Abstract
Endometriosis is associated with chronic inflammation, and reactive oxygen species (ROS) are proinflammatory mediators that modulate cell proliferation. We have investigated whether the dysregulation of ROS production in endometriotic cells correlates with a pro-proliferative phenotype and can explain the spreading of this disease. Stromal and epithelial cells were purified from ovarian endometrioma and eutopic endometrium from 14 patients with endometriosis to produce four primary cell lines from each patient. ROS production, detoxification pathways, cell proliferation, and mitogen-activated protein kinase pathway activation were studied and compared with epithelial and stromal cell lines from 14 patients without endometriosis. Modulation of the proliferation of endometriosis by N-acetyl-cysteine, danazol, and mifepristone was tested in vitro and in 28 nude mice implanted with endometriotic tissue of human origin. Endometriotic cells displayed higher endogenous oxidative stress with an increase in ROS production, alterations in ROS detoxification pathways, and a drop in catalase levels, as observed for tumor cells. This increase in endogenous ROS correlated with increased cellular proliferation and activation of ERK1/2. These phenomena were abrogated by the antioxidant molecule N-acetyl-cysteine both in vitro and in a mouse model of endometriosis. Human endometriotic cells display activated pERK, enhanced ROS production, and proliferative capability. Our murine model shows that antioxidant molecules could be used as safe and efficient treatments for endometriosis.
Endometriosis is a chronic disease characterized by the presence of endometrial tissue outside of the uterine cavity.1 It affects about 10% of women in their reproductive years and causes pelvic pain and infertility.1 The pathophysiology of endometriosis remains poorly understood. The main theory on the pathogenesis of endometriosis, proposed in the 1920s, is the retrograde menstruation of endometrial tissue through fallopian tubes into the peritoneal cavity.1 The development of the disease in the pelvis is attributed to the attachment and the survival of endometrial cells in the peritoneal cavity, progressive invasion of the peritoneum, with neoangiogenesis leading to the spreading of the disease.1,2 Several mechanisms such as metaplasia of the coelomic epithelium, environmental factors, genetic predisposition, immunological abnormalities, and chronic inflammation have been proposed to explain the pelvic development of endometriosis.1,3 Indeed, proinflammatory cytokines and growth factors were found to be elevated in the peritoneal fluid of women with endometriosis and could contribute to the proliferation of endometriotic implants and neoangiogenesis.2,4,5 Chronic inflammation has also been associated with increased oxidative stress.6 In serum and peritoneal fluid of patients with endometriosis, markers of oxidative stress have been found elevated.7,8,9,10 A new role for reactive oxygen species (ROS) as second messenger of cellular proliferation was described. McCubrey et al found that normal cell proliferation correlated with production of endogenous ROS through the activation of growth-related signaling pathways, including the mitogen-activated protein kinase ERK1/2 (extracellular regulated kinase).11 In cancer, modulation of ROS production control tumor cell proliferation,12 and mutations in mitochondrial DNA resulting in respiratory complex I deficiency have been found to increase endogenous ROS production and enhance metastatic potential of tumor cells.13 Endometriosis is considered a benign disease but shares some features with cancer, such as propensity to invasion, unrestrained growth, neoangiogenesis, and distant spreading.14 The known correlation between ROS and proliferation of cancer cells, along with the increased production of ROS in response to chronic inflammation in endometriosis, thus suggests a possible role for ROS in the regulation of endometriotic cell proliferation. We thus hypothesized that the development of endometriosis is not solely due to continuous addition of endometrial tissue through menstrual regurgitation in the peritoneum but rather is due to the acquisition of a pro-proliferative phenotype by ectopic endometrial cells through a dysregulation of endogenous ROS production.
Materials and Methods
Source of Endometrial and Endometriotic Cells
Endometrium and ovarian endometrioma specimens were obtained from 14 patients with endometriosis undergoing surgical treatment. Control endometrial specimens were obtained from 14 patients without macroscopic endometriosis undergoing laparoscopy for other reasons (tubal infertility, non-endometriotic ovarian cyst, myoma). Written informed consent was obtained from each patient.
Purification of Endometrial and Endometriotic Cells
For each of those 14 patients, a biopsy of eutopic endometrium and of the ovarian endometrioma was performed. Epithelial cell lines and stromal cell lines were derived from biopsies of both eutopic endometrium (endometrial cells) and of ovarian endometrioma (endometriotic cells) from those 14 patients. Fourteen other patients without endometriosis were included in the study as a control group. For each control patient, a biopsy of eutopic endometrium was obtained. For each specimen, epithelial and stromal cells were extracted. Thus, 14 primary epithelial and 14 stromal control cell lines were extracted from biopsies of eutopic endometrium from patients without endometriosis.
All of the patients were suffering from chronic inflammation or chronic pelvic pain. C-reactive protein levels, as assessed by the ultrasensitive immunonephelometric method, were within reference ranges for each patient and did not differ from the control group, demonstrating that no patients had acute inflammation at the time of sampling. No differences were observed between the two groups of patients in terms of age, treatment received before surgery, or phase of the menstrual cycle. Patients’ characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Patients with endometriosis (n = 14) | Control (n = 14) | |
|---|---|---|
| Age, mean ± SEM | 31.9 ± 1.22 | 32 ± 1.09 |
| Cycle n (%) | ||
| Anovulation | 9 (64.3) | 4 (28.6) |
| Proliferative phase | 4 (28.6) | 4 (28.6) |
| Secretory phase | 1 (7.1) | 6 (42.8) |
| Treatment before surgery n (%) | ||
| None | 5 (35.7) | 10 (64.3) |
| Estroprogestatives | 2 (14.3) | 2 (14.3) |
| Progestatives | 3 (21.45) | 2 (14.3) |
| Danazol | 1 (7.1) | |
| LHRH agonists | 3 (21.45) | |
| AFS score, mean ± SD | 3 ± 1 | |
| C-reactive protein, mean ± SEM | 2.44 ± 1.01 | 1.52 ± 0.42 |
Cell Isolation and Culture
Primary endometrial and endometrioma cell cultures were prepared from biopsies as described.15 Biopsy specimens were rinsed, minced into small pieces, digested with dispase and collagenase (2 mg/ml, Gibco Invitrogen, Cergy Pontoise, France) for 1 hour at 37°C and separated using serial filtration. Red blood cells were removed by hypotonic lysis (NH4Cl, 0.15 mol/L; KHCO3, 1 mmol/L; Na2 EDTA, 0.1 mmol/L). Debris was removed by 100-μm aperture sieves. Epithelial cells were retained on 40-μm aperture sieves while stromal cells remain in the filtrate. Both cells were plated onto Primaria flasks (Becton Dickinson Labware, Le Pont de Claix, France) and cultured in Dulbecco’s modified Eagle’s medium (Gibco Invitrogen, Cergy Pontoise, France) with 10% fetal calf serum. Purification of stromal and epithelial cells was confirmed by staining with a 1:100 dilution of fluorescein isothiocyanate-labeled anti-cytokeratin and Cy3-labeled anti-vimentin antibodies (Sigma Aldrich, Saint Louis, MO). Fluorescence was analyzed on an Olympus fluorescent microscope (Hamburg, Germany) and images were captured using the CellM Imaging station (Olympus). Both populations were negative for CD3 (T cell), CD45 (leukocyte), and CD11b (monocyte and granulocyte) antibody staining.15
Cellular Production of Reactive Oxygen Species
Cells (104 per well) were seeded in 96-well plates and incubated for 18 hours in triplicate with 6.4 mmol/L N-acetyl-l-cysteine (NAC), 8 mmol/L diethyldithiocarbamate, 40 μmol/L diphenyleneiodonium, 40 μmol/L allopurinol, or culture medium alone. Separately, cells (104 per well) were distributed in triplicates into 96-well plates and, after 18 hours, treated with either 10 μmol/L rotenone or 10 μmol/L antimycin for 30 minutes (all products from Sigma-Aldrich, Saint Louis, MO). Cells were then washed three times in phosphate-buffered saline (PBS) and incubated with 100 μl per well of 5 μmol/L dihydroethidium in PBS for superoxide anion (O⨪2) measurement, or with 100 μl per well of 5 μmol/L 2′,7′-dichlorodihydrofluorescein diacetate in PBS for hydrogen peroxide (H2O2) assay or with 100 μl per well of 5 μmol/L monochlorobimane in PBS for glutathione (GSH) determination. Cellular levels of O⨪2, H2O2 and GSH were assessed by spectrofluorimetry using a Fusion spectrofluorimeter (Packard Bell, Paris, France). Fluorescence intensity was recorded every hour for 5 hours. Fluorescence excitation/emission maxima were for dihydroethidium, 480/610 nm; for 2′,7′-dichlorodihydrofluorescein diacetate, 507/525 nm; and for monochlorobimane, 380/485 nm.16 Fluorescent probes were purchased from Molecular Probes (Eugene, OR). The levels of O⨪2, H2O2, and GSH were calculated in each sample as follows: reactive oxygen species rate (arbitrary units/min/106 cells) = (fluorescence intensity [arbitrary units] at T 300 minutes − fluorescence intensity [arbitrary units] at T 0/300 minutes/number of adherent cells determined by the crystal violet assay as a measure for membrane integrity. In brief, cells were stained with 0.5% crystal violet and 30% ethanol in PBS for 30 minutes at room temperature. After two washes in PBS, the stained cells were resuspended in 50% methanol, and absorbance was measured at 560 nm on an enzyme-linked immunosorbent assay multiwell reader. Production and metabolism of O⨪2, H2O2, and GSH were explored in all of the cell lines extracted from the 14 controls and from the 14 endometriotic patients.
Determination of Enzymatic Activities
The superoxide dismutase (SOD) activities of cells were evaluated by the nitroblue tetrazolium reduction technique according to Beauchamp and Fridovich.17 The catalase activities of cells were determined by ultraviolet spectroscopy at 240 nm according to Aebi.18 SOD and catalase measurements were reported to the amount of proteins in each sample (bovine serum albumin microbiuret assay, Pierce, Bezons, France). The enzymatic activities were explored in all of the cell lines extracted from the 14 controls and from the 14 endometriotic patients.
Modulation of in Vitro H2O2 Production by Drugs Used for Endometriosis Treatment
Cellular production of H2O2 was assessed, as previously described, after treating cells in 96-well plates for 18 hours with various amounts of the following treatments: RU-486 (1 μmol/L, 10 μmol/L, and 100 μmol/L) and danazol (2.5 μmol/L, 25 μmol/L, and 250 μmol/L) (Sigma Aldrich, Saint Louis, MO). The modulation of in vitro H2O2 production by drugs used in the treatment of endometriosis was explored in all of the cell lines extracted from the 14 controls and from the 14 endometriotic patients.
In Vitro Cell Proliferation and Viability Assays
Cells (104 per well) were seeded in 96-well plates and incubated for 48 hours with various amounts of antioxidant and pro-oxidant enzymes or drugs as indicated previously. Cell proliferation was determined by pulsing the cells with [3H]thymidine (1 μCi per well, Amersham, GE HealthCare) during the last 16 to18 hours of culture and measuring the radioactivity incorporated by liquid scintillation counting.16 Cell apoptosis was analyzed using Hoechst 33342 staining (Molecular Probes) and a Fusion spectrofluorimeter (Packard Bell). Cells were incubated in 100 μl of PBS with 1 μg/ml Hoechst 33342 for 30 minutes. Fluorescence intensity was recorded at the end of the incubation with a fluorescence excitation/emission maxima of 350/461 nm.16 Apoptotic cells were detected by the bright staining of condensed chromatin. The rate of cell proliferation and the viability of cells were determined in all of the cell lines extracted from the 14 controls and from the 14 endometriotic patients.
Immunoblotting of Cell Lysates
Cells were lysed in ice-cold RIPA buffer (10 mmol/L Tris-HCl, pH 7.5; NaCl, 150 mmol/L; 1% Triton X-100; 0.1% sodium dodecyl sulfate) supplemented with 25 mmol/L sodium fluoride, anti-protease 1%, and 0.5 mmol/L sodium orthovanadate. Equal amounts of protein (30 μg) were loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer and blocking, nitrocellulose membrane was incubated overnight at 4°C with a 1:200 dilution of a rabbit anti-human ERK 1/2 IgG and a 1:200 dilution of a rabbit anti-human pERK 1/2 IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Specific proteins were detected using a 1:1000 dilution of a horseradish peroxidase-conjugated goat anti-rabbit IgG and visualized by an enhanced chemiluminescence system (Pierce Perbio Sciences, Brebières, France).19 ERK and pERK were explored in all of the cell lines extracted from the 14 controls and from the 14 endometriotic patients.
Animal Model of Endometriosis
Twenty-eight female nude mice (Swiss nu/nu, Charles River Laboratories, L’Arbresle, France) 8 weeks of age were used in this study. Animals received humane care in compliance with institutional guidelines. Mice were anesthetized with intraperitoneal injections of 500 μl of Avertine (2,2-tribromoethanol, 0.04 mol/L; 2 methyl-2-butanol, 2.5%). An incision was made on the ventral midline and one 5-mm2 fragment of human endometrioma was sutured to the parietal peritoneum with 7/0 Proline. The cutis was sutured with a 6/0 nylon thread.20 On day 14 after implantation, mice were operated to confirm the viability of the implant. Treatments started on day 14: the first group received subcutaneous injections of RU-486 (50 mg/kg in 200 μl of sesame oil, three times a week); the second group received subcutaneous injections of danazol (25 mg/kg in 200 μl of sesame oil, three times a week)21,22; and the third group received intraperitoneal injections of NAC (60 mg/mouse, in 300 μl of PBS, three times a week) according to the protocol of Marian et al23 slightly modified. Indeed, to minimize the infectious risks in nude mice, the animals were injected with twice the amount of drug every other day instead of a single amount of drug every day. Moreover, to limit the stress of mice injected intraperitoneally with NAC, we decided not to weigh NAC-injected mice on each day of injection, but to use a medium dose of 60 mg per injection three times a week. Using this regimen, the NAC-treated mice received a cumulative dose of 720 mg. This dose is very similar to the dose that the mice treated with NAC 1000 mg/kg/day received according to the original protocol. Mice of 20 to 22 g were implanted with endometriotic biopsies at day 0. Treatment with NAC started 2 weeks later.
The control group received intraperitoneal injections of 300 μl of PBS or subcutaneous injections of 200 μl of sesame oil three times a week. After 4 weeks of treatment, animals were sacrificed by cervical dislocation. Implants were extracted for histological analysis. Slides were stained with hematoxylin-eosin. Pathology scores were derived as follows: 0 (receded lesion with stromal fibrosis, hemosiderin and absence of glandular structure) to 3 (active lesion with fresh blood, profuse stromal cellular infiltration and developed glandular organization). Scoring was performed by three different scientists unaware of the treatments received in each group.
Statistical Analysis
All of the results of in vitro studies are the means of independent triplicate experiments for each cellular population of each patient. Statistical analysis was performed with StatView 3.0 software (SAS Institute Inc., Cary, NC), according to the practice of the biostatistics department of our institution.24 Means were compared by Student’s t-test. In all figures, error bars represent the SEM. A level of P < 0.05 was accepted as significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Purification of Endometrial and Endometriotic Cells
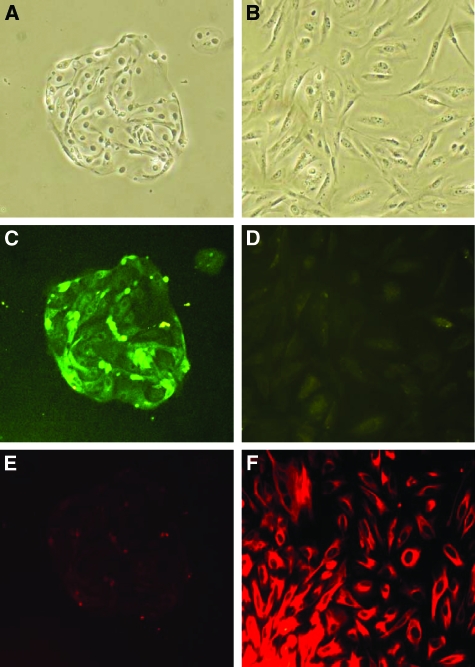
Fourteen patients with ovarian endometrioma were included in the study. For each of those patients, a biopsy of eutopic endometrium and of the ovarian endometrioma was performed. Fourteen patients without endometriosis were included in the study as a control group. For each control patient, a biopsy of eutopic endometrium was obtained. For each specimen, epithelial and stromal cells were extracted. Thus, 14 primary epithelial and stromal cell lines were extracted from biopsies of eutopic endometrium from patients without endometriosis (control cells), and from biopsies of both eutopic endometrium (endometrial cells) and ovarian endometrioma (endometriotic cells) from 14 patients with endometriosis. Examples of stromal and epithelial cell lines derived from the biopsies of patients are shown in Figure 1, A–F.
Figure 1.
Purification of stromal and epithelial cells. A: Epithelial cells in contrast phase microscopy. B: Stromal cells in contrast phase microscopy. C: Epithelial cells stained with fluorescein isothiocyanate anticytokeratin antibodies (green). D: Stromal cells stained with fluorescein isothiocyanate anticytokeratin antibodies (green). E: Epithelial cells stained with Cy3 antivimentin antibodies (red). F: Stromal cells stained with Cy3 antivimentin antibodies (red). Epithelial cells show positive staining for cytokeratin and negative staining for vimentin. Stromal cells show negative staining for cytokeratin and positive staining for vimentin. Original magnification, ×100.
Production and Metabolism of Superoxide Anions
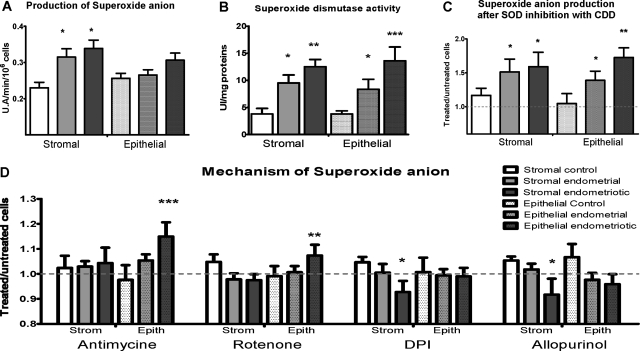
The production of O2− was increased by 39% in stromal endometriotic cells from patients (P < 0.05) and by 35% in stromal endometrial cells (P<0.05) compared with stromal control cells. Production of O2− was not significantly different between the various epithelial cell lines tested (Figure 2A). The treatment received before surgery did not affect the results gained in endometriotic cell lines.
Figure 2.
Production and detoxification pathways of superoxide anion. A: Superoxide anion production. Results are expressed as arbitrary units of fluorescence for 1 minute for 106 cells. B: Superoxide dismutase activity. Results are expressed as SOD activity units/mg of proteins. C and D: Superoxide anion production was measured after treatment with diethyldithiocarbamate (inhibitor of superoxide dismutase), antimycin (inhibitor of complex III of respiratory chain), rotenone (inhibitor of complex I of respiratory chain), diphenyleneiodonium (inhibitor of NADPH oxidase), and allopurinol (inhibitor of xanthene oxidase). Results are expressed as a ratio between treated and untreated cells. Dotted line shows the level of untreated cells. Error bars represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001 as compared with control cells.
O2− is detoxified by SOD to form H2O2. SOD activity was found to be threefold higher in stromal endometriotic cells (P < 0.01) and 2.25-fold higher in stromal endometrial cells from patients (P < 0.05) than in stromal control cells (Figure 2B). SOD activity was 3.4-fold higher in epithelial endometriotic cells (P < 0.001) and twofold higher in epithelial endometrial cells from patients (P < 0.05), than in epithelial control cells (Figure 2B). Treating the cells with diethyldithiocarbamate, a specific inhibitor of SOD activity, increased O2− production by 60% in stromal endometriotic cells (P < 0.05), 50% in stromal endometrial cells (P < 0.05), 70% in epithelial endometriotic cells (P < 0.01), and 40% in epithelial endometrial cells (P < 0.05), compared with untreated cells (Figure 2C).
Thus, the increased production of superoxide anions in endometriotic cells is associated with an increase in SOD activity as a compensatory feedback mechanism to control increased oxidative stress. Inhibiting SOD blocked this adaptive mechanism and induced an oxidative burst.
Superoxide anion is generated by either the mitochondrial respiratory chain or the cytosolic enzymes NADPH oxidase and xanthene oxidase. Several inhibitors of mitochondrial respiratory complexes and of cytosolic enzymes were used to explore the origin of superoxide anions in endometriotic and endometrial cell lines. Rotenone and antimycin blocked the mitochondrial respiratory chain complexes I and III, respectively. This blockage led to the release of electrons from the respiratory chain, which combined with molecular oxygen to produce superoxide anions. Therefore, if a disturbance of complex I or III is responsible for the production of superoxide anions, the treatment with rotenone or antimycin will increase this dysfunction and the release of superoxide anions. By contrast, if superoxide anions originated from the cytosolic enzyme NAD(P)H oxidase, their production would be abrogated by treatment with the selective inhibitor of NAD(P)H oxidase, diphenyleneiodonium. Alternatively, if superoxide anions originated from the cytosolic enzyme xanthene oxidase, their production would be decreased by treatment with the selective inhibitor of xanthene oxidase, allopurinol.25 In stromal endometriotic cells, O2− production was significantly decreased by diphenyleneiodonium (P < 0.05) and by allopurinol (P < 0.05). In epithelial endometriotic cells, O2− production was significantly increased by the inhibition of the mitochondrial respiratory chain complex I by rotenone (P < 0.01) and complex III by antimycin (P < 0.001) (Figure 2D). Thus, these data show that in stromal endometriotic cells, O2− is mostly produced by cytosolic enzymes as in normal cell lines, whereas in epithelial endometriotic cells, it is produced by mitochondrial complex I and III as in tumor cells (Figure 2D).
Production and Metabolism of Hydrogen Peroxide
Hydrogen peroxide is a byproduct of O2− detoxification by SOD. H2O2 production was 2.5-fold higher in stromal endometriotic cells than in stromal control cells (P < 0.05). In epithelial endometriotic cells, production of H2O2 was 7.75-fold higher than in epithelial control cells (P < 0.001) and 3.1-fold higher than in epithelial endometrial cells (P < 0.001) (Figure 3A). The treatment received before surgery did not affect the results gained in endometriotic cell lines.
Figure 3.
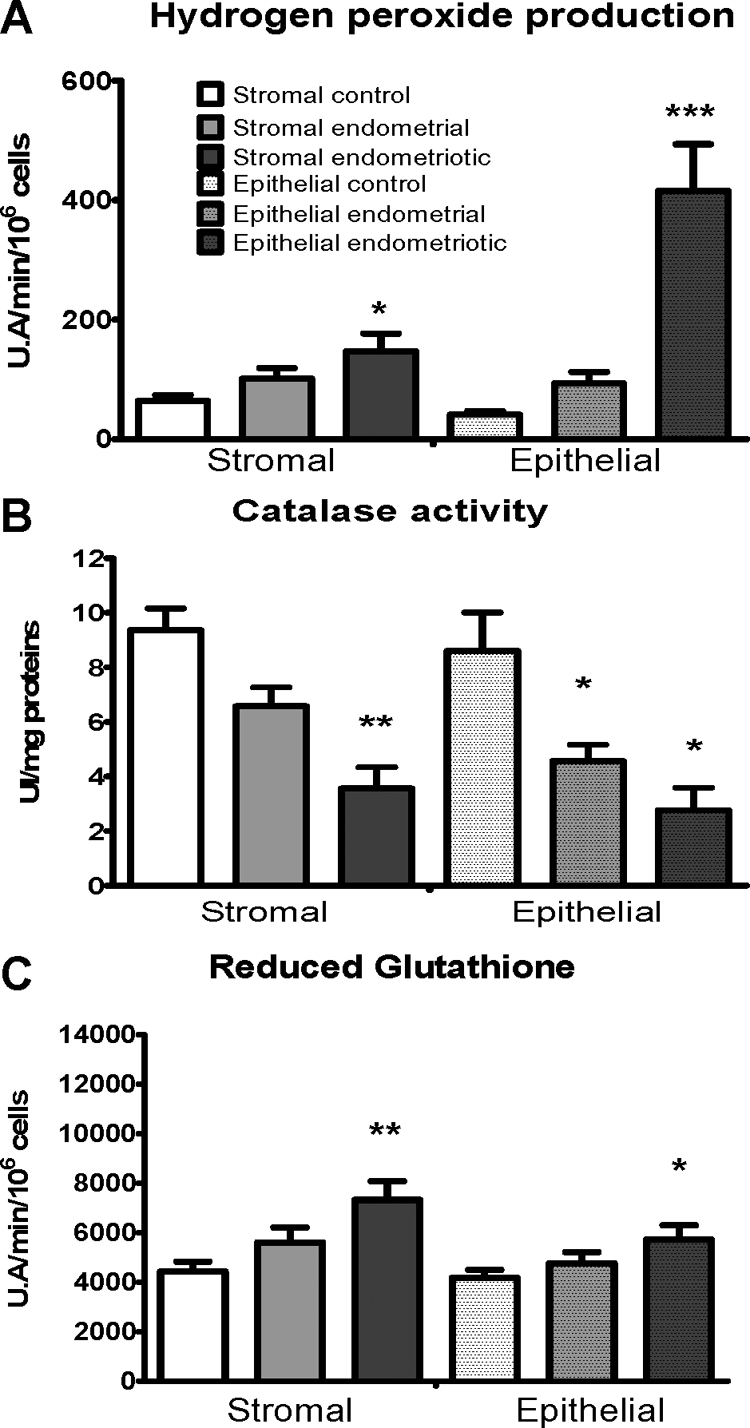
Production and detoxification pathways of hydrogen peroxide. A: Hydrogen peroxide production: results are expressed as arbitrary units of fluorescence for 1 minute for 106 cells. B: Catalase activity results are expressed as catalase activity units/mg of protein. C: Reduced glutathione results are expressed as arbitrary units of fluorescence for 1 minute for 106 cells. Error bars represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001 as compared with control cells.
The detoxification of H2O2 is achieved through two different enzymatic systems, GSH peroxidase and catalase. Glutathione peroxidase is an enzyme that uses reduced glutathione to convert H2O2 into water whereas catalase can do so directly. We found that catalase activity was 2.7-fold lower in stromal endometriotic cells than in stromal control cells (P < 0.01) and 1.8-fold lower than in stromal endometrial cells (P < 0.01) (Figure 3B). Catalase activity was threefold lower in epithelial endometriotic cells (P < 0.01) and 1.5-fold lower in epithelial endometrial cells (P < 0.05) compared with epithelial control cells. The level of GSH was 1.6-fold higher in stromal endometriotic cells than in stromal control cells (P < 0.01). In epithelial endometriotic cells, the level of GSH was 1.2-fold higher when compared with epithelial control cells (P < 0.05) (Figure 3C). These results show that the high level of H2O2 in endometriotic cells results from both an increase in its production by the conversion of superoxide anion by superoxide dismutase and also from a decrease in its detoxification by the drop in catalase activity in those cells. As a consequence of this increase in H2O2 production, GSH concentration is increased but remains insufficient to buffer the increased H2O2 concentration. Taken together, those data indicate that endometriotic cells, as with tumor cells,26,27 display a high endogenous oxidative stress associated with both an increase in ROS production and, simultaneously, a decrease in ROS detoxification.
Control of the Proliferative Properties of Endometriotic Cells
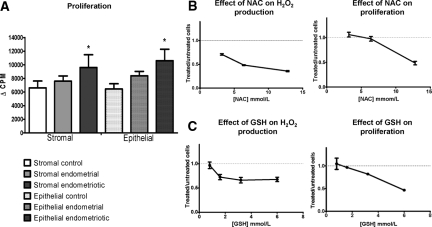
Since the production of endogenous ROS correlates with cellular proliferation, we measured the proliferative capacity of the various cell in culture using thymidine incorporation. The proliferative rate was increased by 50% in stromal endometriotic cells compared with stromal control cells (P < 0.05), and by 65% in epithelial endometriotic cells compared with epithelial control cells (P < 0.05) (Figure 4A). The treatment received before surgery did not affect the results gained in endometriotic cell lines.
Figure 4.
A: Cell proliferation was determined by thymidine incorporation; *P < 0.05, **P < 0.01, ***P < 0.001 compared with control cells. B: Hydrogen peroxide production after treating endometriotic cells with various concentrations of N-acetylcysteine. Proliferation of endometriotic cells treated with N-acetylcysteine was measured by thymidine incorporation. Results are expressed as a ratio between treated and untreated cells. Dotted line shows the level of untreated cells. Error bars represent SEM. C: Hydrogen peroxide production after treating endometriotic cells with various concentrations of reduced GSH. Proliferation of endometriotic cells treated with GSH was measured by thymidine incorporation. Results are expressed as a ratio between treated and untreated cells. Red dotted line shows the level of untreated cells. Error bars represent SEM.
To determine whether the modulation of endogenous H2O2 could control cellular proliferation, endometriotic cells were treated with increasing amounts of NAC, an antioxidant with both catalase-like and glutathione reductase-like activities. As previously described in tumor cells, NAC decreased both the H2O2 intracellular concentrations and the proliferation rate in a dose-dependent manner (Figure 4B). To confirm those findings, endometriotic cells were treated with increasing amounts of reduced GSH. As NAC did, GSH decreased both the intracellular concentrations of H2O2 and the rate of cell proliferation in a dose-dependent manner (Figure 4C).
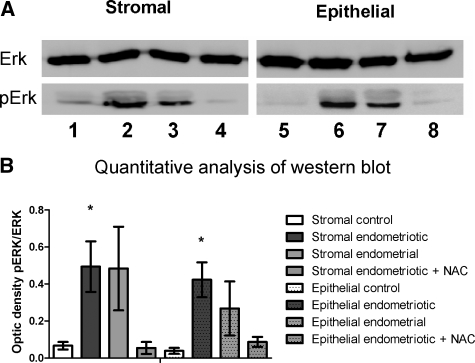
The Raf/MEK/ERK signaling pathway is involved in the proliferative response induced by an endogenous oxidative stress.11 We explored the ERK pathway by performing Western blot on all of the cell lines. The activated form of ERK, pERK, was almost undetectable in control cells (ratio pERK/ERK = 0.067 ± 0.020 in stromal cells and 0.039 ± 0.016 in epithelial cells). In endometrial cells of patients with endometriosis, pERK was increased (ratio pERK/ERK = 0.484 ± 0.226 in stromal cells and 0.268 ± 0.146 in epithelial cells, P = 0.09), while in endometriotic cells pERK was significantly increased (ratio pERK/ERK = 0.494 ± 0.137 in stromal cells and 0.423 ± 0.094 in epithelial cells, P < 0.05) (Figure 5A). Treatment of endometriotic cells with NAC (10 mmol/L) during 2 hours resulted in the normalization of pERK levels between patients and control samples (ratio pERK/ERK = 0.055 ± 0.032 in stromal cells and 0.014 ± 0.008 in epithelial cells P = 0.09) (Figure 5B). Thus, the rate of proliferation of endometriotic cells is increased through the activation of the ERK pathway as a consequence of high constitutive endogenous oxidative stress.
Figure 5.
A: Presence of ERK and pERK was determined by Western blot on lysates of stromal and epithelial control cells (lanes 1 and 5), endometriotic cells (lanes 2 and 6), endometrial cells (lanes 3 and 7), and endometriotic cells treated with NAC 10 mmol/L for 2 hours (lanes 4 and 8). B: Quantitative analysis was done by measuring the optic density ratio pERK/ERK. Those results are the mean of six independent cell lines, *P < 0.05.
Modulation of Endometriosis Development in Vitro and in Vivo
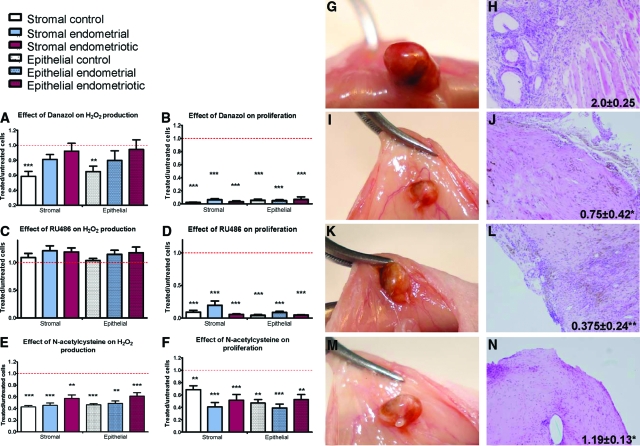
Since we demonstrated that modulation of endogenous H2O2 production can potentially control the growth of endometriosis lesions, we explored the possible use of antioxidant molecule in the treatment of endometriosis. We investigated if the molecules currently used for the medical treatment of endometriosis act as ROS modulators and compared their treatment efficacies to that of the antioxidant molecule NAC. In each cell type, we studied the effects of danazol and mifepristone (RU-486) on H2O2 production and proliferation and compared their effects to NAC. Danazol at 250 μmol/L decreased H2O2 production by 30 to 40% (P < 0.01) only in control cells (Figure 6A) and decreased the proliferation rate of all of the cell types tested by 90% (P < 0.001) (Figure 6B). RU-486 at 100 μmol/L had no effect on H2O2 production (Figure 6C) but decreased the proliferation rate of all of the cell types tested by 60 to 90% (P < 0.001) (Figure 6D). NAC 6.4 mmol/L decreased H2O2 levels by 40 to 60% (P < 0.001) (Figure 6E) and decreased the proliferation rate by 30 to 60% in all of the cell types tested (P < 0.001) (Figure 6F). RU-486 and danazol had stronger effects on cell proliferation than NAC (P < 0.01).
Figure 6.
A–F: Hydrogen peroxide production was measured after treating cells with danazol, 250 μmol/L; RU-486, 100 μmol/L; or NAC, 6.4 mmol/L. Proliferation of cells treated during 48 hours with danazol, RU-486, or NAC was measured by thymidine incorporation. Results are expressed as a ratio between treated and untreated cells. Red dotted line shows the level of untreated cells. Error bars represent SEM. G–N: Mouse model of endometriosis. Macroscopic implants and histology after extraction with pathology score of mice treated with PBS (G and H), danazol (I and J), RU-486 (K and L), and NAC (M and N). Original magnification, ×100.
To confirm our in vitro findings, we tested those treatments in a mouse model of endometriosis. Twenty-eight nude mice received endometriotic implants from ovarian endometriomas. Control mice treated with PBS showed the persistence of active lesions with angiogenic and glandular organization (score 2.0 ± 0.25) (Figure 6, G and H), whereas mice treated with danazol (Figure 6, I and J), RU-486 (Figure 6, K and L) or NAC (Figure 6, M and N) displayed fibrotic and avascular lesions (0.75 ± 0.42, P < 0.05 for danazol; 0.37 ± 0.24, P < 0.01 for RU-486; and 1.19 ± 0.13, P < 0.025 for NAC). Pathology scores were neither significantly different between RU-486 and NAC (P = 0.052), nor were they different between danazol and NAC (P = 0.13). Thus, danazol and mifepristone do not act through ROS modulation, but decreasing endogenous production can reduce the development of endometriosis.
Discussion
Performed ex vivo on stromal and epithelial primary cell lines extracted from biopsies of endometrioma and eutopic endometrium of 14 patients with endometriosis and in vivo in a murine model, this study highlights the role of ROS in the pathogenesis of endometriosis. Endometriotic cells display a high endogenous oxidative stress with a profound alteration of ROS detoxification pathways associated with increased cellular proliferation and activation of the MAP kinase ERK1/2, a phenomenon very close to what is observed in tumor cells. Therefore, our work suggests that endometriosis is a condition that results more from the development of a pseudotumoral disease than from an additive process caused by menstrual regurgitation.
In stromal endometriotic cells, O2− level was significantly increased and produced by the cytosolic NAD(P)H oxidase as already observed in normal dermal fibroblasts28,29 and prostate cancer cells.30,31 The SOD activity was also increased but not enough to overcome this endogenous oxidative stress. In epithelial endometriotic cells, O2− originated from the mitochondria respiratory chain complexes I and III as previously described in colon, ovarian, and prostate cancer cells.12 In epithelial endometriotic cells, O2− levels did not reach the levels observed in stromal endometriotic cells probably because of the significant increase in the SOD activity.
Although O2− is the first radical produced within the cell, H2O2 is the key radical involved in the control of normal and tumor cell proliferation.12 The levels of H2O2 produced in both stromal and epithelial endometriotic cells almost reach those observed in tumor cells.12 Furthermore, in tumor cells, the increase in H2O2 concentration is associated with a drop in catalase activity,32,33,34 which is also observed with endometriotic cells in our study.
ROS play a role in proliferation, in the metastatic potential of tumor cells, and in the control of tumor growth.12,13 In endometriotic cells as in tumor cells, the increased production of endogenous ROS is associated with an increase in the proliferation rate. In addition, the regulation of the oxidative stress in endometriotic cells is close to that described in tumor cells.12 Consistently, as in tumor cells, a marked drop in intracellular H2O2 concentration inhibits endometriotic cell proliferation both in vitro and in vivo.12,13
Other reactive oxygen species such as hydroxyl radical (OH°) and peroxynitrite (ONOO−) can modulate cell proliferation and apoptosis.12,21 The role of OH° and of ONOO− in the control of endometriotic cell proliferation cannot be ruled out but, since they respectively originate from the transformation of H2O2 and O2−, the role of those latter ROS remains crucial.
We also observed that the enhanced oxidative stress and the increased proliferative potential in endometriotic cells are associated with the full activation of pERK as already observed in tumor cells.12 Moreover, increased phosphorylation of ERK has recently been observed in stromal cell lines derived from patients with endometriosis.35 Our work not only extends this finding to endometriotic epithelial cells but also links ERK activation, ROS production and endometriotic cell proliferation for the first time. Compared with control cells, pERK was also overactivated in endometrial cells despite low levels of ROS. This result could be explained by ERK activation in response to interleukin-1ß and tumor necrosis factor-α,36,37 two proinflammatory cytokines increased in the endometrium from patients with endometriosis.4,5 Proinflammatory cytokines may play a role in the pathogenesis of endometriosis and in proliferation of endometriotic cells. Our study does not allow us to rule out the fact that proinflammatory cytokines can be produced by endometriotic cells themselves and act in an autocrine manner through Erk activation. However, manipulating the oxidative properties of the cells dictates their fate in terms of activation and proliferation. Indeed, inhibition of the intracellular level of ROS by antioxidant molecules abrogates ERK phosphorylation and cellular proliferation. Thus, our data suggest that endometriotic cells have a particular phenotype and represent a half-way stage between endometrial cells and tumor cells.
The antiproliferative effect of danazol and mifepristone (RU-486) are not ROS-dependent but linked to the neutralization of both estrogen and progesterone receptors by mifepristone38 and to the antiaromatase and immunomodulating activities of danazol.39 Since those drugs have some unacceptable side effects, our results highlight the possible use of ROS scavenger such as NAC as a new safe and effective treatment of endometriosis and especially in patients who cannot receive hormonal therapy.
Altogether, we show that the dysregulation of endogenous ROS in endometriotic cells shares numerous features with that observed in tumor cells. Our work demonstrates that endometriotic cells from patients with endometriosis have an altered phenotype of ROS production leading to an increase in the proliferative capabilities of the cells that favors the spreading of the disease. While ROS production is up-regulated in endometriotic cells, the origin of this dysregulation is still unclear. Further studies are needed to determine whether ROS are overproduced in response to extracellular stimuli or are the results of intracellular signaling disturbances. This work is not only important because it reveals new insights into the pathogenesis of endometriosis but also because it will allow the development of new therapeutic strategies based on the modulation of the oxidative stress with antioxidant molecules.
Acknowledgments
We thank Dr. Andrew Wang and Dr. Marie Mianowski for reviewing the manuscript.
Footnotes
Address reprint requests to Frédéric Batteux, Université Paris Descartes, Faculté de Médecine, Laboratoire d’immunologie, EA 1833, IFR Alfred Jost, 75679 Paris cedex 14, France. E-mail: frederic.batteux@cch.ap-hop-paris.fr.
Supported by a grant from Fondation pour la Recherche Médicale to C. Ngô.
C.Ché. and C.N. contributed equally to this work. C.Cha. and F.B. contributed equally to the direction of this work.
References
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Healy DL, Rogers PA, Hii L, Wingfield M. Angiogenesis: a new theory for endometriosis. Hum Reprod Update. 1998;4:736–740. doi: 10.1093/humupd/4.5.736. [DOI] [PubMed] [Google Scholar]
- Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. 1996;2:371–384. doi: 10.1093/humupd/2.5.371. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Increased secretion of hepatocyte growth factor by eutopic endometrial stromal cells in women with endometriosis. Fertil Steril. 1997;68:468–472. doi: 10.1016/s0015-0282(97)00226-4. [DOI] [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- Alexandre J, Nicco C, Chereau C, Laurent A, Weill B, Goldwasser F, Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98:236–244. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- Arumugam K, Dip YC. Endometriosis and infertility: the role of exogenous lipid peroxides in the peritoneal fluid. Fertil Steril. 1995;63:198–199. doi: 10.1016/s0015-0282(16)57320-8. [DOI] [PubMed] [Google Scholar]
- Oner-Iyidogan Y, Kocak H, Gurdol F, Korkmaz D, Buyru F. Indices of oxidative stress in eutopic and ectopic endometria of women with endometriosis. Gynecol Obstet Invest. 2004;57:214–217. doi: 10.1159/000076691. [DOI] [PubMed] [Google Scholar]
- Shanti A, Santanam N, Morales AJ, Parthasarathy S, Murphy AA. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil Steril. 1999;71:1115–1118. doi: 10.1016/s0015-0282(99)00145-4. [DOI] [PubMed] [Google Scholar]
- Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79:1288–1293. doi: 10.1016/s0015-0282(03)00266-8. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Swiersz LM. Role of endometriosis in cancer and tumor development. Ann NY Acad Sci. 2002;955:281–295. doi: 10.1111/j.1749-6632.2002.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- Servettaz A, Guilpain P, Goulvestre C, Chereau C, Hercend C, Nicco C, Guillevin L, Weill B, Mouthon L, Batteux F. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann Rheum Dis. 2007;66:1202–1209. doi: 10.1136/ard.2006.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Song JS, Kang CM, Yoo MB, Kim SJ, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir Res. 2007;8:28–40. doi: 10.1186/1465-9921-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. Ann NY Acad Sci. 2002;955:328–342. doi: 10.1111/j.1749-6632.2002.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Majumder S, Zhu Q, Hunzeker J, Datta J, Shah M, Sheridan JF, Jacob ST. Influenza virus infection induces metallothionein gene expression in the mouse liver and lung by overlapping but distinct molecular mechanisms. Mol Cell Biol. 2001;21:8301–8317. doi: 10.1128/MCB.21.24.8301-8317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe K, Morishima S, Nakamuta N, Li ZL, Otsuki Y. Effect of danazol on NK cells and cytokines in the mouse uterus. J Reprod Dev. 2007;53:87–94. doi: 10.1262/jrd.18074. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827–834. doi: 10.1016/j.jacc.2005.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jeng KC, Ping LI. Exogenous cytokine modulation or neutralization of interleukin-10 enhance survival in lipopolysaccharide-hyporesponsive C3H/HeJ mice with Klebsiella infection. Immunology. 1999;98:90–97. doi: 10.1046/j.1365-2567.1999.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Nicco C, Tran Van Nhieu J, Borderie D, Chereau C, Conti F, Jaffray P, Soubrane O, Calmus Y, Weill B, Batteux F. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004;39:1277–1285. doi: 10.1002/hep.20177. [DOI] [PubMed] [Google Scholar]
- Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Poswig A, Wenk J, Brenneisen P, Wlaschek M, Hommel C, Quel G, Faisst K, Dissemond J, Briviba K, Krieg T, Scharffetter-Kochanek K. Adaptive antioxidant response of manganese-superoxide dismutase following repetitive UVA irradiation. J Invest Dermatol. 1999;112:13–18. doi: 10.1046/j.1523-1747.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- Akashi M, Hachiya M, Paquette RL, Osawa Y, Shimizu S, Suzuki G. Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. Possible mechanisms for its accumulation. J Biol Chem. 1995;270:15864–15869. doi: 10.1074/jbc.270.26.15864. [DOI] [PubMed] [Google Scholar]
- Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Amstad P, Peskin A, Shah G, Mirault ME, Moret R, Zbinden I, Cerutti P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991;30:9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S, Hofseth LJ, Moake M, Nagashima M, Forrester KS, Harris CC. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H(2)O(2). Free Radic Biol Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A. ERK activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2008;93:3532–3540. doi: 10.1210/jc.2007-2051. [DOI] [PubMed] [Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918–929. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. 2004;52:306–311. doi: 10.1111/j.1600-0897.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Wu RF, Wang ZH, Sun HC, Xu Z, Xiu HM. Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertil Steril. 2002;77:995–1000. doi: 10.1016/s0015-0282(02)03081-9. [DOI] [PubMed] [Google Scholar]
- Murakami K, Nomura K, Shinohara K, Kasai T, Shozu M, Inoue M. Danazol inhibits aromatase activity of endometriosis-derived stromal cells by a competitive mechanism. Fertil Steril. 2006;86:291–297. doi: 10.1016/j.fertnstert.2005.12.074. [DOI] [PubMed] [Google Scholar]