Abstract
Models of epidermal carcinogenesis have demonstrated that Ras is a critical molecule involved in tumor initiation and progression. Previously, we have shown that RasGRP1 increases the susceptibility of mice to skin tumorigenesis when overexpressed in the epidermis by a transgenic approach, related to its ability to activate Ras. Moreover, RasGRP1 transgenic mice develop spontaneous papillomas and cutaneous squamous cell carcinomas, some of which appear to originate in sites of injury, suggesting that RasGRP1 may be responding to signals generated during the wound-healing process. In this study, we examined the response of the RasGRP1 transgenic animals to full-thickness incision wounding of the skin, and demonstrated that they respond by developing tumors along the wounded site. The tumors did not present mutations in the H-ras gene, but Rasgrp1 transgene dosage correlated with tumor susceptibility and size. Analysis of serum cytokines showed increased levels of granulocyte colony-stimulating factor in transgenic animals after wounding. Furthermore, in vitro experiments with primary keratinocytes showed that granulocyte colony-stimulating factor stimulated Ras activation, although RasGRP1 was dispensable for this effect. Since granulocyte colony-stimulating factor has been recently associated with proliferation of skin cancer cells, our results may help in the elucidation of pathways that activate Ras in the epidermis during tumorigenesis in the absence of oncogenic ras mutations.
The role of Ras activation in non-melanoma skin cancer is well-documented, both from analysis of human squamous cell carcinomas (SCC)1 as well as from studies using mouse models of skin carcinogenesis.2,3 In particular, the multistage carcinogenesis protocol on mouse skin has identified activating mutations in the ras proto-oncogene as the initiation event in skin neoplasms.4 Interestingly, whereas Ras mutations are prevalent in mouse models of skin carcinogenesis, they have only been identified in 12% to 46% of sporadic human SCC samples, despite the fact that Ras is activated in the majority of the human SCC.1,5 This suggests that other mechanisms of Ras activation play a role in the human disease. For example, epidermal growth factor receptor overexpression by amplification is known to occur in human SCC,6,7 and epidermal growth factor receptor could lead to the biochemical stimulation of Ras. In animal models, overexpression of epidermal growth factor receptor ligands like transforming growth factor-α,8 or a dominant form of the Ras exchange factor Sos of seven less (SOS),9 act as an initiation event in the epidermis, further demonstrating that alterations in Ras upstream signals could lead to tumorigenesis in the skin via wild-type Ras activation.
Biochemical activation of Ras in keratinocytes can be triggered by various extracellular stimuli, but in all cases it requires the participation of exchange factors that catalyze the GDP–GTP exchange. The best studied GDP–GTP exchange factor is SOS1, which is activated in response to receptor tyrosine kinase activation.10,11 In recent years, we have identified a new GDP–GTP exchange factor in epidermal keratinocytes, RasGRP1, and demonstrated that it can transduce the activation of Ras by the diacylglycerol analog and potent skin tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA).12 The initial studies prompted us to investigate the role of RasGRP1 in the tumor promotion effects of phorbol esters in the multistage carcinogenesis protocol using a transgenic mouse model for overexpression of RasGRP1 under the keratin 5 promoter (K5.RasGRP1). Surprisingly, RasGRP1 overexpression did not increase the susceptibility to TPA in animals initiated by the carcinogen 7, 12-Dimethylbenz[a]anthracene (DMBA), but caused more malignant tumors than those observed in the wild-type mice, suggesting a role of RasGRP1 in tumor progression.13 This effect appeared strongly related to increases in active Ras, as RasGRP1 overexpression clearly leads to elevated RasGTP levels in mouse epidermal keratinocytes, both under basal as well as TPA stimulated conditions.13 Interestingly, the K5.RasGRP1 mice also produced tumors in response to TPA alone, implying that RasGRP1 overexpression can act as an initiation event. In addition to the response to chemical carcinogens and tumor promoters, we observed that the RasGRP1 transgenic colony developed spontaneous skin tumors over time, more frequently seen in animals housed in groups or with previous episodes of skin abrasion or injury.14 This response resembled that of the Tg.Ac mice, which express v-H-ras under the ζ-globin promoter and generate tumors in response to tumor promoting stimuli like wounding and phorbol esters in absence of carcinogenic/initiation events.15,16
The in vivo studies with the K5.RasGRP1 mice have implicated RasGRP1 as a novel link for Ras activation in epidermal keratinocytes and skin cancer. However, the signaling mechanisms contributing to RasGRP1 activation and spontaneous tumor formation, as well as the direct evidence for a role of skin wounding as a tumor promoter stimulus in the context of RasGRP1 overexpression, remained to be established. To address these questions, we have now characterized the tumorigenic response of the K5.RasGRP1 to full-thickness incision wounding of the skin. Our findings show that RasGRP1 overexpression in the epidermis conferred sensitivity to wounding-induced promotional stimuli. Furthermore, upon wounding, there was a significant increase in the levels of granulocyte colony-stimulating factor (G-CSF) in the circulation of transgenic mice compared with the wild-type animals. In vitro, G-CSF was able to stimulate Ras activation in keratinocytes in a very rapid fashion, although RasGRP1 appeared dispensable for this effect. Since G-CSF has been associated to the growth and progression of skin carcinoma cells, the data presented here may have implications for the understanding of RasGRP1-Ras signaling in skin tumor biology.
Materials and Methods
Mice
The K5.RasGRP1 transgenic mice were previously generated and maintained in the FVB/N background.14 RasGRP1 null mice (KO-RasGRP1), produced by inserting the Escherichia coli β-galactosidase gene and a neomycin cassette in exon 2 of Rasgrp1,17 were originally obtained in 129/J background and backcrossed for >10 generation to the FVB/N background. Wild-type mice were bred in house. All animal studies were done according to Institutional Animal Care and Use Committee guidelines at the University of Hawaii Animal Facility.
Full-Thickness Incision Wounding Protocol
Two cohorts based on genotype (wild-type and K5.RasGRP1 mice) were used for the studies. Each cohort had between 12 and 16 mice of mixed gender (equal number of males and females) between 6 to 8 weeks of age. Two days before wounding, the dorsal skin of the mice was shaved with electric clippers. Then, a full-thickness, 3-cm long incision was performed across the dorsal skin under isoflurane anesthesia. The wound was closed with five to seven surgical clips that were removed 7 days later. Mice were followed twice per week for a total of 10 weeks, to observe wound closure and tumor formation. Tumor size was measured with a caliper at least once a week. At the end of the protocol, the animals were euthanized by CO2 narcosis. Tumor samples were collected and processed for histology.
Histology and Analysis of H-ras Mutations
Skin tumors were fixed in 4% paraformaldehyde for 24 hours, dehydrated, and maintained in 70% ethanol at 4°C until paraffin-embedded. H&E-stained slides were used for descriptive histopathology. Two 10-μm paraffin-embedded tumor sections were used for DNA extraction and ras mutation analysis. Briefly, after deparaffination, samples were digested with proteinase K followed by DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA). One hundred ng of DNA were used for a PCR reaction to amplify the H-ras sequence including codons 12, 13, and 61, using primers described by Vassar et al.8 Genomic DNA from SP-1 cells was used as a positive control for H-ras mutations. These cells (papilloma-derived keratinocytes generated by chemical-induced carcinogenesis in SENCAR mice) were obtained from Dr. Stuart Yuspa (National Cancer Institute, Bethesda, MD) and cultured as described for the primary keratinocytes (below). The PCR products were sequenced by the Advanced Studies in Genomics, Proteomics and Bioinformatics facility at University of Hawaii at Manoa.
Epidermal Keratinocyte Cultures
Primary cultures of mouse skin keratinocytes were prepared as described previously with modifications.14 Briefly, keratinocytes were isolated from newborn epidermis by the trypsin flotation method18 and then plated at a density of 1.5 × 106 cells/60-mm cell culture dish coated with collagen I (Coating Matrix, Cascade Biologics-Invitrogen, Portland, OR) in Minimum Essential Medium (S-MEM) (Invitrogen, Carlsbad, CA) supplemented with 8% fetal bovine serum, non-essential amino acids, antibiotics/antimycotics, and CaCl2 to a final 0.3 mmol/L concentration. After a 24-hour incubation period, cultures were switched to a low calcium medium consisting of medium 154CF (Cascade Biologics-Invitrogen, Portland, OR) containing antibiotics/antimycotics and CaCl2 to a final 50 μmol/L concentration. This medium was supplemented with 2% Chelex-treated fetal bovine serum and a keratinocyte growth factor mix consisting of 0.2% bovine pituitary hormone, 5 μg/ml bovine insulin, 0.18 μg/ml hydrocortisone, 5 μg/ml bovine transferrin, and 0.2 ng/ml human epidermal growth factor (Cascade Biologics-Invitrogen, Portland, OR). Keratinocytes were used within 5 to 6 days after plating.
Southern Blot Analysis
Ten μg of genomic DNA from transgenic K5.RasGRP1 mice were digested with NheI/BamHI overnight and then separated on a 0.7% agarose gel, transferred to a nylon membrane, and hybridized with a probe for detection of the Rasgrp1 transgene. The probe was prepared by PCR of the transgene rat Rasgrp1 plasmid using primers previously described.14 The PCR fragment (∼700 bp) was labeled with [α-32P]dCTP using the DecaLabel kit (Fermentas, Glen Burnie, MD) according to the manufacturer’s instructions, and used for hybridization following standard molecular biology procedures.
Ras Pull-Down Assay
Levels of GTP-loaded Ras (RasGTP) were measured by using the GST-RBD domain of Raf-1 as a probe in a pull-down assay. Briefly, primary keratinocytes were serum starved overnight (0.1% fetal bovine serum), treated with vehicle (0.1% bovine serum albumin in PBS) or 10 ng/ml mouse recombinant G-CSF (Leinco Technologies, Inc., St. Louis, MI) for 3 minutes, and immediately harvested on ice in lysis buffer containing 25 mmol/L Tris-HCl (pH 7.5), 150 mmol/L of NaCl, 5 mmol/L of MgCl2, 1 mmol/L of NaF, 1 mmol/L of sodium orthovanadate, 1% IGEPAL, 5% glycerol, and Mini Complete Roche-protease inhibitors (Roche Applied Science, Indianapolis, IN). Lysates were vortexed, incubated on ice for 5 minutes and then clarified by centrifugation at 13,000 rpm for 15 minutes at 4°C. Five hundred μg of lysate protein were incubated with GST-RBD-Raf-1 conjugated to glutathione beads for 1 hour with rotation in the cold. The affinity complexes were washed thrice with lysis buffer and then resuspended in 2× Laemmli buffer, boiled, and resolved on 15% acrylamide gels. Twenty-five μg of the total lysate protein were run in parallel as measurement of input of total Ras in the assay. Proteins were blotted onto nitrocellulose membranes and immunostaining was done using the pan anti-Ras clone RAS10 antibody (Calbiochem, San Diego, CA). RasGRP1 levels were evaluated by immunostaining using a monoclonal anti-RasGRP1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For the in vitro wound assay, primary keratinocyte monolayers (80% to 90% confluence) were scratched with a pipette tip forming an eight-square grid pattern. Cells were harvested 5 minutes after wounding and used for a Ras pull down assay as described above. Fifty μg of total lysate protein were run in parallel as measurement of input of total Ras for the wound assay.
Mouse Cytokine Antibody Array
Transgenic K5.RasGRP1 and wild-type animals were subjected to a full-thickness incision wound as described above. Blood was extracted from the mice at time 0 and 24 hours post-incision by cardiac puncture under isoflurane anesthesia. Serum was obtained by incubating the blood samples at room temperature for at least 2 hours followed by centrifugation. For the cytokine array experiment, serum samples were diluted 1:4 in dilution media provided in the array kit (TranSignal Mouse Cytokine Antibody Array 1.0, Panomics, Freemont, CA), and incubated for 2 hours at room temperature with gentle rotation. The remaining steps were done according to the manufacturer’s instructions.
Results
K5.RasGRP1 Transgenic Mice Develop Skin Tumors in Response to Full-Thickness Incision Wounding
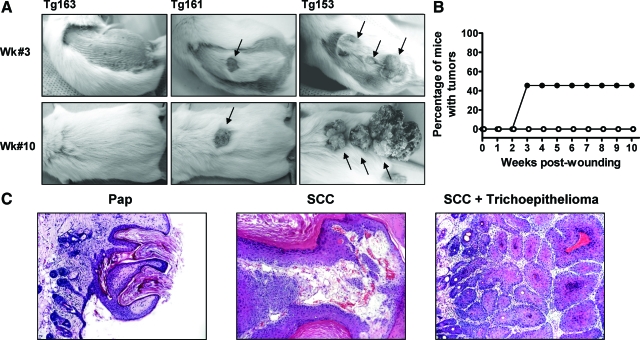
Our previous studies suggested a causal association between wounding and spontaneous skin tumors in the K5.RasGRP1 transgenic mice.14 To investigate this association and gain further insight into the mechanisms of action of RasGRP1 in skin tumorigenesis, we used a full-thickness incision wounding protocol on wild-type and RasGRP1 transgenic mice. Two weeks after the dorsal incisions were performed, the wounds had healed in both groups, but raised lesions began to appear along the margins of the incision in a percentage of the K5.RasGRP1 mice. By the third week after wounding, tumors were clearly evident in the transgenic mice (Figure 1A). The tumor incidence at week #3 reached 50% in the transgenic group and did not vary significantly throughout the remaining 7 weeks of the protocol (Figure 1B). No tumors developed in the wild-type mice. Histopathological analysis revealed that the majority of the tumors generated in the K5.RasGRP1 mice were well-differentiated SCC, with only one papilloma and one trichoepithelioma, the latter observed in conjunction with an SCC (Figure 1C).
Figure 1.
Tumorigenic response of K5.RasGRP1 transgenic mice to skin wounding. A: Representative photographs of skin tumors (arrows) developed in the transgenic mice at week 3 and 10 after a 3-cm full-thickness incision wound was performed on the dorsal skin. B: Tumor incidence (percentage of mice with tumors) in wild-type (open circle) and K5.RasGRP1 transgenic (closed circle) mice subjected to wounding. C: Representative microphotographs of H&E-stained tumors developed in the transgenic mice subjected to wounding. Pap, papilloma; SCC, squamous cell carcinoma.
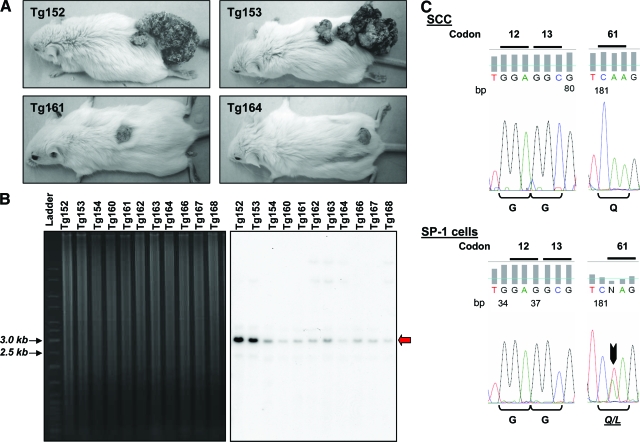
Two transgenic mice in the protocol displayed a significantly elevated tumorigenic response, as compared with the rest of the tumor-bearing mice (Figure 2A). Whereas the transgenic animals used in this protocol were all deemed heterozygous, the differences in response among the animals prompted us to examine transgene copy numbers by Southern blot analysis. The results confirmed the transgenic status of all of the animals in the K5.RasGRP1 cohort (Figure 2B), but also indicated that the two mice with the elevated response had a higher transgene copy number than the rest of the transgenic mice in the group. Whether the nature of the copy number difference is due to homozygosis, remains to be determined; nevertheless, this finding suggests that Rasgrp1 gene dosage influences tumor susceptibility to wounding.
Figure 2.
Analysis of transgene copy number and H-ras mutations in the K5.RasGRP1 mice and skin tumors. A: Photographs of transgenic tumor-bearing mice taken 10 weeks after a full-thickness incision wound was performed on the dorsal skin. Note one animal group with large tumors (top), and the other group with one small dorsal tumor only (bottom). B: Southern blot of NheI/BamHI digested genomic DNA from the K5.RasGRP1 mice subjected to the wounding protocol. The left panel corresponds to an ethidium bromide-stained agarose gel, showing equal DNA loading of the lanes. The right panel is the Southern blot autoradiogram from the same gel, obtained with a probe specific for detection of the Rasgrp1 transgene, and used to determined copy number. The red arrow points at the band containing the transgene (∼2.9 kb). C: H-ras sequencing analysis of genomic DNA obtained from K5.RasGRP1-derived tumors. A representative chromatogram is shown for DNA from SCC. Sequencing results for DNA from SP-1 cells are also shown as a positive control for H-ras mutations (black arrow). The corresponding amino acids for each codon are indicated below the chromatograms.
Since many skin tumors generated in mouse models carry Ras activating mutations that participate as an initiation event, we examined the ras gene status in the tumors developed in K5.RasGRP1 mice in response to wounding. We focused on H-ras, as this is the most commonly mutated form of ras occurring in mouse skin papillomas and squamous cell carcinomas.19 Using a PCR approach followed by sequencing, we analyzed genomic DNA extracted from six SCC from the K5.RasGRP1 group for H-ras mutation at codons 12, 13, and 61. As control, we used genomic DNA extracted from a papilloma-derived keratinocyte cell line (SP-1), which carries a mutation in codon 61 of the H-ras gene.20 While the SP-1 cells showed the expected heterozygous T to A transversion in codon 61 of H-ras, no mutations in the H-ras proto-oncogene were present in the tumors (Figure 2C).
G-CSF Levels Are Increased in the Serum of K5.RasGRP1 Mice in Response to Wounding
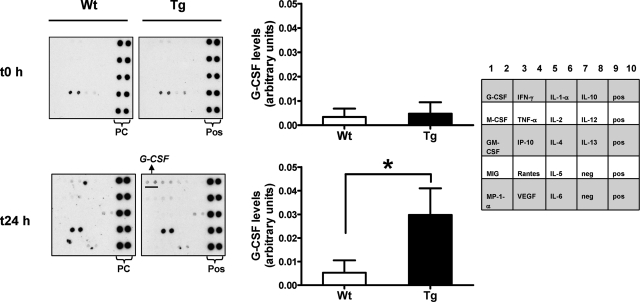
We have previously seen that primary mouse keratinocytes derived from K5.RasGRP1 mice secreted G-CSF on in vitro wounding assays, while levels of this cytokine were negligible in conditioned medium derived from wounded wild-type keratinocyte monolayers.14 This was an interesting observation, since G-CSF is a cytokine associated to skin neoplasia.21 Therefore, we sought to test if K5.RasGRP1 animals could secrete elevated levels of this cytokine on in vivo wounding of the skin. Using a mouse antibody array for cytokine detection, we found that serum levels of G-CSF under non wounded conditions were very low or below the limit of detection in both wild-type and K5.RasGRP1 mice (Figure 3). However, we detected a significant increase in G-CSF levels in the serum of K5.RasGRP1 mice 24-hour post wounding (Figure 3), providing evidence that skin wounding in transgenic RasGRP1 mice leads to increases in G-CSF secretion.
Figure 3.
Cytokine serum levels from mice subjected to skin wounding. A mouse antibody cytokine array was probed with serum derived from wild-type (Wt) or K5.RasGRP1 (Tg) mice before and 24 hours after performing a 3-cm full-thickness incision wound on the dorsal skin of the mice. Pos, positive controls. Representative array images are shown on the middle panel. Densitometry analysis was performed on the G-CSF spots in the arrays, normalized to the positive controls, and assigned arbitrary units. The results were plotted as means ± SE of three to eight independent experiments per group (left panel). Empty columns, wild-type keratinocytes; filled columns, K5.RasGRP1 keratinocytes. *P < 0.05 (one-tailed Student’s t-test). A diagram of the array showing the location of antibodies in the membrane is shown in the right panel. Pos: positive control; neg: negative control.
G-CSF Induces Ras Activation in Mouse Keratinocytes
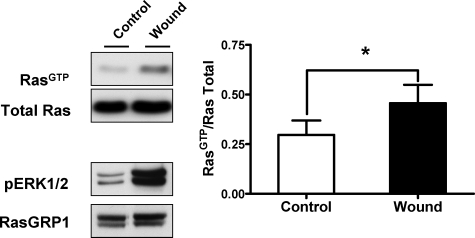
ras oncogenes have been shown to cause induction of G-CSF in different cell types.22 While RasGRP1 transgenic keratinocytes possess elevated levels of active Ras compared with wild-type cells,14 those levels do not appear to be sufficient to trigger G-CSF secretion under control conditions. We then speculated that the increase in G-CSF levels from K5.RasGRP1 keratinocytes observed on wounding could be related to a spike of Ras activation induced by the wound-healing process. In fact, stimulation of Ras by wounding has been shown to occur in primary human keratinocytes.23 To test if that was also the case under our experimental conditions, we subjected primary mouse keratinocytes to in vitro wounding and measured Ras active levels by pull down assay. As shown in Figure 4, levels of RasGTP, as well as active ERK1/2 (phospho ERK1/2), were rapidly increased after wounding. Because RasGTP is elevated in the K5.RasGRP1 cells, the further Ras activation caused by wounding could explain the effect on G-CSF secretion from the transgenic mice.
Figure 4.
Activation of Ras in response to in vitro wounding of primary mouse keratinocytes. Monolayers of primary keratinocytes isolated from newborn mouse skin were wounded as described in Materials and Methods and used to measure levels of active, GTP-loaded Ras (RasGTP) by a pull down assay. The left panel shows representative Western blots for Ras, phospho-ERK1/2, and RasGRP1 for both control (Control) and wounded (Wound) monolayers collected 5-minute post in vitro wounding. Densitometry analysis of RasGTP levels normalized by their corresponding total Ras levels were plotted as mean ± SE of three independent experiments, with each control-wound sample handled in parallel for each experiment (right panel). Empty columns, control keratinocytes; filled columns, wounded keratinocytes. *P < 0.05 (paired Student’s t-test).
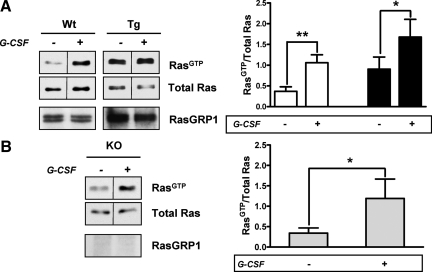
G-CSF is known to stimulate Ras in hematopoietic cells,24 thus G-CSF could also induce further Ras activation in keratinocytes, providing a positive feedback loop between Ras and G-CSF secretion, with RasGRP1 mediating the effect. To test this hypothesis, we compared RasGTP levels in primary keratinocytes derived from wild-type and K5.RasGRP1 animals upon G-CSF treatment. As shown in Figure 5A, G-CSF produced a significant activation of Ras in both wild-type and RasGRP1 transgenic keratinocytes and, as expected from the elevated RasGRP1 levels in the transgenic cells, activation of Ras was overall higher in the K5.RasGRP1 keratinocytes compared with the wild-type counterparts. However, two-way no matching analysis of variance test indicated that there was no effect of the genotype on the response to G-CSF (wild-type versus transgenic, P = 0.92, no significant), suggesting that RasGRP1 was dispensable in the effect of G-CSF on Ras.
Figure 5.
G-CSF-induced activation of Ras in primary mouse keratinocytes. A: Keratinocytes isolated from newborn wild-type (Wt) or K5.RasGRP1 (Tg) mice were treated for 3 minutes with vehicle (−) or 10 ng/ml mouse recombinant G-CSF (+) and then collected for a Ras pull down assay. Representative Western blots for RasGTP, total Ras and RasGRP1 are shown on the left panel. Levels of RasGTP were analyzed by densitometry, normalized by their corresponding total Ras levels, and plotted as mean ± SE of four to six independent experiments per group, with each control-treatment sample handled in parallel for each experiment (right panel). Empty columns, wild-type keratinocytes; filled columns, K5.RasGRP1 keratinocytes. *P < 0.05; **P < 0.003 (paired Student’s t-test). B: Keratinocytes derived from newborn RasGRP1 null mouse (KO) skin were treated with vehicle (−) or G-CSF (+) as indicated above and used for a Ras pull down assay. Representative Western blots are shown on the left panel. After densitometry analysis, results were plotted as mean ± SE of six independent experiments handled as control-treatment pairs per experiment (right panel). *P < 0.05 (paired Student’s t-test). Note that Western blots for RasGTP and total Ras have a dividing line between lanes, as the lanes shown were not contiguous in the gel. The juxtaposed lanes were acquired under the same condition of brightness and contrast, and derived from the same gel.
We have previously noticed that under high basal levels of RasGTP, further stimulation of RasGRP1—even with potent diacylglycerol mimetics—frequently produced only a minimal stimulation of Ras,25 suggesting that Ras activation could reach a plateau under our experimental conditions. Therefore, we wanted to confirm that the lack of effect of genotype on G-CSF-mediated Ras activation was not a result of an experimental artifact due to saturation of a response. To this end, we used primary keratinocytes derived from RasGRP1 null mice (KO-RasGRP1) in Ras pull down assays to evaluate the requirements for RasGRP1 in response to G-CSF. As shown in Figure 5B, the level of Ras stimulation induced by G-CSF in KO-RasGRP1 cells was significant and comparable with the stimulation observed in wild-type keratinocytes. Taken together, the results imply that RasGRP1 was not involved in the stimulation of Ras induced by G-CSF in mouse keratinocytes.
Discussion
In the present study we have demonstrated that transgenic mice for overexpression of RasGRP1 in epidermis are susceptible to wounding as a tumor promotional stimuli, developing cutaneous SCC with a short latency. The findings, together with our previous observations about the role of RasGRP1 in the multistage model of skin carcinogenesis, strongly suggest a role of RasGRP1 in the genesis of skin tumors derived from keratinocytes.
RasGRP1 is one of the members of the RasGRP family, and functions as a guanine nucleotide exchange factor for Ras small GTPases, catalyzing their GDP–GTP exchange and leading to Ras activation.26 In keratinocytes, RasGRP1 overexpression by transient transfection or by transgenic approach, leads to increases in the levels of basal RasGTP.12,14 Therefore, one mechanism for the tumorigenic susceptibility of the K5.RasGRP1 transgenic mice could be the elevated levels of active Ras. Our data revealed no H-ras mutations in tumors originated on wounding of transgenic mice, and although mutations in other ras genes cannot be excluded, the more likely scenario is that the increase in RasGTP levels is a result of biochemical stimulation of wild-type Ras by RasGRP1. Additionally, we observed that Rasgrp1 gene dosage influences the tumor susceptibility and size in response to wounding, further supporting the idea that Ras activation depends on RasGRP1 in our transgenic mouse model.
It has been previously documented that overexpression of active ras mutant genes in the epidermis leads to skin tumors27; however, the localization of the mutant within the epidermis influences the dependency on a tumor promoter stimulus for tumor formation and the malignancy of the tumors.28,29 In this regard, while expression of a mutant ras in suprabasal keratinocytes depends on tumor promotion for papilloma formation, expression in basal keratinocytes using the keratin 5 promoter results in spontaneous tumors.29 One could predict that in our model, in which RasGRP1 is overexpressed under the keratin 5 promoter, tumor promotion could be dispensable. Still, tumor promotion is required. The possibility exists that overexpression of RasGRP1 alone does not lead to full Ras activation—at levels comparable with activation of mutant forms of Ras—and thus, a further stimulus is required to activate RasGRP1 and, in turn, Ras.
The finding that G-CSF was elevated in the circulation of the RasGRP1 transgenic mice after wounding, together with our previous observation with primary keratinocytes subjected to the in vitro wounding assay,14 supports the concept that G-CSF is at least in part produced by the transgenic epidermal keratinocytes in response to wounding. Previous studies have demonstrated that keratinocytes secretion of G-CSF can contribute to tumor growth and progression in SCC.21 Thus, the increase in G-CSF seen in the K5.RasGRP1 mice raises the possibility that this cytokine may be a critical contributing factor in the wounding-induced tumor formation in our transgenic mouse model. The fact that K5.RasGRP1-derived keratinocytes secret this cytokine also suggests that a stimulated RasGRP1-Ras pathway participates in the expression of G-CSF. In fact, ras oncogene expression can induced cytokines like G-CSF in human fibroblast and mesothelioma cells, and while the exact mechanism is still not understood, it involves in part the stabilization of cytokine transcripts.22 Further studies are warranted to examine the role of G-CSF in keratinocytes in our model and the precise sequence of events that triggers RasGRP1-Ras activation in these cells.
Footnotes
Address reprint requests to Patricia S. Lorenzo, Cancer Research Center of Hawaii, 651 Ilalo Street, Room 222-K, Honolulu, Hawaii 96813. E-mail: plorenzo@crch.hawaii.edu.
Supported by the National Institutes of Health, grants R01 CA096841 and R01 CA096841-S (P. S. Lorenzo).
References
- Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis–thirty-third G. H A Clowes Memorial Award lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
- Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Spencer JM, Kahn SM, Jiang W, DeLeo VA, Weinstein IB. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch Dermatol. 1995;131:796–800. [PubMed] [Google Scholar]
- Derynck R. The physiology of transforming growth factor-alpha. Adv Cancer Res. 1992;58:27–52. doi: 10.1016/s0065-230x(08)60289-4. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Izumi H, Oga A, Furumoto H, Murakami T, Ofuji R, Muto M, Sasaki K. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology. 2001;202:203–206. doi: 10.1159/000051637. [DOI] [PubMed] [Google Scholar]
- Vassar R, Hutton ME, Fuchs E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol Cell Biol. 1992;12:4643–4653. doi: 10.1128/mcb.12.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Rambaratsingh RA, Stone JC, Blumberg PM, Lorenzo PS. RasGRP1 represents a novel non-protein kinase C phorbol ester signaling pathway in mouse epidermal keratinocytes. J Biol Chem. 2003;278:52792–52801. doi: 10.1074/jbc.M308240200. [DOI] [PubMed] [Google Scholar]
- Luke CT, Oki-Idouchi CE, Cline JM, Lorenzo PS. RasGRP1 overexpression in the epidermis of transgenic mice contributes to tumor progression during multistage skin carcinogenesis. Cancer Res. 2007;67:10190–10197. doi: 10.1158/0008-5472.CAN-07-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki-Idouchi CE, Lorenzo PS. Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res. 2007;67:276–280. doi: 10.1158/0008-5472.CAN-06-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A, Kuo A, Cardiff RD, Sinn E, Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Nat Acad Sci. 1990;87:9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble MC, Trempus CS, Spalding JW, Cannon RE, Tennant RW. Biological, cellular, and molecular characteristics of an inducible transgenic skin tumor model: a review. Oncogene. 24:8217–8228. doi: 10.1038/sj.onc.1209000. [DOI] [PubMed] [Google Scholar]
- Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303:72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Strickland JE, Greenhalgh DA, Koceva-Chyla A, Hennings H, Restrepo C, Balaschak M, Yuspa SH. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- Obermueller E, Vosseler S, Fusenig NE, Mueller MM. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004;64:7801–7812. doi: 10.1158/0008-5472.CAN-03-3301. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Ernst TJ, Pratt ES, 2nd, Zenzie BW, Rheinwald JG, Griffin JD. Expression of ras oncogenes in cultured human cells alters the transcriptional and posttranscriptional regulation of cytokine genes. J Clin Invest. 1990;86:1261–1269. doi: 10.1172/JCI114833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi L, Chassot AA, Rezzonico R, Yeow K, Loubat A, Ferrua B, Lenegrate G, Ortonne JP, Ponzio G. Dynamic characterization of the molecular events during in vitro epidermal wound healing. J Invest Dermatol. 2002;119:56–63. doi: 10.1046/j.1523-1747.2002.01805.x. [DOI] [PubMed] [Google Scholar]
- Rausch O, Marshall CJ. Tyrosine 763 of the murine granulocyte colony-stimulating factor receptor mediates Ras-dependent activation of the JNK/SAPK mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1170–1179. doi: 10.1128/mcb.17.3.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill MC, Oki CE, Lorenzo PS. Differential effects of bryostatin 1 and 12-O-tetradecanoylphorbol-13-acetate on the regulation and activation of RasGRP1 in mouse epidermal keratinocytes. Mol Cancer Ther. 2006;5:602–610. doi: 10.1158/1535-7163.MCT-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JC. Regulation of Ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34:858–861. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- Brown K, Quintanilla M, Ramsden M, Kerr IB, Young S, Balmain A. v-ras genes from harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986;46:447–456. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- Bailleul B, Surani MA, White S, Barton SC, Brown K, Blessing M, Jorcano J, Balmain A. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]