetics of endometriosis: a role for the progesterone receptor gene polymorphism PROGINS? Clin Endocrinol (Oxf) 2004;61:190–194. doi: 10.1111/j.1365-2265.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- Wieser F, Schneeberger C, Tong D, Tempfer C, Huber JC, Wenzl R. PROGINS receptor gene polymorphism is associated with endometriosis. Fertil Steril. 2002;77:309–312. doi: 10.1016/s0015-0282(01)02984-3. [DOI] [PubMed] [Google Scholar]
- van Kaam KJ, Romano A, Schouten JP, Dunselman GA, Groothuis PG. Progesterone receptor polymorphism +331G/A is associated with a decreased risk of deep infiltrating endometriosis. Hum Reprod. 2007;22:129–135. doi: 10.1093/humrep/del325. [DOI] [PubMed] [Google Scholar]
- Romano A, Delvoux B, Fischer D-M, Groothuis P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol. 2007;38:331–350. doi: 10.1677/jme.1.02170. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Zimmer RL, Griffin WT, Penney LL. Polypeptides synthesized and released by human endometriosis differ from those of the uterine endometrium in cell and tissue explant culture. Fertil Steril. 1993;60:839–851. doi: 10.1016/s0015-0282(16)56285-2. [DOI] [PubMed] [Google Scholar]
- Piva M, Horowitz GM, Shape-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrial-peritoneal interaction in endometriosis. J Clin Endocrinol Metab. 2001;86:2553–2561. doi: 10.1210/jcem.86.6.7613. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Zimmer RL, Khan RS, Penney LL. Proliferative and morphogenic changes induced by the coculture of rat uterine and peritoneal cells: a cell culture model for endometriosis. Fertil Steril. 1992;58:1220–1229. doi: 10.1016/s0015-0282(16)55573-3. [DOI] [PubMed] [Google Scholar]
- lng NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56:1205–1215. doi: 10.1095/biolreprod56.5.1205. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mazella J, Kloosterboer HJ, Tseng L. Effects of tibolone on nuclear receptors in human endometrial cells. Am J Obstet Gynecol. 2006;195:97–102. doi: 10.1016/j.ajog.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Maciel TT, Melo RS, Schor N, Campos AH. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2008;44:370–379. doi: 10.1016/j.yjmcc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RP, Wheeler P, MacNeil S, Haycock JW. α-Melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells. Peptides. 2005;26:1150–1158. doi: 10.1016/j.peptides.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Voisin T, Firar AE, Avondo V, Laburthe M. Orexin-induced apoptosis: the key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology. 2006;147:4977–4984. doi: 10.1210/en.2006-0201. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Yamada T, Horiuchi M, Gibbons GH. Vasoactive substances regulate vascular smooth muscle cell apoptosis: countervailing influences of nitric oxide and angiotensin II. Circ Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GAMS, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Nat Acad Sci (USA) 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14:137–150. doi: 10.1177/1933719106298409. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng Y-H, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Kim JJ. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Verbost P, Groothuis P. Oestrogen and progestin responses in human endometrium. J Steroid Biochem Mol Biol. 2003;84:393–410. doi: 10.1016/s0960-0760(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Schrader WT, O'Malley BW. Progesterone-binding components of chick oviduct: characterization of purified subunits. J Biol Chem. 1972;247:51–59. [PubMed] [Google Scholar]
- Feil PD, Clarke CL, Shine J, Sutherland RL. Progestin-mediated changes in progesterone receptor forms in the normal human endometrium. Endocrinology. 1988;123:2506–2513. doi: 10.1210/endo-123-5-2506. [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Logeat F, Vu Hai MT, Milgrom E. The rabbit progesterone receptor: evidence for a single steroid-binding subunit and characterization of receptor mRNA. J Biol Chem. 1984;259:14196–14202. [PubMed] [Google Scholar]
- Schneider W, Ramachandran C, Satyaswaroop PG, Shyamala G. Murine progesterone receptor exists predominantly as the 83-kilodalton A form. J Steroid Biochem Mol Biol. 1991;38:285–291. doi: 10.1016/0960-0760(91)90099-q. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Hori M, Nishigaki M, Tamaya T. Expression of progesterone receptor form A and B mRNAs in gynecologic malignant tumors. Tumor Biol. 1995;16:254–260. doi: 10.1159/000217942. [DOI] [PubMed] [Google Scholar]
- Misao R, Iwagaki S, Fujimoto J, Sun W, Tamaya T. Dominant expression of progesterone receptor form B mRNA in ovarian endometriosis. Horm Res. 1999;52:30–34. doi: 10.1159/000023429. [DOI] [PubMed] [Google Scholar]
- Wu Y, Shi X, Guo SW. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil Steril. 2008;90:1320–1323. doi: 10.1016/j.fertnstert.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Béliard A, Noël A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82:80–85. doi: 10.1016/j.fertnstert.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Chrobak A, Sieradzka U, Sozanski R, Chelmoríska-Soyta A, Gabryś M, Jerzak M: Ectopic and eutopic stromal endometriotic cells have a damaged ceramide signaling pathway to apoptosis. Fertil Steril 2008. doi: 10.1016/j.fertnstert.2008.09.035 [DOI] [PubMed] [Google Scholar]