Abstract
Therapeutic revascularization with either exogenous angiogenic growth factors or vascular cells has yet to demonstrate efficacy in the clinic. Injection of angiogenic growth factors often produces unstable and abnormal blood vessels. Blood vascular networks derived from implanted endothelial cells persist only transiently due to the insufficient recruitment of perivascular cells. We hypothesize that a combination of the two approaches may act synergistically to yield a better result. To enhance the recruitment of perivascular cells, human umbilical vein endothelial cells were genetically modified to overexpress platelet-derived growth factor (PDGF)-BB. PDGF-BB overexpression promoted both proliferation and migration of perivascular precursor cells (10T1/2 cells) in vitro. When mock-infected endothelial cells were implanted alone in vivo, they formed transient blood vascular networks that regressed by day 30. PDGF-BB overexpression enhanced the survival of endothelial cells in vivo. However, the PDGF-BB-expressing vessel network failed to establish patent blood flow. Co-implantation of PDGF-BB-overexpressing endothelial cells with 10T1/2 cells paradoxically resulted in the rapid regression of the vascular networks in vivo. PDGF-BB stimulated the expression of both chemokine (C-C motif) ligand 2 (CCL2) and CCL7 in 10T1/2 cells and led to the increased accumulation of macrophages in vivo. These results suggest a potential negative interaction between angiogenic growth factors and vascular cells; their use in combination should be carefully tested in vivo for such opposing effects.
Angiogenesis, the growth of new blood vessels, is critical during tissue repair and regeneration.1 In cases of insufficient or impaired angiogenesis, the injured tissue remains dysfunctional and may suffer from irreversible damage.1 To combat this, two broad approaches have been used for enhancing angiogenesis: (i) delivery of pro-angiogenic genes (by gene transfer) or proteins (by bolus injection or controlled release devices) for endothelial and perivascular cell recruitment,2 and (ii) delivery of endothelial cells alone or together with perivascular cells.3
Therapeutic angiogenesis requires temporally and spatially orchestrated delivery of growth factors to form a functional and durable vasculature. A number of studies have shown that the use of a single angiogenic factor fail to form a mature and stable vasculature. For example, injection of an adenoviral vector expressing vascular endothelial growth factor (VEGF) into normal tissue leads to highly disorganized, leaky, and hemorrhagic vessels.4 Furthermore, VEGF can potentiate inflammation by increasing the expression of adhesion molecules or the release of chemokines.5,6,7,8 In addition, the resulting vessels are highly unstable and regress on removal of the angiogenic stimulus. Maturation and stabilization of new vessels requires perivascular cells.2,9 Perivascular cells such as vascular smooth muscle cells and pericytes are thought to provide structural integrity to the blood vessels, lay down the extracellular matrix, and provide necessary survival factors to the endothelial cells.2
One potential way to enhance vessel maturation is by sequentially delivering VEGF and platelet-derived growth factor (PDGF)-BB.10 Such a strategy was shown to induce mature vascular networks. However, the time required for host blood vessels infiltration by this strategy may limit the amount of ischemic tissue rescued. Another limitation with this type of therapeutic angiogenesis is that the neovascular response is reduced with age, and by underlying conditions such as hyperglycemia and atherosclerosis.11 Patients with diabetes mellitus or elevated homocysteine exhibit impaired angiogenic response.12,13
Alternatively, vascular cells can be directly injected or implanted in the ischemic tissue. We have previously shown that human umbilical vein endothelial cells (HUVECs) implanted in a collagen gel in severe combined immunodeficient (SCID) mice form functional engineered vessels. However, the vessels were only transiently perfused, did not recruit adequate number of perivascular cells from the host tissue, and regressed within a few weeks. In contrast, co-implanting HUVECs with mouse perivascular cell precursors allowed pericyte investment and the formation of stable, durable, and functional engineered vessels.14 Thus, providing exogenous perivascular cells overcame the inability of the implanted endothelial cells to recruit host perivascular cells within a critical timeframe for the vessels to become mature and stabilized. Nevertheless, translation of this approach will require sources of both endothelial and perivascular cells in sufficient numbers for cell-based therapy of ischemic tissues. So far, such sources remain elusive, despite promising but preliminary results obtained with bone marrow-derived precursor cells or embryonic stem cells.15,16
We hypothesized that a combination of cellular and molecular engineering approaches will act synergistically to yield the formation of a stable and durable network of blood vessels. To this end, we engineered endothelial cells to overexpress PDGF-BB, a mitogen and a chemoattractant for perivascular cells. We hypothesized that by increasing the levels of PDGF-BB expressed by endothelial cells, it may hasten the recruitment of perivascular cells from the host in an appropriate timeframe to stabilize the nascent blood vessels.
Materials and Methods
Cell Culture
HUVECs were obtained from Center of Excellence in Vascular Biology, Brigham and Women’s Hospital, Boston and maintained in Endothelial Cell Growth Medium (EGM) (Lonza, Basel, Switzerland). 293ET packaging cells were a kind gift from Dr. Brian Seed (Massachusetts General Hospital, Boston). 10T1/2 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). All cells were maintained at 37°C in a humidified 5% CO2 incubator.
Retrovirus Plasmid Construction
The enhanced green florescent protein (EGFP) retrovirus vector, PBMN-I-EGFP was kindly provided by Dr. Gary Nolan (Stanford, CA). Human PDGF-B cDNA was purchased from InvivoGen (San Diego, CA). Full length PDGF-B cDNA was subcloned into the retroviral vector by first using PCR to add BamHI and NotI restriction sites with the following set of primers: forward primer-5′-GAATTCGGATCCATGAATCGCTGCTGGGCG-3′ and reverse primer-5′-AAGCTTGCGGCCGCCTAGGCTCCGAGGGTCTC-3′. The PCR product was cut with BamHI and NotI restriction enzymes and then inserted into the multicloning sites of PBMN-I-EGFP vector.
Retrovirus Packaging and Transduction
For retrovirus packaging, the plasmids of PBMN-I-EGFP, Gag-pol, and VSVG (15 μg, 7 μg, and 5 μg, respectively) were mixed and co-transfected into 293ET cells with lipofectamine 2000 (Invitrogen, Carlsbad, CA) per manufacturer’s protocol. After overnight incubation, the 293ET cells were washed with PBS and then given Dulbecco’s Modified Eagle Medium 10% fetal bovine serum. The next day, the supernatant containing retrovirus was collected and fresh media was added; this step was repeated three more times. After the supernatant was collected, it was passaged through a 0.45 μm filter (Whatman, Brentford, UK) and was either used immediately for infection or kept at −80°C. For the transduction of HUVECs, the supernatant was first diluted 1:1 with fresh EGM and supplemented with polybrene (8 μg/ml). The diluted supernatant was then added to a subconfluent monolayer of HUVECs and allowed to incubate for 4 hours. Fresh EGM medium was exchanged at the end of the incubation period and this step was repeated 2 to 3 times on consecutive days. After 2 to 3 rounds of infection, more than 90% of HUVECs expressed the gene of interest as assessed by the expression of EGFP. HUVECs expressing EGFP only or co-expressing PDGF-BB and EGFP will be referred to as HUVEC-EGFP and HUVEC-PDGF-BB hereafter.
Protein Measurements by Enzyme-Linked Immunosorbent Assay
HUVECs were grown in gelatin coated 10 cm dishes. After 4 days of culture, secreted and cell-retained fractions of PDGF-BB were quantified by enzyme-linked immunosorbent assay per manufacturer’s instruction (R&D Systems, Minneapolis, MN). For measuring secreted PDGF-BB, supernatant was collected and filtered through a 0.22-μm filter before measurement. For cell retained PDGF-BB, HUVECs were first washed with PBS, and then incubated with 1M/L solution of NaCl for 30 minutes on ice. The salt solution disrupts the charge–charge interaction between PDGF-BB and the extracellular matrix. The salt solution was then filtered with a 0.22-μm filter before measurement.
Western Blot for PDGF-Rβ and VEGFR-2 Phosphorylation
For PDGF receptor (PDGFR) phosphorylation, 10T1/2 cells were starved overnight in 0.5% fetal bovine serum. The cells were then exposed to conditioned media from HUVEC-PDGF-BB for 5 minutes to stimulate the phosphorylation of PDGF receptor (PDGFR). The cells were washed three times with PBS containing 1 mmol/L NaVO4 and 50 mmol/L NaF and then were lysed with RIPA buffer containing protease inhibitor and phosphatase inhibitor. Immunoprecipitation was performed by the addition of 5 μl of anti-PDGFR-β antibodies (#3162) (Cell Signaling, Danvers, MA) to cell lysates containing 0.5 mg of total protein and was incubated overnight at 4°C. The next day, the antigen-antibody conjugate was precipitated with Agarose A/G. Phosphorylated PDGFR-β was immunoblotted with an anti-phosphotyrosine antibody conjugated with horseradish peroxidase (clone 4G10) (Upstate, Charlottesville, VA) and total PDGFR-β was immunoblotted with an anti-PDGFR-β antibody (#3162) (Cell Signaling). For VEGF receptor 2 (VEGFR-2) phosphorylation, EGFP-HUVEC and PDGF-BB-HUVEC cells were incubated in serum-free medium for 1 hour and then incubated with or without 50 ng/ml VEGF for 2 to 5 minutes. Then the cells were scraped from plates, pelleted, and resuspended in lysis buffer; 60 μg of protein per sample was separated on a 4% to 15% acrylamide gradient gel (Bio-Rad, Hercules, CA). The expression of phopho-VEGFR2, VEGFR2, and actin were detected by polyclonal antibodies against VEGFR2 (1:1000) and phosphorylated VEGFR2 (1:2000) (Cell Signaling), and by monoclonal antibodies against actin (1:5000) (Sigma).
Cell Proliferation Assay
The activity of the ectopically expressed soluble PDGF-BB in HUVECs was assayed by testing the ability of cell culture supernatant to induce the proliferation of 10T1/2 cells. Briefly, confluent monolayers of HUVECs that had been transduced with PDGF-BB or EGFP were incubated for 12 hours with Dulbecco’s Modified Eagle Medium containing 1% fetal bovine serum. The conditioned medium was filtered with a 0.22 μm filter and stored in −80°C until ready for use. 10T1/2 cells were plated in a 96 well plate at a density of 5000 cells/well. The next day, the medium in each well was removed and replaced with 100 μl of HUVECs conditioned medium and allowed to incubate for 24 hours. After 24 hours, 20 μl of 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (20 μg/ml, Sigma-Aldrich, St. Louis, MO) was added to each well and the plate was incubated further for 2 hours. The medium was then removed and replaced with 100 μl of dimethyl sulfoxide. The absorbance was read with a colorimetric plate reader at 550 to 655 μm wavelength.
Transwell Migration Assay
Cell migration was assessed using Falcon HTS FluoroBlok 24-well inserts (BD Biosciences, San Jose, CA) with 3-μm pores. EGFP-10T1/2 cells (2 × 104) suspended in 250-μl Basal Medium Eagle (Invitrogen) with 0.5% fetal bovine serum were placed inside each insert and 5 × 104 per well HUVECs suspended in 800 μl EGM (Lonza) were plated on a 24-well plate. The cells were kept overnight. The next day, the cell culture media of both HUVECs and 10T1/2 cells were changed to Basal Medium Eagle with 0.5% fetal bovine serum, and then the inserts were placed in the respective wells. At 4, 8, and 12 hours, the bottom of each insert was imaged in fluorescence using an inverted fluorescence microscope (Olympus IX70, Center Valley, PA) equipped with a motorized stage and motorized filter wheel (Improvision Inc, Lexington, MA). Transmigrated cells were quantified using ImageJ (http://rsb.info.nih.gov/ij/) by using threshold function and measuring the area covered by the migrated cells.
In Vitro Tube Formation Assay
In vitro tube formation was studied using previously described procedures.16 Matrigel (Collaborative Biomedical Products, Bedford, MA) was diluted with Endothelial Cell Growth Medium in 1:1 ratio. Next, 60 μl of the solution were added to each well of a 96-well plate and allowed to form a gel at 37°C for 30 minutes. EGFP-HUVEC and PDGFBB-HUVEC (20,000 cells) cells in 200 μl of complete medium were subsequently added to each well and incubated overnight at 37°C in 5% CO2. Under these conditions, both EGFP- and PDGFBB-HUVEC cells formed delicate networks of tubes that were fully developed after 16 hours.
TdT-Mediated dUTP Nick End Labeling Assay of HUVEC
In situ TdT-mediated dUTP nick end labeling (ApopTag peroxidase In situ detection kit, Chemicon, Temecula, CA) was used according to manufacturer’s instructions to identify apoptotic cells. EGFP-HUVEC and PDGFBB-HUVEC cells were cultured in serum-free medium, in complete medium, or under hypoxic condition (1% O2 to 5% CO2 balance N2 was used).
Tissue Engineered Blood Vessel Constructs
One million endothelial cells and 2 × 105 10T1/2 cells were suspended in 1 ml solution of rat-tail type 1 collagen (1.5 mg/ml) (BD Biosciences) and human plasma fibronectin (90 μg/ml) (Sigma) in 25 mmol/L Hepes (Sigma) buffered EGM medium at 4°C. The pH was adjusted to 7.4 by using 1N NaOH (Fisher Science, City, NJ). The cell suspension was pipetted into 12-well plates and warmed to 37°C for 30 minutes to allow polymerization of collagen. Each solidified gel construct was covered by 1 ml of warmed EGM medium. After 1 day culture in 5% CO2, a skin puncher was applied to create circular disk-shape pieces of the construct (4-mm diameter), and they were implanted into the cranial windows in SCID mice.14,17 Multiphoton laser-scanning microscopy was used to visualize and quantify the morphological changes in EGFP-expressing HUVECs. The perfused vessels were highlighted by tail vein injection of 1% tetramethylrhodamine-labeled dextran (MW 2,000,000), indicating the formation of functional engineered vessels14,17
The cord formation assay was performed identically as above except that the tissue engineered construct was allowed to culture in vitro. Images of the tissue-engineered construct were taken randomly at 3, 7, and 12 days with an inverted fluorescence microscope. The formation of vessel-like network was quantified by thresholding the image and measuring the area of vessel-like structures with Image J software.
PCR Array and Quantitative Real-Time PCR
10T1/2 cells were serum starved overnight and stimulated with 50 ng/ml of recombinant human PDGF-BB (R&D Systems) for 2 or 4 hours. Total RNA was isolated from 10T1/2 cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). Quantity and purity of RNA were determined by UV absorbance at 260 and 280 nm. For PCR array analysis, cDNA was first synthesized with RT2 First Strand Kit Array (SABiosciences, Frederick, MD) and the samples were then analyzed with Mouse Inflammatory Cytokines & Receptors RT2 Profiler PCR Array (SABiosciences). All procedures were performed according to manufacturer’s instructions. For real-time quantitative PCR, cDNA was synthesized using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). qRT-PCR was performed using the 7300 Real-Time PCR System and Power SYBR Green PCR Master Mix (Applied Biosystems). Primers were designed using Primer Express (Applied Biosystems) and purchased from Integrated DNA Technologies (Coralville, IA). Primer specificity for each gene of interest was confirmed by comparison with known sequences in the BLAST database (National Center for Biotechnology Information). Samples were analyzed in triplicates, and the gene expression level for each sample was normalized to the corresponding glyceraldehyde-3-phosphate dehydrogenase expression level, to control for loading differences. Negative controls were performed for each sample using non-reverse-transcribed RNA.
Results
Overexpression of PDGF-BB in HUVECs Enhanced 10T1/2 Cell Proliferation and Migration in Vitro
HUVECs were transduced with retroviral constructs to stably overexpress either PDGF-BB and EGFP or EGFP alone (Figure 1, A and B). The expression of PDGF-BB was quantified by enzyme-linked immunosorbent assay in the transduced HUVECs (Figure 1, C and D). In the case of HUVECs overexpressing PDGF-BB, we observed a trend of a higher ratio of soluble to cell-associated fraction of PDGF-BB, possibly due to saturation of binding sites on the cell surface (Figure 1E). Alternatively, the amount of cell-associated PDGF-BB might be underestimated since majority of PDGF-BB is secreted basally in endothelial cells.18 Overexpression of PDGF-BB did not significantly change the phenotype of the endothelial cells (ie, migration rate, VEGFR2 signaling, tube formation) (see supplemental Figures S1 and S2 at http://ajp.amjpathol.org). However, PDGF-BB overexpression reduced the proliferation rate of HUVECs (see supplemental Figure S1a at http://ajp.amjpathol.org).
Figure 1.
Retroviral transduction of HUVECs to overexpress PDGF-BB. HUVECs were transduced with retrovirus either to express EGFP only (A) or to co-express EGFP and PDGF-BB (B). Fluorescence images of EGFP expressing cells (green) with corresponding bright field image demonstrate that almost all of the HUVECs expressed EGFP. The amount of PDGF-BB secreted in the culture media (C) and bound to cell surface (D) was quantified by enzyme-linked immunosorbent assay. Ratio of secreted PDGF-BB in culture media to that on the cell surface showed more PDGF-BB was release in the media in endothelial cells overexpressing PDGF-BB (E). Scale bar = 50 μm. *P < 0.0001, **P < 0.05, N.S. = not significant.
Next, we examined the paracrine effects of PDGF-BB overexpression in endothelial cells on 10T1/2 cells, a line of mouse embryonic fibroblast that mimics the behavior of pericyte. HUVEC-PDGF-BB-conditioned medium stimulated the phosphorylation of the PDGF-Rβ in 10T1/2 cells and enhanced cell proliferation compared with control medium (Figure 2, a and b). Using Boyden chamber migration assay, we found that overexpression of PDGF-BB in HUVECs promoted the migration of 10T1/2 cells in vitro (Figure 2c). These findings demonstrated that the PDGF-BB overexpressed in HUVECs was functionally active on 10T1/2 perivascular cell precursors in vitro.
Figure 2.
In vitro activity of PDGF-BB on perivascular cells proliferation and migration. HUVEC conditioned media (CM) stimulated the phosphorylation of PDGF-Rβ in 10T1/2 cells (A). Endothelial growth medium was used as control. Conditioned medium from HUVECs overexpressing PDGF-BB enhanced the proliferation of 10T1/2 cells as quantified by 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide assay compared with mock transfected HUVECs (EGFP only) (B). Transwell migration assay was performed to assess HUVECs-induced migration of 10T1/2 cells (C). **P < 0.0001, *P < 0.005.
PDGF-BB Overexpression Enhanced HUVECs Survival in Vivo
To create engineered vessels, we implanted HUVEC-PDGF-BB cells in a fibronectin/collagen matrix onto the pial surface of the brain in a cranial window in SCID mice.14 For control, we implanted HUVEC expressing EGFP alone. We tracked the cells in vivo with multiphoton laser scanning microscopy and monitored the kinetics of blood vessel formation. In the group implanted with HUVEC-EGFP cells, the endothelial cells became elongated and interconnected, forming a mesh-like network on day 3 (Figure 3A). Multiple vacuoles were observed to have formed within the endothelial cells. By day 7, some of these vacuoles had coalesced into luminal structure (Figure 3A). On day 18, blood flow was seen within the lumen of the endothelium suggesting that the engineered blood vessels had formed functional connection to the host circulatory network (Figure 3A). Similar to previous results,14 engineered blood vessels derived from HUVEC only were unstable and most of the vessels had regressed by day 30 (Figure 3, A and C). In contrast, in animals implanted with HUVEC-PDGF-BB, the endothelial cells persisted beyond 30 days. Similar to the control group, HUVEC-PDGF-BB formed a mesh-like network with lumen inside it. However, few of these vascular structures were perfused with blood at day 30 (Figure 3, B and D).
Figure 3.
Effects of PDGF-BB overexpression on engineered blood vessels. HUVEC-EGFP (A) and HUVEC-PDGF-BB (B) were implanted in a collagen gel in cranial windows in SCID mice. Images were taken 3, 7, 11, 18, and 30 days after implantation with multiphoton laser scanning microscope to monitor the in vivo dynamics of neovascularization by the implanted endothelial cells (n = 5 for each experimental group). Densities of total engineered blood vessels (C) and functional engineered blood vessels (D) were quantified. Total engineered blood vessels denote all vessel-like structure derived from the implanted HUVECs. Functional engineered blood vessels denote HUVEC derived vessels that had blood flow as revealed by intravenously injected rhodamine-dextran contrast agent. Green, HUVECs expressing EGFP; red, functional blood vessels contrast-enhanced with rhodamine-dextran. Scale bar = 50 μm.
Co-Implantation of 10T1/2 Cells Promoted the Regression of PDGF-BB Overexpressing Endothelial Cells in Vivo
Next, we co-implanted HUVECs with 10T1/2 cells to determine whether exogenously supplied perivascular cell precursors could enhance the formation of functional engineered blood vessels and enhanced the anastamosis of HUVEC-PDGF-BB-derived vessels to the host circulation. In the control group with HUVEC-EGFP and 10T1/2 cells, HUVEC-EGFP formed a vessel-like network at day 4. By day 8, blood flow was detectable inside the newly formed lumens (Figure 4A). Nevertheless, we found that co-implantation of HUVEC-PDGF-BB and 10T1/2 cells led to an accelerated reduction in the density of HUVECs in vivo compared with the control (Figure 4B). Quantification of the total vessel length density showed a reduction in the number of HUVEC-derived vessels at day 4, and the density dropped precipitously thereafter (Figure 4, C and D).
Figure 4.
Effect of PDGF-BB overexpression and 10T1/2 cells on engineered blood vessels. HUVECs expressing EGFP only (A) or HUVECs co-expressing EGFP and PDGF-BB (B) were co-implanted with 10T1/2 cells in a collagen gel in cranial windows in SCID mice. Images were taken 4, 8, and 12 days after implantation with multiphoton laser scanning microscope to monitor the in vivo dynamics of vascularization by the implanted endothelial cells (n = 4 for each experimental group). Green, HUVECs expressing EGFP; red, functional blood vessels contrast-enhanced by rhodamine-dextran. Scale bar = 50 μm. Densities of total engineered blood vessels (C) and functional engineered blood vessels (D) were quantified. Total engineered blood vessels denote all vessel-like structure derived from the implanted HUVECs. Functional engineered blood vessels denote HUVEC derived vessels that had blood flow as revealed by rhodamine-dextran contrast agent. *P < 0.005, **P > 0.05, ***P < 0.0001.
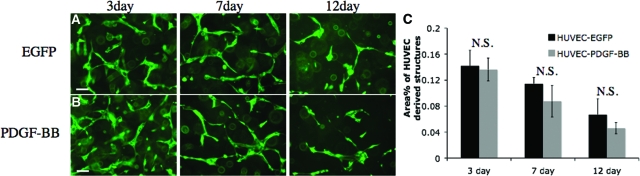
Next, we sought to determine whether this unexpected in vivo phenotype was due directly to the presence of 10T1/2 cells or indirectly through a change in the in vivo microenvironment. HUVEC-PDGF-BB and HUVEC-EGFP were incorporated with 10T1/2 cells in fibronectin/collagen matrix and cultured in vitro. After 3 days, we observed the formation of vessel-like structures by the HUVECs similar to those formed in vivo (Figure 5, A and B). There was no significant difference in the ability of HUVEC-PDGF-BB and HUVEC-EGFP to morph into the vessel-like structures. The vessel-like structures in both groups regressed at similar rate with the progression of time and by day 12, only a few of these structures remained in the collagen gel (Figure 5C). These data suggest HUVEC-PDGF-BB and HUVEC-EGFP—when co-cultured with 10T1/2 cells—morphed into vessel-like structures with similar efficiency in vitro. The inability of co-implanted HUVEC-PDGF-BB and 10T1/2 cells to form stable blood vessels in vivo is likely secondary to in vivo alterations of the local microenvironment by the overexpression of PDGF-BB.
Figure 5.
In vitro vessel formation by endothelial cells. HUVECs expressing EGFP only (A) or HUVECs co-expressing EGFP and PDGF-BB (B) were incorporated with 10T1/2 cells in collagen/fibronectin gel. Images were taken with fluorescence microscope at days 3, 7, and 12. No apparent difference was observed in the ability of HUVEC-EGFP and HUVEC-PDGF-BB to form vessel-like structure in vitro. The vessel-like structures were quantified (C). Scale bar = 50 μm.
PDGF-BB Stimulation of 10T1/2 Cells Altered the in Vivo Microenvironment by Increasing Local Inflammation
We next tested the new hypothesis that the PDGF-BB-induced change in microenvironment caused the regression of the implanted endothelial cells. We implanted two groups of mice with genetically modified endothelial cells. In the experimental group, we co-implanted HUVEC-PDGF-BB, HUVEC-DsRed, and 10T1/2 cells. In the control group, we co-implanted HUVEC-EGFP, HUVEC-DsRed, and 10T1/2 cells. In the control group, we observed that both HUVEC-EGFP and HUVEC-DsRed formed vessel-like network and persisted for more than 11 days (Figure 6). In contrast, HUVEC-DsRed co-implanted with HUVEC-PDGF-BB and 10T1/2 cells showed a much smaller number of cells surviving at day 11. This result suggests that 10T1/2 cells stimulated by PDGF-BB caused the regression of the implanted endothelial cells irrespective of their specific genetic modification.
Figure 6.
Combined effects of PDGF-BB and 10T1/2 cells on endothelial cells in vivo. HUVECs expressing DsRed express and 10T1/2 cells were implanted either with HUVEC-EGFP or HUVEC-PDGF-BB in a collagen gel in cranial windows in SCID mice. Images were taken 4, 7, and 11 days after implantation with multiphoton laser scanning microscope. Green, HUVECs expressing EGFP only or co-expressing PDGF-BB and EGFP; red, HUVECs expressing DsRed express. Scale bar = 100 μm.
To examine the effects of PDGF-BB stimulation on 10T1/2 cells, we performed PCR array analysis on 10T1/2 cells with or without PDGF-BB stimulation (see supplementary Table S1 at http://ajp.amjpathol.org). Initial screening revealed that CCL2 (MCP-1), CCL7 (MCP-3), and Spp1 were up-regulated. We confirmed the up-regulation of CCL2 and CCL7 by quantitative real-time PCR (Figure 7, A and B). Since both CCL2 and CCL7 are critical chemokines for monocyte/macrophage recruitment, we performed immunohistochemistry for infiltration of inflammatory cells. We found a significantly higher number of F4/80 positive macrophages in animals implanted with HUVEC-PDGF-BB and 10T1/2 cells (Figure 7, C–E). This finding suggests that PDGF-BB overexpression leads to an increased in local inflammation.
Figure 7.
Effects of PDGF-BB stimulation on 10T1/2 cells Quantitative real-time PCR analysis of 10T1/2 cells simulated with 50 ng/ml of rhPDGF-BB for 2 and 4 hours (A, B). Glyceraldehyde-3-phosphate dehydrogenase was used to normalize gene expression level. F4/80 staining of macrophage in collagen gel implanted with either HUVEC-EGFP (C) and 10T1/2 cells or HUVEC-PDGF-BB (D) and 10T1/2 cells. Collagen gels were extracted for immunostaining 8 days after implantation. Quantification of F4/80 stained area fraction in collagen gel (E). Scale bar = 100 μm. *P < 0.05.
Discussion
Therapeutic angiogenesis has great promise to alleviate tissue ischemia and to help repair damaged tissues. Presently, a number of clinical trials are testing different angiogenic agents, such as VEGF and basic fibroblast growth factor, to induce new blood vessels in poorly perfused tissue with the ultimate goal of improved tissue and organ function.11 In parallel, cell-based therapy with bone marrow-derived progenitor and/or embryonic stem cells is also being investigated for regenerative medicine.15,16 However, it is currently unknown whether a combination of these two approaches can be synergistic, and how to combine them to generate functional and stable vessels. In this report, we tested the hypothesis that implanting endothelial cells that overexpress PDGF-BB—a factor known to promote perivascular cell recruitment—can create a stable and long-lasting network of blood vessels. Our in vitro results demonstrated that PDGF-BB overexpression in HUVEC promoted, as expected, the proliferation and migration of 10T1/2 cells. Moreover, implantation of HUVEC-PDGF-BB alone showed an increase in the survival of vessel-like structure in vivo. However, such structures were rarely functional even after 30 day in vivo. In addition, co-implantation of HUVEC-PDGF-BB with 10T1/2 cells led to an accelerated regression of the endothelial cell cords in vivo. Almost all of the implanted endothelial cells disappeared by day 12.
PDGF-BB was previously implicated to mediate vessel maturation. In vitro studies suggest that PDGF-BB is involved in the recruitment of perivascular cells.19 This was further confirmed by genetic models of knockout of the ligand (PDGF-BB) and the receptor (PDGF-Rβ).20 These two knockout mice share similar phenotypes. Both knockout mice die in utero and their blood vessels are hemorrhagic and contain a reduced number of perivascular cells. Specific ablation of PDGF-BB in endothelial cells causes abnormality in the vasculature of the heart, kidney, and brain.21 These data suggest that endothelial cell derived PDGF-BB is necessary for the maturation of blood vessels during development. Recent study has further shed light into complexity of the regulation of PDGF-BB activity—it was shown that the spatial distribution of PDGF-BB is critical for proper perivascular cell recruitment.22,23 PDGF-BB contains, at its carboxyl terminal end, a sequence of positively charged amino acids that is termed the retention sequence. It is believed that the retention sequence of PDGF-BB interacts with the negatively charged components of extracellular matrix such as heparan sulfate on the cell surface. Normally, most of the secreted PDGF-BB is retained at the cell surface, thereby limiting their actions to nearby cells only. Mice that have been genetically engineered to have PDGF-BB deficient in retention sequence exhibit marked abnormality in the brain blood vessels due to a reduction in perivascular cells.22
Based on these observations, we reasoned that incorporating PDGF-BB directly in the tissue-engineered construct might disrupt the spatial distribution of PDGF-BB. Disabling the local chemotactic gradient of PDGF-BB has been shown to reduce vascularization.24 Therefore, we engineered the implanted endothelial cells to overexpress PDGF-BB to establish a local chemotactic gradient of PDGF-BB that is critical for pericyte recruitment in nascent vessels.25
However, unexpectedly, we found a contextual effect of perivascular cell precursors in this system. We found a substantial difference in the vascular network formation in vitro and in vivo. These differences may be in part explained by the recruitment of bone marrow-derived cells. Stimulation of 10T1/2 cells with PDGF-BB significantly up-regulated the expression of MCP-1 and this in turn led to an increase in the accumulation of cells of the monocyte/macrophage lineage. Monocytes/macrophages may promote or inhibit angiogenesis, depending on their subtypes (M1/M2). M2 cells are pro-angiogenic and have been implicated in promoting tumor growth through the release of angiogenic factors (ie, VEGF and PDGF).26,27 In contrast, M1 cells are cytotoxic with the production of high levels of tumor necrosis factor-α, and they directly induce apoptosis in endothelial cells through the release of reactive oxygen radicals.27,28,29
The level and the timing of PDGF-BB expression may also not be optimal for vascular engineering (Figure 8A–C). First, increased expression of PDGF-BB may saturate the protein binding sites on the cell surface and potentially disrupt the gradient of PDGF-BB near endothelial cells. Second, the increase in 10T1/2 cell proliferation in response to PDGF-BB altered the optimal ratio of 10T1/2 cells to HUVECs. 10T1/2 cells suppressed proliferation/survival of HUVECs when they were co-cultured in a 1:1 ratio.30 Third, PDGF-BB is a well-known dedifferentiating factor for smooth muscle cells.31 Stimulation of smooth muscle cells with PDGF-BB reduces the expression of smooth muscle markers including α-smooth muscle actin and SM-MHC. PDGF-BB was also shown to stimulate the expression of VEGF in 10T1/2 cells.32 Recent data suggest VEGF may negatively regulate pericyte coverage in nascent blood vessels by disrupting PDGF-Rβ signaling in pericytes.33 Thus, the constitutive expression of PDGF-BB in endothelial cells may potentially inhibit mural cell differentiation and result in the destabilization of nascent blood vessels. Temporally and spatially regulated PDGF-BB expression may overcome some of these problems. These unclear mechanisms notwithstanding, we showed that local and sustained expression of PDGF-BB in endothelial cells resulted in the rapid regression of the engineered microvascular network.
Figure 8.
Schematic of the effects of PDGF-BB overexpression HUVECs when implanted alone regressed spontaneously due to insufficient pericyte recruitment (AI, BI, CI). Co-implantation of 10T1/2 cells with HUVECs led to the formation of a stable and functional microvascular network by differentiating into perivascular cells (AIII, BIII, CIII). HUVECs overexpressing PDGF-BB remained viable in vivo, however they did not form functional anastamoses to the host circulation (AII, BII, CII). Co-implantation of 10T1/2 cells with PDGF-BB expressing HUVECs did not rescue the phenotype. Instead, they hastened the regression of the implanted HUVECs (AIV, BIV, CIV). Constitutive expression of PDGF-BB in HUVECs stimulated the expression of MCP1 (purple dots) in 10T1/2 cells. MCP1 attracted the infiltration of monocytes/macrophages (blue cells) into the collagen matrix and they potentially induced the regression of HUVECs by an unknown mechanism.
In summary, our findings reveal the complexity of the in vivo systems even when well-characterized cells and growth factors are used in combination. They further demonstrate a need to determine the optimal growth factors as well as the dosage, and spatial and temporal distribution of cellular and molecular players for therapeutic angiogenesis.
Supplementary Material
Footnotes
Address reprint requests to Rakesh K. Jain, Department of Radiation Oncology, Massachusetts General Hospital, 100 Blossom Street, Cox-7, Boston, MA 02114. E-mail: jain@steele.mgh.harvard.edu.
Supported in part by American Heart Association Predoctoral Fellowship (PA) and National Institutes of Health grants P01-CA80124 (RKJ, DF), R01-CA96915 (DF), R01-CA115767 (RKJ), R01-CA126642 (RKJ), R01-CA85140 (RKJ), and Federal Share/NCI Proton Beam Program Income Grants (RKJ, DGD).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nature Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;277:10445–10451. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- Heil M, Clauss M, Suzuki K, Buschmann IR, Willuweit A, Fischer S, Schaper W. Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur J Cell Biol. 2000;79:850–857. doi: 10.1078/0171-9335-00113. [DOI] [PubMed] [Google Scholar]
- Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2:992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, Shimada T, Imaizumi T. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2000;20:2579–2585. doi: 10.1161/01.atv.20.12.2579. [DOI] [PubMed] [Google Scholar]
- Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nature Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- Zerwes HG, Risau W. Polarized secretion of a platelet-derived growth factor-like chemotactic factor by endothelial cells in vitro. J Cell Biol. 1987;105:2037–2041. doi: 10.1083/jcb.105.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF. TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest. 2003;112:1134–1136. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetow BS, Crosby JR, Kaminski WE, Ramachandran RK, Lindahl P, Martin P, Betsholtz C, Seifert RA, Raines EW, Bowen-Pope DF. Platelet-derived growth factor B-chain of hematopoietic origin is not necessary for granulation tissue formation and its absence enhances vascularization. Am J Pathol. 2001;159:1869–1876. doi: 10.1016/S0002-9440(10)63033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wu KH, Poon MC, Han ZC. Endothelial progenitor cells transfected with PDGF: cellular and molecular targets for prevention of diabetic microangiopathy. Medical Hypotheses. 2006;67:1308–1312. doi: 10.1016/j.mehy.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Berger MS, Pieper RO. Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growth and angiogenesis. J Biol Chem. 2006;281:37256–37264. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- Woller G, Brandt E, Mittelstadt J, Rybakowski C, Petersen F. Platelet factor 4/CXCL4-stimulated human monocytes induce apoptosis in endothelial cells by the release of oxygen radicals. J Leukoc Biol. 2008;83:936–945. doi: 10.1189/jlb.0907592. [DOI] [PubMed] [Google Scholar]
- McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, Bucana C, Ellis LM. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117:2114–2122. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.