Abstract
Heme oxygenase-1 (HO-1) catalyzes the conversion of heme into carbon monoxide (CO), iron, and biliverdin. In preliminary studies, we observed that the absence of HO-1 in aortic allograft recipients resulted in 100% mortality within 4 days due to arterial thrombosis. In contrast, recipients normally expressing HO-1 showed 100% graft patency and survival for more than 56 days. Abdominal aortic transplants were performed using Balb/cJ mice as donors and either HO-1+/+ or HO-1−/− (C57BL/6×FVB) mice as recipients. Light and electron microscopy revealed extensive platelet-rich thrombi along the entire length of the graft in HO-1−/− recipients at 24 hours. Treatment of recipients with CORM-2, a CO-releasing molecule (10 mg/kg of body weight intravenously), 1 hour prior and 1, 3, and 6 days after transplantation, significantly improved survival (62% at >56 days, P < 0.001) compared with HO-1−/− recipients treated with inactive CORM-2 (median survival 1 day). Histological analyses revealed that CO treatment markedly reduced platelet aggregation within the graft. Adoptive transfer of wild-type platelets to HO-1−/− recipients also conferred protection and increased survival. Aortic transplants from either HO-1−/− or HO-1+/+ C57BL/6 donors into HO-1+/+ (Balb/cJ) mice did not develop arterial thrombosis, surviving more than 56 days. These studies demonstrate an important role for systemic HO-1/CO for protection against vascular arterial thrombosis in murine aortic allotransplantation.
Heme oxygenase-1 (HO-1) is an inducible enzyme that catalyzes the rate-limiting step in heme degradation, leading to the generation of equimolar amounts of iron, biliverdin, and carbon monoxide (CO). Biliverdin is then converted to bilirubin by biliverdin reductase.1,2 HO-1 is highly up-regulated in mammalian tissues in response to a wide variety of conditions including vascular injury, ischemia, inflammation, immune injury, oxidative stress, cell cycle dysregulation, and sublethal and lethal cell damage.3,4,5 The wide range of inducers of HO-1 provides support for a vital role in maintenance of cellular homeostasis under different pathophysiological conditions including inflammatory diseases such as septic shock and asthma,6,7 cardiovascular diseases such as myocardial infarction and atherosclerosis,8,9 ischemia-reperfusion injury in multiple organ systems,8,10 and transplant rejection.11,12
One of the products of HO-1-mediated heme degradation, CO, is known to be toxic at high concentrations due to its high affinity for hemoglobin. However, there is substantial evidence that lower concentrations of CO endogenously generated from the breakdown of heme by HO serves essential regulatory roles in a variety of physiological and pathophysiological processes.13 Exogenous or endogenous CO can confer some of the cytoprotective effects attributed to HO-1.14,15
Transitional metal carbonyls, CO-releasing molecules (CORMs), have been used to deliver CO in a controlled manner without altering carboxyhemoglobin levels.16,17,18 A wide range of CORMs containing manganese (CORM-1), ruthenium (CORM-2 and −3), boron (CORM-A1), and iron (CORM-F3) are currently being investigated to facilitate the pharmaceutical use of CO for the prevention of vascular dysfunction, inflammation, ischemia-reperfusion injury, and transplant rejection.19,20,21,22,23
Thrombosis is a major complication during multiple vascular pathological conditions during which HO-1 and its byproduct CO could provide significant protection through attenuation of inflammation, endothelial cell damage, and apoptosis, as well as modulation of vascular tone.6,8,9 However, very little is known regarding the potential roles of HO-1 and CO in modulating platelet-dependent effects after vascular injury in the setting of transplantation. In these studies, we show that expression of HO-1 plays a critical role in the development of post-transplant arterial thrombosis immediately following abdominal aortic transplantation. We tested the hypothesis that CO, a product of the HO-1 reaction, mediates anti-thrombotic effects in vivo by inhibition of platelet mediated thrombus formation within the graft. We found that HO-1-deficient mice develop vascular thrombosis following aortic transplantation and that the development of thrombosis can be prevented by systemic administration of CORM-2.
Materials and Methods
Reagents
Purified mouse anti-eNOS/NOS type III was obtained from BD Biosciences. Anti-HO-1 antibody (SPA-896) and anti-HO-2 antibody (SPA-897) were obtained from StressGen Biotechnologies. OptiPrep (60% w/v iodixanol) was obtained from Axon Laboratories AG (Le Mont-sur-Lausanne, Switzerland). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Balb/cJ mice (H-2d) were purchased from Jackson Laboratories Inc. (Bar Harbor, ME). HO-1+/+ and HO-1−/− mice were maintained in breeding colonies on a C57BL/6 (H-2b) × FVB (H-2q) background.24 All animals were used at an age of 8 to 12 weeks and maintained on standard rodent chow and allowed free access to water. All surgical procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Surgical Procedure
The transplant procedure was performed using a technique as previously described.25,26 Briefly, donor and recipient mice were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg body weight). A segment of the donor abdominal aorta was isolated and removed, and preserved in chilled normal saline. The recipient aortic segment below the renal arteries and above the iliac bifurcation was transected between two vascular clamps (B-1 Clamp, Fine Science Tools, Foster City, CA), followed by end-to-end anastomosis of the donor aortic segment into recipient’s abdominal aorta using 11–0 nylon interrupted sutures (AROS Surgical, Newport Beach, CA). Hind-limb paralysis was monitored postoperatively and scored from 1 to 4: grade 1 = complete paralysis; grade 2 = moderate to severe hind-limb paralysis; grade 3 = mild hind-limb weakness; grade 4 = no paralysis.
Experimental Groups
Groups were established using Balb/cJ mice as donors and HO-1+/+ mice or HO-1−/− littermates as recipients. Experimental groups were treated with multiple doses of CO-releasing molecule, tricarbonyldichlororuthenium(II) dimer (CORM-2, Sigma-Aldrich) at 10 mg/kg intravenously 1 hour before transplantation, and 1, 3, and 6 days after transplantation. Control groups were treated using the same dose schedule except they received inactive CORM-2 that had been previously depleted of CO (iCORM-2) by exposure to air for 24 hours before use. Within each group, eight animals were followed until death or 8 weeks after transplantation; another five animals were sacrificed at 24 hours after transplantation for analysis. Two additional control groups (n = 5 per group) were performed using HO-1+/+ or HO-1−/− mice as donors and Balb/cJ mice as recipients. No immunosuppression was used.
Histology
For determination of histological changes, aortic grafts and major organs including the liver, lungs, heart, kidney, brain and large bowel were isolated and harvested, fixed in 10% neutral buffered formalin (Fisher Scientific), and embedded in paraffin. Five-micrometer serial sections were stained using hematoxylin and eosin. Histomorphometric analyses were performed on images acquired with a DMR Leica microscope (Leica, Bannockburn, IL) and IMAGE PRO software (Media Cybernetics, Silver Spring, MD). The patency of aortic grafts was calculated by subtracting the area of thrombus from the lumen area. The areas were calculated in three to five sections per aortic graft. Immunohistochemical staining for mouse eNOS/NOS type III was performed on paraffin-embedded tissue sections. Negative controls without the primary or secondary antibody were also used as previously described.27
Transmission Electron Microscopy
A segment of aortic graft was harvested 24 hours after transplantation and immediately fixed in 2.5% glutaraldehyde in phosphate-buffered saline, postfixed in 4% osmium tetroxide, and embedded in Epon resin. Semithin sections (1–2 μm thick) were used to locate vascular tissue in the adventitia of the graft. Ultrathin sections (50–80 nm thick) were prepared, stained with lead citrate and uranyl acetate, and observed with a Zeiss EMI transmission electron microscope.
Western Blot
Splenic tissue was lysed in a buffer containing a broad spectrum mixture of protease inhibitors (Roche Diagnostics, Indianapolis, IN) and Triton X-100. Immunoblot analysis was performed as described24 by using anti-HO-1 antibody (1:5000 dilution) and anti-HO-2 antibody (1:2000) followed by incubation with the corresponding peroxidase-conjugated secondary antibody (1:10,000 dilution) for 1 hour. The membranes were reprobed with an anti-actin antibody (1:1000; Sigma) to confirm equal loading.
HO Enzyme Activity
Heme oxygenase activity was measured by bilirubin generation in microsomal preparations from mouse spleen as described previously.28,29 Spleen microsomes were incubated with rat liver cytosol, a source of bilirubin reductase (3 mg), hemin (20 μmol/L), glucose-6-phosphate (2 mmol/L), glucose-6-phosphate dehydrogenase (0.2 units), and NADPH (0.8 mmol/L) for 1 hour at 37°C in the dark. The formed bilirubin was extracted with chloroform and the change in optical density, 464 to 530 nm, was measured (extinction coefficient, 40 mmol/L−1. cm−1 for bilirubin). Enzyme activity was expressed as nmol of bilirubin formed per 60 minutes/mg protein.
Adoptive Transfer of Platelets
Platelets were isolated using the method described previously.30 OptiPrep (5 volumes) was diluted with 0.85% (w/v) NaCl, 1 mmol/L ethylenediamine tetraacetic acid, and 20 mmol/L HEPES-NaOH, pH 7.4 (22 volumes) to produce a 1.063 g/ml solution. In a 15-ml centrifuge tube, 0.8 ml of whole mouse blood was layered over an equal volume of the 1.063 g/ml solution and centrifuged at 350 × g for 15 minutes at 20°C in a swinging-bucket rotor (Eppendorf centrifuge 5810R). Platelets were harvested from the band just above the interface and counted in a Neubauer hemocytometer. After washing three times with phosphate-buffered saline, platelets from HO-1+/+ mice (1 × 107/g of body weight) or equal volume of vehicle (saline) were injected into HO-1−/− recipient mice through the tail vein 30 minutes before receiving a Balb/cJ abdominal aortic graft.
Statistical Analysis
All data are presented as the mean ± SEM. Statistical analysis was performed by using analysis of variance and the Student-Neuman-Keuls post-test analysis. Survival was determined by the Kaplan-Meier method and the log-rank test was used to analyze the differences among groups. Statistical significance was defined as P < 0.05.
Results
Arterial Thrombosis and Mortality in HO-1-Deficient Mice Receiving Allogeneic Aortic Grafts
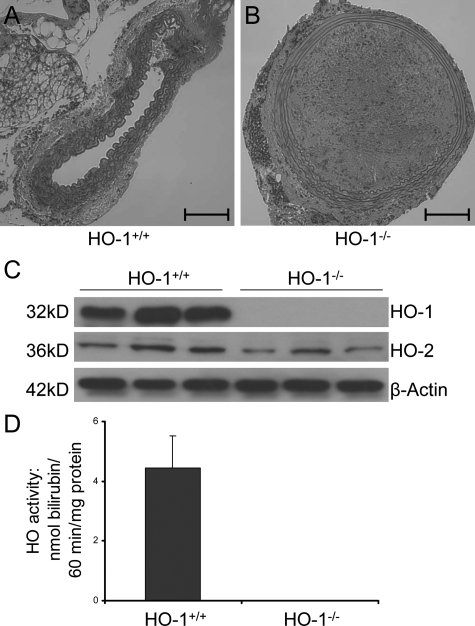
All HO-1+/+ recipients of Balb/cJ aortic allografts survived at least 8 weeks after transplantation, but HO-1−/− recipients died within 4 days after transplantation (n = 5 per group, P < 0.001). All deaths were preceded by hind-limb paralysis, which was due to thrombosis of the arterial graft (Figure 1B). HO-1+/+ mice showed patent grafts for >56 days (Figure 1A). The thrombus consisted of stiff, fibrous material consistent with coagulation of blood components or platelets. Histological analysis of vital organs including the liver, heart, lungs, and brain indicated no evidence of thrombus formation or deposition in these organs sufficient to be the cause of death in the HO-1−/− animals. The occlusion of the graft did, however, result in ischemia and necrosis of the large bowel, and the kidneys showed evidence of acute tubular necrosis and thrombus formation in the renal arteries. The blood supply of each of these systems is proximal to the large thrombus found in the aortic graft. Therefore, it is likely that these abnormalities contributed to death in the HO-1 knockout recipients. Western blots confirmed a lack of HO-1 expression in the HO-1−/− mice and showed no compensatory increase in HO-2 expression (Figure 1C). Splenic HO enzyme activity was not detectable in HO-1−/− mice (Figure 1D).
Figure 1.
Thrombus formation in allogeneic abdominal aortic grafts in HO-1−/− and HO-1+/+ mice at 24 hours after transplantation. A: Allogeneic aortic grafts from Balb/cJ mice were 100% patent in HO-1+/+ recipient animals (n = 5), but in B, dense arterial thrombi were found in allogeneic aortic grafts in HO-1−/− mice (n = 5). H&E staining. Original magnification for A and B, ×200; scale bar = 100 μm. C: Genotyping of the HO-1+/+ and HO-1−/− mice used in the experiments was confirmed by Western blot performed with splenic tissue as described in the methods. D: HO enzyme activity in splenic microsomes from HO-1+/+ and HO-1−/− mice. HO activity was measured by bilirubin generation (nmol/60 minutes/mg protein) as described in Materials and Methods.
Carbon Monoxide-Rescued HO-1-Deficient Recipients by Preventing Arterial Thrombosis
Since arterial thrombosis only occurred in HO-1−/− mice and CO, a product of HO-1, has been implicated in venous thrombosis,31 we determined whether administration of CO using CORM-2, a CO-releasing molecule, would ameliorate the thrombotic complications following surgery. As shown in Figure 2, five of eight CORM-2-treated HO-1−/− mice (62.5%) survived at least 8 weeks (median survival time >56 days). In comparison, control HO-1−/− graft recipients treated at the same concentration and volume with iCORM-2, which does not release CO, quickly developed arterial thrombosis, as indicated by hind-limb paralysis and all died within 4 days (median survival time = 1 day, P < 0.01).
Figure 2.
Kaplan-Meier analysis of HO-1+/+ and HO-1−/− recipients of allogeneic aortic grafts following treatment with CORM-2 or iCORM-2. All HO-1+/+ recipient mice treated with CORM-2 or iCORM-2 survived the entire 8-week follow-up period. In contrast, all eight HO-1−/− recipient mice treated with iCORM-2 died with arterial thrombosis within 4 days after transplantation (median survival time = 1 day). Five of eight CORM-2-treated HO-1 knockout mice (62.5%) survived to 8 weeks (median survival time = 56 days, *P < 0.01 in comparison with iCORM-2-treated animals). There was no statistical difference between CORM-2-treated group and HO-1+/+ groups (#P = 0.08).
Histological analyses indicated no arterial thrombosis at 24 hours following abdominal aortic transplantation in the allogeneic grafts of HO-1+/+ recipient animals treated with either CORM-2 or iCORM-2 (Figure 3A and B). In contrast, allografts from HO-1−/− mice treated with iCORM-2 showed histological evidence of arterial thrombosis within 24 hours after transplantation with occlusion of the lumen (Figure 3C) and CORM-2 treatment resulted in patent grafts with no occlusion (Figure 3D). Morphometric analysis revealed that the mean patency of the aortic grafts in the HO-1−/− CORM-2-treated group was 67 ± 12.2% in comparison with 5.0 ± 4.8% in HO-1−/− iCORM-2-treated recipients (Figure 3E, P < 0.01).
Figure 3.
Histological evaluation of aortic grafts. No arterial thrombus can be seen 24 hours after transplantation in the allogeneic grafts of HO-1+/+ recipient animals treated with either iCORM-2 (A, n = 5) or CORM-2 (B, n = 5). C: All iCORM-2-treated HO-1−/− recipients contained arterial thrombi at 24 hours after transplantation (n = 5) H&E staining; original magnification, ×200; scale bar = 100 μm. D: HO-1−/− mice treated with CORM-2 showed little or no arterial thrombi at 24 hours after transplantation (n = 5). E: Mean patency of allogeneic aortic grafts in HO-1+/+ and HO-1−/− recipient animals treated with iCORM-2 or CORM-2. CORM-2 treatment significantly increased the mean patency of grafts in HO-1−/− recipients (67 ± 12.2%) in comparison with HO-1−/− mice treated with iCORM-2 (5.0 ± 4.8%, P < 0.01). F: Hind-limb function at 24 hours after transplantation. The severity of hind-limb paralysis was graded using a scoring system as described in Materials and Methods.
As shown in Figure 3F, little or no hind-limb paralysis was observed in the CORM-2-treated animals compared with the iCORM-2 group (hind-limb function score 3.0 ± 0.22 versus 1.8 ± 0.25, P < 0.01). As shown in Figure 2 and Figure 3F, all HO-1+/+ recipient mice, whether treated with CORM-2 or iCORM-2, survived for the full 8-week follow-up period without hind-limb paralysis, indicating that the effects observed in the HO-1−/− mice could not be attributed to CORM-2 or iCORM-2 treatment.
Aortic Allograft Endothelial Cell Cytoarchitecture and eNOS Expression in CORM-2-Treated HO-1-Deficient Recipients
To address the mechanism of protection against vascular thrombosis by CORM-2, we hypothesized that CO released from CORM-2 or from HO-1 expression may reduce the extent of endothelial cell damage caused by ischemia/reperfusion injury during surgery. Aortic grafts were harvested 24 hours following allogeneic transplantation. Electron microscopy revealed that the endothelial cell layer appeared to be similar in HO-1−/− recipients treated with CORM-2 or iCORM-2, with a largely intact endothelial cell layer and occasional small regions of denudation (Figure 4, A and B). However, in iCORM-2-treated HO-1−/− mice, a proteinaceous matrix and platelets were seen adhering to the endothelium. Immunohistochemical staining indicated that the graft endothelium in HO-1+/+ and HO-1−/− mice receiving either CORM-2 or iCORM-2 maintained similar levels of eNOS expression (Figure 5). These results suggested that the differences observed in graft thrombosis as a function of the presence or absence of HO-1 were likely due to a systemic response to the graft by the host, rather than the graft endothelium per se.
Figure 4.
Thrombus formation and endothelial cell morphology in allogeneic aortic grafts in HO-1−/− recipients. Transmission electron microscopy of allogeneic aortic grafts 24 hours after transplantation in HO-1−/− recipients with iCORM-2 (A) or CORM-2 (B) treatment. Arrows denote platelets. Original magnification for A, ×4500; B, ×3000.
Figure 5.
Endothelial nitric oxide synthase (eNOS) expression in aortic grafts in CORM-2 and iCORM-2-treated HO-1+/+ and HO-1−/− recipients. Immunohistochemical staining of eNOS in allogeneic aortic grafts at 24 hours following transplantation in HO-1+/+ (left) and HO-1−/− (right) recipients treated with iCORM-2 (top) or CORM-2 (lower). Original magnification, ×200; inset, ×400.
To test this conclusion, we determined the role of local HO-1 expression in the arterial graft on the development of thrombus formation. We transplanted HO-1+/+ or HO-1−/− mouse aortic grafts to HO-1+/+ Balb/cJ recipients (n = 5/group) and found that local expression of HO-1 had no measurable effect on thrombosis (Figure 6, A and B) or survival (Figure 6C).
Figure 6.
Aortic transplantation from HO-1+/+ or HO-1−/− donors to Balb/cJ recipients. A and B, H&E staining of allogeneic aortic grafts 8 weeks after transplantation. Inflammation and fibrosis of the adventitia can be found in both groups. Intimal hyperplasia, narrowing and denucleation of the media were not significant. Original magnification , ×200; scale bar = 100 μm. C: Kaplan-Meier survival analysis of these two groups. All Balb/cJ recipients receiving either HO-1+/+ or HO-1−/− donors (n = 5 per group) survived to 8 weeks following transplantation.
Adoptive Transfer of Wild-Type Platelets Prolonged Survival of HO-1-Deficient Aortic Graft Recipients
Based on our findings that systemic CO is important, the presence of large aggregates of platelets in the vascular lumen, and on previous studies by others that platelet aggregation is inhibited by CO,32,33 we hypothesized that a lack of HO-1 expression in platelets may be a key factor in the development of post-transplant thrombosis in the HO-1−/− mice receiving HO-1+/+ Balb/cJ aortic grafts. To test this hypothesis, we performed adoptive transfer of HO-1+/+ platelets to HO-1−/− mice via tail vein injection before aortic transplantation. As shown in Figure 7A, two of four HO-1−/− mouse recipients survived to 8 weeks (P < 0.05, compared with HO-1−/− mice without platelet transfusion). Accordingly, the HO-1−/− recipients that received wild-type platelets also showed significantly less hind-limb paralysis after transplantation (Figure 7B, P < 0.05).
Figure 7.
Survival of HO-1−/− recipients receiving wild-type platelets and hind-limb function at 24 hours after transplantation. A: Kaplan-Meier survival analysis of HO-1−/− aortic allograft recipients treated with HO-1+/+ platelets (solid line) and of control HO-1−/− recipients that did not receive platelets but an equal volume of saline (dashed line, *P < 0.01 versus treated group). B: Hind-limb function at 24 hours after transplantation. #P < 0.05 versus control group.
Discussion
We have previously shown that the protective effects of systemic administration of interleukin-10 are mediated through a HO-1-dependent mechanism in a Dark Agouti to Lewis rat model of aortic transplantation.34 To confirm this finding we embarked on further studies to test the role of HO-1 in the protective effect of interleukin-10 on the development of vascular lesions in a mouse aortic transplantation model using HO-1−/− mice. Surprisingly, we found that all of the HO-1−/− recipient animals, irrespective of interleukin-10 or control treatment, died within 4 days with significant thrombosis located in the allogeneic aortic grafts. In contrast, wild-type recipients showed 100% graft patency and survival for >56 days. This report describes our findings that treatment of HO-1-deficient recipients with CORM-2, a CO-releasing molecule, markedly reduced local platelet aggregation in the allografts and resulted in significantly improved survival in comparison with HO-1−/− recipients treated with iCORM-2. These results indicate that application of CO significantly ameliorates thrombus formation in the absence of HO-1. This requirement for CO or HO-1 is systemic because transplantation of aorta from wild-type mice into HO-1−/− mice produced similar results. In addition, adoptive transfer of wild-type platelets into HO-1 deficient recipients also resulted in increased survival and improved hind-limb function compared with control animals.
Thrombosis is a critical event in vascular diseases associated with myocardial infarction and stroke, and in venous thromboembolic disorders, which are a major cause of morbidity and mortality in postoperative patients.35 Thrombus formation involves the interaction of vascular wall injury and circulating blood cells including leukocytes and platelets. Following endothelial cell damage, tissue factors are released and collagen in the subendothelial matrix becomes exposed, which then triggers platelet activation and aggregation, resulting in thrombus formation.36 Mice with complete HO-1 deficiency by genetic deletion37,38 or mice in which HO activity has been inhibited by chemical means (eg, tin protoporphyrin-IX),36,39 exhibit an accelerated thrombotic response to vascular injury compared with wild-type mice. In this report, we showed accelerated thrombus formation in the setting of allogeneic aortic transplantation, a model that differs from previously reported murine models of vena cava thrombosis and carotid arterial thrombosis induced by oxidant, electric, or photochemical injury.31,36,38,40
HO-1 is well known for protective effects against cellular damage, smooth muscle cell proliferation, and vascular constriction caused by vascular inflammation and ischemia-reperfusion injury.8,9,10 Previous studies have addressed the role of HO-1 expression and/or CO in modulation of vascular endothelial cell damage, which is a key factor in thrombus formation following hypoxia or oxidant injury,38,41 irradiation-induced apoptosis,42 and interleukin-18-dependent inflammation.43 HO-1 expression or endogenously generated CO inhibits production of proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1, and monocyte chemoattractant protein-1, most likely through inhibition of NF-κB activation.13,20 These same mechanisms also limit the expression of adhesion molecules such as E- and P-selectin, and the expression of tissue factor and plasminogen activator inhibitor-1, which can facilitate intravascular thrombus formation.38 In this study, we did not observe significant differences in the morphology of vascular endothelial cells within allografts in HO-1 wild-type and deficient recipients treated with either iCORM-2 or CORM-2. Moreover, experiments in which we transplanted HO-1 wild-type or deficient aortic grafts to Balb/cJ recipients suggested the expression of HO-1 by local vascular endothelial cells had no effect on thrombosis or survival. Therefore, protection of the endothelium by HO-1 or its products may not be the primary mechanism of defense against thrombosis.
Previous studies have shown that platelets possess functional HO-1,44 and hemin, an inducer of HO-1, dramatically suppresses platelet activation in vitro and prevents platelet-dependent thrombosis in vivo.36,37,39 True et al found that platelet counts, bleeding time, platelet aggregation and prothrombin time were not significantly different in HO-1 wild-type and deficient mice,38 suggesting that HO-1 may not be essential for megakaryopoiesis or platelet production. In our experiments, all of the HO-1−/− recipient animals receiving allogeneic aortic grafts died within 4 days after transplantation due to platelet-dependent thrombosis. In contrast, adoptive transfer of wild-type platelets and CO administration rescued HO deficient animals from acute thrombus formation indicating that loss of HO-1 expression in platelets might play an important role in the accelerated thrombosis that contributed to poor survival of the HO-1-deficient recipients.
To deliver CO, we used CORM-2, a chemical compound used by several investigators to demonstrate cytoprotection by CO in models of microbial sepsis, liver inflammation, and renal ischemia-reperfusion injury,6,20,22 In this model, systemic administration of CO was required to protect aortic grafts from thrombosis. Similar protection was also produced by administration of HO-1-expressing wild-type platelets.
The role of CO in platelets is not clearly understood. CO has been previously reported to attenuate platelet aggregation following elevation of cGMP-mediated activation of guanylate cyclase.32,45 In contrast, Chlopicki et al found CO inhibits platelet aggregation by a mechanism independent of soluble guanylate cyclase, possibly by affecting calcium-activated potassium channels, cytochrome P450, the mitochondrial respiratory chain, or p38MAPK.33 CO can selectively promote phosphorylation of vasodilator-stimulated phosphoprotein , which is a critical actin motor protein required for platelet aggregation.46 Apart from direct effects on platelet function, it is possible that platelets serve as a source of CO, leading to direct vascular relaxation by CO or CO-dependent suppression of plasminogen activator inhibitor-1.37,47 In addition, wild-type platelets could ameliorate thrombosis through selective delivery of HO-1, biliverdin, or CO to the graft site.
In summary, we demonstrate that HO-1 expression plays an important role in prevention of vascular arterial thrombosis in murine aortic allotransplantation, and systemic administration of a byproduct of HO-1 activity, CO, or adoptive transfer of HO-1-expressing platelets rescues HO-1-deficient recipients from thrombosis after transplantation. We suggest these findings may offer mechanistic insights into the therapeutic effects of HO-1 induction or CO administration in organ transplantation.
Footnotes
Address reprint requests to James F. George, Ph.D., Department of Surgery, Room 790 LHRB, or Anupam Agarwal, M.D., Department of Medicine, Room 647 THT, 1530 3rd Avenue South, University of Alabama at Birmingham, Birmingham, AL 35294. E-mail: jgeorge@uab.edu and agarwal@uab.edu.
Supported by National Institutes of Health grants DK75332 and DK59600 (to A.A.), American Heart Association grant 0655318B (to J.G.), and the core resource of the National Institutes of Health P30 O'Brien Center (DK 079337) for assistance with the animal microsurgeries.
References
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- Ferrandiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Okamoto M, Takei S, Sakazaki Y, Iwanaga T, Aizawa H. Redox-regulated mechanisms in asthma. Antioxid Redox Signal. 2008;10:769–783. doi: 10.1089/ars.2007.1936. [DOI] [PubMed] [Google Scholar]
- Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- Kotsch K, Martins PN, Klemz R, Janssen U, Gerstmayer B, Dernier A, Reutzel-Selke A, Kuckelkorn U, Tullius SG, Volk HD. Heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxid Redox Signal. 2007;9:2049–2063. doi: 10.1089/ars.2007.1801. [DOI] [PubMed] [Google Scholar]
- Soares MP, Bach FH. Heme oxygenase-1 in organ transplantation. Front Biosci. 2007;12:4932–4945. doi: 10.2741/2439. [DOI] [PubMed] [Google Scholar]
- Buis CI, van der Steege G, Visser DS, Nolte IM, Hepkema BG, Nijsten M, Slooff MJ, Porte RJ. Heme oxygenase-1 genotype of the donor is associated with graft survival after liver transplantation. Am J Transplant. 2008;8:377–385. doi: 10.1111/j.1600-6143.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Invest Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- Ott MC, Scott JR, Bihari A, Badhwar A, Otterbein LE, Gray DK, Harris KA, Potter RF. Inhalation of carbon monoxide prevents liver injury and inflammation following hind limb ischemia/reperfusion. FASEB J. 2005;19:106–108. doi: 10.1096/fj.04-2514fje. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Jr, Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res. 2006;54:24–29. doi: 10.1016/j.phrs.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Cepinskas G, Katada K, Bihari A, Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G184–G191. doi: 10.1152/ajpgi.00348.2007. [DOI] [PubMed] [Google Scholar]
- Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85:576–581. doi: 10.1097/TP.0b013e318160516a. [DOI] [PubMed] [Google Scholar]
- Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benza RL, Anderson PG, Lyle K, Barchue J, de Oliveira AL, Cavender MA, Pinderski LJ, George JF. Donor PAI-1 expression inhibits the intimal response of early allograft vascular disease. J Heart Lung Transplant. 2003;22:515–518. doi: 10.1016/s1053-2498(02)00662-9. [DOI] [PubMed] [Google Scholar]
- Koulack J, McAlister VC, Giacomantonio CA, Bitter-Suermann H, MacDonald AS, Lee TD. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 1995;16:110–113. doi: 10.1002/micr.1920160213. [DOI] [PubMed] [Google Scholar]
- Chen B, Kapturczak MH, Joseph R, George JF, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Tisher CC, Flotte TR, Agarwal A, Chen S. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007;7:1112–1120. doi: 10.1111/j.1600-6143.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- Bagamery K, Kvell K, Landau R, Graham J. Flow cytometric analysis of CD41-labeled platelets isolated by the rapid, one-step OptiPrep method from human blood. Cytometry A. 2005;65:84–87. doi: 10.1002/cyto.a.20133. [DOI] [PubMed] [Google Scholar]
- Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol. 2008;173:1882–1890. doi: 10.2353/ajpath.2008.080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- Chlopicki S, Olszanecki R, Marcinkiewicz E, Lomnicka M, Motterlini R. Carbon monoxide released by CORM-3 inhibits human platelets by a mechanism independent of soluble guanylate cyclase. Cardiovasc Res. 2006;71:393–401. doi: 10.1016/j.cardiores.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Desbuards N, Rochefort GY, Schlecht D, Machet MC, Halimi JM, Eder V, Hyvelin JM, Antier D. Heme oxygenase-1 inducer hemin prevents vascular thrombosis. Thromb Haemost. 2007;98:614–620. [PubMed] [Google Scholar]
- Peng L, Mundada L, Stomel JM, Liu JJ, Sun J, Yet SF, Fay WP. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid Redox Signal. 2004;6:729–735. doi: 10.1089/1523086041361677. [DOI] [PubMed] [Google Scholar]
- True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol. 2004;24:601–606. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Whittaker P. Adaptation of a photochemical method to initiate recurrent platelet-mediated thrombosis in small animals. Lasers Med Sci. 2007;22:42–45. doi: 10.1007/s10103-006-0410-1. [DOI] [PubMed] [Google Scholar]
- Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing P, Wilke A, Eissner G, Holler E, Andreesen R, Gerbitz A. Expression of heme oxygenase-1 protects endothelial cells from irradiation-induced apoptosis. Endothelium. 2005;12:113–119. doi: 10.1080/10623320500189814. [DOI] [PubMed] [Google Scholar]
- Zabalgoitia M, Colston JT, Reddy SV, Holt JW, Regan RF, Stec DE, Rimoldi JM, Valente AJ, Chandrasekar B. Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic Biol Med. 2008;44:284–298. doi: 10.1016/j.freeradbiomed.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell SA, Leakey JE, Warren JF, Lang NP, Frame LT. Identification of enzymes responsible for the metabolism of heme in human platelets. J Biol Chem. 1998;273:33342–33346. doi: 10.1074/jbc.273.50.33342. [DOI] [PubMed] [Google Scholar]
- Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- Li Calzi S, Purich DL, Chang KH, Afzal A, Nakagawa T, Busik JV, Agarwal A, Segal MS, Grant MB. Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation: evidence for blunted responsiveness in diabetes. Diabetes. 2008;57:2488–2494. doi: 10.2337/db08-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]