Abstract
Alzheimer’s disease (AD) is thought to be caused by the accumulation of amyloid beta (Aβ) peptide within the brain. Endothelin-converting enzyme-2 (ECE-2), which is expressed in neural tissues, cleaves ‘big endothelin’ to produce the vasoconstrictor endothelin-1. ECE-2 also degrades Aβ. We have examined ECE-2 expression in the temporal cortex of brain tissue from patients with AD, vascular dementia, and controls. Immunohistochemistry with specific antibodies showed ECE-2 to be abundant within pyramidal neurons in both the hippocampus and neocortex, but also to be present in certain astrocytes and microglia, particularly in AD brains. Quantitative real-time PCR showed ECE-2 mRNA to be markedly elevated in AD but not in vascular dementia. ECE-2 protein concentration, measured by sandwich enzyme-linked immunosorbent assay, was also significantly elevated in AD but not in vascular dementia. Exposure of SH-SY5Y human neuroblastoma cells to monomeric or oligomeric Aβ1–42 caused an initial decrease in ECE-2 mRNA at 4 hours, but a marked increase by 24 hours. Our findings indicate that Aβ accumulation in AD is unlikely to be caused by ECE-2 deficiency. However, ECE-2 expression is up-regulated, perhaps to minimize Aβ accumulation, but this may also be a mechanism through which endothelin-1 production is increased and cerebral blood flow is reduced in AD. Our findings suggest that endothelin-1 receptor antagonists, already licensed for treating other diseases, could be of benefit in AD therapies.
Alzheimer’s disease (AD) is thought to be caused by the accumulation of Aβ peptide within the brain, leading to dysfunction and loss of synapses and eventually of neurons.1 The amount of Aβ in the brain is determined by the balance between its production and clearance. Familial autosomal dominant forms of AD, which either increase overall production of Aβ or the ratio of Aβ1–42 to Aβ1–40, account for fewer than 5% of cases. The mechanisms by which Aβ accumulates in late-onset sporadic AD, the major form of the disease, are unknown. There is little evidence of increased Aβ production in late-onset sporadic AD, except perhaps in the later stages of disease, when β-site APP-cleaving enzyme (BACE) activity may be increased.2,3 The initiating abnormality and perhaps the main cause of Aβ accumulation in late-onset sporadic AD seems to be an impairment of Aβ clearance. Several potential pathways of Aβ clearance have been identified. These include perivascular drainage,4,5 receptor-mediated transport across the blood-brain barrier,6,7 and proteolytic degradation of Aβ within the brain.8,9,10
The endothelin-converting enzymes (ECEs) are a class of type II integral membrane-bound proteases, belonging to the M13 family of zinc metallopeptidases. The ECEs are named for their ability to convert the inactive precursor ‘big endothelin’ to its potent vasoactive peptide endothelin-1 (ET-1). In addition, ECEs have been reported to hydrolyze several biologically active peptides in vitro, including bradykinin, neurotensin, substance P, and oxidized insulin B chain, by cleavage on the amino side of hydrophobic residues,11,12 and have been shown to degrade Aβ.13 The two ECE isoforms, ECE-1 and ECE-2, have 59% sequence homology and similar catalytic activity; however, in contrast to the neutral pH optimum of ECE-1, ECE-2 has an pH optimum of pH 5.5 and is active within a narrow pH range.14 Neural tissues are the most abundant site of ECE-2 expression.14 ECE-2 has been localized to intracellular secretory compartments.15 With a pH 5.5 optimum, a predominantly neural distribution and very little expression in peripheral tissues, ECE-2 is thought to have a role in the non-classical processing of regulatory peptides.16
ECE-2 homozygous knock-out mice17 show decreased degradation of Aβ and significantly elevated levels of Aβ1–40 and Aβ1–42 in the brain.18 ECE-2 knock-out mice are deficient in learning and memory, without having impaired balance or motor function.19 Although these studies suggest that ECE-2 is a physiologically relevant Aβ-degrading enzyme with a role in learning and memory, little information is available on ECE-2 expression in AD: to our knowledge only a single report has been made of decreased ECE-2 mRNA levels in the inferior part of the parietal lobe, in six cases of AD.20 Our aim in the present study was to investigate the distribution of ECE-2, and the levels of ECE-2 mRNA and protein in the temporal neocortex of AD and non-demented control brains. Because ECE-2 activity leads to the production of the vasoconstrictor peptide ET-1, we thought it would be of interest to measure ECE-2 mRNA and protein levels in vascular dementia (VaD) too. Finally we also examined the relationship between Aβ exposure and ECE-2 expression in SH-SY5Y neuroblastoma cells in vitro.
Materials and Methods
Study Cohort
We studied brain tissue from 14 cases of neuropathologically confirmed sporadic AD, 15 matched controls and 15 cases of VaD (Table 1). The tissue was obtained from the Human Tissue Authority licensed South West Dementia Brain Bank, University of Bristol. The brains had been divided midsagittally at autopsy: the left half was sliced and frozen at −80°C and the right half was fixed in formalin for detailed neuropathological assessment. The AD cases were 57 to 93 years in age (mean 77.8, SD 9) and were selected on the basis of a diagnosis according to the Consortium to Establish a Registry for Alzheimer’s Disease of ‘definite AD’ and a Braak tangle stage of V or VI.21 The postmortem (PM) delays ranged from 4 to 36 hours (mean 20.5, SD 11.2). The controls were 58 to 93 years in age (mean 78, SD 9.8), had no history of dementia, few or no neuritic plaques, a Braak tangle stage of III or less, and no other neuropathological abnormalities, and had similar PM delays, of 3 to 36 hours (mean 21.3, SD 11.7). The VaD cases had a clinical history of dementia, no more than occasional neuritic plaques, a Braak tangle stage of II or less, histopathological evidence of multiple infarcts/ischemic lesions, moderate to severe atheroma and/or arteriosclerosis, and an absence of histopathological evidence of other disease likely to cause dementia. The VaD cases were 67 to 97 years in age (mean 83.4, SD 7.7) and had PM delays of 20 to 70 hours (mean 42.9, SD 17.4). For ECE-2 protein and mRNA level studies, we dissected samples of unfixed frozen cortex from Brodmann area 22 in the left temporal lobe. For immunohistochemistry, we used formalin-fixed, paraffin-embedded tissue from the right temporal lobe. The studies had local Research Ethics Committee approval.
Table 1.
Cases Studied
| *Case | Age | Sex | Post-mortem delay (h) | Braak tangle stage |
|---|---|---|---|---|
| C1 | 82 | M | 3 | II |
| C2 | 90 | M | 5.5 | II |
| C3 | 75 | M | 6 | II |
| C4 | 76 | F | 12 | 0 |
| C5 | 64 | M | 12 | II |
| C6 | 93 | F | 18 | II |
| C7 | 76 | M | 23 | II |
| C8 | 58 | M | 20 | 0 |
| C9 | 79 | M | 24 | 0 |
| C10 | 83 | F | 24 | II |
| C11 | 82 | M | 30 | II |
| C12 | 73 | M | 33 | I |
| C13 | 73 | M | 35 | III |
| C14 | 93 | M | 38 | III |
| C15 | 73 | M | 36 | II |
| AD1 | 81 | M | 4 | VI |
| AD2 | 89 | F | 4 | VI |
| AD3 | 76 | M | 11 | VI |
| AD4 | 78 | F | 9 | VI |
| AD5 | 69 | M | 12 | V |
| AD6 | 93 | M | 20 | VI |
| AD7 | 78 | F | 21 | VI |
| AD8 | 57 | F | 24 | V |
| AD9 | 74 | M | 24 | V |
| AD10 | 82 | F | 24 | VI |
| AD11 | 79 | M | 28 | VI |
| AD12 | 71 | M | 30 | VI |
| AD13 | 75 | F | 40 | VI |
| AD14 | 87 | M | 36 | VI |
| VaD1 | 84 | F | 60 | III |
| VaD2 | 81 | M | 66 | 0 |
| VaD3 | 84 | F | 20 | II |
| VaD4 | 93 | M | 30 | III |
| VaD5 | 80 | F | 70 | II |
| VaD6 | 72 | M | 41 | III |
| VaD7 | 86 | F | 28 | II |
| VaD8 | 90 | F | 31 | I |
| VaD9 | 89 | M | 30 | III |
| VaD10 | 67 | M | 54 | III |
| VaD11 | 97 | F | 66 | II |
| VaD12 | 84 | M | 30 | II |
| VaD13 | 76 | M | 40 | III |
| VaD14 | 79 | M | 56 | II |
| VaD15 | 89 | F | 22 | I |
C = control, AD = Alzheimer’s disease, VaD = vascular dementia. The numbering reflects the matching of control and AD cases (ie, C1 was matched with AD1, C2 with AD2 etc). The VaD cases were not matched individually with control or AD cases.
Characterization of ECE-2 Antibodies
We used two antibodies against ECE-2, both provided by R&D Systems (Minneapolis, MN). One was raised in goats against recombinant human ECE-2, amino acids 104 to 787. The other was raised in rats against recombinant human ECE-2, amino acids 104 to 787. We assessed the specificity of these antibodies against full-length recombinant human ECE-1 and ECE-2 protein (R&D Systems). The recombinant proteins were denatured and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4% to 20% Tris-HCL precast gels (Bio-Rad Hercules, CA). The proteins were transferred to nitrocellulose membrane (Bio-Rad) overnight at 4°C. The binding of non-specific protein was reduced by blocking with 10% nonfat dry milk in Tris-buffered saline-Tween (0.05%) for 1 hour. The membrane was incubated with each antibody at 1:500 and 1:1000 dilutions in 5% nonfat dry milk/Tris-buffered saline-Tween for 1 hour at room temperature on a shaking platform. The membrane was washed three times in Tris-buffered saline-Tween, incubated with peroxidase-conjugated, species-specific, secondary antibodies (goat, Vector labs, Burlingame, CA; rat, Cell Signaling Technology, Boston, MA) diluted 1:5000 in 5% nonfat dry milk/Tris-buffered saline-Tween for 1 hour at room temperature on a shaking platform, and immunolabeled bands were visualized using enhanced chemiluminescence (ECL Plus kit, Amersham Biosciences, Buckinghamshire, UK). Band density was measured with the aid of Image-J software. Both antibodies detected recombinant ECE-2 at the anticipated size. The goat antibody showed ∼5% cross-reactivity to ECE-1. The rat antibody showed no cross-reactivity to ECE-1.
Immunoperoxidase Staining of Paraffin Sections
Paraffin sections 7 μm in thickness were cut through temporal lobe in the coronal plane of the lateral geniculate body. These sections were collected on 3-amino-propyl-triethoxy-silane coated slides and left to dry overnight in a 40°C oven. Before staining, slides were incubated overnight in a 60°C oven, dewaxed, and rehydrated through graded alcohols. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide in methanol for 30 to 45 minutes, followed by a 10 minutes wash in running water. Sections were microwaved in citrate buffer (9 mmol/L tri-sodium citrate, pH 6.0) until boiling, left to cool for 5 minutes, microwaved again until boiling, and left to cool for 15 minutes before being washed in continuously running water for 10 minutes and rinsed in PBS. Non-specific binding of antibody was blocked by incubation of the sections in 20% normal goat serum (Vector labs) for 20 minutes before application of antibody to ECE-2 (rat, 1:200, R&D Systems) overnight at room temperature. Sections were washed in two changes of PBS and biotinylated Universal Antibody (Vector labs) applied for 20 minutes. Sections were washed in two changes of PBS, then VectaElite ABC complex (Vector labs), which had been prepared and left to sit at room temperature for 30 minutes, was applied for 20 minutes. Sections were washed in two changes of PBS, incubated for 7 to 10 minutes in 3,3′-diaminobenzidine containing <0.1% H2O2 (Vector labs) and washed in continuously running water for 10 minutes. Sections were immersed in copper sulfate for 4 minutes, washed in running water, counterstained with Harris’ hematoxylin, and washed in running water for at least 10 minutes, before being dehydrated, cleared, and mounted.
Double Immunoperoxidase Staining of Paraffin Sections
Sections were immunolabeled for ECE-2 with 3,3′-diaminobenzidine as described for single immunolabeling. After sections had been immersed in copper sulfate and washed in water, they were again immersed in 3% hydrogen peroxide in methanol for 45 minutes, rinsed for 10 minutes in running water, and incubated in 20% normal goat serum for 20 minutes. Sections were then incubated with antibody either to glial fibrillary acidic protein (1:1200, Dako, Glostrup, Denmark) or human leukocyte antigen DR-1 (1:800, Dako) overnight at room temperature. Sections were washed in two changes of PBS and biotinylated Universal Antibody (Vector labs) was applied for 20 minutes. Sections were washed in two changes of PBS, then VectaElite ABC complex (Vector labs), which had been prepared and left to sit at room temperature for 30 minutes, was applied for 20 minutes. Sections were washed in two changes of PBS, incubated for 7 to 10 minutes in Vector Very Intense Purple Substrate Kit (Vector labs) and washed in running water for 10 minutes. Sections were immersed in copper sulfate for 4 minutes, washed in running water, counterstained with Harris’s hematoxylin, and washed in running water for at least 10 minutes, before being dehydrated, cleared, and mounted.
Assessment of Post-Mortem Stability of ECE-2 Protein in Frozen Brain Tissue
The effects of PM delay were simulated using tissue from a brain removed 5.5 hours after the death of a control patient. A 50 mg sample of anterior frontal cortex was subdivided into 10 aliquots. The individual aliquots were stored for 0, 6, 12, 18, 24, 48, or 72 hours at room temperature, or 24, 48, or 72 hours at 4°C. They were then homogenized in 250 μl lysis buffer (0.1 mmol/L NaCl, 10 mmol/L Tris pH 6, 1 μmol/L phenylmethylsulphonyl fluoride, 1 μg/ml aprotinin, and 1% SDS in distilled water) in a Precellys 24 homogenizer (Stretton Scientific, Stretton, UK) with 2.3-mm silica beads (Biospec, Thistle Scientific, Glasgow, UK). ECE-2 protein levels were measured by sandwich enzyme-linked immunosorbent assay, as described below.
cDNA Generation and Real-Time PCR on Brain Tissue
Brain tissue from the temporal neocortex was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA), in a Precellys 24 homogenizer (Stretton Scientific). The homogenates were incubated for 3 minutes in chloroform then centrifuged at 12,000 × g for 15 minutes at 4°C. The aqueous phase was mixed with an equal volume of isopropyl alcohol and 30 μg of glycogen, incubated for 10 minutes and centrifuged at 12,000 × g for 10 minutes at 4°C to precipitate the RNA. The RNA pellet was washed with 75% ethanol, resuspended in water (Sigma-Aldrich, Gillingham, UK), and treated with DNase-I (40U, Roche Diagnostics Ltd, West Sussex, UK) to remove any DNA. RNA concentration was determined using a Ribogreen RNA quantification kit (Invitrogen) and a fluorescence plate reader (FLUOstar OPTIMA, BMG Labtech, Aylesbury, UK). cDNA was produced using the High Capacity c-DNA Archive Kit from Applied Biosystems (Foster City, CA): 100 ng of RNA in a total volume of 100 μl was incubated at 25°C for 10 minutes, 37°C for 2 hours, followed by inactivation at 85°C for 5 seconds. cDNA concentration was determined with the Picogreen DNA quantification kit (Invitrogen, UK). Real-time reverse transcription PCR (RT-PCR) was performed using the ABI 7000 sequencing detection system (ABI Prism, Applied Biosystems) with Assay-on-demand Gene Expression Products for ECE-2, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), microtubule-associated protein-2, and neuron-specific enolase (TaqMan MGB probes, FAM dye-labeled, Applied Biosystems), SensiMix dT (Quantace, London, UK), and 10 ng of cDNA in a total volume of 20 μl: 50°C for 2 minutes; 95°C for 10 minutes; and 40 cycles of 95°C for 15 minutes and 60°C for 1 minute. All samples were analyzed in triplicate. Relative gene expression (expressed as fold difference of each subject relative to the average of control tissue) was calculated using the 2−ΔΔCt method,22 and the geometric mean taken for each group. Separate calculations were made using GAPDH, microtubule-associated protein-2, or neuron-specific enolase as the reference ‘housekeeping’ gene.
Measurement of ECE-2 Protein Levels Using Sandwich Enzyme-Linked Immunosorbent Assay
Five hundred milligram samples of temporal neocortex were homogenized in lysis buffer with the aid of a Precellys 24 homogenizer (Stretton Scientific), aliquoted, and stored at −80°C until required. Total protein concentrations were determined using the Sigma-Aldrich total protein kit according to the manufacturer’s instructions; absorbance values were read at 590 nm on a FLUOstar Optima plate reader (BMG Labtech, Aylesbury, UK). Ninety-six-well immunoplates (Nunc Maxisorp, obtained from Fisher Scientific, Loughborough, UK) were coated with a goat anti-human ECE-2 antibody (1:20, R&D Systems) in coating buffer (0.1 M/L sodium carbonate, 0.3 M/L sodium bicarbonate, pH 9.6) and left at 4°C overnight. The wells were drained, washed thoroughly with wash buffer (0.05% Tween20/PBS) and incubated with 300 μl blocking buffer (0.5% nonfat dry milk powder in PBS) for 90 minutes at room temperature on a shaking platform. Serial dilutions of recombinant human ECE-2 (R&D Systems) in blocking buffer were used to produce a standard curve with final concentrations ranging from 27 to 432 ng/ml. Homogenates were diluted in 50 μl blocking buffer to a final concentration of 1 mg/ml total protein, added in triplicate and left at room temperature for 2 hours while shaking. The plates also included blank wells that contained blocking buffer and lysis buffer without tissue. After further washes, 50 μl of rat anti-human ECE-2 (1 μg/ml, R&D Systems) in blocking buffer was added to each well and left for 90 minutes at room temperature while shaking. The wells were washed and incubated with 50 μl peroxidase anti-rat conjugate (1:500, Cell Signaling Technology) in wash buffer for 60 minutes at room temperature while shaking. Wells were again washed, peroxidase substrate (R&D Systems) added, and plates left to incubate for 20 minutes in the dark before the addition of Stop solution (R&D Systems). Absorbance was read by a plate reader with a 450 nm filter. The mean readings from the blank wells were subtracted from the readings obtained from the wells containing recombinant protein or brain homogenate. A standard curve was generated and ECE-2 protein concentration calculated for each homogenate. Each sample was repeated on two different plates and measured in duplicate on each plate.
Preparation of Monomeric and Oligomeric Aβ1–42
Lyophilized Aβ1–42 (Anaspec, CA) was reconstituted in 35% acetonitrile. This 1.6 mmol/L stock solution of monomeric Aβ1–42 was diluted to 10 μmol/L in Dulbecco’s Modified Eagle Medium (D6546 Sigma-Aldrich, Dorset, UK) before its addition to cell cultures. For oligomerization, the stock solution was diluted to 300 μmol/L in Dulbecco’s Modified Eagle Medium and incubated overnight at 37°C. We previously showed that this leads to formation of a mixture of trimeric, tetrameric and higher molecular weight species of Aβ1–42.23 Immediately before its addition to cell cultures, the oligomeric Aβ1–42 was further diluted to 10 μmol/L in Dulbecco’s Modified Eagle Medium.
Cell Culture and cDNA Generation for RT-PCR
SH-SY5Y human neuroblastoma cells (European Collection of Cell Cultures, Porton Down, UK) were grown in Dulbecco’s Modified Eagle Medium supplemented with 2 mmol/L l-glutamine (Sigma-Aldrich) and 15% fetal calf serum (Autogen Bioclear, Wiltshire, UK) at 37°C in 5%CO2/95% air. The cells were differentiated in 10 μmol/L retinoic acid (Sigma-Aldrich) for 6 days and treated with 10 μmol/L monomeric or 10 μmol/L oligomeric Aβ1–42 for 4 and 24 hours. Control cells were incubated for the same duration in Dulbecco’s Modified Eagle Medium supplemented with 0.2% acetonitrile. Following treatment, protein and RNA were extracted from the cells.
RNA was extracted and cDNA produced using the TaqMan gene expression cells-Ct-kit (Applied Biosystems) according to the manufacturer’s instructions. 103 cells were added to lysis solution plus DNase I (5 minutes) before the addition of the stop solution (2 minutes). To synthesize cDNA, the extracted RNA was placed in a thermal cycler with the reverse transcription buffer and RT enzyme mix and incubated at 37°C for 1 hour and 95°C for 5 minutes. The cDNA concentration was measured and RT-PCR was performed as described previously, with GAPDH as housekeeping gene.
Statistical Analysis
The relationship between simulated PM delay and ECE-2 concentration was assessed by Pearson correlation analysis. Comparisons of ECE-2 mRNA and protein levels between the AD, VaD, and control groups were made by Dunn’s multiple comparison test. Wilcoxon signed rank test was used to compare ECE-2 mRNA levels in SH-SY5Y human neuroblastoma cells treated with Aβ and in untreated cells. The statistical tests were performed using GraphPad v5 for Windows. P values of <0.05 were considered significant.
Results
Distribution of ECE-2 in Human Temporal Neocortex
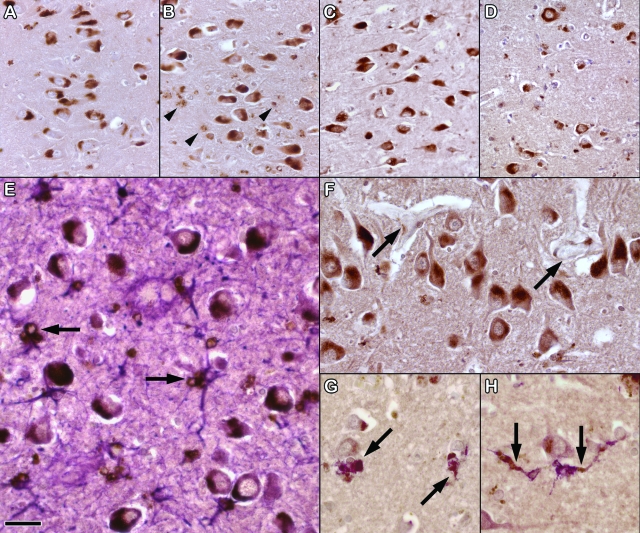
Pyramidal neurons in the hippocampus in AD, VaD, and control brains were strongly immunopositive for ECE-2 (Figure 1, A–H). The pattern of immunolabeling was similar for the two antibodies. The neurons showed a combination of finely granular and diffuse labeling of the somatic cytoplasm. Scattered astrocytes and possibly microglia contained coarsely granular immunopositive cytoplasmic material; this was particularly prominent in the AD brains. Pyramidal neurons and scattered astrocytes and microglia (including some perivascular microglia) were also immunopositive for ECE-2 within the neocortex, but the staining tended to be weaker than that in the hippocampus. A few leptomeningeal arteries showed very weak ECE-2 positivity in the tunica media but most blood vessels were not labeled. However, most intracortical blood vessels were close to ECE-2 positive neurons.
Figure 1.
Labeling of ECE-2 in neurons and glia. Sections through the CA1 field of the hippocampus in control (A), AD (B), and VaD brain (C). Most neurons show a combination of fine granular and diffuse labeling of the somatic cytoplasm. There are also coarse granular aggregates of immunopositive material in some cells (arrowheads in B), particularly in the AD brain. D: Labeling of neurons for ECE-2 in the temporal neocortex (Brodmann area 22). A few small non-neuronal cells also contain scanty immunopositive material. E: Double-labeling for ECE-2 (brown) and glial fibrillary acidic protein (purple) shows that the coarse granular aggregates of ECE-2 are within glial fibrillary acidic protein-positive astrocytes (arrows). F: Shows the close relationship of blood vessels (arrows) to ECE-2 labeled neurons in the hippocampus. G, H: Double-labeling for ECE-2 (brown) and human leukocyte antigen DR-1 (purple) shows ECE-2 to be present in occasional macrophages or microglia (arrows). Scale bar = 20 μm in (A–D), 10 μm in (E), and 15 μm in (F–H).
Measurement of ECE-2 mRNA in Human Temporal Neocortex
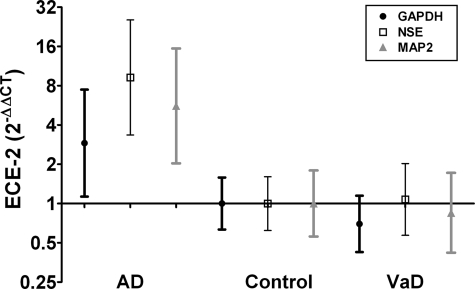
ECE-2 mRNA levels differed significantly between AD, VaD, and control brains (Figure 2). Calibration of ECE-2 mRNA levels with reference to GAPDH revealed an approximately threefold increase in temporal neocortex from AD, as compared with normal controls (P = 0.01). ECE-2 mRNA levels were not significantly increased in the VaD brains.
Figure 2.
ECE-2 mRNA levels were measured by RT-PCR. The fold difference relative to the average in control tissue was calculated for each sample by the 2−ΔΔCt method, with GAPDH, neuron-specific enolase, and microtubule-associated protein-2 as calibrators. The geometric mean and 95% confidence intervals are shown for each group on a logarithmic scale (to the base 2). The ECE-2 mRNA level is significantly elevated in AD but not VaD.
GAPDH is often used as the housekeeping gene for RT-PCR, as it is expressed in all cell types. However, because of this fact, it may not be an accurate calibrator when measuring RNA levels within neurons in diseased brain tissue, as increases in the number of GAPDH-expressing non-neuronal cells, particularly astrocytes and microglia, may mask changes in expression of the gene of interest by neurons. As ECE-2 is predominantly expressed in neurons, we therefore also analyzed ECE-2 mRNA levels with respect to two other neuron-specific transcripts: neuron-specific enolase and microtubule-associated protein-2. Calculations based on use of neuron-specific enolase and microtubule-associated protein-2 mRNA levels as calibrators indicated approximately ninefold (P < 0.001) and an approximately sixfold (P = 0.01) increases respectively, in ECE-2 mRNA levels in AD compared to control temporal neocortex. Use of these calibrators indicated no increase in ECE-2 mRNA in VaD.
ECE-2 Protein Levels in Human Temporal Neocortex
ECE-2 protein level was significantly higher in temporal neocortex from AD than control (P < 0.001) brains (Figure 3). The mean concentration in AD brains was 108.9 ng/ml (SD = 53.5), in VaD brains 74.3 ng/ml (SD = 39.4) and controls 42.2 ng/ml (SD = 29.2). The difference between ECE-2 protein level in VaD and control brains was not significant.
Figure 3.
ECE-2 protein levels were measured by sandwich enzyme-linked immunosorbent assay. ECE-2 protein concentration is significantly elevated in AD compared to controls, but not in VaD.
Simulated Effects of Post-Mortem Delay on Stability of ECE-2
When we measured ECE-2 level in brain tissue that had been stored at different times and temperatures, we found no significant change over periods of up to 72 hours, either at room temperature or 4°C (see supplementary Figure S1 available at http://ajp.amjpathol.org). It is therefore unlikely that differences in ECE-2 protein measurements were due to differences in postmortem delay.
Expression of ECE-2 mRNA Following Exposure of SH-SY5Y Cells to Aβ
To investigate whether the increase in ECE-2 in AD was a response to the accumulation of Aβ, we measured ECE-2 mRNA expression after 4 hours and 24 hours treatment of SH-SY5Y cells with monomeric or oligomeric Aβ1–42, compared to non-treated cells. There was a decrease in ECE-2 mRNA levels after 4 hours treatment with either monomeric (P = 0.0002) or oligomeric Aβ1–42 (P < 0.0001), and an increase in ECE-2 mRNA levels after 24 hours treatment with monomeric (P = 0.0023) or oligomeric Aβ1–42 (P = 0.0037) (Figure 4).
Figure 4.
ECE-2 mRNA from SH-SY5Y neuroblastoma cells was measured by RT-PCR after treatment with both monomeric and oligomeric Aβ1–42. The ECE-2 (2−ΔΔCt) values represent the fold difference in ECE-2 mRNA level in treated cells over control cells. The geometric mean and 95% confidence intervals are shown. Gene expression was found to be reduced after 4 hours treatment, and increased after 24 hours treatment, with both species of Aβ1–42.
Discussion
Although ECE-2 has been shown to be a physiologically relevant Aβ-degrading enzyme in mouse models,17,18 as far as we are aware, this is the first investigation of ECE-2 protein in AD and only a single previous study has been reported of ECE-2 mRNA in AD.20 We have now shown that ECE-2 mRNA and protein levels are markedly elevated in the temporal neocortex in AD but not VaD, indicating that the increased expression of ECE-2 in AD is unlikely to be simply secondary to tissue damage. Our in vitro findings of a significant increase in ECE-2 mRNA expression after 24 hours treatment of SH-SY5Y cells with both monomeric Aβ1–42 and oligomeric Aβ1–42 supports our findings of increased ECE-2 in AD, suggesting that ECE-2 gene expression is up-regulated in response to Aβ. The decrease in ECE-2 mRNA expression after 4 hours treatment with Aβ42 could indicate a delay in the response to Aβ, however this apparent feedback mechanism is unknown, and warrants further investigation.
Our findings differ from those of Weeraratna et al20 who found a decrease in ECE-2 mRNA in inferior parietal cortex in AD. However, the studies are not directly comparable. Unlike Weeraratna et al,20 we examined temporal cortex. Our sample size was higher (14 AD cases and 15 controls, as compared with 6 AD cases and 6 controls). In addition, it is unclear from their paper how the data were calibrated with respect to housekeeping/reference gene expression. Immunohistochemistry was only performed on one case, and no data were presented as to the specificity of the antibody to ECE-2.
Multiple proteolytic enzymes have the capacity to degrade Aβ, but their relative contributions to Aβ degradation in vivo are not known. Present findings indicate that Aβ accumulation is not likely to be caused by ECE-2 deficiency. Previous studies have shown the expression of some other putative Aβ-degrading enzymes to be decreased in AD. Immunolabeling of insulin-degrading enzyme (IDE) was reduced in hippocampal neurons24 and labeling of neprilysin (NEP) was reduced in cerebrocortical neurons and blood vessels.25,26,27 Other studies have found lower NEP mRNA levels25,28 and lower IDE mRNA, protein levels, and enzyme activity in AD.29,30,31 However, these findings cannot be construed as evidence of a primary deficiency in the expression of either NEP or IDE. None of these studies has adjusted for the potential impact of neuronal loss or damage in established AD, or for the possible effects of other processes such as microglial inflammation or astrocytosis, and the studies have mostly been of relatively small cohorts. Western blot analysis of NEP in human brain tissue homogenates has yielded inconsistent results: some studies have shown a reduction of NEP in AD28,32 and others have shown no significant change.27,33,34
Recently, Vepsalainen et al35 reported that IDE mRNA and protein levels were significantly higher in APPswe/PSEN(dE9) mice than in non-transgenic littermates. NEP protein levels were also higher, but this did not reach statistical significance. The positive correlation between IDE mRNA and levels of Aβ1–40 and Aβ1–42 suggested that IDE mRNA production might be induced by Aβ, possibly in activated astrocytes as a result of Aβ-related inflammation.36 Similarly, up-regulation of NEP has been shown in activated astrocytes surrounding plaques.36,37 Other studies have examined the effect on several Aβ-degrading enzymes of incubating neuronal, glial, or smooth muscle cell lines with fibrillar Aβ42 in vitro. This was shown to up-regulate IDE,36 MMP-2, -3, and -9,38,39,40 and plasminogen activators.41 Our in vitro data provide evidence that the up-regulation of ECE-2 expression is similarly likely to be caused by the accumulation of Aβ in AD, perhaps as a physiological feedback response or protective mechanism whereby increased Aβ degradation minimizes further Aβ accumulation.
Several lines of evidence suggest that intracerebral accumulation of Aβ has the potential to produce cerebrovascular dysfunction. Synthetic Aβ has been shown to impair endothelium-dependent relaxation and enhance vasoconstriction in vivo and in vitro.42,43,44 Cerebral blood flow is reduced by 20% to 40% in mice that over-express APP45 and regulation of cerebral blood flow tends to be most impaired in transgenic mice with highest intracerebral levels of Aβ.46 It has long been speculated that cerebrovascular dysfunction contributes to AD.47 Patients with AD have reduced cerebral blood flow,48 and cerebral blood flow correlates inversely with performance of AD patients upon mini-mental state examination.49,50,51 Impairment of cerebrovascular autoregulation precedes neuropathological alterations in APP transgenic mice, supporting the hypothesis that reduced cerebral perfusion may play a pathogenic role in AD.46
ET-1 plays a central role in the regulation of blood flow. It is produced by ECE-mediated cleavage of big ET-1. ET-1 can act on ETA receptors to cause vasoconstriction and ETB receptors to cause vasodilatation.52 Intravenous administration of ET-1 in different species leads to a short-lived decrease in vascular resistance (ie, vasodilation), followed by a long-term increase (ie, vasoconstriction).53 The overall physiological effect of ET-1 is to increase blood pressure54 by an increase in vascular tone.55 Endothelin is the major vasoconstrictor in cerebral blood vessels and can induce marked and sustained cerebral vasoconstriction.56 Aβ1–40 and Aβ1–42 were shown to enhance ET-1 induced vasoconstriction in rat aorta.57
An increase in ECE-2 may be a mechanism through which Aβ accumulation affects cerebrovascular blood flow and the capacity of the central nervous system to increase blood flow in response to regional increases in metabolic demand. Increased Aβ in AD may induce ECE-2 expression, proteolytic cleavage of big-ET-1 and release of ET-1, including by perivascular nerve terminals, with the result that cerebrovascular tone is increased and blood flow is reduced. This raises the possibility that endothelin-1 receptor antagonists, already licensed for the treatment of other diseases such as pulmonary hypertension, could be of benefit in AD, as a way to counteract overproduction of ET-1 and to increase cerebral perfusion.
Supplementary Material
Footnotes
Address reprint requests to Jennifer C. Palmer, Dementia Research Group, John James Building, Frenchay Hospital, Bristol BS16 1LE, United Kingdom. Email: jen.palmer@bristol.ac.uk.
Supported by a PhD studentship (J.C.P.) from Bristol Research into Alzheimer’s and Care of the Elderly (BRACE), by a project grant from the James Tudor Foundation (S.B.), and by a fellowship from the Sigmund Gestetner Foundation (P.G.K.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yao RQ, Beach T, Sue L, Sabbagh M, Cai HB, Wong P, Price D, Shen Y. Amyloid-β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan RQ, Citron M, Xia WM, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Preston SD, Steart PV, Wilkinson A, Nicoll JAR, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid-β from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy—Amyloid-β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FC, Liu RH, Lu PH, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. β-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-β(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. Aβ-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Comery TA, Martone RL, Elokdah H, Crandall DL, Oganesian A, Aschmies S, Kirksey Y, Gonzales C, Xu J, Zhou H, Atchison K, Wagner E, Zaleska MM, Das I, Arias RL, Bard J, Riddell D, Gardell SJ, Abou-Gharbia M, Robichaud A, Magolda R, Vlasuk GP, Bjornsson T, Reinhart PH, Pangalos MN. Enhanced clearance of Aβ in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci USA. 2008;105:8754–8759. doi: 10.1073/pnas.0710823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lee CYD, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang MV, Turner AJ. Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem J. 1997;327:23–26. doi: 10.1042/bj3270023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GD, Stevenson T, Ahn KH. Hydrolysis of peptide hormones by endothelin-converting enzyme-1-A comparison with neprilysin. J Biol Chem. 1999;274:4053–4058. doi: 10.1074/jbc.274.7.4053. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer’s amyloid-β peptide by endothelin-converting enzyme. J Biol Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound. Phosphoramidon-sensitive metalloprotease with acidic Ph optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Cellular expression of isoforms of endothelin-converting enzyme-1 (ECE-1c, ECE-1b, and ECE-1a) and endothelin-converting enzyme-2. J Cardiovasc Pharmacol. 2000;36:S12–S14. doi: 10.1097/00005344-200036051-00006. [DOI] [PubMed] [Google Scholar]
- Mzhavia N, Pan H, Che FY, Fricker LD, Devi LA. Characterization of endothelin-converting enzyme-2—Implication for a role in the nonclassical processing of regulatory peptides. J Biol Chem. 2003;278:14704–14711. doi: 10.1074/jbc.M211242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease β-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Gadnidze K, Ragnauth A, Dorr N, Yanagisawa M, Wetsel WC, Devi LA. Animals lacking endothelin-converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2008;7:418–426. doi: 10.1111/j.1601-183X.2007.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT, Kalehua A, DeLeon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer’s disease tissues. Exp Cell Res. 2007;313:450–461. doi: 10.1016/j.yexcr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) Method. Methods (Duluth) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- van Helmond Z, Heesom K, Love S. Characterisation of two antibodies to oligomeric Aβ and their use in ELISAs on human brain tissue homogenates. J Neurosci Methods. 2009;176:206–212. doi: 10.1016/j.jneumeth.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Aβ-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL. Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid β-protein (Aβ) deposition. Brain Res. 2001;902:277–281. doi: 10.1016/s0006-8993(01)02390-3. [DOI] [PubMed] [Google Scholar]
- Carpentier M, Robitaille Y, DesGroseillers L, Boileau G, Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:849–856. doi: 10.1093/jnen/61.10.849. [DOI] [PubMed] [Google Scholar]
- Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65:1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Akiyama H, McGeer EG, McGeer PL. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of β-amyloid peptide. Neurosci Lett. 2001;297:97–100. doi: 10.1016/s0304-3940(00)01675-x. [DOI] [PubMed] [Google Scholar]
- Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon 4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Morelli L, Cresto JC, Castano EM. Degradation of soluble amyloid β-peptides 1–40, 1–42, and the Dutch variant 1–40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem Res. 2000;25:247–255. doi: 10.1023/a:1007527721160. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Xiang ZM, Haroutunian V, Buxbaum JD, Stetka B, Pasinetti GM. Insulin degrading enzyme activity selectively decreases in the hippocampal formation of cases at high risk to develop Alzheimer’s disease. Neurobiol Aging. 2007;28:824–830. doi: 10.1016/j.neurobiolaging.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Wang DS, Lipton RB, Katz MJ, Davies P, Buschke H, Kuslansky G, Verghese J, Younkin SG, Eckman C, Dickson DW. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J Neuropathol Exp Neurol. 2005;64:378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Ravid R, Nordberg A. Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiol Aging. 2008;29:210–221. doi: 10.1016/j.neurobiolaging.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Russo R, Borghi R, Markesbery W, Tabaton M, Piccini A. Neprylisin decreases uniformly in Alzheimer’s disease and in normal aging. FEBS Lett. 2005;579:6027–6030. doi: 10.1016/j.febslet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Vepsalainen S, Hiltunen M, Helisalmi S, Wang J, van Groen T, Tanila H, Soininen H. Increased expression of Aβ degrading enzyme IDE in the cortex of transgenic mice with Alzheimer’s disease-like neuropathology. Neurosci Lett. 2008;438:216–220. doi: 10.1016/j.neulet.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Leal MC, Dorfman VB, Gamba AF, Frangione B, Wisniewski T, Castano EM, Sigurdsson EM, Morelli L. Plaque-associated overexpression of insulin-degrading enzyme in the cerebral cortex of aged transgenic Tg2576 mice with Alzheimer pathology. J Neuropathol Exp Neurol. 2006;65:976–987. doi: 10.1097/01.jnen.0000235853.70092.ba. [DOI] [PubMed] [Google Scholar]
- Apelt J, Ach K, Schliebs R. Aging-related down-regulation of neprilysin, a putative β-amyloid-degrading enzyme, in transgenic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of β-amyloid plaques. Neurosci Lett. 2003;339:183–186. doi: 10.1016/s0304-3940(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Deb S, Zhang JWJ, Gottschall PE. Activated isoforms of MMP-2 are induced in U87 human glioma cells in response to β-amyloid peptide. J Neurosci Res. 1999;55:44–53. doi: 10.1002/(SICI)1097-4547(19990101)55:1<44::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jung SS, Zhang WB, Van Nostrand WE. Pathogenic Aβ induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem. 2003;85:1208–1215. doi: 10.1046/j.1471-4159.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- Lee JM, Yin KJ, Hsin I, Chen SW, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S. Tissue plasminogen activator requires plasminogen to modulate amyloid-β neurotoxicity and deposition. J Neurochem. 2000;75:2172–2177. doi: 10.1046/j.1471-4159.2000.0752172.x. [DOI] [PubMed] [Google Scholar]
- Niwa K, Carlson GA, Iadecola C. Exogenous Aβ 1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol-Heart Circul Physiol. 2001;281:H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol-Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Jagust WJ. Neuroimaging in dementia. Neurol Clin. 2000;18:885–901. doi: 10.1016/s0733-8619(05)70231-0. [DOI] [PubMed] [Google Scholar]
- Dekosky ST, Shih WJ, Schmitt FA, Coupal J, Kirkpatrick C. Assessing utility of single photon emission computed tomography Spect Scan in Alzheimer Disease correlation with cognitive severity. Alzheimer Dis Assoc Disord. 1990;4:14–23. doi: 10.1097/00002093-199040100-00002. [DOI] [PubMed] [Google Scholar]
- Imran MB, Kawashima R, Awata S, Sato K, Kinomura S, Ono S, Sato M, Fukuda H. Tc-99m HMPAO SPECT in the evaluation of Alzheimer’s disease: correlation between neuropsychiatric evaluation and CBF images. J Neurol Neurosurg Psych. 1999;66:228–232. doi: 10.1136/jnnp.66.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H, Chang CC, Kanno H, Yamamoto I. The relationship between cerebral blood flow and cognitive function in patients with brain insult of various etiology. J Clin Neurosci. 2004;11:138–141. doi: 10.1016/j.jocn.2003.02.013. [DOI] [PubMed] [Google Scholar]
- Shah R. Endothelins in health and disease. Eur J Int Med. 2007;18:272–282. doi: 10.1016/j.ejim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Wright CE, Fozard JR. Regional vasodilation is a prominent feature of the haemodynamic response to endothelin in anaesthetized, spontaneously hypertensive rats. Eur J Pharmacol. 1988;155:201–203. doi: 10.1016/0014-2999(88)90425-6. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Ferro CJ, O'Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone.[see comment]. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Schilling L. New developments in the understanding of cerebral vasoregulation and vasospasm—the Endothelin Nitric-Oxide Network. Cleve Clin J Med. 1995;62:105–116. doi: 10.3949/ccjm.62.2.105. [DOI] [PubMed] [Google Scholar]
- Crawford F, Suo ZM, Fang CH, Mullan M. Characteristics of the in vitro vasoactivity of β-amyloid peptides. Exp Neurol. 1998;150:159–168. doi: 10.1006/exnr.1997.6743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.