Abstract
Tumor hypoxia directly promotes genomic instability and facilitates cell survival, resulting in tumors with a more aggressive phenotype. The proto-oncogene pim-1 regulates apoptosis and the cell cycle by phosphorylating target proteins. Overexpression of Pim-1 can cause genomic instability and contribute to lymphomagenesis. It is not clear whether Pim-1 is involved in hypoxia-mediated tumor survival in solid tumors. Here, we show that hypoxia can stabilize Pim-1 by preventing its ubiquitin-mediated proteasomal degradation and can cause Pim-1 translocation from the cytoplasm to the nucleus. Importantly, overexpression of Pim-1 increases NIH3T3 cell transformation exclusively under hypoxic conditions, suggesting that Pim-1 expression under hypoxia may be implicated in the transformation process of solid tumors. Also, blocking Pim-1 function by introduction of dominant negative Pim-1 resensitizes pancreatic cancer cells to apoptosis induced by glucose-deprivation under hypoxia. Introduction of short interfering RNAs for Pim-1 also resensitizes cancer cells to glucose deprivation under hypoxic conditions, while forced overexpression of Pim-1 causes solid tumor cells to become resistant to glucose deprivation. Moreover, dominant negative Pim-1 reduces tumorigenicity in pancreatic cancer cells and HeLa xenograft mouse models. Together, our studies indicate that Pim-1 plays a distinct role in solid tumor formation in vivo, implying that Pim-1 may be a novel target for cancer therapy.
Low oxygen partial pressure is a characteristic of almost all types of solid tumors. Tumor hypoxia is a result of the abnormal process of neoplastic growth, which has a crucial dependence on oxygen and nutrients from the host. The supply of oxygen and nutrients is mainly achieved through neo-angiogenesis, a process by which new blood vessels are formed from pre-existing ones (angiogenesis) and endothelial precursor cells (vasculogenesis).1 Tumor hypoxia directly promotes genetic instability, thereby accelerating the incidence of genetic changes, which could be favored by the tumor microenvironment. Tumor hypoxia also selects for genetic mutations in tumor cells, in particular, mutations in genes involved in the process of apoptosis. In fact, it has been demonstrated that repeated exposure to low oxygen tension promotes p53 mutations.2
The pim-1 oncogene, first identified as an oncogene activated in retroviral-induced murine T-cell lymphomas,3 has weak oncogenic activity when expressed as a transgene in a lymphoid-specific fashion. When newborn pim-1 transgenic mice were infected with Moloney murine leukemia virus, c-myc or N-myc were activated by proviral insertion resulting in faster development of T-cell lymphomas, when compared with nontransgenic mice.4 Moreover, Allen et al reported that pim-1 transgenes induced lymphomas in mice in a dose-dependent manner and that pim-1, collaborated with myc family oncogenes, bmi-1, or gfi-1/pal-1, to accelerate experimental T-cell lymphomagenesis.5 Recent reports define a novel role for elevated Pim-1 expression in promoting genomic instability in human prostate tumors.6,7,8 It has become evident that Pim-1 kinases may also have important roles outside the hematopoietic system.9 However, it is yet to be determined how Pim-1 transforms cells.
Pim-1 encodes a serine/threonine protein kinase that has been shown to phosphorylate several proteins, including p21cip1/waf1,10 p27 Kip1,11 CDC25A,12 NuMA,13 Pim-1 associated protein (PAP-1),14 and Bad.15 However, the molecular mechanism regulating Pim-1 expression is still not clear. Recent reports demonstrate that Pim-1 is degraded through the ubiquitin-proteasome pathway and is stabilized by heat shock protein (Hsp) 90, which is a regulator in the stability and activity of hypoxia-induced transcription factor-1 α (HIF-1α).16 Moreover, Pim-1 is increased in a neonatal hypoxia-ischemia rat model.17 Thus, we hypothesize that hypoxia may stabilize Pim-1 protein by blocking its ubiquitin-mediated proteasomal degradation and enhance Pim-1 activity to contribute to tumor formation.
In this study, we examine the roles of Pim-1 in solid tumor formation in vivo, as well as the molecular mechanism regulating Pim-1 expression under hypoxia. We show that Pim-1 is induced and is stabilized by hypoxia through reduction in the ubiquitin-mediated proteasomal degradation process. Importantly, overexpression of Pim-1 increases NIH3T3 cell transformation exclusively under hypoxia, suggesting that Pim-1 expression under hypoxia may be implicated in the transformation process of solid tumors. Introduction of dominant negative Pim-1 increases apoptosis and inhibits tumor angiogenesis in a pancreatic cancer xenograft mouse model. Together, our studies indicate that Pim-1 has a critical role in solid tumor formation, implying that Pim-1 may well be a novel target for solid tumor therapy.
Materials and Methods
Cell Lines and Reagents
Pancreatic ductal adenocarcinoma cell lines PCI-10 and PCI-43 cells were kindly provided by Dr. Hiroshi Ishikura (The First Department of Pathology, Hokkaido University School of Medicine). Human colon cancer HCT-116 cells, human cervical cancer HeLa cells, and human embryonic kidney 293 cells were provided by Dr. Jun-ichi Hamada (Division of Cancer-Related Genes, Institute for Genetic Medicine, Hokkaido University). FLAG-Pim-1 plasmids were kindly provided by Dr. Naoya Fujita (Division of Experimental Chemotherapy, The Cancer Chemotherapy Center of the Japanese Foundation for Cancer Research). HA-suppressor of cytokine signaling-1 (SOCS-1) plasmids were kindly provided by Dr. Paul Rothman. PCI-43 cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM)/F-12 1:1 (Sigma, Saint Louis, MO) supplemented with 10% fetal bovine serum (Gemini Biological Products, Woodland, CA) and penicillin/streptomycin (Mediatech, Inc., Herndon, VA; 100 units/ml and 100 μg/ml, respectively). HCT-116, HeLa, NIH3T3, and 293 cells were maintained in DMEM (Sigma) supplemented with 10% fetal bovine serum and penicillin/streptomycin. All cell lines were grown in the presence of 5% CO2 at 37°C. Cells were maintained under hypoxic conditions at 37°C in a modular incubator chamber (Billups-Rothenberg Inc., San Diego, CA) filled with 5% CO2 and 1% O2 (balanced with N2).
Small interfering RNAs (siRNAs) for Pim-1 were synthesized with the Silencer siRNA Construction kit (Ambion, Austin, TX, Cat. 1620). Sequences were as follows: RNAsi-Pim-1, sense: 5′-AATGATGAAGTCGAAGAGATCCCTGTCTC-3′, anti- sense: 5′-AAGATCTCTTCGACTTCATCACCTGTCTC-3′. GFP-control-siRNA: 5′-GGCTACGTCCAGGAGCGCACC-3′.
Annexin-V-FLUOS staining kit was purchased from Japan Roche Diagnostic Co. Ltd. (Tokyo, Japan). The 26S proteasome inhibitor N-acetyl leucyl-leucyl norlucinal (ALLnL) was purchased from Calbiochem Inc (Darmstadt, Germany). The proteasome inhibitor MG132 was purchased from Peptide Institute Inc (Osaka, Japan). The Hsp 90 inhibitor geldanamycin was purchased from AG Scientific (San Diego, CA). PCI-43, HCT-116, HeLa, NIH3T3, and 293 cells were transfected with FLAG-Pim-1 plasmids or FLAG-dominant negative (dn)Pim-1 plasmids by using Lipofectamine TM 2000 (Invitrogen, Carlsbad, CA). At 24 hours post-transfection, cells were initiated to incubate under hypoxia.
Western Blot Analysis
Cells were harvested and lysed with lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 0.15M NaCl, 1%NP-40, 1 mmol/L EDTA, Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), and were then sonicated. After centrifugation at 15,000 rpm, supernatants were collected as a whole lysate protein sample. Total proteins were separated on 10% polyacrylamide SDS-polyacrylamide electrophoresis gels and electrotransferred to polyvinylidene difluoride membranes. The membranes were incubated with anti-Pim-1 antibody (ABGENT, San Diego, CA), anti-Pim-2 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Pim-3 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-FLAG M2 monoclonal antibody (Sigma, Saint Louis, MO), anti-actin antibody (Santa Cruz Biotechnology), and anti-HA antibody (Santa Cruz Biotechnology).
To extract nuclear proteins, HeLa cells were first transfected with Pim-1 plasmid and then incubated under normoxia or hypoxia for 16 hours. The cells were then harvested and incubated in buffer A (10 mmol/L Hepes, pH 7.8, 10 mmol/L KCl, 0.1 mmol/L EDTA, 1 mmol/L dithiothreitol, 2 mg/ml aprotinin, 0.5 mmol/L phenylmethyl sulfonyl fluoride, and 0.5% Triton X-100). After centrifugation at 5000 rpm, buffer A was collected, including cytoplasmic proteins, as the cytoplasmic protein sample. Buffer C (50 mmol/L Hepes, pH 7.8, 420 mmol/L KCl, 0.1 mmol/L EDTA, 5 mmol/L MgCl2, 10% glycerol, 1 mmol/L dithiothreitol, 2 mg/ml aprotinin, and 0.5 mmol/L phenylmethyl sulfonyl fluoride) was added to the pellet. After rotation for 30 minutes and centrifugation at 15,000 rpm, supernatants were collected as the nuclear protein sample.
Ubiquitination Assays
PCI-43 cells were pre-treated with 26S proteasome inhibitor ALLnL (Calbiochem Inc., Darmstadt, Germany) for 16 hours, and ubiquitinated proteins collected by means of an ubiquitinated protein enrichment kit (Cat. No.662200, Calbiochem Inc., Darmstadt, Germany). The expression of ubiquitinated proteins was detected with anti-Pim-1 antibody. For the transfection experiment, 293 cells were co-transfected with FLAG-Pim-1 (3 μg) and pCI-neo-HA-ubiqutin (1 μg). At 24 hours post-transfection, cells were incubated under hypoxia or normoxia for 16 hours. Cells were harvested and lysed with lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 0.15 M/L NaCl, 1%NP-40, 1 mmol/L EDTA, Complete Protease Inhibitor Cocktail (Roche Applied Science), 10 μmol/L proteasome inhibitor MG132 (Peptide Institute, Inc. Osaka, Japan), and 10 mmol/L deubiquitination inhibitor N-ethylmaleimide (Sigma)]. Proteasome inhibitor MG132 was used in ubiquitination assays for preventing protein degradation and for accumulation of ubiquitinated proteins. HA-ubiquitinated FLAG-tagged Pim-1 proteins were pulled down using anti-FLAG M2 beads (Sigma) twice. The protein complexes were then separated on 4% to 12% gradient SDS-polyacrylamide gels and probed with anti-HA to reveal the HA-ubiquitinated Pim-1.
Immunofluorescence Analysis
HeLa cells were prepared and seeded onto chamber slides at 2 × 104 cells per well for 12 hours. Cells were then transfected with FLAG-Pim-1 (wild-type Pim-1) for 24 hours and cultured under hypoxia or normoxia for 16 hours. For protecting Pim-1 degradation under normoxia, all cells were treated with MG132 for 6 hours before harvesting cells. For immunostaining, cells were fixed with 4% paraformaldehyde at room temperature for 30 minutes and treated with 2% Triton X-100 for 2 minutes. Cells were then stained with mouse anti-FLAG M2 monoclonal antibody (Sigma) overnight, followed by 1 hour incubation with fluorescein isothiocyanate-conjugated anti-mouse antibody (Jackson ImmunoResearch). For all staining experiments, cells were incubated with 0.5 μg/ml of propidium iodide to stain the nuclei. For immunostaining of endogenous Pim-1, HeLa cells were used and were stained with Pim-1 antibody (ABGENT, San Diego, CA). The figure photographs were taken by an Olympus microscope (Olympus IX81, magnification= original × 200).
Soft-Agar Colony Formation Assays
To compare the effect of hypoxia on anchorage-independent growth, NIH3T3 cells were transiently transfected with 3 μg pCMV-3xFLAG-Pim-1 expression plasmid, and transfectants selected with G418 for 2 weeks. Selected NIH3T3/Pim-1 cells were seeded at a density of 1000 cells per 3.5 cm plate in 0.3% agarose solution in DMEM containing 10% fetal bovine serum over a cushion of 0.5% agarose solution also in DMEM growth medium. After culturing in either hypoxic or normoxic conditions for 3 weeks, colonies exceeding 0.25 μm were scored and photographed.
Fluorescence-Activated Cell Sorting Analysis
Sensitivity of tumor cell lines to apoptosis was determined by two-color analysis using PI and fluorescein isothiocyanate-conjugated anti-annexin V according to the manufacturer’s instructions. After incubation with low glucose (16 mg/dL) medium for 48 hours, under normoxia or hypoxia, the cells were stained with PI and fluorescein isothiocyanate-conjugated anti-annexin V and then analyzed with a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Establishment of Dominant Negative Pim-1 Transfectants
A cDNA for dominant negative Pim-1, which lacks a kinase activation domain,18 was amplified from reverse transcription products of mRNAs purified from PCI-10 cells and cloned into PCR4-TOPO. Plasmids were sequenced with a DyeDeoxy Terminator kit (Perkin-Elmer, Urayasu, Japan) on an ABI 377 automated sequencer (Applied Biosystems, Urayasu, Japan) according to the manufacturer’s protocol. Cloned fragments were ligated into PcDNA3.1+ (Invitrogen, Carlsbad, CA). PCI-43 cells were transfected with the dominant negative (dn)Pim-1 expression vector with the use of Lipofectamine (Life Technologies, Tokyo, Japan). Transfectants were cloned by limiting dilution method following selection with G-418 (1200 mg/ml). The transfectants were then maintained in the presence of 600 μg/ml of G-418. PCR primers were as follows: dnPim-1 forward, 5′-GTAGAATTCGCCACCATGCCTGCCTAATGGCACTCGAGTG-3′; reverse, 5′-GTACTATTTGCTGGGCCCCGGCGAC-3′.
Preparation of a HeLa Cell Line Expressing dnPim-1 in the Presence of Doxycycline
The tetracycline-inducible expression system and the HeLa/Tet-On cell line, a HeLa clone expressing a reverse tetracycline-controlled transactivator, were purchased from BD Biosciences Clontech (Palo Alto, CA). HeLa Tet-On cells were transfected with pTRE/dominant negative Pim-1 and PTK-Hyg, a plasmid expressing the hygromycin resistance gene (BD Biosciences Clontech). The cells were cultured for more than 4 weeks in medium containing hygromycin (200 μg/ml) to obtain stable transfectants.
Immunocytochemical Staining
Expression of Pim-1 was analyzed by immunocytochemical staining using the streptavidin-biotin technique (Histofine SAB-PO kit, Nichirei, Tokyo) according to the manufacturer’s instructions. Diaminobenzidine was used as a chromogen to visualize the reaction products. Finally, all slides were counterstained with hematoxylin and anti-Pim-1 antibody (Transduction Laboratories, Inc., Lexington, KY).
Immunohistochemistry Analysis
At 9 days after inoculation with dnPim-1-transfected cells, mice were sacrificed and tumors were removed for detection of apoptosis and angiogenesis. Terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) and CD31 analyses were performed by immunohistochemical staining using the streptavidin-biotin technique (Histofine SAB-PO kit; Nichirei, Tokyo, Japan) according to the previously described method.19 Snap-frozen tissue specimens were used for CD31 analysis and paraffin-embedded tissue specimens were used for TUNEL staining analysis. The tissue sections were pre-incubated for 30 minutes with PBS containing 1% bovine serum albumin. Endogenous peroxidase was inactivated with 3% H2O2 in methanol for 15 minutes. Sections were incubated overnight at 4°C with anti-mouse CD31 antibody (BD Pharmingen, San Diego, CA) at concentrations of 5 μg/ml in PBS. After washing with PBS, sections were incubated for 1 hour at room temperature with the biotin-conjugated anti-rat second antibody (DAKO, Tokyo, Japan), which was followed by the avidin-biotin-peroxidase reaction. Diaminobenzidine was used as a chromogen to visualize the reaction products. Finally, the sections were counterstained with hematoxylin and methyl green.
Apoptosis in tumors was determined by TUNEL staining. Perfusion-fixed, paraffin-embedded tissues were stained for apoptotic nuclei with an in situ apoptosis detection kit (Takara, Otsu Sihga, Japan). Following treatment with proteinase K, slides were incubated with a TUNEL reaction mixture, treated with converter-peroxidase solution, and exposed to diaminobenzidine black nickel chromagen. Slides were counterstained with hematoxylin and analyzed by light microscopy for total and TUNEL-positive cells. The photographs were taken by an Olympus microscope (Olympus IX70, magnification= original ×200). The densities of apoptotic cells were expressed as the average of the five areas of greatest intensity identified within a single field.
For immunohistochemistry analysis of Pim-1 and CA IX, nude mice xenograft model was used (n = 5). Five million cells of HCT116 cells were injected subcutaneously into the right flank of each mouse. After tumors grew to 600 mm3, the animals were sacrificed and the tumors were excised. Expression levels of CA IX, and Pim-1 were evaluated by a standard indirect immunoperoxidase procedure (ABC-Elite; Vector Laboratories, Burlingame, CA). In brief, antigen retrieval was performed by treatment in a steamer for 35 minutes. Anti-CAIX (Novus Biologicals, Inc.) was used at 1: 500 dilution at 4°C overnight. Anti-Pim-1 polyclonal antibody (ABGENT, San Diego, CA) was used at 1:50 dilution at 4°C overnight. Secondary antibody incubation was performed at room temperature for 60 minutes. Mayer’s hematoxylin nuclear staining was used as a counterstain.
In Vivo Tumorigenicity
Severe combined immunodeficient (SCID) mice were housed in Assessment and Accreditation of Laboratory Animal Care-approved barrier facilities on a 12-hour light/dark cycle, with food and water ad libitum. Mice were treated under approved protocols in compliance with the animal care and use guidelines and in accordance with international standards including the National Institutes of Health. Five million cells of dnPim-1 transfectants were injected subcutaneously into the right flank of each mouse (n = 5). Tumor growth was observed every 3 days for 3 weeks after inoculation. Tumor volumes were measured with this formula: tumor volume = 0.5 × ab2 (a, major axis; b, minor axis).20 Body weight, feeding behavior and motor activity were monitored thrice weekly as indicators of general health. Animals with the following conditions were euthanized: >10% weight loss, motor retardation, inability to obtain food or water, ruffled hair, or largest diameter of the tumor >15 mm.
Statistical Analyses
All statistical analyses, except the analysis of tumor sizes in the xenograft study, were performed using an unpaired Student’s t-test to evaluate the significance between the means of two groups and one-way analysis of variance with posthoc intergroup comparison (Tukey test) to evaluate the significance among the means of more than two groups. The xenograft data were analyzed by linear mixed models (SPSS for Windows, version 12.0, SPSS, Inc. Chicago, IL) with comparisons of fixed effects using the restricted maximum likelihood method.
Results
Hypoxia Induces Pim-1 Expression
We previously found that pim-1 was expressed at a higher level under hypoxia than under normoxia in a pancreatic cancer cell line (PCI-10) with the use of a cDNA microarray system.21 Although we confirmed that hypoxia up-regulates the expression of Pim-1 mRNA (unpublished), the post-transcriptional regulation of Pim-1 under hypoxia is still unclear. For this reason, we investigated Pim-1 protein levels under hypoxia and normoxia. Figure 1A shows that Pim-1 protein was strongly induced under hypoxia in HCT-116 cells by immunohistochemical staining. To confirm whether Pim-1 expression is elevated in hypoxic areas in vivo, we investigated the expression of Pim-1 and CA IX, a hypoxia marker, in xenografts of HCT-116 cells. Figure 1B shows that Pim-1 is highly expressed near necrotic tumors, where the hypoxia marker CA IX also clearly expressed (arrows). However, in tumor areas where CA IX is not expressed, indicating nonhypoxic tumors, the expression of Pim-1 also does not express (Figure 1B, bottom). The observed colocalization of Pim-1 and CA IX suggest that Pim-1 is overexpressed in hypoxic tumors. These results are consistent with a recent report that shows the expression of Pim-1 increased significantly in pancreatic malignancies, which are always considered to be hypoxic tumors.22 Western blot analysis also showed higher levels of Pim-1 expression under hypoxia compared with normoxia in HCT-116, PCI-10, and PCI-43 (Figure 1C). Pim-2 and Pim-3, other Pim family members have a similar function to that of Pim-1, including phosphorylation of its targets.23,24 However, hypoxia induced Pim-1, but not Pim-2 or Pim-3 (Figure 1D), suggesting that only pim-1 is an hypoxia-inducible gene.
Figure 1.
Hypoxia induces Pim-1 expression. A: Immunocytochemical staining of Pim-1 proteins in HCT-116 cells cultured under hypoxia or normoxia for 16 hours. B: Immunohistochemical staining of Pim-1 and CA IX in HCT-116 xenografts. Nude mice (n = 5) were inoculated with 5 × 106 HCT-116 cells. Mice were sacrificed and tumors were removed for detection of Pim-1 and CA IX at 21 days post-inoculation. Representative results of the HCT-116 xenografts are shown. C: Western blot analyses of Pim-1 in HCT116, PCI-10, and PCI-43 cells following incubation under hypoxia at the indicated times. D: Western blot analyses of Pim-1, Pim-2, and Pim-3 in 293, HeLa, and PCI-43 cells following incubation under hypoxia or normoxia for 16 hours. N: normoxia, H: hypoxia. The arrowheads indicate the positive signals of Pim-1 or CAIX.
Hypoxia Blocks the Ubiquitin-Mediated Proteasomal Degradation of Pim-1
Pim-1 is a short half-life protein degraded through the ubiquitin-proteasome pathway and is stabilized by Hsp90, which is also a regulator for HIF-1α stability and activity.16,25 It is possible that hypoxia decreased the degradation of Pim-1 protein through the ubiquitin-proteasome pathway. To test this hypothesis, we treated PCI-43 cells with ALLnL (50 μmol/L), a 26S proteasome inhibitor, and found that Pim-1 was increased in the presence of proteasome inhibitor ALLnL (Figure 2A). Furthermore, we examined the half-life of Pim-1 under normoxia and hypoxia (Figure 2B). 293 cells transfected with FLAG-Pim-1 were cultured under normoxia or hypoxia for 16 hours and were then treated with cycloheximide to halt protein synthesis. Figure 2B shows that hypoxia promoted the stabilization of Pim-1 by increasing its half-life, suggesting that hypoxia can prevent the degradation of Pim-1. To elucidate the mechanisms of hypoxia-regulated stabilization of Pim-1, we investigated whether Hsp90 is involved in hypoxia-mediated stabilization of Pim-1. Cells treated with Hsp90 inhibitor geldanamycin, which disassociate Hsp90 from its targets, were consistent with previous studies16 where geldanamycin was found to increase Pim-1 turnover under normoxic condition. Moreover, inhibition of Pim-1 association with Hsp90 by geldanamycin under hypoxia also compromised hypoxia-mediated Pim-1 stabilization, suggesting that Hsp90 binding to Pim-1 is required for hypoxia-mediated stabilization of Pim-1.
Figure 2.
Hypoxia up-regulates Pim-1 and blocks its degradation by the ubiquitin-proteasome pathway. A: Western blot analyses of Pim-1 in PCI-43 cells treated with ALLnL (50 mmol/L) under normoxia for 6 hours. B: Western blot analyses for Pim-1 half-life studies. 293 cells were transfected with FLAG-Pim-1 for 48 hours and were then treated with cycloheximide at the indicated times under normoxia or hypoxia. After transfection of FLAG-Pim-1, the cells were treated with 2μmol/L geldanamycin for 4 hours to test the half-life of Pim-1 under normoxia or hypoxia. Anti-FLAG antibody was used to detect the expression of transfected Pim-1. The Western blot signal at each time point was measured using a densitometer and the level of Pim-1 at time 0 was set at 100%. The percentages of Pim-1 remaining are indicated graphically. C: PCI-43 cells were pre-treated with a 26S proteasome inhibitor (ALLnL, 50 mmol/L and 100 mmol/L) for 8 hours, and ubiquitinated Pim-1 was detected by immunoprecipitating with ubiquitin antibody followed by immunoblotting with Pim-1 antibody. D: 293 cells were co-transfected with FLAG-Pim-1 and HA-ubiquitin for 24 hours and were then incubated under hypoxia or normoxia for 16 hours. After immunoprecipitation using anti-FLAG M2 beads (Sigma) to pull down Pim-1 proteins, the ubiquitinated Pim-1 proteins were detected using anti-HA antibody.
To investigate ubiquitination of Pim-1, PCI-43 cells were treated with ALLnL for 16 hours. We found that ubiquitinated Pim-1 protein is increased in an ALLnL dose-dependent manner (Figure 2C). To further determine whether the ubiquitin-proteasome degradation of Pim-1 is reduced by hypoxia, 293 cells co-transfected with FLAG- Pim-1 and HA-ubiquitin were cultured under normoxia or hypoxia for 16 hours. The amount of polyubiquitinated Pim-1 in cells cultured under hypoxia was reduced compared with cells cultured under normoxia (Figure 2D). Together, these results indicate that Pim-1 is degraded by the ubiquitin-proteasome pathway under normoxia and that hypoxia inhibited this process.
Hypoxia Induces Nuclear Localization of Pim-1 Protein
To further investigate the molecular mechanism of how hypoxia protects Pim-1 degradation, we examined the subcellular localization of Pim-1 protein by examining its expression in whole cell lysate, cytoplasmic lysate, and nuclear lysates of HeLa cells (Figure 3A). After transfection of FLAG-tagged Pim-1 in 293 cells, the cells were placed under normoxic or hypoxic conditions for 16 hours. For protecting Pim-1 degradation under normoxia, all cells were treated with MG132 for 6 hours before harvesting cells. Pim-1 protein was observed in both the nuclear and cytoplasmic lysates under normoxia, but after incubation under hypoxia for 16 hours, Pim-1 protein distribution in the nucleus markedly increased compared with in the cytoplasm. The extra bands above Pim-1 on the immunoblots were likely to be ubiquitinated Pim-1, since the cells had been treated with MG132. Next, we also examined the localization of Pim-1 protein by immunofluorescence in HeLa cells. Cells were transfected with FLAG-tagged Pim-1 and incubated under normoxia or hypoxia. Figure 3B shows that Pim-1 protein is localized both in the cytoplasm and nucleus under normoxia, whereas it is mainly localized in the nucleus under hypoxia. These results suggest that hypoxia caused the localization of Pim-1 protein to shift from the cytoplasm to the nucleus.
Figure 3.
Hypoxia induces nuclear localization of Pim-1 protein. A: Western blot analysis of Pim-1 in HeLa cells transfected with Pim-1. HeLa cells were transfected with FLAG-Pim-1 for 24 hours and incubated under hypoxic or normoxic conditions for 16 hours. Cells were treated with MG132 for 6 hours before harvesting cells. Anti-FLAG antibody was used to detect the expression of Pim-1 in the whole cell lysate, nuclear lysate, and cytoplasmic lysate. B: Immunofluorescence staining of Pim-1 in normoxia and hypoxia. HeLa cells were transfected with FLAG-Pim-1 for 24 hours and incubated under hypoxia for 16 hours. Cells were treated with MG132 for 6 hours before staining, followed by fixing with anti-FLAG antibody to detect the subcellular localization of Pim-1. Nuclei were stained with PI. C: Immunofluorescence staining of endogenous Pim-1 under normoxia or hypoxia. HeLa cells were incubated with 2 μmol/L geldanamycin under hypoxia for 16 hours. Cells were fixed and stained with anti-Pim-1 antibody to detect the subcellular localization of Pim-1. Nuclei were stained with PI.
Further, we investigated the effects of Hsp90 inhibitor geldanamycin on subcellular localization of endogenous Pim-1 under hypoxia in HeLa cells. Figure 3C shows that major localization of Pim-1 is in the nucleus under hypoxic conditions, while geldanamycin compromises Pim-1 nuclear translocation under hypoxia. These results suggest that Pim-1 binding with Hsp90 is required for the process of Pim-1 translocation to the nucleus under hypoxia.
Hypoxia Promotes Transformation of NIH3T3 Cells Transfected with Pim-1
In a previous study, pim-1 transgenic mice were found to develop T-cell lymphomas with low incidence (5% to 10%) and long latency (at least 7 months), indicating that overexpression of the pim-1 gene alone is insufficient for transformation.26 Additionally, when Eμ-Pim-1 (Pim-1 over-expression via the Eμ heavy chain promoter) mice were exposed to viruses, chemical carcinogens, or X-ray radiation, the development of lymphomas was significantly accelerated.27 Here, we examined the effect of hypoxia on the transformation activity of Pim-1 in NIH3T3 cells. NIH3T3 cells were transfected with Pim-1 and clones selected with G-418 for 2 weeks. NIH3T3 Pim-1 expressing cells were incubated under hypoxic and normoxic conditions for 16 hours to confirm the expression levels of transfected Pim-1. In both the vector-transfectants and Pim-1-transfectants, Pim-1 protein expression was elevated under hypoxia when compared with normoxia (Figure 4A). We then examined the colony forming activity of NIH3T3 cells that were transfected with Pim-1, by incubating them in soft-agar under hypoxia and normoxia for 3 weeks. Under normoxia, transfection with Pim-1 alone could not enhance colony formation of NIH3T3 cells in soft-agar. By contrast, colony formation of NIH3T3 cells transfected with Pim-1 was significantly enhanced under hypoxia compared with the vector-transfectants. Figure 4B shows a representative photograph of colonies formed in soft-agar under hypoxia and normoxia. Numbers of colonies having a diameter exceeding 20 μm are displayed in Figure 4C. These data indicate that Pim-1 induced by hypoxia is efficient in facilitating cell transformation.
Figure 4.
Hypoxia promotes transformation of NIH3T3 cells following Pim-1 transfection. A: Pim-1 transfection of NIH3T3 cells in normoxia and hypoxia. NIH3T3 cells were transfected with Pim-1 and selected with G-418 for 2 weeks. Pim-1 antibody was used for the Western blot. N: normoxia, H: hypoxia. Asterisk indicates a non-specific band. B: A representative photograph of the soft-agar colony formation assay (Magnification = original ×40; Scale bar = 20 μm). Selected NIH3T3/Pim-1 cells were seeded in agarose. After culture in either hypoxic or normoxic conditions for 3 weeks, colony numbers exceeding 20 μm in size were counted. C: Mean ± SD colony numbers in three different wells for each condition is shown.
Effects of dnPim-1, siRNA for Pim-1, and Overexpression of Pim-1 on the Sensitivity of Pancreatic Cancer Cells to Apoptosis
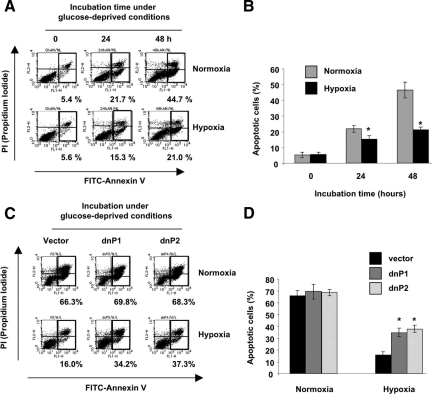
As a tumor expands, vigorous growth of cancer cells creates a hypoxic microenvironment in which tumors have an insufficient supply of oxygen and glucose. If alleviated, a hypoxic microenvironment with low glucose supply may restrict tumor growth or even cause cell death.28 Hypoxic conditions induce cellular responses such as angiogenesis, increased glycolysis and up-regulation of survival or apoptotic related molecules to improve cell survival. The glucose-deprived condition is a pivotal contributing factor for the death of cancer cells in intrinsic tumor hypoxia.29,30 In Figure 4A and 4B, glucose-deprivation induced apoptosis in PCI-43 cells under normoxia, while apoptosis was reduced under hypoxia. These results, as well as our previous reports,19,31 indicate that hypoxia can reduce apoptosis even under glucose-deprivation conditions.
Since Pim-1 is an anti-apoptotic factor in hematopoietic cells, we further investigated the role of Pim-1’s anti-apoptotic function in PCI-43 cells under hypoxia. To do this, we antagonized the function of Pim-1 by transfecting PCI-43 cells with dnPim-1, which lacks a kinase activation domain (1 to 80 aa), and is known to inhibit the kinase activity of wild-type Pim-1 (supplemental Figure S1A, see http://ajp.amjpathol.org).18 The expression of dnPim-1 was evaluated in three stable dnPim-1 transfectants (supplemental Figure S1B, see http://ajp.amjpathol.org). Since Pim-1 has been reported to phosphorylate SOCS-1 protein,32 we confirmed the antagonistic effect of dnPim-1 on Pim-1 mediated phosphorylation of SOCS-1 (supplemental Figure S1C, see http://ajp.amjpathol.org). Phosphorylated SOCS-1 (detected as a slower-migrating band compared with the unphosphorylated SOCS-1 protein) was observed in cells that over-express Pim-1, and was reduced in dnPim-1-transfected cells. The phosphorylated SOCS-1 band also disappeared in the dnPim-1 transfectants under normoxia and hypoxia (supplemental Figure S1D, see http://ajp.amjpathol.org). These results confirm that the disruption of wild-type Pim-1 by dnPim-1 works in dnPim-1-transfectants. Next, we examined the sensitivity of dnPim-1-transfectants to apoptosis. Hypoxia inhibited glucose-deprived induction of apoptosis in vector-transfected control cells, but glucose-deprived inductions of apoptosis was restored in dnPim-1 transfectants even under hypoxia (dnP1 and dnP2) (Figure 5, A–D). Therefore, dnPim-1 expression resensitized cells to glucose-deprivation under hypoxia.
Figure 5.
dnPim-1 transfectants sensitize cells to apoptosis induced by glucose-deprivation. Representative results of fluorescence-activated cell sorting (FACS) analysis for three independent experiments are shown. A: Apoptotic analysis of PCI-43 cells cultured in glucose-deprived (16 mg/dL) conditions for 24 and 48 hours in the presence of normoxia or hypoxia. Apoptotic cells were determined by PI and annexin V staining using FACS analysis. B: Mean ± SD apoptotic cell percentages in three different experiments of (A). *P < 0.01 compared with normoxic condition. C: Apoptotic analysis of dnPim-1-transfectants under glucose-deprived conditions for 48 hours in the presence of normoxia or hypoxia. Apoptotic cells were determined as described in (A). D: Mean ± SD apoptotic cell percentages in three independent experiments of (C). *P < 0.01 compared with vector control.
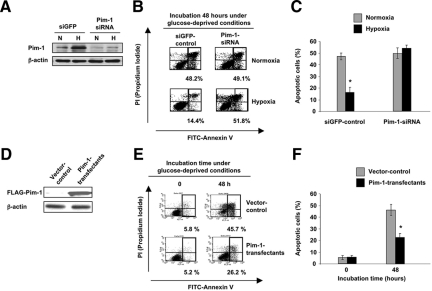
To further confirm the role of Pim-1 in solid tumor survival, we also used molecular methods to knockdown Pim-1 levels. Under hypoxia, siRNA for Pim-1 effectively suppressed Pim-1 protein expression in PCI-43 cells (Figure 6A), and siRNA suppression of Pim-1 restored sensitivity to glucose-deprivation (Figure 6, B and C). Conversely, we established Pim-1-overexpressing stable transfectant PCI-43 cells (Figure 6D) and found that over-expression of Pim-1 rendered the cells resistant to glucose-deprivation, even under normoxia (Figure 6, E and F). These results demonstrate that Pim-1 induced by hypoxia plays a central role in the intrinsic improvement of solid tumor survival.
Figure 6.
Pim-1 siRNA or Pim-1 overexpression has an impact on glucose-deprivation induced apoptotic response. Representative results of FACS analysis for three independent experiments are shown. A: Expression of Pim-1 proteins in PCI-43 cells treated with Pim-1 siRNAs under normoxia and hypoxia (12 hours). N: normoxia, H: hypoxia. B: Apoptotic analysis of cells treated with Pim-1 siRNAs, followed by glucose deprivation for 48 hours in the presence of normoxia and hypoxia. Apoptotic cells were quantified by PI and annexin V staining via FACS analysis. C: Apoptotic cell percentages in three independent experiments of (B). The error bars represent SD. * indicates significance (P < 0.01). D: Western blot of Pim-1 proteins in PCI-43 Pim-1 transfectants. Anti-FLAG immunoblot is shown. E: Apoptotic analysis of Pim-1 transfectants treated with glucose deprivation for 48 hours under normoxia. Apoptotic cells were quantified as described in (B). F: Apoptotic cell percentages in three independent experiments of (E). The error bars represent SD. *P < 0.01.
Suppression of Pim-1 Activity Inhibits Pancreatic Cancer Growth in a Murine Xenograft Model
To investigate the role of Pim-1 in pancreatic cancer growth in vivo, we analyzed the tumorigenicity of dnPim-1-transfectants in SCID mice (n = 5, in each group). Vector transfectants formed tumors, whereas all dnPim-1 transfectants (dnP1, dnP2, and dnP3) failed to grow beyond 60 mm3 and were resorbed, suggesting that inhibiting the activity of Pim-1 leads to tumor suppression (Figure 7A). To explore the mechanisms by which dnPim-1 inhibited tumor formation, mice from separate group were resacrificed and tumors extracted at 9 days post inoculation (before resorption) with dnPim-1-transfected cells. We performed immunohistochemical staining of TUNEL and CD31/PECAM to estimate the apoptotic fractions and angiogenesis in tumors, respectively (Figure 7B). TUNEL staining showed that there were more apoptosis positive cells in dnPim-1-transfected tumor tissues than in the vector-transfectants, suggesting that inhibiting the activity of Pim-1 leads to apoptosis (Figure 7C). CD31 staining showed that there were many more CD31-positive tumor cells in the vector-transfectants than in the dnPim-1-transfectants, suggesting that inhibiting the activity of Pim-1 decreased tumor angiogenesis (Figure 7D). Only a few studies have been reported on the role of Pim-1 in vasculogenesis or angiogenesis. These results, in combination with a recent report that demonstrated Pim-1 is required for vascular endothelial growth factor-A-dependent proliferation and migration of endothelial cells,33 implies that Pim-1 may modulate vascular endothelial growth factor-induced angiogenesis.
Figure 7.
Introduction of dominant negative Pim-1 reduces tumorigenicity of pancreatic cancer cells. A: Tumorigenesis analysis of dnPim-1 transfectants. SCID mice (n = 5) were inoculated with 5 × 106 dnPim-1 transfectants or vector transfected control cells. Tumor volume was measured every 3 days post-inoculation (mean ± SD). B: Immunohistochemical staining of tumor tissues for TUNEL and CD31. SCID mice (n = 5) were inoculated with 5 × 106 dnPim-1 transfectants or vector transfected control cells as described in (A). Mice were sacrificed and tumors were removed for detection of apoptosis, and angiogenesis at 9 days post-inoculation. Representative results of the dnPim-1-transfected (dnP2) and vector-transfected tumors are shown. C: Mean ± SD TUNEL positive cell numbers in three different tumor tissues for each group is shown. D: Mean ± SD of microvessel counts in three different tumor tissues for each group. Magnification = original ×200.
Introduction of dnPim-1 Reduces Tumorigenicity of HeLa Cells
To confirm the role of Pim-1 in in vivo tumor formation in other types of solid tumors such as cervical carcinoma, we used Tet-On HeLa cell transfectants, which express dnPim-1 in the presence of doxycycline (Figure 8A). Consistent with previous observations in pancreatic cancer cells, tumor volumes of HeLa Tet-On dnPim-1 transfectants in SCID mice were reduced in dnPim-1-expressing cells, when compared with dnPim-1 transfectants in the absence of doxycycline (Figure 8B). Additionally, weights of tumors obtained from dnPim-1 transfectants were less than their respective controls (Figure 8C). We also examined the apoptotic fractions and angiogenesis in tumors by immunohistochemical staining of TUNEL and CD31/PECAM (Figure 8D). The results show a significant increase in apoptosis and a decrease in angiogenesis (Figure 8E). Together, these experiments provide important evidence that suppressing the activity of Pim-1 in cancer cells leads to solid tumor suppression.
Figure 8.
Introduction of dominant negative Pim-1 reduces tumorigenicity of HeLa cells by administration of tetracycline. A: Western blot analyses of dnPim-1 in Tet-on dnPim-1-transfectants. Anti-HA antibody was used to detect the expression of tetracycline induced dnPim-1 in HeLa cells. * indicates a non-specific band. B: Growth of Tet-On dnPim-1 transfectants in SCID mice by doxycycline administration. Five mice in each group were inoculated with 5 × 106 tumor cells on Day 0. Doxycycline administration was initiated on Day 4 via drinking water at 2 μg/ml delivering approximately 13 mg/kg/day. Tumor sizes were measured every 3 to 4 days following inoculation. Error bars represent SD. *P < 0.01 compared with dnPim-1/doxycycline(−) control. C: Terminal tumor weights were measured (mean ± SD). *P < 0.01 compared with dnPim-1/doxycycline(−) control. D: Immunohistochemical staining of tumor tissues for TUNEL and CD31. Terminal tumors were removed for detection of apoptosis, and angiogenesis at 21 days after inoculation. Representative results of dnPim-1 and vector-transfected tumors are shown. E: Mean ± SD TUNEL positive cell numbers and microvessel counts in three different tumor tissues for each group. Magnification = original ×200. *P < 0.01.
Discussion
Hypoxic conditions induce cellular responses to improve cell oxygenation and survival through several mechanisms such as angiogenesis, enhanced anaerobic metabolism, and up-regulation of molecules related to cell survival or apoptosis.34 In this study, we found that hypoxia can stabilize Pim-1 by preventing its ubiquitin-proteasomal degradation and can facilitate Pim-1 nuclear translocation. High transformation activity of Pim-1 in NIH3T3 cells under hypoxic conditions indicates that Pim-1 is highly activated under hypoxia and can induce tumor formation.
Our present results demonstrate that degradation of Pim-1 protein is mediated through the ubiquitin-proteasome pathway, and that this degradation is suppressed by hypoxia. Two factors, Hsp9016,35 and PP2A,36,37 have been reported to be involved in the degradation of Pim-1 at the posttranscriptional level. Interestingly, both of these two molecules are regulated by hypoxia. Hypoxia increased nuclear accumulation of Hsp90 for HIF-1α stability,25 while hypoxia significantly decreased the protein levels and activities of PP2A in the neuronal nuclei.38 These observations suggest that these two molecules might be involved in hypoxia-regulated Pim-1 polyubiquitination. To address the possibility that Hsp90 might participate in hypoxia-mediated Pim-1 degradation, we investigated the effects of the specific Hsp90 inhibitor, geldanamycin, on Pim-1 stability and subcellular localization under hypoxia. We found that geldanamycin blocked the nuclear translocation of Pim-1 under hypoxia (Figure 3C) and suppressed hypoxia-mediated stability of Pim-1 (Figure 2B). Our preliminary experiments also showed that Pim-1 is not degraded through prolyl hydroxylation (data not shown), which is the case for HIF-1. Interestingly, even though HIF-1α is a major factor in adaptation to hypoxia, Pim-1 mRNA and protein were still induced under hypoxia in HIF-1α knock-down cells (unpublished data). This suggests that there is an HIF-1α-independent role in hypoxia-mediated Pim-1 regulation. So far, the mechanism behind Pim-1 degradation has not been well characterized. For example, the E3 ligase for Pim-1 ubiquitination remains unknown. It would be important to characterize the regulation of this E3 ligase in hypoxia-mediated Pim-1 stabilization.
Pim-1 is located in both the cytoplasm and the nucleus, but the distinct roles of Pim-1 in these two locations have not yet been clarified. Some binding proteins and phosphorylation target proteins of Pim-1 have been identified in the nucleus, including p100,39 CDC25A,12 HP-1,40 and PAP-1.14 It has been shown that nuclear localization of Pim-1 protein was essential for regulating MDM2 protein in Burkitt’s lymphoma.41 Also, binding of Pim-1 protein to TFAF2/SNX6 (tumor necrosis factor receptor-associated factor 4-associated factor 2/sorting nexin 6) led to the translocation of TRAF2/SNX6 from the cytoplasm to nucleus.42 These reports suggest that translocation of Pim-1 protein from the cytoplasm to the nucleus might be important for its biological function. The mechanism behind hypoxia-mediated Pim-1 nuclear import is not clear. The peptide sequences corresponding to the typical nuclear localization signal remain to be characterized in Pim-1. It is therefore not clear whether Pim-1 nuclear localization involves a nuclear localization signal. However, it is possible that some hypoxia-associated proteins facilitate the process of Pim-1 nuclear translocation under hypoxia. Our present results indicate that Hsp90 is involved in Pim-1 nuclear translocation. Both wild-type Pim-1 and an N-terminal deletion form, dnPim-1 (81 to 333 aa), can translocate from the cytoplasm to the nucleus under hypoxia (supplemental Figure S2, see at http://ajp.amjpathol.org), suggesting that the C-terminal of Pim-1 is responsible for its nuclear translocation. These results are consistent with Pim-1-mediated TFAF2/SNX6 studies,41 in which 222 to 333 aa of Pim-1 is necessary for Pim-1 nuclear translocation. On the basis of our results, it is possible that the translocation of Pim-1 protein under hypoxia may protect itself from degradation through the ubiquitin-proteasome pathway. Thus, the translocation of Pim-1 protein might enable Pim-1 to phosphorylate some nuclear target proteins required for adaptation to hypoxia. We are in the process of identifying the target proteins phosphorylated by Pim-1 in the nucleus under hypoxia.
Mutations in the Pim-1 gene have been found in diffuse large-B cell lymphomas in a 1.2-kb stretch of the first 2 kb from the transcription initiation site, which results in an altered structure and function of Pim-1.43 Also, overexpression of Pim-1 leads to genomic instability in prostate epithelial cells.6 These findings suggest an important role for Pim-1 in the initiation or progression of human cancer. However, Pim-1-transgenic mice develop T-cell lymphomas with low incidence (5% to 10%) and long latency (∼7 months), indicating that overexpression of the Pim-1 gene alone is insufficient for the transformation.4 Furthermore, transformation by Pim-1 is accelerated by the activation of c-myc and c-myb.27,44 It is possible that degradation of Pim-1 under normoxia may be responsible for the weak transformation activity of Pim-1, as we found that Pim-1 is unable to facilitate cell transformation under normoxia. Importantly, our in vitro experiments confirm that the transfection of Pim-1 leads to high transformation activity under hypoxia, suggesting that hypoxia’s impact on Pim-1 stabilization plays an important role in tumorigenesis.
In conclusion, this study demonstrates that Pim-1 is stabilized by hypoxia through reduction of its polyubiquitination and that Pim-1 is translocated from the cytoplasm to the nucleus under hypoxia. It is important to note that Pim-1 has a higher transforming activity under hypoxia. Collectively, our results indicate that Pim-1 is hypoxia-inducible and plays an important role in cell transformation and tumorigenesis. A more comprehensive analysis of the physiological role of Pim-1 and the regulation of Pim-1 protein stabilization is underway to provide a better understanding of Pim-1-mediated tumorigenesis.
Supplementary Material
Acknowledgments
We thank Dr. Jun-ichi Hamada (Division of Cancer Related genes, Institute for Genetic Medicine), Dr. Hiroshi Ishikura (The First Department of Pathology, Hokkaido University School of Medicine), and Dr. Naoya Fujita (Division of Experimental Chemotherapy, The Cancer Chemotherapy Center of the Japanese Foundation for Cancer Research) for providing us with cell lines and plasmids.
Footnotes
Address reprint requests to Mong-Hong Lee, PhD, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd. Box 79, Houston, TX 77030. E-mail: mhlee@mdanderson.org or Sai-Ching Jim Yeung, MD, PhD, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd. Box 1465, Houston, TX 77030. E-mail: syeung@mdanderson.org.
Supported by the NIHRO1CA (089266 M.H.L.), Grants-in-Aid for Scientific Research from Japan Society for the Promotion Science (J.C.), U. S. Department of Defense Breast Cancer Research Program of the Office of the Congressionally Directed Medical Research Programs (DOD SIDA BC062166 S.J.Y. & M.H.L.) and Cancer Center Core Grant (CA16672).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.K. School of Nursing & Social Services, Health Science University of Hokkaido, Sapporo, Japan.
References
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Cuypers HT, Selten G, Berns A, Geurts van Kessel AH. Assignment of the human homologue of Pim-1, a mouse gene implicated in leukemogenesis, to the pter-q12 region of chromosome 6. Hum Genet. 1986;72:262–265. doi: 10.1007/BF00291892. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- Allen JD, Berns A. Complementation tagging of cooperating oncogenes in knockout mice. Semin Cancer Biol. 1996;7:299–306. doi: 10.1006/scbi.1996.0038. [DOI] [PubMed] [Google Scholar]
- Roh M, Gary B, Song C, Said-Al-Naief N, Tousson A, Kraft A, Eltoum IE, Abdulkadir SA. Overexpression of the oncogenic kinase Pim-1 leads to genomic instability. Cancer Res. 2003;63:8079–8084. [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Yuan L, Breant C, Alitalo K, Koskinen PJ. Developmental expression of pim kinases suggests functions also outside of the hematopoietic system. Oncogene. 2000;19:1215–1224. doi: 10.1038/sj.onc.1203355. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593:45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68:5076–5085. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999;274:18659–18666. doi: 10.1074/jbc.274.26.18659. [DOI] [PubMed] [Google Scholar]
- Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma. 2002;111:80–95. doi: 10.1007/s00412-002-0192-6. [DOI] [PubMed] [Google Scholar]
- Maita H, Harada Y, Nagakubo D, Kitaura H, Ikeda M, Tamai K, Takahashi K, Ariga H, Iguchi-Ariga SM. PAP-1, a novel target protein of phosphorylation by pim-1 kinase. Eur J Biochem. 2000;267:5168–5178. doi: 10.1046/j.1432-1327.2000.01585.x. [DOI] [PubMed] [Google Scholar]
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Shay KP, Wang Z, Xing PX, McKenzie IF, Magnuson NS. Pim-1 kinase stability is regulated by heat shock proteins and the ubiquitin-proteasome pathway. Mol Cancer Res. 2005;3:170–181. doi: 10.1158/1541-7786.MCR-04-0192. [DOI] [PubMed] [Google Scholar]
- Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007;1145:227–238. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18:4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao S, Nakada K, Kuge Y, Tamaki N, Okada F, Wang J, Shindo M, Higashino F, Takeda K, Asaka M, Katoh H, Sugiyama T, Hosokawa M, Kobayashi M. Dominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am J Pathol. 2003;162:1283–1291. doi: 10.1016/s0002-9440(10)63924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Fukazawa M, Ushio H, Aiyoshi Y, Soeda S, Ito K. Study of cell kinetics in anaplastic thyroid carcinoma transplanted to nude mice. J Surg Oncol. 1989;41:1–4. doi: 10.1002/jso.2930410104. [DOI] [PubMed] [Google Scholar]
- Niizeki H, Kobayashi M, Horiuchi I, Akakura N, Chen J, Wang J, Hamada JI, Seth P, Katoh H, Watanabe H, Raz A, Hosokawa M. Hypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cells. Br J Cancer. 2002;86:1914–1919. doi: 10.1038/sj.bjc.6600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser-Erkan C, Erkan M, Pan Z, Bekasi S, Giese NA, Streit S, Michalski CW, Friess H, Kleeff J. Hypoxia-inducible proto-oncogene Pim-1 is a prognostic marker in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2008;7:1352–1359. doi: 10.4161/cbt.7.9.6418. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- Katschinski DM, Le L, Heinrich D, Wagner KF, Hofer T, Schindler SG, Wenger RH. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J Biol Chem. 2002;277:9262–9267. doi: 10.1074/jbc.M110377200. [DOI] [PubMed] [Google Scholar]
- von Lindern M, van Agthoven T, Hagemeijer A, Adriaansen H, Grosveld G. The human pim-1 gene is not directly activated by the translocation (6;9) in acute nonlymphocytic leukemia. Oncogene. 1989;4:75–79. [PubMed] [Google Scholar]
- Breuer M, Wientjens E, Verbeek S, Slebos R, Berns A. Carcinogen-induced lymphomagenesis in pim-1 transgenic mice: dose dependence and involvement of myc and ras. Cancer Res. 1991;51:958–963. [PubMed] [Google Scholar]
- Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res. 2005;65:3171–3178. doi: 10.1158/0008-5472.CAN-04-3395. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Brosius FC., 3rd Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem. 1999;274:12567–12575. doi: 10.1074/jbc.274.18.12567. [DOI] [PubMed] [Google Scholar]
- Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–6554. [PubMed] [Google Scholar]
- Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci USA. 2002;99:2175–2180. doi: 10.1073/pnas.042035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: pim-1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004;103:4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Shirogane T, Shinohara A, Iwamatsu A, Hibi M, Hirano T. Regulation of Pim-1 by Hsp90. Biochem Biophys Res Commun. 2001;281:663–669. doi: 10.1006/bbrc.2001.4405. [DOI] [PubMed] [Google Scholar]
- Ma J, Arnold HK, Lilly MB, Sears RC, Kraft AS. Negative regulation of Pim-1 protein kinase levels by the B56beta subunit of PP2A. Oncogene. 2007;26:5145–5153. doi: 10.1038/sj.onc.1210323. [DOI] [PubMed] [Google Scholar]
- Losman JA, Chen XP, Vuong BQ, Fay S, Rothman PB. Protein phosphatase 2A regulates the stability of Pim protein kinases. J Biol Chem. 2003;278:4800–4805. doi: 10.1074/jbc.M208246200. [DOI] [PubMed] [Google Scholar]
- Truttmann AC, Ashraf Q, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on protein phosphatase 2A activity, subcellular distribution and expression in cerebral cortex of newborn piglets. Neuroscience. 2004;127:355–363. doi: 10.1016/j.neuroscience.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol Cell. 1998;2:417–425. doi: 10.1016/s1097-2765(00)80141-0. [DOI] [PubMed] [Google Scholar]
- Koike N, Maita H, Taira T, Ariga H, Iguchi-Ariga SM. Identification of heterochromatin protein 1 (HP1) as a phosphorylation target by Pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of HP1(1). FEBS Lett. 2000;467:17–21. doi: 10.1016/s0014-5793(00)01105-4. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Maita H, Yano M, Koike N, Tamai K, Ariga H, Iguchi-Ariga SM. Pim-1 translocates sorting nexin 6/TRAF4-associated factor 2 from cytoplasm to nucleus. FEBS Lett. 2001;506:33–38. doi: 10.1016/s0014-5793(01)02881-2. [DOI] [PubMed] [Google Scholar]
- Ionov Y, Le X, Tunquist BJ, Sweetenham J, Sachs T, Ryder J, Johnson T, Lilly MB, Kraft AS. Pim-1 protein kinase is nuclear in Burkitt’s lymphoma: nuclear localization is necessary for its biologic effects. Anticancer Res. 2003;23:167–178. [PubMed] [Google Scholar]
- Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, Dalla-Favera R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- van der Houven van Oordt CW, Schouten TG, van Krieken JH, van Dierendonck JH, van der Eb AJ, Breuer ML. X-ray-induced lymphomagenesis in E mu-pim-1 transgenic mice: an investigation of the co-operating molecular events. Carcinogenesis. 1998;19:847–853. doi: 10.1093/carcin/19.5.847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.