Abstract
Acute respiratory distress syndrome is characterized by loss of lung tissue as a result of inflammation and fibrosis. Augmenting tissue repair by the use of mesenchymal stem cells may be an important advance in treating this condition. We evaluated the role of term human umbilical cord cells derived from Wharton’s jelly with a phenotype consistent with mesenchymal stem cells (uMSCs) in the treatment of a bleomycin-induced mouse model of lung injury. uMSCs were administered systemically, and lungs were harvested at 7, 14, and 28 days post-bleomycin. Injected uMSCs were located in the lung 2 weeks later only in areas of inflammation and fibrosis but not in healthy lung tissue. The administration of uMSCs reduced inflammation and inhibited the expression of transforming growth factor-β, interferon-γ, and the proinflammatory cytokines macrophage migratory inhibitory factor and tumor necrosis factor-α. Collagen concentration in the lung was significantly reduced by uMSC treatment, which may have been a consequence of the simultaneous reduction in Smad2 phosphorylation (transforming growth factor-β activity). uMSCs also increased matrix metalloproteinase-2 levels and reduced their endogenous inhibitors, tissue inhibitors of matrix metalloproteinases, favoring a pro-degradative milieu following collagen deposition. Notably, injected human lung fibroblasts did not influence either collagen or matrix metalloproteinase levels in the lung. The results of this study suggest that uMSCs have antifibrotic properties and may augment lung repair if used to treat acute respiratory distress syndrome.
An enduring problem in respiratory and critical medicine is the treatment of acute respiratory distress syndrome (ARDS)/acute lung injury, a condition that is characterized by refractory hypoxemia in patients with bilateral lung infiltrates in the absence of pulmonary edema.1 A National Institutes of Health study estimated the incidence of acute respiratory distress syndrome/acute lung injury to be 75 per 100,000 population in the United States with 40 to 60% mortality.2 ARDS may be the end result of several conditions that directly injure the lung such as pneumonia, pulmonary contusion, inhalational injury, and near drowning.3 Generic injury to the lung results in damage to the epithelial and endothelial cells and a compromised alveolar-capillary barrier. There is exudation of fluid into the alveolar space followed by inflammatory cells, a process driven by cytokines such as interleukin (IL)-8, tumor necrosis factor (TNF)-α and IL-1. The progression of acute lung injury to fibrosis portends a poor prognosis and may be observed as early as 5 to 7 days after injury.4 Many strategies have been directed at augmenting repair of ARDS. These include improved ventilation techniques, surfactant therapy, vasodilators, and anti-inflammatory agents.1 Notably, there has been an increasing focus on the acceleration of resolution by epithelial restitution and the consequent reduction in fibrosis of ARDS.
To this end, new stem cell therapies have raised the possibility of improving lung repair. Mesenchymal stem cells (MSCs)5 are multipotent and differentiate into a range of cell types and are being tested for their regenerative potential, particularly in myocardial infarction and some neurodegenerative disorders.6 MSCs are adherent cells and a common MSC immuno-phenotype can be identified in cells from many sources including bone marrow, umbilical cord blood, and adult organs.7
The role of MSCs in the treatment of lung injury has been the subject of several studies. Indeed, MSCs have displayed the potential to improve lung function in pulmonary disease through several mechanisms. Murine bone marrow MSCs (bmMSCs) have been shown to selectively home to sites of injury through the chemokine receptor CXCR4 and chemokine, Stromal derived factor as well as Flk surface receptors8,9 and improved respiratory capacity in bleomycin, lipopolysaccharide, and monocrotaline-induced models of lung injury.10,11,12 Furthermore, both in vivo and in vitro studies have shown that murine and human bmMSCs and human umbilical cord blood cells may differentiate into cells with markers of lung epithelium.13,14 Aguilar et al supported the safety profile of human MSCs by demonstrating that murine bmMSCs but not human bmMSCs differentiated into osteosarcomas when injected into the lung.15
Based on these studies, we hypothesized that MSCs derived from the Wharton’s jelly of the umbilical cord (uMSCs) would repair lung injury and prevent fibrosis. The umbilical cord is derived from the extraembryonic mesoderm and develops from the proximal epiblast during the formation of the embryonic primitive streak.16 The umbilical cord contains two arteries and a vein that are surrounded by a matrix rich in hyaluronic acid known as Wharton’s jelly (WJ). Recently, groups have cultured MSCs from the WJ of the umbilical cord and differentiated them in vitro into several tissue types.17,18 These cells have the advantage of ready availability, do not require invasive bone marrow biopsies, and are more plentiful than umbilical cord blood-derived MSCs. In the present study, we examined the therapeutic potential of uMSCs in a bleomycin-induced model of lung injury that shares many features in common with the phenotype of ARDS in human subjects.
Materials and Methods
The Human and Animal Research Ethics Committees of the Alfred Hospital and Monash University, Melbourne, Australia approved the study.
Isolation and Culture of uMSCs
Human uMSCs were isolated from the Wharton’s jelly of the umbilical cord by methods previously described.18 Umbilical cords were obtained with consent from women undergoing normal vaginal delivery at term (n = 12). The cords were dissected and the blood vessels removed. The remaining WJ was cut into small pieces (1–2 cm2) and placed in six-well plates (12 cm) in the presence of Dulbecco’s modified Eagle’s medium supplemented with 20% fetal calf serum (Invitrogen, Sydney, Australia) and antibiotics (penicillin, 100 μg/ml; streptomycin, 10 μg/ml; and amphotericin, 250 μg/ml) (Invitrogen). After 5 days in culture, adherent cells were observed proliferating from individual explanted tissue of the WJ. At this time, the WJ was removed from culture and the adherent cells were cultured to confluence. The cells were then trypsinized (0.25% trypsin:EDTA solution; Invitrogen), washed in phosphate-buffered saline and sorted for a homogenous CD73+CD31−CD34−CD45− phenotype by fluorescent activated cell sorting (FACS F1000, Becton Dickinson). The CD73+CD31−CD34−CD45− cells were expanded in cell culture and then further characterized by flow cytometry studies (Table 1). Primary antibodies were purchased from Santa Cruz Biotechnology and secondary antisera from Becton Dickinson (North Ryde, Australia). Hematopoeitic cells were used as positive controls. The expanded populations of CD73+CD31−CD34−CD45− cells that demonstrated all of the markers of MSCs (Table 1) were then subjected to in vitro differentiation into osteoblastic or lung epithelial phenotypes17 and injection into the bleomycin-induced model of lung injury.
Table 1.
Positive and Negative Markers on the Surface of uMSCs
| Marker | Positive |
|---|---|
| CD45 | Absent |
| CD34 | Absent |
| CD31 | Absent |
| CD73 | Present |
| CD105 | Present |
| CD14 | Absent |
| HLA A,B,C | Present |
| HLA DR | Absent |
| CD28 | Absent |
| CD80 | Absent |
| CD66 | Absent |
| CD90 | Present |
| CD166 | Present |
| CD86 | Absent |
In Vitro Differentiation Assays
At passage 3 to 6, uMSCs were placed on 13-mm culture dishes (Sigma-Aldrich, St. Louis, MO) and propagated to 80% confluence in Dulbecco’s modified Eagle’s medium and 20% fetal calf serum. The medium was then changed to the osteogenic stem cell kit containing Mesencult MSC basal media and osteogenic supplements β-glycerol sulfate, ascorbic acid, and dexamethasone (Stem Cell Technologies, Vancouver, Canada). The medium was changed every 2 days and cultures observed for bone nodule formation. The cells were then fixed in Karnovsky fixative and subjected to Von Kossa staining.17
For differentiation into lung epithelial cells, uMSCs (105 per well) were seeded onto type IV collagen (Roche Diagnostics, Indianapolis, IN) coated six-well plates and cultured in small airway growth medium (SAGM) (Clonetics, Baltimore, MD). Control cultures were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal calf serum. Cultures were maintained for 4 weeks. Following culture in SAGM, uMSCs (2.5 × 105) were incubated with antibodies against caveolin (1:100), aquaporin-5 (1:100), and surfactant protein-C (1:50), markers of alveolar epithelium, for 1 hour at room temperature. After several washes, cells were incubated with donkey anti-goat (aquaporin-5, surfactant protein-C) secondary antibodies for 30 minutes at room temperature. Cells were washed to remove excess secondary antibodies and analyzed by flow cytometry. Primary antibodies were purchased from Santa Cruz Biotechnology and secondary antisera from Becton Dickinson.
Injection of uMSCs into SCID Mice
There were five treatment groups of mice. Group 1 received saline intranasally and saline via the tail vein (tv), group 2 received saline (intranasally) and uMSCs (tv), group 3 received bleomycin (intranasally) and saline (tv), group 4 received bleomycin (intranasally) and uMSCs (tv), and group 5 received bleomycin (intranasally) and fibroblasts (tv). Each group had n = 8 mice and were analyzed at 7, 14, and 28 days post-bleomycin injection making a total of 40 × 3 = 120 mice. Bleomycin sulfate (0.15 mg) in saline (Calbiochem, San Diego, CA) was administered intranasally under weak ether anesthesia to induce pulmonary fibrosis in 8-week-old SCID mice.19 Twenty-four hours after bleomycin or saline instillation, mice were injected intravenously with uMSCs (1 × 106 in 0.2 ml of saline), saline (0.2 ml), or lung fibroblasts (1 × 106 in 0.2 ml of saline) via the tail vein. The injection within 24 hours of bleomycin injection was chosen to optimize cell incorporation into the lung during early inflammation. In preliminary experiments, 1 × 106 was the maximum number of cells that could be injected into the bleomycin-treated mice without significantly affecting mortality. There was no mortality associated with bleomycin administration or tail vein injection of uMSCs. The uMSCs were used at early passage 3 to 6 and comprised the CD73+CD31−CD34−CD45− expanded populations that were fluorescence-activated cell sorted as previously described. The human lung fibroblast cells were obtained from lung resections and a generous gift obtained from Prof. Philip Bardin, Monash Medical Centre, Melbourne. Human lung fibroblasts were expanded in culture in 10% fetal calf serum and Dulbecco’s modified Eagle’s medium and used as cell injection controls, since they exhibit a mesenchymal phenotype and are central to lung repair. As such, the fibroblasts may induce a similar response to uMSCs. Animals were sacrificed after 7, 14, or 28 days of treatment, since these time points represent the phases of maximal inflammation (7 and 14 days) and fibrosis (28 days). Lobes of the lung tissue were weighed, snap frozen, and stored at −80°C or fixed in 10% formalin for paraffin embedding. The lobes from each pair of lungs obtained were divided into the various assays; therefore each mouse was analyzed for all of the assays described.
Immunohistochemical Analysis of Lung Tissue
Paraffin-embedded tissue sections of 3 μm in thickness were dewaxed and rehydrated. Antigen retrieval was performed by heating in citrate buffer. After quenching endogenous peroxidase activity and blocking nonspecific binding, sections were incubated with antibodies against human anti-mitochondrial antibodies (1:10, Serotec) and human surfactant protein-C (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 37°C. Isotype-matched mouse IgG2a and goat IgG were applied to sections serving as negative controls. Antibody binding was detected using horseradish peroxidase-labeled biotin-streptavidin secondary antibodies (LSAB kit, Dako, CA, and immunostaining visualized using 3,3′-diaminobenzidine chromogen (Dako). Co-localization of anti-mitochondrial antibodies and surfactant protein-C was assessed by examining serial tissue sections. For fibrosis, slides were scored by the Ashcroft method for fibrosis,20 and the score for alveolar and interstitial infiltration of the lung was based on a method by Raisberg et al.21
Analysis of Cytokine and Collagen I mRNA Expression
Total RNA was extracted from lungs of SCID mice after 2 weeks of receiving bleomycin with or without uMSCs (n = 8/group), since this represented a phase of maximal inflammation. Following DNase treatment, 1 μg of RNA was converted to cDNA with Superscript III as described by the manufacturer (Invitrogen). The cDNA (diluted 1:20) was amplified with polymerase chain reaction (PCR) primers against IL-1: reverse, 5′-CATCAGAGGCAAGGAGGAAA-3′; forward, 5′-CCCAAGCAATACCCAAAGAA-3′; IL-2 forward, 5′-ATGTACAGCATCCAGCTCGCATC-3′; reverse, 5′-GGCTTGTTGAGATGATGCTTTGACA-3′; IL-6 forward, 5′-TTCCATCCAGTTGCCTTCTT-3′; reverse, 5′-ATTTCCACGATTTCCCAGAG-3′; IL-10 forward, 5′-TTGAGTCTGCTGGACTCCAGGACCTAGACA-3′; reverse, 5′-GCAGCCAAACAATACACCATTCCCAGAGG03′; IFNγ forward, 5′-CCCAAGCAATACCCAAAGAA-3′; reverse, 5′-CATCAGAGGCAAGGAGGAAA-3′; TNFα forward, 5′-GCCTCTTCTCATTCCTGCTT-3′; reverse, 5′-CACTTGGTGGTTTGCTACGA-3′; TGFβ forward, 5′-CTACTGCTTCAGCTCCACAG-3′; reverse, 5′-GCACTTGCAGGAGCGCAC-3′; MIF forward, 5′-TGACTTTTAGCGGCACGAAC-3′; reverse, 5′-CTCAAGCGAAGGTGGAAC-3′; MIP forward, 5′-CCACTGCCCTTGCTGTTCTTCTCT-3′; reverse, 5′-CAGGCATTCAGTTCCAGGTCAGTG-3′. For mouse collagen type 1 α 1 (col 1a) the TaqMan probe was obtained from Applied Biosystems (Mm 00801666_g1). Cycling parameters used for quantitative PCR were: denaturation at 95°C for 7 seconds, annealing 60°C for 7–15 seconds, and extension at 72°C for 15 seconds for 40 cycles. After normalizing data to β-actin, expression relative to saline treated control mice was calculated by the ΔΔCt method.22
Enzyme-Linked Immunosorbent Assay for Transforming Growth Factor (TGF)-β1
Mouse lung tissue was lysed in buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Triton X-100, 0.5% Tween 20, 0.1% sodium dodecyl sulfate, 1 mmol/L EDTA) containing a protease inhibitor cocktail (Roche, Mannheim, Germany). Samples were then sonicated, followed by centrifugation at 14,000 × g for 15 minutes to remove insoluble debris. TGF-β1 content of lung lysates was measured using a mouse TGF-β1 enzyme-linked immunosorbent assay kit (R&D Systems Inc., Minneapolis, MN) The total protein content of the lysates was determined using a bicinchoninic acid assay (Thermo Scientific, Rockford, IL), and results are expressed as TGF-β1 concentration standardized to total lung weight (pictograms per milligram of lung).
Western Blot Analysis
Total protein was extracted from similar regions of the lung using Trizol reagent according to the manufacturer’s instructions (Life Technologies, Gaithersburg, MD) and analyzed by the Bio-Rad dye-binding protein assay (Bio-Rad, Richmond, CA). Protein extracts (in 1% sodium dodecyl sulfate; 30 μg of total protein/lane) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12.5% acrylamide gels, as previously described.23,24 Western blot analysis was performed with antibodies to phospho-Smad2 and Smad2 (Cell Signaling Technology, Danvers, MA), matrix metalloproteinase (MMP)-13 (Calbiochem, San Diego, CA), and a goat anti-rabbit secondary antibody (1:1000 dilution; Bio-Rad). Blots were probed with the ECL detection kit (Amersham Pharmacia Biotech Ltd, Buckinghamshire, UK), according to the manufacturer’s instructions, before being quantified by densitometry, using a Bio-Rad GS710 calibrated imaging densitometer and Quantity-One software (Bio-Rad). Equal loading of samples was verified by Coomassie blue staining of proteins.
Collagen Concentration
The collagen content in lungs of mice was measured using the hydroxyproline assay as described previously.25 Briefly, lungs were lyophilized, hydrolyzed in HCl, and the absorbance measured at 558 nm using a Varian series 634 digital spectrophotometer (Varian Australia Pty Ltd, Melbourne, Australia). Collagen content was calculated by multiplying the hydroxyproline measurements by 6.94 and then expressed as a proportion of the dry weight tissue to yield collagen concentration.24
MMP Activity and Tissue Inhibitors of Matrix Metalloproteinase (TIMP) Expression
MMPs were extracted from tissue obtained from the five groups of mice (n = 6/group). MMP-1, MMP-2, and MMP-9 activity was assessed by gelatin zymography as described previously.26 Zymographs were analyzed by densitometry and gelatinase activity quantified as described above. The zymography densitometry data were expressed as the relative mean ± SEM of the saline-treated control group, which was expressed as 1.
TIMP-1, TIMP-2, TIMP-3, and TIMP-4 mRNA expression in lung tissue was measured by quantitative real-time PCR as described above. Reagents, primers, and TaqMan probes were purchased from Applied Biosystems (Mm00441818_m1, Mm00441825_m1, Mm00441826_m1, Mm00446568_m1). After normalizing data to β-actin, expression relative to saline-instilled mice was calculated by the ΔΔCt method.26
Reverse zymography of lung tissue extracts from the various groups was also performed to measure TIMP-1 and TIMP-2 protein expression, as described previously.27 Purified TIMP-1 (Calbiochem, Kilsyth, Victoria, Australia) and TIMP-2 (Chemicon International, Temecula, CA) standards were included in all zymographs, while densitometry of the TIMP bands was performed as described above.
Statistical Analyses
Analysis of treatment effects between the different groups was performed using a one-way analysis of variance analysis and Dunnet’s post hoc test with significance accorded when P < 0.05.
Results
uMSC Phenotype
After 5 days of culture, cells were observed proliferating from individual explanted tissue of the WJ. Initial cultures contained a population of adherent fibroblast-like cells with long and short processes (Figure 1A). Fluorescence-activated cell sorting demonstrated that 98 ± 1% of the adherent cells expressed the typical mesenchymal pattern of markers CD73+CD31−CD34−CD45− (Figure 1B). The cells were further characterized with several other markers (Table 1).
Figure 1.
The expansion and characterization of umbilical cord mesenchymal stem cells. A: Wharton’s jelly was dissected from the umbilical cord and placed in culture wells. The cells derived from the Wharton’s jelly were trypsinized following growth from the explanted tissue and cell sorted for a CD31−CD34−CD45−CD73+ population that were further propagated in culture. Characterization of the uMSCs demonstrates a fibroblastic appearance. B: Flow cytometry confirms the uMSCs are a homogenous cell population of CD31−CD34−CD45−CD73+ cells. C: Following propagation to confluence, uMSCs were cultured in conditioned media that would encourage the growth of osteoid tissue. Following 14 days in culture, Von Kossa staining (black) demonstrates calcium deposition that is suggestive of osteoblastic activity and bone formation. D: uMSCs were injected into the tail vein 24 hours following bleomycin injury. Using a specific anti-human anti-mitochondrial antibody, we isolated the injected uMSCs to the fibrotic areas of injury (brown staining and arrows) and to alveolar areas of the lung. Magnifications: ×50, ×100, and ×200.
Osteogenic differentiation was demonstrated between passages 3 to 6. After 7 to 14 days in conditioned media of osteogenic supplements, bone nodule formation was observed. The cells surrounding the nodule were fibroblastic in appearance, while the cells in the center were polygonal. In addition, the Von Kossa stain for calcium deposition revealed strong staining (Figure 1C).
uMSCs Do Not Differentiate into Lung Epithelial Cells in Vitro
Noting the differentiation properties of uMSCs, these cells were cultured in SAGM for 14 and 28 days. SAGM has been shown to induce the differentiation of embryonic and umbilical cord blood-derived mesenchymal stem cells into type II pneumocytes.28,29 This medium contains hydrocortisone, human epidermal growth factor, and retinoic acid, which are factors that are required for lung development.30 uMSCs did not survive beyond 14 days in SAGM. The cellular extensions of the uMSCs retracted and the cells detached from the culture wells.
uMSCs in Lungs of Mice 14 Days after Injection
The bleomycin-induced model of lung fibrosis was established in the SCID model as demonstrated histologically by a significant pneumonitis, inflammatory exudates, fibroblastic foci, and distortion of the normal architecture of the lung at 14 days (Figure 1D and Figure 2A) and 28 days (Figure 2A). The uMSCs (1 × 106) injected into the tail veins of mice were identified by a specific human anti-mitochondrial antibody. uMSCs were found in the mouse lung at 14 days, but there was no evidence of their presence at 28 days following tail vein injection. Of note, uMSCs were localized to fibrotic foci and nonfibrotic alveolar regions of the lung at 14 days following bleomycin injury (Figure 1D). The cells constituted 15 ± 5 cells/200 cells counted within fibrotic foci and 5 ± 1 cells/200 cells in the nonfibrotic alveolar regions (Figure 1D). Initial optimization of the antibody demonstrated that the antibody does not cross-react with mouse lung tissue, thereby confirming the specificity of the antibody (data not shown). uMSCs administered to healthy mice that were not treated with bleomycin were not identified in lung tissue, and no human control lung fibroblasts were identified in the bleomycin injured mouse lung at 14 days. Furthermore, uMSCs did not differentiate into morphologically typical pneumocytes in SAGM culture, nor did any of the human cells stain positively for SPC.
Figure 2.
uMSCs inhibit lung fibrosis. A: Histology of lung obtained from a SCID mouse at 7, 14, and 28 days following bleomycin lung injury and treatment with uMSCs. The control demonstrates normal lung architecture. There are mild infiltrates involving 10 to 25% of the lung at 7 days post-bleomycin injury. These changes are attenuated by uMSC injection at 7 days. At 14 days there is a significant increase in lung infiltrates involving 25 to 50% of the lung with some distortion of the lung architecture and collagen deposition. These changes are also attenuated by uMSC injection. At 28 days post-bleomycin injury, infiltrates occupy more than 75% of the lung with significant distortion of lung architecture and the presence of fibrotic foci. uMSC injection reduced the number of infiltrates as well as the number of fibrotic foci. uMSCs also preserved the lung architecture at 28 days. Magnification (all sections), ×100 (n = 8). B: The Ashcroft score of fibrosis shows a progressive elevation as lung injury evolves from 7 to 28 days after bleomycin injury. The levels of fibrosis at each time point are significantly reduced by uMSC treatment. Furthermore, alveolar and interstitial infiltrates progressively increased from 7 to 28 days following bleomycin lung injury. At each time point the number of infiltrates was significantly reduced by uMSC treatment. *P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone for each time point of analysis (n = 8).
Attenuation of Bleomycin-Induced Pneumonitis and Fibrosis after uMSC Injection
The pneumonitis induced by bleomycin was attenuated by uMSC treatments. An alveolar and interstitial exudate involved between 10 and 25% of the lung at 7 days following bleomycin exposure. The exudate increased to between 25 to 50% at 14 days and 50 to 75% at 28 days post-bleomycin (Figure 2A). The extent of the exudate was significantly reduced by uMSC injection at all time points (Figure 2A). Furthermore, the Ashcroft score for the presence of lung fibrosis demonstrated minimal fibrosis and alveolar wall thickening at 7 days, with a rapid increase in fibrosis and progressive distortion of the lung architecture at days 14 and 28. These fibrotic changes were reduced at each time point by uMSC injection (Figure 2B). Notably, uMSC injection reduced the exudative infiltration in the lung and early fibrosis at 7 days post-bleomycin (Figure 2). This suggests that the early blunting of inflammation may result in the attenuation of the downstream events leading to collagen deposition.
Cytokines and uMSC Treatment
In demonstrating the reduction in inflammation and fibrosis of bleomycin-induced lung injury by uMSCs, we studied possible mechanisms that may mediate these effects such as activation of cytokine expression. mRNA was extracted from whole lung suspensions obtained from mice analyzed at 14 days post-bleomycin. The transition from inflammation to fibrosis occurs at this time point; hence the cytokine analysis reflects the important influences on subsequent collagen deposition. Transcripts were analyzed by quantitative PCR. The quantification of transcripts of both inflammatory and fibrotic cytokines was standardized to healthy control tissue. There was a significant increase in the expression of all cytokines mediating inflammation (IL-1, IL-10, IL-2, IL-6, TNF-α, and MIF) and fibrosis [(TGF-β, interferon (IFN)-γ] at 14 days after bleomycin treatment compared with healthy controls. There was a reduction in the expression of those cytokines involved in inflammation such as IL-10 and TNF-α as well as cytokines mediating fibrosis (TGF-β and IFN-γ) following uMSC treatment (Figure 3A).
Figure 3.
uMSCs inhibit the expression of profibrotic and proinflammatory cytokines. A: Transcripts for cytokines extracted from whole lung suspensions and analyzed by quantitative PCR demonstrate a significant increase in all cytokines at 14 days following bleomycin lung injury with significant attenuation of TGF-β, IFN-γ, and MIF following uMSC injection. *P < 0.05 comparing bleomycin plus uMSCs versus bleomycin alone (n = 6). The results are expressed as fold expression over normal lung. B: The enzyme-linked immunosorbent assay for TGF-β1 demonstrated a significant increase in TGF-β1 following bleomycin at 14 days with a significant reduction in levels with uMSCs C: Densitometry of the Western blot analysis for p-SMAD-2 a transcription factor mediating the actions of TGF-β1, done on whole lung protein extracts (14 days) demonstrate an increase in TGF-β1 following bleomycin-induced lung injury and a fall with uMSC treatment (n = 8). *P < 0.05 compared with healthy controls. +P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone.
TGF-β is central to lung fibrosis and was therefore chosen to compare the trends in mRNA expression to that of protein expression in the mouse lung. The down-regulation of TGF-β mRNA expression by uMSCs paralleled the uMSC-induced reduction in TGF-β protein expression by enzyme-linked immunosorbent assay of mouse lung tissue (Figure 3B). Furthermore, phospho-Smad2, a transcription factor associated with TGF-β1 activity, was up-regulated by bleomycin but down-regulated by uMSC treatment (Figure 3C).
Collagen Deposition and uMSC Treatment
Collagen deposition was assessed by the hydroxyproline assay performed on whole lung cell suspensions at 7, 14, and 28 days post-bleomycin. There was a significant up-regulation in collagen deposition following bleomycin injury compared with healthy control mice at days 14 and 28 (Figure 4, A and B). In addition, uMSC administration did not affect basal collagen deposition in healthy control mice. However, uMSC treatment of bleomycin-injured mice resulted in a significant attenuation of collagen deposition (P < 0.05) when compared with mice treated with bleomycin alone. Of note, there was no reduction in collagen levels following injection of human lung fibroblasts into mice exposed to bleomycin, as compared with mice treated with bleomycin alone (Figure 4A).
Figure 4.
The uMSCs reduce collagen deposition. A: The hydroxyproline assay is a marker of collagen deposition. There is a significant elevation of collagen deposition following bleomycin-induced lung injury at 14 and 28 days but not at 7 days with significant abrogation by uMSC injection at 14 and 28 days. Notably there is no influence on collagen levels in the lung with injection of uMSCs into healthy mice. In contrast to uMSCs, injection of primary human lung fibroblasts into bleomycin-injured mice does not reduce the levels of collagen deposition. *P < 0.05 compared with healthy controls. +P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone (n = 8). B: Transcripts for mouse collagen type 1 α 1, a marker for collagen deposition, extracted from whole lung suspensions and analyzed by quantitative PCR demonstrate a significant increase in collagen type 1 α 1 following 14 days of bleomycin injury. The expression of collagen type 1 α 1 mRNA in the bleomycin plus uMSC group was reduced in comparison with the bleomycin alone treated group. *P < 0.05 compared with healthy controls. +P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone (n = 8). C: Masson’s trichrome staining for collagen deposition (blue) at 28 days demonstrates minimal collagen within control lung. There is an increase in collagen deposition with bleomycin that is reduced by uMSC treatment.
To determine if the fall in collagen was due to reduced synthesis, whole lung transcripts were analyzed by quantitative real-time PCR for collagen type 1 α 1. There was a significant increase in collagen type 1 α 1 mRNA expression at 14 days post-bleomycin-induced lung injury. At 14 days post-bleomycin the levels of collagen type 1 α 1 mRNA were lower in mice treated with uMSCs than the mice treated with bleomycin alone (Figure 4B). This suggests that the reduction in lung hydroxyproline levels was due to a fall in collagen type 1 synthesis. However, there was still a fivefold increase in mRNA synthesis of collagen type 1 in bleomycin-injured mice that were treated with uMSCs as compared with healthy controls (Figure 4B). Masson’s trichrome staining confirmed the up-regulation of collagen deposition by bleomycin administration and subsequent reduction by uMSCs (Figure 4C).
Up-Regulation of MMP-2 and Down-Regulation of TIMPs by uMSCs
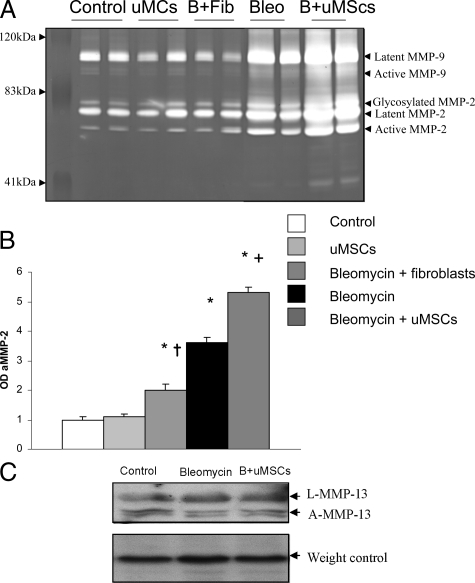
The balance between MMPs and their endogenous inhibitors (TIMPs) is of significance in determining the extent of fibrosis following injury. The expression of MMPs in whole lung suspensions was determined at 28 days after bleomycin treatment by zymography. Figure 5, A and B, shows the up-regulation of active MMP-2 following bleomycin injury. Notably, there is also a significant up-regulation of MMP-2 but not MMP-9 following uMSC treatment. There was no up-regulation of MMP-13 following bleomycin injury as compared with controls and no change in expression with uMSC treatment (Figure 5C). Furthermore, there was no increase in MMP expression following injection of uMSCs in bleomycin untreated control mice. Injection of human fibroblasts into bleomycin injured mice resulted in an increase in MMP-2 levels above healthy controls but was significantly lower when compared with the bleomycin alone or bleomycin and uMSC treatment cohorts.
Figure 5.
uMSCs augment the expression of MMPs in the injured lung. A: Zymographic analysis of whole lung suspensions shows expression of latent and active forms of MMP-9 and MMP-2 by all samples including healthy controls, healthy mice plus uMSC injection, bleomycin plus fibroblasts, bleomycin alone, as well as bleomycin plus uMSCs. We suggest that the product at 41 kd is a nonspecific catalytic product. B: Analysis of the protein bands on zymography by densitometry demonstrates an increase in MMP-2 activity with bleomycin lung injury. The increase in MMP-2 activity following bleomycin treatment was unaffected by fibroblast injection but further elevated above bleomycin levels with uMSC injection. *P < 0.05 compared with healthy controls. +P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone. †P < 0.05 comparing bleomycin plus fibroblasts to bleomycin alone. (n = 6). C: There is no difference in MMP-13 levels between bleomycin and bleomycin plus uMSC-treated mice as analyzed by Western blots.
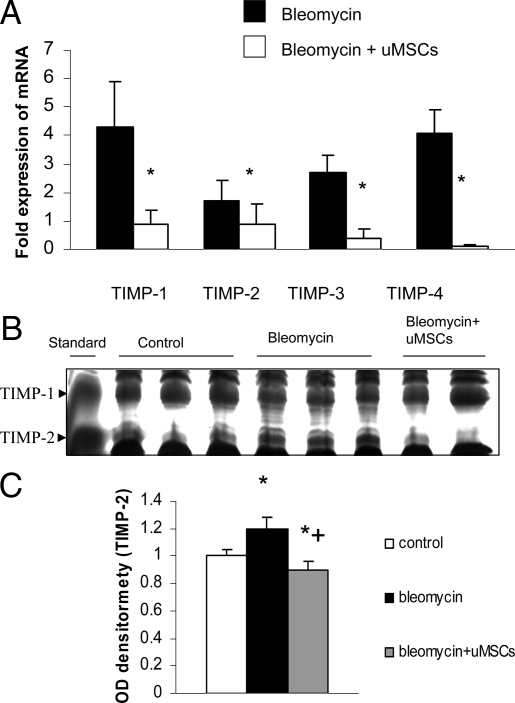
The corresponding expression of TIMPs was examined in lung tissues. This provides insights into the composite influences on collagen breakdown in the lung. mRNA was extracted from whole lung suspensions following various treatments and the transcripts were analyzed by quantitative PCR. The quantification of transcripts of TIMPs 1–4 was standardized to healthy untreated control tissue. There was increased expression of TIMPs 1–4 in bleomycin-injured mice as compared with healthy controls not treated with bleomycin. However, uMSC treatment resulted in reduced expression of TIMPs 1–4 compared with that measured in the bleomycin alone-treated group (Figure 6A). Reverse zymography for protein expression of TIMP-1 and -2 showed that bleomycin did not alter TIMP-1 protein expression, but significantly up-regulated TIMP-2 expression over a 2-week period (Figure 6B). The application of uMSCs to bleomycin-treated mice was able to significantly down-regulate TIMP-2 protein levels. This suggests that there is an increase in the MMP-2/TIMP-2 ratio following uMSCs, and this augments a pro-degradative environment for extracellular matrix breakdown following lung injury.
Figure 6.
A: There is inhibition of TIMP expression following uMSC treatment. Transcripts for TIMPs extracted from whole lung suspensions and analyzed by quantitative PCR demonstrate a significant increase in TIMPs 1–4 at 2 weeks following bleomycin lung injury with attenuation of TIMPs 1, 3, and 4 by uMSC injection. The results are expressed as fold expression over normal lung. *P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone (n = 8). B and C: Densitometry of the lung TIMP-1 and TIMP-2 bands analyzed by reverse zymography demonstrated a significant up-regulation of TIMP-2 protein levels following bleomycin-induced lung injury but a significant down-regulation of TIMP-2 protein expression by uMSC injection to bleomycin-treated mice. There are no significant changes in TIMP-1 levels between the groups. *P < 0.05 compared with healthy controls. +P < 0.05 comparing bleomycin plus uMSCs to bleomycin alone (n = 8).
Discussion
Mice were treated with bleomycin to induce lung injury. Hydrolyzed metabolites of bleomycin cleaves DNA of lung cells resulting in epithelial apoptosis and necrosis with the resultant early increase in capillary permeability, followed by the inflammatory response (maximum at 1–2 weeks) and subsequent fibrosis (maximum at 2–4 weeks), which closely mimics the pathogenesis of acute respiratory distress syndrome/acute lung injury.31 In keeping with previous studies, we established the pulmonary fibrosis model in SCID mice thereby minimizing potential rejection reactions to human cells.19 uMSCs were detected in both the fibrotic and nonfibrotic areas of the bleomycin-injured lung at 14 days but they were not detected by 28 days after injection. Notably, uMSCs were not detected in the lungs of healthy mice at 14 to 28 days following injection, implying that tissue injury is necessary to attract and retain these cells. The repair of injured lung, despite the transient presence of uMSCs, is consistent with previous reports involving other organs.32,33 Therefore, the paracrine release of trophic factors by, or induced by, MSCs may be important mediators of tissue repair.
Notably, both in vitro and in vivo assays in the present study demonstrated that uMSCs did not differentiate into typical type II pneumocytes. Studies examining MSCs derived from bone marrow and umbilical cord blood have shown that these cells differentiate into alveolar epithelial cells, albeit at a very low frequency. Although uMSCs did not differentiate into lung tissue, these cells, however, did modify the fibrotic response in the bleomycin-induced model of lung injury. Notably, uMSC injection reduced the exudative infiltration in the lung and early fibrosis at 7 days post-bleomycin (Figure 2). This suggests that the early blunting of inflammation by uMSCs leads to an attenuation of the downstream events leading to collagen deposition and fibrosis. The reduction in inflammatory cytokine expression at 14 days supports the assertion that uMSCs reduce the inflammatory response in bleomycin injured lung. At this stage the mechanism of uMSC-induced lung regeneration and repair are unclear but may include the activation or inhibition of key cytokines. IFN-γ is generally elevated during bleomycin lung injury and is secreted by lymphocytes and in SCID mice by natural killer cells.19,34 This cytokine promotes inflammation by activating expression of the major histocompatibility antigens on macrophages and up-regulating IL-6 and TNF-α production.34 Therefore it is likely that the inhibition of IFN-γ by uMSCs attenuates the inflammatory and fibrotic outcomes of bleomycin lung injury. TNF-α and IFN-γ are significantly elevated in response to bleomycin in mouse strains (C57/Bl6) prone to develop injury as compared with the resistant strains of mice such as Balb/C and supports the role for these cytokines in the progression of lung fibrosis.35,36 The inhibition of TNF-α has been shown to limit the inflammation and fibrosis of bleomycin-induced lung injury.
TGF-β is a key cytokine in the pathogenesis of lung fibrosis.37 At 14 days following bleomycin administration, TGF-β is increased in macrophages, epithelial cells, fibroblasts, and myofibroblasts.37,38 Indeed, several studies have demonstrated that TGF-β has a bimodal effect on fibroblast proliferation, stimulating monocyte production of cytokines and is a potent stimulator of collagen deposition.39 Khalil et al37 have shown conclusively that the amelioration of bleomycin-induced fibrosis is dependent on the inhibition of TGF-β. There was an up-regulation of TGF-β mRNA and protein levels following bleomycin-induced lung injury, while TGF-β expression was significantly reduced by uMSCs. Furthermore, there was up-regulation of p-Smad2, a transcription factor mediating the actions of TGF-β following bleomycin injury, and a reduction in protein levels with uMSC injection. The inhibition of TGF-β and the antifibrotic role of uMSCs as evidenced by the reduced fibrosis score on histology, collagen synthesis, and collagen deposition as indicated by hydroxyproline levels suggests the regenerative influence of uMSCs may act through this important cytokine. Although IL-R1 antagonist, angiopoientin-1, and nitric oxide are also implicated in the pro-reparative mechanisms of murine bone marrow-derived MSCs, the factors mediating human uMSC repair needs to be further elucidated.10,11,12
Bleomycin induces excessive collagen deposition and fibrosis, which is at a maximum between 3 and 4 weeks after injury, resulting in the development of fibroblastic foci.31 Collagen deposition showed the expected increase in mice treated with bleomycin but there was a reduction in collagen deposition in the cohort subjected to bleomycin and uMSC injection. There was no influence of uMSCs injected into healthy mice on collagen deposition. Furthermore, the reduction in collagen was specific to uMSCs, since the injection of primary human lung fibroblasts into bleomycin-treated mice did not have any influence on collagen levels. Quantitative real-time PCR on transcripts for collagen type 1 α 1, a sensitive indicator of collagen type 1 synthesis, demonstrated at 14 days post-bleomycin the levels of collagen type 1 α 1 mRNA were lower in mice treated with uMSCs than the mice treated with bleomycin alone. This is possibly due to uMSC inhibition of TGF-β levels. MMPs and their endogenous inhibitors (TIMPs) are primarily responsible for the degradation of extracellular matrix proteins such as collagen.40 Gelatin zymographic analysis demonstrated that MMP-2 and MMP-9 were up-regulated following uMSC treatment in bleomycin-injured mice, in keeping with previous studies.41 Notably, uMSC injection into bleomycin-exposed mice resulted in a significant increase in MMP levels when compared with bleomycin-treated mice alone or healthy controls. This is a unique response to lung injury, since there was no elevation of MMPs in lung samples from controls injected with uMSCs. In addition, there was a significant increase in MMP levels following the injection of uMSCs when compared with primary human lung fibroblasts. There are correlative studies linking MMP-2 and MMP-9 to lung repair following bleomycin-induced lung repair. MMP-2 is responsible for the degradation of most extracellular matrix proteins and is also implicated in alveolar regeneration.41 Ortiz et al demonstrated down-regulation of MMP-2 when using murine bmMSCs to reduce the fibrotic response to bleomycin injury.42 This difference may be due to the source of MSCs and the mouse strains (black 6/C57 versus SCID) used.
TIMPs are major endogenous inhibitors of MMPs and bind these molecules in a 1:1 stoichometry. There are four homologues of TIMPs, TIMPs 1–4, each having specific functions.43 Inhibition of TIMP-1 augmented the inflammatory response to bleomycin but not an increase in fibrosis.43 Similarly, TIMP-2 and TIMP-3 are also elevated in response to bleomycin,44 as shown in the present study. Lung transcripts demonstrated a down-regulation of TIMP-1, -2, -3, and -4 in mice treated with uMSCs at 28 days following bleomycin lung injury. Comparatively, TIMP-2 protein levels were significantly elevated in response to bleomycin but were also markedly reduced by uMSCs treatment. There is a causal relationship between TIMP elevation and fibrosis, since TIMP concentrations increase before the phase of collagen deposition in the bleomycin mouse model at 14 to 28 days after injury.45 Furthermore, mouse strains resistant to bleomycin-induced fibrosis do not demonstrate an increase in TIMP-1 levels following injury.44 Taken together, there is an increase in the MMP-2/TIMP-2 ratio that is induced by uMSC injection. This may result in a microenvironment that favors extracellular matrix degradation in response to uMSC injection that may ameliorate fibrosis.
To date, there are no safe and effective therapeutic agents to augment lung repair. A stem cell-based therapy using uMSCs obtained from discarded placental tissue may impact on several pathways in the pathogenesis of major lung injury. Furthermore, MSCs derived from umbilical cord blood has been successfully used in the treatment of HLA mismatched recipients.32 This suggests that uMSCs may not need to be exactly matched with the recipient to avoid a significant graft-versus-host reaction. This study has yielded several novel findings, namely that systemically administered uMSCs are present in the bleomycin-injured lung at 2 weeks. These cells inhibit inflammation and fibrosis. In addition they down-regulate lung cytokine and TIMP expression while up-regulating MMPs.
Acknowledgments
We thank Dr. Jinhua Li, Department of Anatomy and Cell Biology, Monash University, for assistance with tail vein injections. We are grateful for the generous gift of primary human lung fibroblasts obtained from Prof. Philip Bardin, Department of Respiratory Medicine, Monash Medical Centre. We also thank Dr. Carla Thomas, Anatomical Pathology Research Laboratory, Pathwest Laboratory Medicine WA, for performing the col I real-time PCR.
Footnotes
Address reprint requests to Dr. Yuben Moodley, Dept. of Respiratory Medicine, Monash Medical Centre, Clayton, Victoria 3168, Australia, E-mail: yuben.moodley@med.monash.edu.au.
Y.M., A.T., R.B., and U.M. are recipients of a collaborative grant from Monash University. C.S.S. is supported by a National Heart Foundation of Australia/National Health & Medical Research Council of Australia R.D. Wright Fellowship.
Current address of Y.M.: Southern Clinical School of Medicine, Monash University, Melbourne, Australia. Current address of A.T.: California Institute for Regenerative Medicine, 210 King Street, San Francisco, CA 94107.
References
- Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest. 2001;120:1347–1367. doi: 10.1378/chest.120.4.1347. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Petty TL, Ashbaugh DG. The adult respiratory distress syndrome: clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome: association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med. 1995;122:17–23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann NY Acad Sci. 2003;996:152–157. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- Albera C, Polak JM, Janes S, Griffiths MJ, Alison MR, Wright NA, Navaratnarasah S, Poulsom R, Jeffery R, Fisher C, Burke M, Bishop AE. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng. 2005;11:1115–1121. doi: 10.1089/ten.2005.11.1115. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2007;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- Bouwstra H, Dijck-Brouwer DJ, Decsi T, Boehm G, Boersma ER, Muskiet FA, Hadders-Algra M. Relationship between umbilical cord essential fatty acid content and the quality of general movements of healthy term infants at 3 months. Pediatr Res. 2006;59:717–722. doi: 10.1203/01.pdr.0000215013.19164.57. [DOI] [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65:187–195. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisfeld IH. Bleomycin-induced pulmonary toxicity: a model for study of pulmonary fibrosis. Hecht SM, editor. New York: Springer-Verlag,; In Bleomycin, Chemical, Biochemical and Biological Aspects. 1979:pp 324–335. [Google Scholar]
- Provenzano M, Mocellin S. Complementary techniques: validation of gene expression data by quantitative real time PCR. Adv Exp Med Biol. 2007;593:66–73. doi: 10.1007/978-0-387-39978-2_7. [DOI] [PubMed] [Google Scholar]
- Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol. 2003;163:345–354. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–3890. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- Hewitson TD, Mookerjee I, Masterson R, Zhao C, Tregear GW, Becker GJ, Samuel CS. Endogenous relaxin is a naturally occurring modulator of experimental renal tubulointerstitial fibrosis. Endocrinology. 2007;148:660–669. doi: 10.1210/en.2006-0814. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr Quantification of matrix metalloproteinases in tissue samples. Methods Enzymol. 1995;248:510–528. doi: 10.1016/0076-6879(95)48033-1. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Kerchner LJ, Fisher MC, McGuane JT, Doty KD, Conrad KP. Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J Appl Physiol. 2006;100:1955–1963. doi: 10.1152/japplphysiol.01330.2005. [DOI] [PubMed] [Google Scholar]
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- Berger MJ, Adams SD, Tigges BM, Sprague SL, Wang XJ, Collins DP, McKenna DH. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480–487. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- Warburton D, Lee MK. Current concepts on lung development. Curr Opin Pediatr. 1999;11:188–192. doi: 10.1097/00008480-199906000-00002. [DOI] [PubMed] [Google Scholar]
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfect. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel MJ, Izbicki G, Cohen PY, Or R, Christensen TG, Wallach-Dayan SB, Breuer R. Role of interferon-gamma in the evolution of murine bleomycin lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1255–L1262. doi: 10.1152/ajplung.00303.2002. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170:727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- Khalil N, Parekh TV, O'Connor RN, Gold LI. Differential expression of transforming growth factor-beta type I and II receptors by pulmonary cells in bleomycin-induced lung injury: correlation with repair and fibrosis. Exp Lung Res. 2002;28:233–250. doi: 10.1080/019021402753570527. [DOI] [PubMed] [Google Scholar]
- Parks WC. Matrix metalloproteinases in lung repair. Eur Respir J Suppl. 2003;44:36s–38s. doi: 10.1183/09031936.03.00001203. [DOI] [PubMed] [Google Scholar]
- Yaguchi T, Fukuda Y, Ishizaki M, Yamanaka N. Immunohistochemical and gelatin zymography studies for matrix metalloproteinases in bleomycin-induced pulmonary fibrosis. Pathol Int. 1998;48:954–963. doi: 10.1111/j.1440-1827.1998.tb03866.x. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madtes DK, Elston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001;24:599–607. doi: 10.1165/ajrcmb.24.5.4192. [DOI] [PubMed] [Google Scholar]
- Kim KH, Burkhart K, Chen P, Frevert CW, Randolph-Habecker J, Hackman RC, Soloway PD, Madtes DK. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol. 2005;33:271–279. doi: 10.1165/rcmb.2005-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggionni T, Morbini P, Inghilleri S, Palladini G, Tozzi R, Vitulo P, Fenoglio C, Perlini S, Pozzi E. Time course of matrix metalloproteases and tissue inhibitors in bleomycin-induced pulmonary fibrosis. Eur J Histochem. 2006;50:317–325. [PubMed] [Google Scholar]