Abstract
This study systematically analyzes platelet-derived growth factor (PDGF) receptor expression in six types of common tumors as well as examines associations between PDGF β-receptor status and clinicopathological characteristics in breast cancer. PDGF receptor expression was determined by immunohistochemistry on tumor tissue microarrays. Breast tumor data were combined with prognostic factors and related to outcome endpoints. PDGF α- and β-receptors were independently expressed, at variable frequencies, in the tumor stroma of all tested tumor types. There was a significant association between PDGF β-receptor expression on fibroblasts and perivascular cells in individual colon and prostate tumors. In breast cancer, high stromal PDGF β-receptor expression was significantly associated with high histopathological grade, estrogen receptor negativity, and high HER2 expression. High stromal PDGF β-receptor expression was correlated with significantly shorter recurrence-free and breast cancer-specific survival. The prognostic significance of stromal PDGF β-receptor expression was particularly prominent in tumors from premenopausal women. Stromal PDGF α- and β-receptor expression is a common, but variable and independent, property of solid tumors. In breast cancer, stromal PDGF β-receptor expression significantly correlates with less favorable clinicopathological parameters and shorter survival. These findings highlight the prognostic significance of stromal markers and should be considered in ongoing clinical development of PDGF receptor inhibitors.
Platelet-derived growth factor (PDGF) α- and β-tyrosine kinase receptors exert important control functions in mesenchymal cells, such as pericytes, fibroblasts and vascular smooth muscle cells during development.1 PDGF receptor activation has also been shown to be involved in multiple dimensions of cancer growth.2 The clinical relevance of these findings is enhanced by the recent approval of tyrosine kinase inhibitors with PDGF receptor inhibitory activity, eg, imatinib, sunitinib, and sorafenib.
PDGF receptor-dependent growth stimulation is well documented in malignant cells of some solid tumors, such as glioblastomas,3,4,5,6,7 dermatofibrosarcoma protuberans8,9 and a subset of gastrointestinal stromal tumors.10,11 Also, in hematological malignancies such as chronic myelomonocytic leukemia and idiopathic eosinophilic syndrome, PDGF α- or β-receptor signaling has been shown to be activated through translocations or deletions of the PDGF receptor genes.12,13,14 However, in most common solid tumors PDGF receptor signaling appears to be most important for the pericytes of the tumor vessels, and for the fibroblasts of the tumor stroma.
Concerning the role of PDGF β-receptor signaling in pericytes, a series of experimental studies have demonstrated that stimulation of PDGF receptors on pericytes increases pericyte coverage of vessels in a manner that is associated with increased vessel function and, in some cases, also increased tumor growth.15,16,17 Furthermore, vascular endothelial growth factor receptor-targeted antiangiogenic approaches in experimental tumor models appear to be most efficient on immature pericyte-poor vessels.18 Finally, combinations of vascular endothelial growth factor receptor- and PDGF-receptor inhibitors have been demonstrated to exert synergistic antiangiogenic effects.19,20
Studies in experimental tumor models have demonstrated that paracrine activation of PDGF receptors on fibroblasts acts as a potent signal for tumor stroma recruitment.21,22 Other studies with PDGF antagonists have also demonstrated direct antitumoral effects of stromal PDGF receptor inhibition,23,24 as well as beneficial effects on tumor drug uptake.25,26,27,28
The biological effects of PDGF receptors in tumor fibroblasts and pericytes, together with the advent of drugs with PDGF receptor-inhibitory activity thus motivates a systemic characterization of the expression pattern of PDGF α- and β-receptors in human tumors. In this study we have characterized the fibroblast and pericyte expression of PDGF α- and β-receptors in lymphomas and in colon, ovarian, prostate, lung and breast cancers. Furthermore the relationship between stromal PDGF β-receptor status and prognostic parameters and survival was analyzed in breast cancer.
Materials and Methods
Paraffin Embedding of Cultured Cells
Porcine aortic endothelial (PAE) cells transfected with the PDGF α- or β-receptor (PAE/PDGFαR and PAE/PDGFβR cells) were kept in F12 (Sigma-Aldrich, Stockholm, Sweden) media containing 10% fetal calf serum Sigma-Aldrich, Stockholm, Sweden 1% glutamine, and antibiotics (penicillin, 100 units/ml, Sigma-Aldrich) and streptomycin (100 μg/ml, Sigma-Aldrich). PAE/PDGFαR and PAE/PDGFβR cells were starved overnight in medium containing 1% fetal calf serum following stimulation with or without 100 ng/ml PDGF-BB (Peprotech, London, UK) on ice for 1 hour. Cells were then washed in cold phosphate-buffered saline, (Medicago, Uppsala, Sweden) removed from the plate, and centrifuged at 2000 rpm for 10 minutes. The phosphate-buffered saline was discarded and cell pellets incubated in 4% phosphate-buffered paraformaldehyde (WWR, Stockholm, Sweden) solution overnight. The pellet was placed in a tissue embedding box in 70% ethanol and then placed in higher grades of alcohol for dehydration before being embedded in paraffin, sectioned, and put on Superfrost Plus slides (Menzel-Gläser, Braunschweig, Germany).
Tissue Microarrays (TMAs)
A tumor TMA (TARP 4) containing 450 tumor biopsies were obtained from the National Cancer Institute Tissue Array Research Program, National Institutes of Health. The TARP 4 comprises 0.6 mm core punch biopsies of normal tissues, glioblastoma multiforme, malignant melanomas, lymphomas, breast, colon, lung, ovarian, and prostate adenocarcinomas with 25 to 75 samples of each tumor type. Clinical data were not available for these specimens.
The breast cancer TMA was made from 512 consecutive cases of primary breast cancer diagnosed between 1988 and 1992 at the Department of Pathology, Malmö University Hospital. Median age at diagnosis was 64.2 years (range, 27–96) and median follow-up time from diagnosis until first breast cancer event was 106 months (range, 0–207). Follow-up data regarding recurrence-free survival and death was available for 507 patients. This cohort is described in detail elsewhere.29 Complete information regarding adjuvant systemic treatment was available for 389 patients. Of these, 157 had received adjuvant tamoxifen, 19 adjuvant chemotherapy, 209 had not received any adjuvant treatment, and 4 patients had received a combination of tamoxifen and chemotherapy.
Before TMA construction, all breast cancer cases were histopathologically re-evaluated on hematoxylin and eosin-stained slides. Areas representative of cancer were then marked and TMAs constructed as described previously.30
PDGF Receptor Immunohistochemistry
PDGF receptor immunohistochemistry was done as described31 using either anti-PDGF β-receptor rabbit monoclonal (3169, Cell Signaling Technology, Danvers, MA) (2 μg/ml)) or anti-PDGF α-receptor rabbit polyclonal (3164, Cell Signaling Technology) (1:50) antibodies. Nonimmune rabbit serum was used as a negative control. The PDGF receptor staining was scored independently in fibroblasts and pericytes as negative (0), weak (1), moderate (2), or strong (3). The fibroblast PDGF β-receptor expression in breast tumors were dichotomized (0–2 and 3) for all statistical analyses.
Statistics
Analyses of PDGF α- and β-receptor expression dependency in fibroblasts and pericytes were done in STATISTICA by the Yates corrected χ2 test using 2 × 2 contingency tables (StatSoft Inc., Tulsa, OK). The χ2 test and Spearman’s correlation test were used for comparison of PDGF β-receptor expression and relevant patient and tumor characteristics in breast tumors. The Kaplan-Meier method and log rank test were used to compare recurrence-free survival and overall survival in different strata. Recurrence-free survival considered loco-regional and distant metastasis or breast cancer specific death as primary event, whereas contralateral events and non-breast cancer deaths were excluded. A Cox proportional hazards model was used for estimation of relative risks in both univariate and multivariate analyses including other relevant risk and therapy predictive factors as age, size, nodal status, histopathological grade , estrogen receptor (ER), progesterone receptor (PgR), and HER2 status in the model. All statistical tests were two-sided and P values <0.05 considered significant. Calculations were performed with the statistical package SPSS 15.0 (SPSS Inc.).
Results
Characterization of the Specificity of PDGF Receptor Antibodies
A screen was performed on a series of in-house and commercial PDGF α- and β-Receptor antibodies to characterize their specificity and sensitivity. For this screen, sections of paraformaldehyde-fixed paraffin-embedded cultured cells of known PDGF receptor status were used. One pair of PDGF α- and β-receptor antibodies was selected for further use. The two selected antibodies showed an isoform-specific staining of PDGF α- and β-receptors (see Supplemental Figure S1 at http://ajp.amjpathol.org). Furthermore, the staining pattern in non-stimulated or ligand-stimulated cells was similar (see Supplemental Figure S1 at http://ajp.amjpathol.org), demonstrating that antibody-recognition was not affected by the activation status of receptors.
PDGF Receptor Expression in Tumor Stroma
For an initial description of the pattern of PDGF α- and β-receptor expression in human malignancies, immunohistochemistry analyses were performed on a TMA of lymphomas, ovarian, colon, lung and prostate cancers with the two antibodies. For each sample the staining in malignant cells, tumor fibroblasts and perivascular cells were scored separately as being negative or positive.
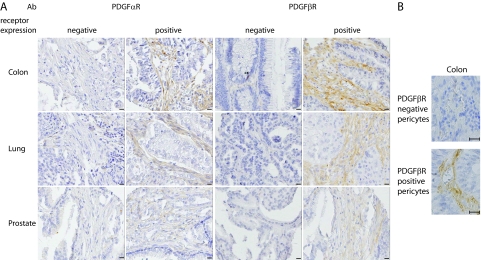
In general, PDGF β-receptor was found on perivascular cells and on stromal fibroblasts but not on the malignant cells (Table 1 and Figure 1A). However, the frequency of tumors with PDGF receptor positive tumor fibroblasts varied largely between tumor groups (Table 1). Among these malignancies, PDGF β-receptor expression in fibroblasts was most common in lung and colon cancer, where 58% to 68% of tumors were positive (Table 1 and Figure 1, A and B). In contrast, only 11% of ovarian cancers had PDGF β-receptor positive fibroblasts (Table 1). Lymphomas and prostate cancers showed an intermediate frequency with around 20% of cases being positive (Table 1). The frequencies of positivity for perivascular PDGF β-receptor staining were in general higher, but again differences between tumor types were noted (Table 1). For example, 80% of colon tumors showed perivascular PDGF β-receptor staining, whereas only 31% of prostate cancers were positive.
Table 1.
PDGF α- and β-Receptor Expression in Fibroblasts and Pericytes of Different Tumor Types
| Tumor type | PDGFαR
|
PDGFβR
|
||
|---|---|---|---|---|
| Fibroblasts (%) | Pericytes (%) | Fibroblasts (%) | Pericytes (%) | |
| Lymphoma (n = 19–42) | 16 | 25 | 19 | 50 |
| Ovarian (n = 31–40) | 3 | 5 | 11 | 58 |
| Colon (n = 53–59) | 65 | 53 | 68 | 80 |
| Lung (n = 39–43) | 24 | 33 | 58 | 74 |
| Prostate (n = 58–62) | 2 | 0 | 21 | 31 |
Figure 1.
PDGF receptor expression in three common solid tumors. A: Examples of colon, lung, and prostate tumors with different patterns of stromal fibroblast PDGF α- and β-receptor expression (scale bar = 50 μm). B: Examples of colon tumors with PDGF β-receptor negative and positive pericytes. Magnification, ×400. Scale bar = 25 μm.
PDGF α-receptor expression was not as frequent as the PDGF β-receptor in tumor fibroblasts, with frequencies ranging from 2% in prostate tumors to 65% in colon tumors (Table 1). Also, perivascular PDGF α-receptor expression was less common than perivascular PDGF β-receptor and ranged between 0% in prostate tumors to 53% in colon cancers.
Relationships between PDGF α- and β-Receptor Expression and between the Expression of PDGF Receptors in Fibroblasts and Perivascular Cells
Analyses of the PDGF α- and β-receptors in the different tumor types failed to identify a significant association between the PDGF α- and β-receptor expression in tumor fibroblasts. Among all of the different tumor types analyzed, individual tumors were found that expressed none of the receptors, the β-receptor alone, or the β-receptor together with the α-receptor.
Subsequent analyses focused on the relationship between PDGF β-receptor status of pericytes and fibroblasts in individual tumors. Both in colon and prostate tumors significant positive associations were observed between the PDGF β-receptor status of perivascular cells and stromal fibroblasts in individual tumors (Table 2).
Table 2.
Significant Associations of PDGF β-Receptor Expression in Pericytes and Fibroblasts of Colon and Prostate Tumors Analyzed by the χ2 Test
| Colon (n = 57) | Pericytes
|
||
|---|---|---|---|
| PDGFβR− | PDGFβR+ | ||
| Fibroblasts | |||
| PDGFβR− | 15.8% | 17.5% | |
| PDGFβR+ | 5.3% | 61.4% | P = 0.0019 |
| Pericytes
|
|||
| Prostate (n = 61)
|
PDGFβR−
|
PDGFβR+
|
|
| Fibroblasts | |||
| PDGFβR− | 62.3% | 16.4% | |
| PDGFβR+ | 6.6% | 14.8% | P = 0.0027 |
These observations thus demonstrate that PDGF α- and β-receptors are independently regulated. Furthermore, the relationship between the expression of PDGF β-receptors in fibroblasts and perivascular cells suggest a functional link between the PDGF receptor status of fibroblasts and perivascular cells.
PDGF β-Receptor Expression and Clinicopathological Parameters in Breast Cancer
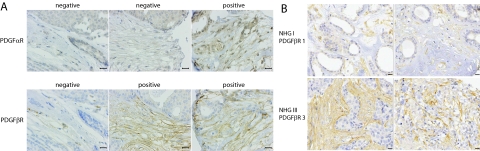
In breast cancer, we performed an extended analysis on TMAs with tumor samples linked to clinicopathological and outcome data. In this material 34.6% of samples were scored as strongly positive for fibroblast expression of PDGF β-receptor (Figure 2A and Supplemental Table S1 at http://ajp.amjpathol.org). Analyses of PDGF α-receptor identified only 1% of cases with moderate or strong staining (see Figure 2A and Supplemental Table S1 at http://ajp.amjpathol.org).
Figure 2.
Differential PDGF receptor expression in fibroblasts of breast tumors. A: Three examples of breast tumors with different patterns of PDGF α- and β-receptor expression in tumor fibroblasts. Scale bar = 25 μm. B: Four examples of breast tumors selected to illustrate the association between histological grade and PDGF β-receptor expression. The two upper pictures show tumors with low grade and low stromal PDGF β-receptor expression whereas the lower panel shows high grade tumors with high stromal PDGF β-receptor expression. Scale bar = 50 μm.
Significant positive associations were observed between strong PDGF β-receptor expression in the tumor fibroblasts and high histological grade, ER and progesterone receptor negativity, HER2 expression, proliferation rate and tumor size (Table 3 and Figure 2B). Together these analyses thus clearly demonstrated a previously unrecognized relation between PDGF β-receptor expression in tumor fibroblasts and factors associated with an impaired prognosis.
Table 3.
Associations between Stromal Fibroblast PDGF β-Receptor Expression and Clinicopathological Parameters in Breast Cancer
| Number (PDGFβR data) PDGFβR expression n (%) |
n = 512 (289)
|
P value | |
|---|---|---|---|
| 0–2 189 (65.4) | 3 100 (34.6) | ||
| Age | |||
| ⩽50 | 24 (8.3) | 9 (3.1) | 0.07 |
| >50 | 165 (57.1) | 70 (24.2) | |
| Tumor size | |||
| ⩽20 mm | 127 (43.9) | 57 (19.7) | 0.09 |
| >20 mm | 62 (21.5) | 43 (14.9) | |
| Missing | 0 (0) | 0 (0) | |
| Nottingham histological grade | |||
| I | 53 (18.3) | 17 (5.9) | 0.001** |
| II | 82 (28.4) | 35 (12.1) | |
| III | 53 (18.3) | 48 (16.6) | |
| Missing | 1 (0.4) | 0 (0) | |
| Node status | |||
| Negative | 109 (37.7) | 53 (18.3) | 0.29 |
| Positive | 59 (20.4) | 39 (13.5) | |
| Missing | 21 (7.3) | 9 (3.1) | |
| ER status | |||
| Negative | 20 (6.9) | 19 (6.6) | 0.05* |
| Positive | 165 (57.1) | 81(28.0) | |
| Missing | 4 (1.4) | 0 (0) | |
| Progesterone receptor status | |||
| Negative | 11 (3.8) | 16 (5.5) | 0.007** |
| Positive | 168 (58.1) | 84 (29.1) | |
| Missing | 10 (3.5) | 0 (0) | |
| HER2 immunohistochemistry | |||
| 0 | 136 (47.1) | 47 (16.3) | 0.01** |
| 1 | 39 (13.5) | 24 (8.3) | |
| 2 | 19 (6.6) | 12 (4.2) | |
| 3 | 12 (4.2) | 15 (5.2) | |
| Missing | 13 (4.5) | 2 (0.7) | |
| Ki67 | |||
| 0–10% | 81 (28.0) | 31 (10.7) | 0.001** |
| 11–25% | 68 (23.5) | 25 (8.7) | |
| >25% | 35 (12.1) | 39 (13.5) | |
| Missing | 5 (1.7) | 5 (0) | |
χ2 test for linear trend. Hormone receptor status was assessed by immunohistochemistry using a cutoff at 10% positive nuclei, according to current clinical guidelines in Sweden.
P < 0.05.
P < 0.01.
PDGF β-Receptor Expression and Breast Cancer Survival
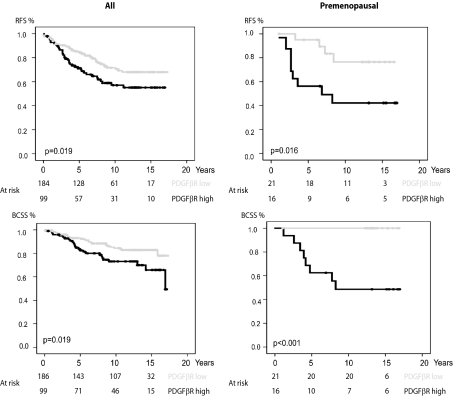
The results from the analyses of stromal fibroblast expression of PDGF β-receptor were used together with clinical data to investigate possible prognostic significance of PDGF β-receptor expression. As shown in Figure 3, high PDGF β-receptor expression was associated with a significantly shorter recurrence-free survival (P = 0.019) and breast cancer specific survival (P = 0.019). In a Cox proportional hazards model high PDGF β-receptor expression in fibroblasts increased the risk of recurrence (relative risk = 1.67 [1.08–2.58] 95% confidence interval; P = 0.02) and breast cancer specific death (relative risk = 2.02 [1.16–3.49] 95% confidence interval; P = 0.01). This did not remain significant in multivariate analysis (see Supplemental Table S2 at http://ajp.amjpathol.org).
Figure 3.
Correlations between survival and fibroblast expression of PDGF β-receptor in breast cancer. Recurrence-free survival (RFS, upper panels) and breast cancer specific survival (BCSS, lower panels) in groups defined by PDGF β-receptor expression in fibroblasts. Analyses were performed on all cases of the breast cancer cohort (left panels) or restricted to the subset of premenopausal women (right panels).
To investigate if the prognostic significance was more pronounced in certain patient subsets, survival analyses were performed in strata according to menopausal status, node status, HER2 status, histological grade or endocrine treatment. As shown in Figure 3 and Supplemental Table S2 at http://ajp.amjpathol.org, the prognostic significance of PDGF β-receptor expression in fibroblasts was particularly prominent in premenopausal women. In this subset, PDGF β-receptor expression was also a significant predictor of recurrence-free survival in multivariate analysis (relative risk = 5.49 [1.33–22.74] 95% confidence interval; P = 0.02) (Supplemental Table S2 at http://ajp.amjpathol.org). These analyses thus identified a prognostic significance of stromal fibroblast PDGF β-receptor expression in an unselected breast cancer cohort, which was most prominent in the subset of premenopausal women.
Discussion
The analyses of PDGF receptor expression in stromal fibroblasts of different types of solid tumors and malignant lymphoma revealed large differences between tumor types (Table 1 and Figure 1). It is noteworthy that PDGF α-receptor expression was most common in lung and colon cancer, since previous studies have demonstrated particularly important roles for PDGF α-receptor signaling in mesenchymal cells of these organs.32,33 This finding is thus compatible with the notion that stromal fibroblasts of solid tumors are derived from local mesenchymal cells, and share their particular pattern of growth factor receptor dependency. Additional studies should be done to further explore this issue.
PDGF receptor expression in human tumors has been analyzed in previous studies with some conflicting results; some of these studies have reported common epithelial expression of PDGF receptors.34,35,36 However, most of these earlier studies have not subjected antibodies to specificity controls of the same stringency as in the present study. Also in breast cancer some studies have suggested frequent expression on epithelial cells.37,38 This was not observed in the present study. There could still exist a minor subset of breast cancer where PDGF receptors are expressed eg, as a consequence of genetic alterations including amplifications or translocations. Future studies are likely to provide additional information concerning such subsets. However, at present stage, and based on the analyses of this and earlier studies,39,40 it should be concluded that in the large majority of breast cancers PDGF receptors are exerting their effects in stromal cells such as fibroblast and pericytes.
The analyses of PDGF α- and β-receptor expression indicated that the two proteins are independently regulated in the examined human cancers. This is in agreement with previous studies in tissue culture models, and with analyses of the developmental roles of these two related receptors.1 It is presently not known whether consistent functional differences exist between these two types of fibroblast populations. Continued analyses of the unique prognostic and response-predicative significance of PDGF α- and β-receptor expression are thus warranted.
Significant associations between expression of PDGF β-receptors on fibroblasts and perivascular cells were observed in colon and prostate cancer (Table 2). This could either be due to interdependency of these two cell populations or, alternatively, reflect that these cell populations are derived from a common source. Some support for the latter has been provided from animal studies. These have shown that PDGF β-receptor positive mouse embryo fibroblasts contribute to both the fibroblast population and the population of perivascular cells in experimental tumors following coinjection with malignant cells.41
The analyses of breast cancer revealed previously un-recognized associations between stromal PDGF β-receptor expression and tumor characteristics such as histological grade, tumor cell proliferation, ER status and HER2 status (Table 3). The mechanisms underlying these relationships remain to be clarified in future studies. However, previous experimental studies have demonstrated protumorigenic effects of stromal PDGF β-receptors.21,22 Multiple mechanisms might be involved in these effects. A recent study implied osteopontin production as one PDGF receptor-dependent tumorigenic mechanism of stromal cells.42 PDGF receptor activation in stromal cells have also, based on animal model studies, been shown to control tumor drug uptake and tumor oxygenation.26,28,43 It has also been shown in tissue culture studies that some fibroblast types can reduce the tamoxifen sensitivity of ER-positive breast cancer cells.44 Whether such effects are dependent on PDGF receptor activation remains to be determined. Concerning the strong correlation with ER negativity and high HER2 expression, this might also reflect a particular dependency of these types of tumor cells on a stroma characterized by PDGF β-receptor positivity. Obviously, these issues should be further explored in experimental settings.
A major finding of the present study is the prognostic role of stromal PDGF β-receptor expression in breast cancer (Figure 3 and Supplemental Table S2 at http://ajp.amjpathol.org). The observation that stromal PDGF β-receptor status displayed most prominent prognostic significance in the subset of tumors from premenopausal women is also intriguing. One possibility suggested by these findings is that PDGF receptor status and activity in stromal cells is controlled by ER activity in epithelial or stromal cells. In this context it should also be noted that experimental studies have suggested that some of the therapeutic effects of antiestrogens are mediated by targeting of ER in stromal cells.45 These findings also prompt continued studies in other hormone dependent tumors, eg, prostate cancer. Interestingly stromal androgen receptor status was recently shown to be a prognostic marker in this tumor type.46 It will thus be interesting in future studies to investigate if any relationships exist between stromal expression of PDGF receptors and androgen receptor in prostate cancer.
Identification of prognostic markers in breast cancer, and other solid tumors, has focused on properties of the malignant cells, such as hormone receptor status, proliferation index and HER2 status. Our study illustrates, in general terms, an additional prognostic significance of stromal characteristics. Such findings are in agreement with advances in tumor biology, which emphasize the importance of the tumor microenvironment.47,48,49 Other recent studies have also identified prognostic significance of stroma-derived markers. In the case of breast cancer a recent study demonstrated prognostic significance of a stroma-derived gene expression signature of 26 genes.50 Other studies have also identified prognostically significant genetic alterations in cells of breast cancer stroma.51,52 Additionally, recent reports have demonstrated prognostic significance of stromal characteristics in colorectal, head and neck, and pancreatic cancer.53,54,55 Together these studies encourage continued analyses of the prognostic and predicative role of stromal markers.
The findings of this study also impact the ongoing clinical development of approved tyrosine kinase inhibitors with PDGF receptor inhibitory activity, such as imatinib, sorafenib and sunitinib. The highly variable and independent PDGF α- and β-receptor expression in solid tumors, which is revealed in this study, suggests that the antistromal impact of these drugs will show large variation. The findings in the present study of robust staining procedures for PDGF receptors should motivate continued studies of the response-predicative value of stromal PDGF receptor status. Such studies should also be stimulated by the recent identification of a chemotherapy response-predicative stroma-derived gene expression signature.56
Supplementary Material
Acknowledgments
We acknowledge Margareta Rodensjö and Liss Garberg for excellent technical assistance and Kristian Pietras for contributions during the initiation of this project.
Footnotes
Address reprint requests to Arne Östman, Cancer Center Karolinska, R8:03, Karolinska Institutet, 171 76 Stockholm, Sweden. E-mail: arne.ostman@ki.se.
Supported by Swedish Cancer Society research grant 070617, “Cancerföreningen” research grant 074271, and Swedish Research Council grant 60016001. The groups of A.Ö. and J.B. are supported by the Swedish Cancer Society and are recipients of the STARGET Linné grant from the Swedish Research Council.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Ostman A, Heldin CH. PDGF Receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT: PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207:224–231. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, Ali IU. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- Hagerstrand D, Hesselager G, Achterberg S, Wickenberg Bolin U, Kowanetz M, Kastemar M, Heldin CH, Isaksson A, Nister M, Ostman A. Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene. 2006;25:4913–4922. doi: 10.1038/sj.onc.1209497. [DOI] [PubMed] [Google Scholar]
- Kilic T, Alberta JA, Zdunek PR, Acar M, Iannarelli P, O'Reilly T, Buchdunger E, Black PM, Stiles CD. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60:5143–5150. [PubMed] [Google Scholar]
- Strawn LM, Mann E, Elliger SS, Chu LM, Germain LL, Niederfellner G, Ullrich A, Shawver LK. Inhibition of glioma cell growth by a truncated platelet-derived growth factor-beta receptor. J Biol Chem. 1994;269:21215–21222. [PubMed] [Google Scholar]
- Shimizu A, O'Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, Heldin CH, Dumanski JP, Ostman A. The dermatofibrosarcoma protuberans-associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res. 1999;59:3719–3723. [PubMed] [Google Scholar]
- Sjoblom T, Shimizu A, O'Brien KP, Pietras K, Dal Cin P, Buchdunger E, Dumanski JP, Ostman A, Heldin CH. Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res. 2001;61:5778–5783. [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, Cross NC, Tefferi A, Malone J, Alam R, Schrier SL, Schmid J, Rose M, Vandenberghe P, Verhoef G, Boogaerts M, Wlodarska I, Kantarjian H, Marynen P, Coutre SE, Stone R, Gilliland DG. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ, Baxter EJ, Chase A, Chessells JM, Colombat M, Dearden CE, Dimitrijevic S, Mahon FX, Marin D, Nikolova Z, Olavarria E, Silberman S, Schultheis B, Cross NC, Goldman JM. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347:481–487. doi: 10.1056/NEJMoa020150. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Sjoblom T, Abramsson A, Ellingsen J, Micke P, Li H, Bergsten-Folestad E, Eriksson U, Heuchel R, Betsholtz C, Heldin CH, Ostman A. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64:2725–2733. doi: 10.1158/0008-5472.can-03-1489. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Valyi-Nagy I, Heldin CH, Herlyn M, Westermark B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci USA. 1993;90:393–397. doi: 10.1073/pnas.90.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Fusenig NE. Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc Natl Acad Sci USA. 1998;95:1050–1055. doi: 10.1073/pnas.95.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169:2054–2065. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- Pietras K, Stumm M, Hubert M, Buchdunger E, Rubin K, Heldin CH, McSheehy P, Wartmann M, Ostman A. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res. 2003;9:3779–3787. [PubMed] [Google Scholar]
- Baranowska-Kortylewicz J, Abe M, Pietras K, Kortylewicz ZP, Kurizaki T, Nearman J, Paulsson J, Mosley RL, Enke CA, Ostman A. Effect of platelet-derived growth factor receptor-beta inhibition with STI571 on radioimmunotherapy. Cancer Res. 2005;65:7824–7831. doi: 10.1158/0008-5472.CAN-04-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgquist S, Holm C, Stendahl M, Anagnostaki L, Landberg G, Jirstrom K. Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J Clin Pathol. 2008;61:197–203. doi: 10.1136/jcp.2006.040378. [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Nupponen NN, Paulsson J, Jeibmann A, Wrede B, Tanner M, Wolff JE, Paulus W, Ostman A, Hasselblatt M. Platelet-derived growth factor receptor expression and amplification in choroid plexus carcinomas. Mod Pathol. 2008;21:265–270. doi: 10.1038/modpathol.3800989. [DOI] [PubMed] [Google Scholar]
- Bostrom H, Gritli-Linde A, Betsholtz C. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn. 2002;223:155–162. doi: 10.1002/dvdy.1225. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate. 1996;29:282–286. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008;3:963–970. doi: 10.1097/JTO.0b013e3181834f52. [DOI] [PubMed] [Google Scholar]
- Apte SM, Bucana CD, Killion JJ, Gershenson DM, Fidler IJ. Expression of platelet-derived growth factor and activated receptor in clinical specimens of epithelial ovarian cancer and ovarian carcinoma cell lines. Gynecol Oncol. 2004;93:78–86. doi: 10.1016/j.ygyno.2003.12.041. [DOI] [PubMed] [Google Scholar]
- Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005;7:R788–R795. doi: 10.1186/bcr1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Morandi P, Krishnamurthy S, Reuben JM, Lee BN, Francis D, Booser DJ, Green MC, Arun BK, Pusztai L, Lopez A, Islam R, Valero V, Hortobagyi GN. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-beta: clinical activity and biological correlations. Ann Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj B, Klassen J, Cossette N, Sterns E, Tuck A, Deeley R, Sengupta S, Elliott B. Localization of platelet-derived growth factor beta receptor expression in the periepithelial stroma of human breast carcinoma. Clin Cancer Res. 1996;2:773–782. [PubMed] [Google Scholar]
- Coltrera MD, Wang J, Porter PL, Gown AM. Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahovic G, Rabbani ZN, Herndon JE, 2nd, Dewhirst MW, Vujaskovic Z. Treatment with Imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer. 2006;95:1013–1019. doi: 10.1038/sj.bjc.6603366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Santner S, Carolin KA, Tait L. Direct involvement of breast tumor fibroblasts in the modulation of tamoxifen sensitivity. Am J Pathol. 2007;170:1546–1560. doi: 10.2353/ajpath.2007.061004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- Wikström PMJ, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumour prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- Fukino K, Shen L, Patocs A, Mutter GL, Eng C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA. 2007;297:2103–2111. doi: 10.1001/jama.297.19.2103. [DOI] [PubMed] [Google Scholar]
- Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- Weber F, Xu Y, Zhang L, Patocs A, Shen L, Platzer P, Eng C. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297:187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, Andre S, Piccart M, Campone M, Brain E, Macgrogan G, Petit T, Jassem J, Bibeau F, Blot E, Bogaerts J, Aguet M, Bergh J, Iggo R, Delorenzi M. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.