Abstract
Non-alcoholic fatty liver disease (NAFLD), which includes steatosis and its progression to non-alcoholic steatohepatitis, is a liver disorder of increasing clinical significance. Here we characterize a murine model of high fat diet-induced NAFLD with progression from liver steatosis to histological features compatible with steatohepatitis and more advanced stages of NAFLD in humans, including chronic portal inflammation, pericellular and bridging fibrosis, Mallory body formation, and bile ductular reaction. Chronic changes induced by the prolonged consumption of a high-fat diet alone culminate in the development of primary liver dysplasias. Importantly, we extend these studies to demonstrate that even the early stages of uncomplicated steatosis provide a permissive microenvironment for the growth of colon cancer cells that are metastatic to the liver. High fat diet-induced steatosis, coupled with a splenic injection model of experimental liver metastasis using syngeneic MC38 colon cancer cells, resulted in an increased number of secondary tumor nodules and metastatic burden in steatotic livers. Metastatic nodules were associated with focal peritumoral areas of infiltrating inflammatory cells and associated apoptotic cell populations. These results suggest that the modulation of specific host factors in the steatotic liver contributes to tumor progression in the microenvironment of NAFLD.
Obesity and non-alcoholic steatohepatitis (NASH), have emerged as two independent risk factors for the development of cirrhosis and hepatocellular carcinoma distinct from well established risk factors including viral hepatitis and alcohol consumption.1,2,3 Nonalcoholic fatty liver disease (NAFLD) can result from a high-fat diet, lack of exercise, hepatitis infection, type II diabetes, or a combination of these factors. NAFLD is commonly associated with central obesity, insulin resistance, and hyperlipidemia, and characterized clinically by the metabolic syndrome.4,5 Obesity is clinically associated with an increased prevalence of certain malignancies including colon cancer and breast cancer.6,7 NASH associated with obesity is a recognized risk factor for the development of primary hepatocellular carcinoma.2,8 Recently, a small case study has identified hepatocellular carcinoma in subset of patients with NAFLD in the absence of cirrhosis, supporting NAFLD as permissive environment for the development of hepatocellular carcinoma.9 Further, since systemic disease represents the major cause of cancer mortality, the rising incidence of obesity, NAFLD, and NASH is likely to have an enormous influence on cancer-associated deaths in the United States.
In humans, liver steatosis represents an early reversible stage of disease that is histologically characterized by the accumulation of triglycerides in hepatocytes.10,11 Histologically, the broad term NALFD may include steatosis alone or it can include progressive changes associated with non-alcoholic steatohepatitis such as inflammation, hepatocyte ballooning, necrosis, Mallory’s hyaline, and even fibrosis.11,12,13 NASH can only be definitively diagnosed by liver biopsy, but it is suspected in patients with obesity and/or syndromes of insulin resistance accompanied by elevated serum aminotransferase levels.14,15 Naturally occurring genetic mutations such as of the leptin gene (ob/ob), genetically modified mice, environmental dietary models, and toxins such as ethanol have been shown to increase hepatic fat uptake leading to steatosis and steatohepatitis.16,17
Improved murine models that corroborate human disease are important for the understanding of microenvironmental factors that contribute to tumor promotion in the liver. It has been suggested through the use of genetic, as well as dietary mouse models, that obesity-related hepatic steatosis might increase the susceptibility of the liver to malignancy.18,19,20 It is recognized that other than high-fat diets, alcohol consumption, inflammatory cytokines, oxidative stress, alterations in the extracellular matrix, as well as viral mediators, can participate in development of steatohepatitis in humans and can be contributing risk factors for hepatocellular carcinoma. For example, changes in the inflammatory cytokines and extracellular matrix remodeling proteases have been associated with increased metastatic risk in multiple model systems and organs.21,22,23,24,25 Significant steatotic changes and or steatohepatitis cause increases in a number of signaling molecules, including transforming growth factor β and select matrix metalloproteinases that may be important in tumor promotion and growth.26,27,28
The microenvironmental effects of high-fat diet induced hepatic steatosis on tumor growth in our data indicate that early uncomplicated steatosis of the liver microenvironment enhances the number of metastatic foci and tumor burden in an experimental murine model of colorectal cancer metastasis to the liver. We demonstrate that changes in the steatotic liver establish a more susceptible microenvironment for metastasis as compared with normal liver. Additionally, multifocal dysplasia of the liver develops with prolonged consumption of high fat diet concordant with progressive histological changes analogous to NASH in this murine model.
Materials and Methods
Animals and Diet Study
C57bl/6J male mice were obtained from Jackson Research Laboratories (Bar Harbor, ME) at 8 weeks of age and housed in an accredited laboratory animal facility. On receipt, the mice were separated into appropriate cages and fed either a 13.5% fat “regular” diet (RD, 5001, LabDiet: 13.5% calories from fat, 58% from carbohydrates, and 28.5% from protein) or fed a 42% fat “high fat/western-style” diet (HF, TD.88137, Harlan Teklad (North America): 42% calories from fat, 42.7% from carbohydrates, and 15.2% from protein) ad libitum. Numbers of mice at specific time points on diet are as follows: at 1 month (3RD, 3HF), 3 month (3RD, 3HF), 7 month (4RD, 5HF), 9 month (3RD, 2HF), 14 month (2RD, 3HF), and 20 month (3HF). At the time of sacrifice, all mice were weighed, the livers were removed and weighed, and samples of each liver were either frozen in Tissue-Tek OCT compound (Sakura), fixed in buffered formalin, preserved in RNAlater (Qiagen, Valencia, CA), homogenized in RIPA buffer (10 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton) with addition of a complete Mini protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN) for protein analysis, or snap frozen in liquid nitrogen for storage. All samples not immediately used were stored at −80°C.
Histology
Gross liver images were obtained using a Konica Minolta 3.2MP camera. Oil Red O (ORO) staining was performed on 8 μm, freshly cut liver sections from samples preserved in Tissue-Tek on a Microm cryostat set to −19°C. Hydrated sections were fixed in buffered formalin for 30 minutes at room temperature. Following fixation, the slides were rinsed in indirect running tap water for 10 minutes, equilibrated in 60% isopropanol for 5 minutes, and stained for 15 minutes with ORO working solution. The working solution was prepared from stock solution, consisting of 5g ORO (Sigma, Aldrich, St. Louis, MO) in 100% isopropanol, at 3:2 with distilled water and subsequently filtered to remove particulates. Post staining, slides were rinsed for 30 seconds in 60% isopropanol, 5 minutes in distilled water, Mayer’s hematoxylin for 5 minutes, Tris-buffered saline for 1 minute, and then distilled water for 5 minutes. Slides were mounted with an aqueous mounting media (EMS Biomeda) and imaged with a Q Imaging Micropublisher color digital camera mounted to a Zeiss Axioplan 2 microscope using MetaMorph software. Additionally, formalin-fixed paraffin-embedded tissue sections were stained with Mayer’s Hematoxylin (Sigma) and Eosin/Phloxine B or stained with trichrome performed according to Gomori’s method and then imaged. Reticulin Staining was performed by the Vanderbilt Immunohistochemistry Core Facility.
For immunohistochemistry, formalin-fixed, paraffin-embedded tissue samples were cut at 6 μm on a Leica microtome, dried, and then re-hydrated with xylenes and a decreasing ethanol series. For antigen retrieval, hydrated sections were boiled in a citric acid solution (10 mmol/L trisodium salt dihydrate pH 6.0, 0.5% Tween-20) for 8 minutes or treated for 25 minutes at 37°C with 0.1% Trypsin in 0.05 M/L Tris, pH 7.8 with 0.1% CaCl. Post-retrieval, slides were stained with antibodies against either ubiquitin at 1:50 (Covance, PRB-268C), glial fibrillary acid protein (GFAP) premixed solution (Dako, N1506, Carpinteria, CA), smooth muscle actin (αSMA) at 1:500 (Abcam, ab32575), CK19 at 1:1000 (Developmental Studies Hybridoma Bank, Troma III clone, Iowa City, IA), CK8 at 1:100 (Abcam, ab9287), CD45 at 1:100 (BD Pharmingen, 30-F11), phospho-histone H3 at 1:500 (Upstate, 06-570, Lake Placid, NY), cleaved caspase-3 at 1:400 (Cell Signaling Technology, Asp175, Beverly, MA), anti-neutrophil at 1:100 (AbD Serotec, MCA771G, Raleigh, NC), or with F4/80 at 1:100 (AbD Serotec, MCA497GA). For diaminodenzidine, sections were labeled with appropriate species specific biotinylated secondary antibody (Vector Labs, Burlingane, CA), processed with a Vectastain kit (Vector Labs) and developed in chromogen solution (0.1 M/L Tris-HCl pH 7.4, 1.125 mmol/L diaminobenzidine, 0.01% H2O2) for 6 minutes. Images of diaminobenzidine-processed sections were acquired as stated above. For immunofluorescence, sections were labeled with appropriate species specific Alexa Fluor 488 or 568 conjugated secondary antibodies. Fluorescent images were acquired with a Hamamatsu Orca ER CCD camera mounted to a Zeiss Axioplan 2 Microscope using MetaMorph software (Molecular Devices, Dowington, PA). The staining index of each antibody was performed to determine the amount of staining per section by thresholding images to a preset background level through MetaMorph software and measuring the total area, average intensity, the thresholded area, and the percent thresholded area. The average intensity was multiplied by the percent thresholded area to determine the staining index per sample. Statistical analysis of the staining index was performed as non-parametric Mann-Whitney t-test using GraphPad Prism 4.03 software.
ORO Quantification
Images from ORO stained sections were calibrated using MetaMorph analysis software and the threshold was set according to the relative range of staining intensities. The threshold was kept constant for all images and a percent threshold area was calculated for each image. Eight images were obtained for each sample and percent area was averaged to give the relative staining level for that sample. ORO staining level for western diet and regular diet samples were then plotted over time using GraphPad, and statistical significance was calculated using one-way analysis of variance.
Experimental Liver Metastasis
C57bl/6J mice were maintained on either a 42% fat diet (n = 17) or on 13.5% standard rodent chow (n = 22) for a period of 12 weeks. Metastatic tumors were initiated using a splenic injection model of liver metastasis with syngeneic MC38 murine colon cancer cells provided by Dr. Steven Libutti, National Cancer Institute. MC38 cells were grown in culture conditions of 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) in Dulbecco’s Modified Eagle Media (Gibco BRL, Carlsbad, CA) at 37°C and harvested at 75% confluence for experimental metastatic splenic injections. Splenic injections were performed following previously described methods29 on 5-month-old mice that had been on diet for 3 months at the time of injection. In brief, 1 × 105 cells were injected into the spleen and allowed to perfuse to the liver for 2 minutes before resecting the spleen via cauterization, suturing the abdominal cavity, and allowing the mouse to recover. Mice were sacrificed at 21 days post-injection and the burden of metastatic liver tumors were compared between the two groups. At the time of sacrifice the mice were weighed, the livers were removed and weighed, and the livers were processed for histology as described above. Gross liver weights of 14 regular diet and 9 high-fat diet, non-injected animals were taken for comparison with MC38 injected gross liver weights. Graphical representation of metastatic burden was calculated using GraphPad software to compare the total liver weights, as well as the percentage of liver weight relative to the total animal weight between 14 injected regular diet mice and 11 injected high-fat diet mice. Statistical significance was determined using a nonparametric test with Bonferroni’s multiple comparison. Further, in-depth quantitative analysis of tumor burden was assessed on the left lateral lobe of the liver, which was removed and fixed whole in buffered formalin overnight at 4°C. An additional 8 regular-diet mice and 6 high-fat diet mice with MC38 injections were used for quantitative section analysis. The fixed liver was cut sagittally into four parts and paraffin-embedded. Fully reconstructed liver cross sections were obtained from multiple images for each H&E-stained section of the four parts of each liver sample. Metamorph software was used to quantify the size and number of tumors per section. Statistical significance was determined using nonparametric Mann-Whitney analytical t-tests.
Results
Induction of Steatosis in Mice through a High Fat Diet
Mice were maintained on their respective diets until they were sacrificed at specified time points of 1, 3, 7, 9, 14, and 20 months on diet. Steatosis in the cohorts was assessed by gross liver appearance, liver weight, liver weight as a percentage of body weight, and ORO staining for lipid accumulation.
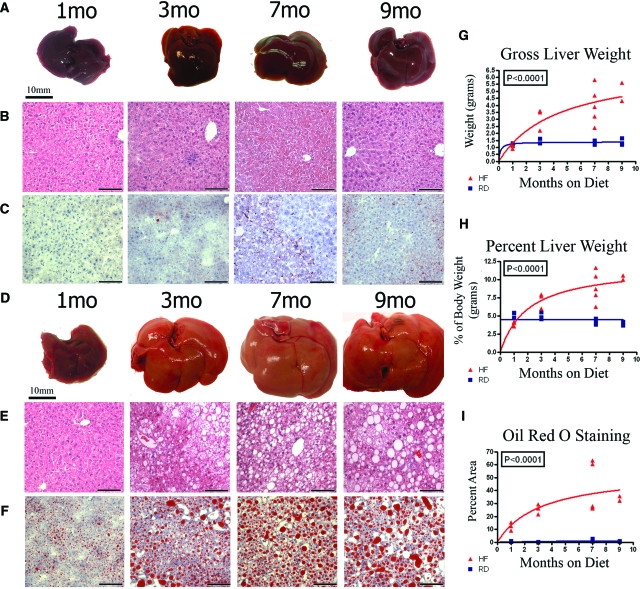
The high fat diet generated a dramatic increase in the liver size and weight, as well as in the percentage of lipid accumulation compared with controls (Figure 1). Gross livers from mice maintained on regular diet for 1, 3, 7, and 9 months retained dark red coloration of the liver and showed very little change in either the gross appearance or the size and weight (Figure 1A). Consumption of a high fat diet resulted in an increase in gross liver size and yellowish coloration of the liver progressively over time (Figure 1D). Graphical comparison of the gross liver weights over time, as well as liver as a percentage of the total body weight showed a significant threefold (P < 0.016) and twofold (P < 0.016) increase respectively by 7 months in the mice fed high-fat diet when compared with fed mice regular diet (Figure 1, G and H). H&E stained sections from livers at respective time points demonstrate the enlarged hepatocytes and an increasing degree of steatosis represented by the vacuolation in hepatocytes, which correlated with increasing amount of time consuming the high fat diet (Figure 1, B and E). ORO staining was used to distinguish and quantify the accrual of lipid deposits in the liver over time (Figure 1, C and F). Consumption of a high fat diet induced rapid accumulation of lipids such that an average of 25% of the liver area showed lipid positive staining by 3 months on diet and 40% of liver area had lipid positive staining by 7 months on diet. Even after 7 months on regular diet the liver lipids accumulate to only a very minimal 1.8% of the liver area as determined by ORO staining. Quantification of hepatic steatosis through ORO staining showed a significant increase over time in livers after consumption of high fat diet compared with regular diet (Figure 1I, P < 0.0001).
Figure 1.
Mice were maintained on a regular diet (RD: A–C) of 13.5% fat or on a high fat diet (HF: D–F) consisting of 42% fat through a time course of 1 to 9 months. Even after 9 months, gross livers from mice on the regular diet remain dark, red, free of lipid accumulation, and show little change in size over time (A). Gross liver images from mice on high fat diet show an increase in the gross size, along with yellowish appearance as a result of steatosis over time (D). The degree of hepatic steatosis increases with time in the livers of mice on high fat diet and appears as vacuolation in H&E stain (E), which is absent at all stages in mice maintained on regular diet (B). Steatosis is distinguished by ORO (C, F) staining of lipids (red droplets). The graphs respectively plot gross liver weight (G, P < 0.0001, one-way ANOVA), the percent liver weight as compared against total body weight (H; P < 0.0001, one-way ANOVA), and the percent area of ORO staining (I; P < 0.0001, one-way ANOVA) in livers of mice fed regular diet (blue box/line) and mice fed the high fat diet (red triangle/line). ORO staining in the liver increased 23-fold at 7 months on high fat diet, as compared with regular diet (P < 0.016 nonparametric Mann-Whitney). Scale bars are 10 mm for all gross liver images and 100 μm for H&E and ORO images.
Progression to Pathology Compatible with NASH and Early-Stage Cirrhosis
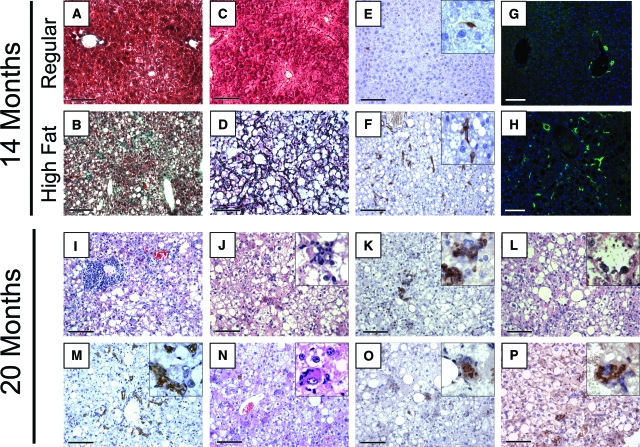
To determine whether mice exposed to a high fat diet would eventually develop features comparable with NASH, a subset of mice were maintained on the 42% fat diet for late stage time points of 14 and 20 months. Two control mice maintained on regular diet were followed out to 14 months as well. Gross morphology indicated that all livers maintained on high fat diet exhibited an abnormal appearance with atypical yellowish coloration. Trichrome, blue-green staining of collagen fibers (Figure 2, A and B), and reticulin, black staining of reticular fibers (Figure 2, C and D), was performed to determine the relative extent of fibrosis in the high fat diet livers compared with regular diet livers. The mice fed a 42% high fat diet exhibited prominent pericellular fibrosis by 9 months on diet that progressed into Zones 2 and 3 by 14 months on diet. By 14 months, livers of mice fed the high fat diet revealed advanced stages of fibrosis included classical signs of pericellular ‘chicken wire’ fibrotic appearance (Figure 2, B and D) and even delicate bridging fibrosis was noted. The mice fed regular diet had very minimal fibrosis indicated by trichrome and reticulin (Figure 2, A and C).
Figure 2.
Prolonged consumption of high fat diet leads to the development of conditions associated with NASH in mice consuming high fat diet for 14 months (B, D, F, H) and 20 months (I–P). Comparison of fibrosis and presence of activated stellate cells between mice fed regular diet (A, C, E, G) and mice fed high fat diet (B, D, F, H) for 14 months. Consumption of high fat diet for 14 months results in the development of prominent pericellular and portal fibrosis in high fat livers compared with regular livers as revealed through trichrome (A, B) or reticulin staining (C, D). At the 14-month stage, classical chicken wire fibrosis is demonstrated by reticulin staining in high fat livers (D), which was absent in regular diet livers (C). Increased detection of activated hepatic stellate cells is seen through α smooth muscle actin (αSMA; E, F) or glial fibrillary acidic protein (GFAP; G, H) staining (green) in livers of mice on high fat diet (F, H), as compared with regular diet (E, G). Consumption of high fat diet for 20 months results in the development of portal inflammation (I), as well as lobular inflammation (J) seen in H&E or through staining for CD45 (K). Features of ballooning degeneration of hepatocytes were detected via H&E (L). Bile ductal reaction was identified with anti-cytokeratin 19 staining (M). Further, pathological inclusions compatible with Mallory bodies were detected within the hepatocytes as pink accumulations evident in H&E stained sections (N), or as brown precipitate from diaminobenzidine reaction after anti-ubiquitin staining (O), or anti-cytokeratin 8 staining (P). Scale bars = 100 μm; insets magnification = original ×63.
Hepatic stellate cells are typically associated with fibrotic phenotypes and increased deposition of collagen in the liver. An increased number of stellate cells was detected in livers after 14 months’ consumption of high fat diet, compared with regular diet, as determined by immunohistochemistry for αSMA or immunofluorescence for GFAP (Figure 2, E and F, and G and H, respectively). The average relative staining index at 14 months on diet increased between regular diet and high fat diet for both αSMA from 20 ± 7 to 111 ± 46; P < 0.002 and GFAP from 475 ± 362 to 3136 ± 255; P < 0.05.
Additional histological features of fatty liver disease were observed at 14 months on diet and became more prominent and with increased incidence by 20 months on high fat diet (Figure 2, I–P). By 20 months on diet, chronic inflammatory cell infiltration was widely seen associated with the portal triads (Figure 2I). At these times there was also lobular inflammation and the association of CD45 positive cells around hepatocytes (Figure 2, J and K). The high fat content made ballooning hepatocytes difficult to identify, however they were noted at 14 months as well as 20 months (Figure 2L). Bile ductular reaction was revealed by cytokeratin 19 immunohistochemistry (Figure 2M). In addition, pink inclusions comparable with Mallory’s hyaline were seen in H&E sections (Figure 2N), which was further confirmed through immunohistological staining for ubiquitin (Figure 2O) as well as for cytokeratin 8 (Figure 2P). Both ubiquitin and cytokeratin 8 have been noted as positive indicators of pathological inclusions, consistent with Mallory bodies.30,31,32 The results seen in our mouse model are similar to typical histological features seen in humans diagnosed with late stage NAFLD and NASH, including stages commonly associated with pre-cirrhotic complications.11,12
Progression to Primary Dysplasia
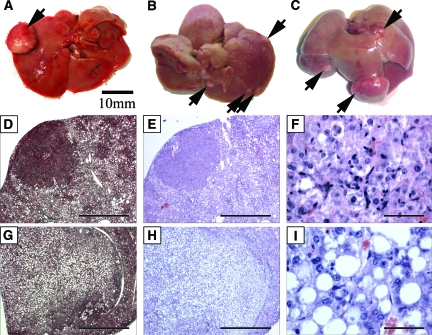
Gross and histological examination of livers from mice fed the high fat diet for 9, 14, and 20 months revealed multiple atypical nodules, as well as focal masses in the livers. Histological analysis detected dysplastic features in these nodules. Spontaneous dysplastic tumors were detected in our experiments as early as 9 months in a mouse maintained on high fat diet (Figure 3A), but were not seen in control mice maintained on regular diet at the same time points. There were detectable dysplastic lesions in 1/2 mice at 9 months, 3/3 mice at 14 months, and 3/3 mice at 20 months in mice maintained on high fat diet. In distinction, none of the mice (0/3 at 9 months and 0/2 at 14 months) maintained on regular diet were observed with gross or microscopic abnormal dysplasia. An increase in the number of dysplastic foci correlated with time on diet and multiple tumors were observed in mice on high fat diet for 14 and 20 months (Figure 3, B and C).
Figure 3.
Prolonged consumption of high fat diet leads to dysplastic tumor formation. Dysplastic tumor nodules were identified in high fat diet livers as early as 9 months on diet (A: 1/2 mice). Gross images of livers maintained on a high fat diet for 14 months (B: 3/3 mice), and 20 months (C: 3/3 mice) respectively display multiple dysplastic nodules visible on the liver surface while no dysplasias were detected in mice maintained on regular diet for 9 or 14 months (0/3, 0/2 mice), even after microscopic sectional analysis. Histological analysis of liver sections revealed two different types of dysplastic nodules as either solid and non-fatty (D, E, F) or as tumors containing large droplets of lipid (G, H, I). Histological sections were stained with trichrome (D, G), as well as H&E (E, F, H, I) for analysis. High power magnification of H&E stained sections shows the cellular context of the two types of tumors (F, I). Scale bar = 10 mm (A, B, C); 1 mm (D, E, G, H); and 50 μm in (F, I).
Serial sections of livers were stained with Trichrome, as well as H&E to enhance the detection and identification of tumor nodules on the surface and within the liver parenchyma. Histologically, nodules displayed either a solid dysplastic morphology (Figure 3, D–F) or a dysplastic morphology associated with lipid and protein globules (Figure 3, G–I). High power magnification demonstrates the histology of the cellular differences between the two types of dysplastic tumors (Figure 3, F and I). These dysplasias were noted to be non-invasive as determined by pathological assessment and no further testing was done to determine the extent of molecular transformation in these cells. No tumors were detected in any major organs outside of the liver, including the lungs, heart, epididymal fat, intestine, or mesentery.
Increased Colorectal Liver Metastases in a Murine Model
The dysplasia observed in the diseased livers of mice maintained on prolonged high fat diet suggested that this microenvironment was favorable for tumor formation. Next, we asked whether the hepatic steatosis, without the marked inflammatory cell infiltration and other histological features associated with late stage NAFLD, might be conducive to the establishment of metastases from distant sites. The liver represents the most important site for colorectal cancer metastasis.
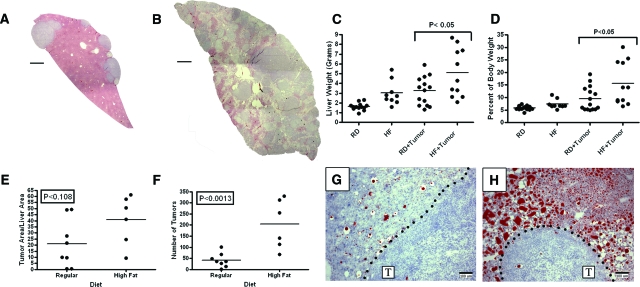
Mice maintained on the high fat diet showed an increase in metastatic tumor burden when compared with mice maintained on a regular diet. High resolution composite illustrations from multiple images of liver sections from MC38 injected mice show the extent of metastatic tumor burden in the steatotic high fat diet livers (Figure 4B), as compared with regular-diet livers (Figure 4A). Tumor burden was assessed as total liver weight in grams, as well as liver weight as a percentage of total body weight (Figure 4, C and D). Both graphical analyses showed statistically significant increases in liver and tumor weights for injected mice fed the high fat diet, as compared with the regular diet (P < 0.05).
Figure 4.
Liver steatosis resulting from a high fat diet contributes to an increase in metastatic tumor burden. Mice fed either the regular diet (RD) or the high fat diet (HF) for 12 weeks were subsequently injected via the spleen with 100,000 MC38 syngeneic colon carcinoma cells. Representative reconstructed sections of liver from injected mice are shown for regular diet (A) and high fat diet (B). Graphs reflect the changes in liver weight for uninjected mice (RD n = 14, HF n = 9), as well as MC38 injected mice (RD+Tumor n = 14, HF+Tumor = 11) through assessment of metastatic tumor burden as absolute liver weights (C) or liver weights as a percentage of total body weight (D). Injected mice fed the high fat diet showed a 1.6-fold increase in total liver weight and a 1.4-fold increase in liver weight normalized as a percentage of total body weight (P < 0.05 Bonferroni’s multiple comparison), as compared with injected regular diet mice. To detect the average percent tumor area as well as tumor number, the upper left lobe of the liver was sectioned into four parts and composite images were assembled for mice on regular (n = 8) and high fat diet (n = 6). Graphs from composite image analysis show a trending but non-significant increase in values for the tumor area per section (E; P < 0.108), but result in a dramatic increase in the number of tumors per section (F; P < 0.0013) for high fat diet livers compared with regular diet livers. ORO with hematoxylin-stained sections from the tumor-stromal interface of a postinjected mouse maintained on regular diet (G) or the high fat diet (H). Metastatic tumor is labeled T and the dotted line represents the tumor-stromal interface in each image. Statistical significance in all graphs was determined using the Nonparametric Mann-Whitney analytical t-test. Scale bar = 1 mm (A, B) and 100 μm = (G, H).
To further assess the extent of tumor burden, histological analysis was performed on additional injected mice by measuring tumor number and tumor area in multiple sections of a single lobe of the liver. The percent area occupied by tumor per section and the average number of tumors are shown in a graphical representation (Figure 4, E and F). Although the percent tumor area was not statistically different (P < 0.108, Mann-Whitney, Figure 4E), the average number of tumors per mouse was statistically increased by 4.8-fold in mice maintained on high fat diet compared with regular diet (P < 0.0013; Figure 4F). Histologically, hepatic steatosis persisted throughout tumor development in the high fat diet mice as evidenced by ORO staining of lipids among tumors that appeared blue from the hematoxylin counterstain (Figure 4, G and H).
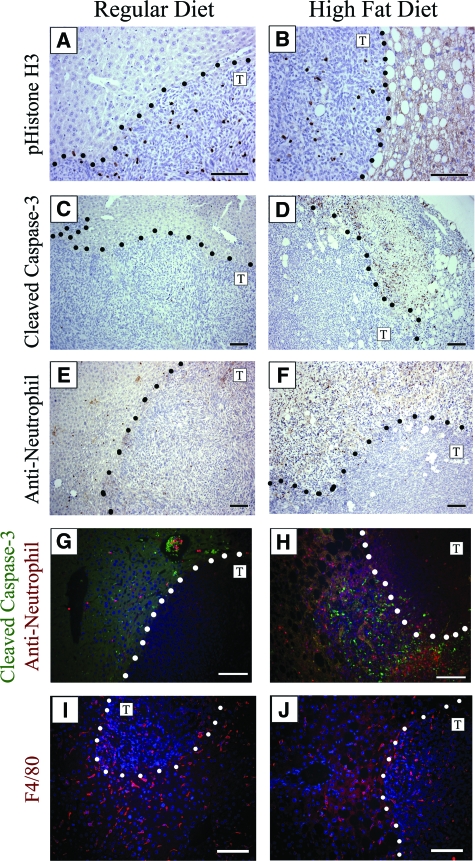
To assess whether the steatotic liver provided a proliferative growth advantage for the MC38 cells, we performed phospho-histone H3 immunostaining (Figure 5, A and B). The proliferative index showed similar levels of proliferation between metastatic tumors in the regular and high fat livers (P < 0.178). The non-tumor bearing areas were also similar in their basal levels of proliferation (data not shown). To determine whether there was more apoptotic cell death associated with either of the tumors, sections were stained with a cleaved caspase-3 antibody (Figure 5, C and D). There was no detectable difference in the number of apoptotic cells within the tumors of either the high fat diet or regular diet livers. However, there were areas of densely positive cleaved caspase-3 stained cells adjacent to tumors in the high fat diet livers (6/6, Figure 5D), which was not seen in the regular diet livers (0/8, Figure 5C). These areas of dense apoptotic cells next to tumor nodules in high fat diet livers also showed a high number of infiltrating inflammatory cells as determined by staining with an anti-neutrophil antibody (Figure 5F). Co-immunofluorescence revealed that the neutrophils (red) had infiltrated into the same area as the cleaved caspase-3 (green)-positive cells, but represented distinct cell populations (Figure 5H). Neutrophils were sparsely observed in the host tissue and in the tumor nodules of livers from regular diet mice (Figure 5, E and G). Further, macrophages (red) were detected in the tumor nodules as well as the non-tumor liver tissue from both regular diet and high fat diet mice (Figure 5, I and J). We speculate that infiltrating inflammatory cells may contribute to cellular apoptosis in peri-tumoral regions of metastatic lesions in the steatotic but not in the normal liver microenvironment.
Figure 5.
Cellular responses to metastatic tumors in livers of mice maintained on regular diet or high fat diet. Dotted lines represent the tumor/liver boundary and the T designates the tumor side of the interface. No difference in cellular proliferation was detected from the staining index when comparing MC38 metastatic tumors or surrounding liver tissue using a phospho-histone H3 (pHistone-H3) antibody (A, B). Tumors contained very low numbers of apoptotic cells regardless of diet (C, D), while focal areas adjacent to MC38 induced tumors in the livers of 6/6 mice maintained on the high fat diet were found to contain high levels of cleaved caspase-3 staining indicating increased apoptosis (D). These densely apoptotic areas seen in the steatotic livers also contain high levels of inflammatory cells, demonstrated with anti-neutrophil staining (F), while neutrophils in regular diet livers were sparse (E). Co-immunofluorescence microscopy of cleaved caspase-3 (green) and anti-neutrophil (red) shows sparse staining near tumors in the liver of a mouse maintained on the regular diet (G), whereas mice maintained on high fat diet show high levels of distinct areas of peri-tumoral staining for both antibodies (H). Macrophages were found associated with the surrounding host tissue as well as within the tumor nodules in both sets of mice after staining with anti-F4/80 antibodies (red) and Hoechst for nuclei (blue) (I, J). Scale bar =50 μm (C, D, E, F) and 100 μm (A, B, G, H, I, J).
Discussion
NAFLD encompasses a spectrum of liver disease and complex metabolic disorders that can eventually lead to end stage hepatic dysfunction and cirrhosis, complicated by malignancy. Notably, the murine model described in this study corroborates findings of human liver disease with the clinically relevant high fat diet induction of fatty liver disease. We have shown that consumption of a 42% fat content diet causes accumulation of lipids within the hepatocytes of mice. Other studies have reported the same genetic strain of mice and the same diet used in our study also leads to increased visceral fat mass, serum insulin, and serum leptin levels, as early as 8 weeks on diet.33 The lipid accumulation in our mouse model became prominent within zone 1 at 3 months on diet and eventually occupied 75% of hepatocytes by 7 months on diet. In addition, prolonged consumption of high fat diet in our mouse model leads to the development of features comparable with the human condition of NASH. These features include fibrosis, increased stellate cell activation, inflammation, hepatocellular ballooning degeneration, bile ductular reaction, and even the formation of pathological inclusions.
Our findings using only a high fat diet suggest that the steatotic microenvironment is favorable for the development of primary tumors. We demonstrated that prolonged consumption of a high fat diet alone culminates over time in the development of primary dysplastic nodules in the liver. In the setting of a steatotic microenvironment, we observed two histologically different types of dysplastic nodules, solid or lipid associated, as early as 32 weeks. This result corroborated published observations where male mice fed a choline deficient diet showed similar findings that pre-neoplastic foci consisted of both acidophilic (solid) as well as vacuolated (fat) adenomas by 65 weeks.20 Interestingly, adenomas containing fat droplets also developed in adiponectin deficient mice fed a choline deficient diet after just 24 weeks.34 Liver tumors can develop due to a loss of leptin (ob/ob mouse model) and accompanied over-nourishment.18 Our mouse model has demonstrated the development of dysplastic hepatic tumors from high fat diet alone without genetic manipulations, although these tumors did not have features of invasion. This is notable since the murine C57Bl/6J genetic background has been recognized as having a low susceptibility to spontaneous tumor formation (The Jackson Laboratory, http://jaxmice.jax.org/strain/000664.html, 07.30.2008).35 Additionally, no liver tumors were detected in any of the mice in our study maintained on regular diet. Overall, this model shows that alterations in diet and fat metabolism can shift the balance between lipid production and lipid catabolism, affecting the liver microenvironment and its susceptibility to develop tumors.
These studies further demonstrated that the steatotic liver is a favorable stromal microenvironment for metastasis. The role of the stromal microenvironment has been suggested to act by influencing both the proliferation of the tumor cells, as well as their differentiation.36 Although we saw similar levels of proliferation in our metastatic model, the number of tumors was increased in the high-fat diet livers as compared with controls. The percent tumor area involved was not statistically different (P < 0.108, Mann-Whitney, Figure 4E), but the average number of tumors per mouse was statistically increased by 4.8-fold in mice maintained on high fat diet, as compared with regular diet (P < 0.0013; Figure 4F). This suggests a possible role of the steatotic microenvironment that is favorable for tumor initiation.
Histological examination showed the metastatic colon cancer tumors in steatotic livers were associated with areas of high inflammatory cell infiltration and peri-tumoral cell death. Inflammatory cells such as neutrophils may be recruited in response to the metastatic tumors in the steatotic livers and contribute to their growth. This same inflammatory cell recruitment did not occur around tumors in livers maintained on the regular diet, suggesting that the steatotic microenvironment incurred a heightened cytokine response after introduction of metastatic cells. Neutrophils are reported to be a source of T-cell recruiting chemokines37 and can additionally produce cytokines to attract mature dendritic cells and macrophages.38 The invading neutrophils that are seen, may be responsible for the high levels of peri-tumoral cell death, however, the exact mechanism is not known at this time. Importantly, it has been suggested that tumor cells may actually commandeer inflammatory cell mediators such as chemokines and growth factors for their own growth advantage.39,40 Although inflammatory infiltrates are often associated with tumors, the type and functional relevance of infiltrate varies among tumors.41 Tumor associated macrophages of the M2 pathway are more likely to promote tumors than the classical M1 type macrophages.42
The epidemic of obesity and NAFLD has many clinical implications. Our data demonstrates that a diet consisting of 42% calories from fat results in a steatotic microenvironment in the murine liver that is conducive to the establishment of metastatic tumors and the eventual development of primary dysplastic nodules in the setting of chronic disease. We demonstrate that signals from the steatotic host microenvironment likely set the stage for tumor development even in the initial reversible and treatable stages of fatty liver disease. As steatosis progresses to exhibit an inflammatory component, the susceptibility for tumor establishment increases. Dietary-induced NAFLD, superimposed on any predisposition for hereditary or other non-hereditary cancers may additionally incur an even greater risk for the development of hepatic neoplasias, whether primary or metastatic. This model appears to be a good system in which potential therapeutic agents might be tested and lead to a better understanding of mechanisms involved in the progression of non-alcoholic fatty liver disease.
Acknowledgments
We acknowledge Dr. Stacey Huppert in the Department of Cell and Developmental Biology at Vanderbilt University for gracious technical help with the CK19 immunostaining.
Footnotes
Address reprint requests to D. Lee Gorden, 1313 21st. Ave. S., 801 Oxford House, Vanderbilt University Medical Center, Nashville, TN 37212. E-mail: lee.gorden@vanderbilt.edu.
Supported by grants from the American Cancer Society #PF-05-167-01-CSM (M.V.S.); Vanderbilt Ingram Cancer Center P50CA095103 (in support of the Immunohistochemistry Core Facility). Core Services performed through Vanderbilt University Medical Center’s Digestive Disease Research Center were supported by NIH grant P30DK058404 (M.K.W.). D.L.G. was supported in part by Grant Number P50CA095103 from National Cancer Institute and a K08 DK70708-01 grant from the National Institute of Health. Additional support was from NIH grant R01CA060867 (L.M.) and Vanderbilt University Discovery Grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the ACS, NCI, or NIH.
References
- Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291–293. [PubMed] [Google Scholar]
- Cuadrado A, Orive A, Garcia-Suarez C, Dominguez A, Fernandez-Escalante JC, Crespo J, Pons-Romero F. Non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma. Obes Surg. 2005;15:442–446. doi: 10.1381/0960892053576596. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Plat J, Schrauwen P. Diet and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:25–29. doi: 10.1097/MOL.0b013e3282f382ea. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Tabuchi M, Tsurita G, Ishikawa M, Otani K, Nagawa H. Adiposity and gastrointestinal malignancy. Digestion. 2009;79 Suppl 1:26–32. doi: 10.1159/000167863. [DOI] [PubMed] [Google Scholar]
- Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4:173–177. [PubMed] [Google Scholar]
- Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–1133. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- Bondini S, Kleiner DE, Goodman ZD, Gramlich T, Younossi ZM. Pathologic assessment of non-alcoholic fatty liver disease. Clin Liver Dis. 2007;11:17–23. doi: 10.1016/j.cld.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Mendler MH, Kanel G, Govindarajan S. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver Int. 2005;25:294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65:S57–S63. doi: 10.1111/j.1753-4887.2007.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Bjornsson E, Angulo P. Non-alcoholic fatty liver disease. Scand J Gastroenterol. 2007;42:1023–1030. doi: 10.1080/00365520701514529. [DOI] [PubMed] [Google Scholar]
- Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- Koteish A, Mae Diehl A. Animal models of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:679–690. doi: 10.1053/bega.2002.0332. [DOI] [PubMed] [Google Scholar]
- Heston WE, Vlahakis G. Genetic obesity and neoplasia. J Natl Cancer Inst. 1962;29:197–209. [PubMed] [Google Scholar]
- Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61:5016–5023. [PubMed] [Google Scholar]
- Denda A, Kitayama W, Kishida H, Murata N, Tsutsumi M, Tsujiuchi T, Nakae D, Konishi Y. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient. L-amino acid-defined diet. Jpn J Cancer Res. 2002;93:125–132. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- Swain MG. Hepatic NKT cells: friend or foe? Clin Sci (Lond) 2008;114:457–466. doi: 10.1042/CS20070328. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes and Development. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta 1 release. Biochem J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK, Rogers DD, 2nd, Wyatt TA, Sorrell MF, Tuma DJ. Transforming growth factor-beta induces contraction of activated hepatic stellate cells. J Hepatol. 2004;41:60–66. doi: 10.1016/j.jhep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Gorden DL, Fingleton B, Crawford HC, Jansen DE, Lepage M, Matrisian LM. Resident stromal cell-derived MMP-9 promotes the growth of colorectal metastases in the liver microenvironment. Int J Cancer. 2007;121:495–500. doi: 10.1002/ijc.22594. [DOI] [PubMed] [Google Scholar]
- Manetto V, Abdul-Karim FW, Perry G, Tabaton M, Autilio-Gambetti L, Gambetti P. Selective presence of ubiquitin in intracellular inclusions. Am J Pathol. 1989;134:505–513. [PMC free article] [PubMed] [Google Scholar]
- Pei RJ, Danbara N, Tsujita-Kyutoku M, Yuri T, Tsubura A. Immunohistochemical profiles of Mallory body by a panel of anti-cytokeratin antibodies. Med Electron Microsc. 2004;37:114–118. doi: 10.1007/s00795-003-0240-1. [DOI] [PubMed] [Google Scholar]
- Ohta M, Marceau N, Perry G, Manetto V, Gambetti P, Autilio-Gambetti L, Metuzals J, Kawahara H, Cadrin M, French SW. Ubiquitin is present on the cytokeratin intermediate filaments and Mallory bodies of hepatocytes. Lab Invest. 1988;59:848–856. [PubMed] [Google Scholar]
- Bullen JW, Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2007;292:E1079–E1086. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: seventh listing for the International Committee on Standardized Genetic Nomenclature for Mice. Cancer Res. 1980;40:2083–2128. [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Molesworth-Kenyon SJ, Oakes JE, Lausch RN. A novel role for neutrophils as a source of T cell-recruiting chemokines IP-10 and Mig during the DTH response to HSV-1 antigen. J Leukoc Biol. 2005;77:552–559. doi: 10.1189/jlb.0904485. [DOI] [PubMed] [Google Scholar]
- Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, Cassatella MA. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981–1988. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Le Bitoux MA, Stamenkovic I. Tumor-host interactions: the role of inflammation. Histochem Cell Biol. 2008;130:1079–1090. doi: 10.1007/s00418-008-0527-3. [DOI] [PubMed] [Google Scholar]
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection, Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]