Abstract
Human hepatic lipase (HL) is an interfacial enzyme that must be liberated from cell surface proteoglycans to hydrolyze lipoprotein triglyceride. Both high-density lipoprotein (HDL) and apolipoprotein (apo)A-I can displace HL from cell surface proteoglycans, much like heparin. HL displacement is inhibited by HDL-apoE content. Postprandial HDL is approximately twofold better at displacing HL than is fasting HDL, but only has approximately one-half the apoE content. Enriching native HDL with triglyceride decreases HDL-apoE content and increases HL displacement. Incubation of HDL with the anti-apoE antibody, 6C5, also increases HL displacement. In contrast, enrichment of synthetic HDL with apoE significantly inhibits HL displacement. HDL from fasted female normolipidemic subjects displaces HL approximately twofold better than HDL from male subjects. HDL from female subjects also has significantly less apoE than HDL from males. Normolipidemic females have increased circulating HDL-bound HL. Hyperlipidemia has little effect on the HL displacement ability of HDL from men, whereas HDL from hypercholesterolemic females exhibits impaired HL displacement. HL displacement from liver heparan sulfate proteoglycans therefore appears to be linked to interlipoprotein apoE exchange. Decreased HL displacement is associated with higher HDL-apoE levels and may therefore affect vascular triglyceride hydrolysis.
Hepatic lipase (HL) is an interfacial enzyme that is bound to the surface of hepatocytes by heparan sulfate proteoglycans (HSPG).1 HL hydrolyzes both triglycerides (TG) and phospholipids in plasma lipoproteins. HL activity is undetectable in fasted plasma and can only be measured after HL is released from the liver with an injection of heparin. Elevated postheparin HL activities are considered to be a risk factor of heart disease and correspond to a pro-atherogenic lipid profile. Patients with familial hyperlipidemia2 and type 2 diabetes patients,3 who are at a higher risk for heart disease, also have elevated postheparin HL activity. Other heart disease risk factors have also been linked to a higher postheparin HL activity including smoking,4 visceral obesity,5 and sedentary lifestyle.6 An increase in androgenous hormones, such as testosterone, leads to an increase in postheparin HL activity7,8 and an increase in estrogen can lower postheparin HL activity.9,10 Women consequently tend to have a lower postheparin activity than men.11
HL must be liberated from cell surface HSPG to hydrolyze lipoprotein TG.12 A higher postheparin activity therefore appears to be a marker for a larger liver depot of inactive HSPG-bound HL. High-density lipoprotein (HDL) has been shown to displace HL from the cell surface much like heparin12,13,14; however, the composition of the HDL can affect its ability to displace HL. While apolipoprotein A-I (apoA-I), apolipoprotein A-II (apoA-II) and apolipoprotein C-I (apoC-I) appear to stimulate HL displacement, other apoproteins had the opposite effect and blocked HDL from displacing cell surface HL.12,13,14 Apolipoprotein E (apoE) is an exchangeable apolipoprotein present on both HDL- and TG-rich lipoproteins. Plasma apoE levels appear to correlate to postheparin HL activity.15,16 It has been shown that women have lower plasma apoE levels than men15,16 and that hyperlipidemic patients have higher amounts of plasma apoE than normolipidemic subjects.17,18,19 Subjects with very high levels of plasma apoE have also been shown to be hypertriglyceridemic17 and high levels of apoE can increase cardiovascular disease mortality, specifically in the older population.20
In this study, we show that increasing apoE content of synthetic HDL directly inhibits HL displacement. Conversely, HDL isolated at the peak of a postprandial response contains less apoE and is more effective at displacing HL, than HDL isolated from fasted plasma. HDL isolated from females contains lower levels of apoE and displaces more HL from cell-surface HSPG, than HDL isolated from males. Women were also shown to have significantly more HDL-bound HL circulating in their bloodstream. Interlipoprotein apoE movement therefore plays central role in regulating the displacement and hydrolytic activity of HL.
Materials and Methods
Chemicals
The mouse monoclonal HL antibody was a kind gift from Dr. Bensadoun, Cornell University. The mouse monoclonal apoA-I and apoE antibodies were obtained from Dr. Marcel, University of Ottawa Heart Institute. The biotinylated apoE polyclonal antibody was purchased from Biodesign (Saco, ME). The colorimetric triglyceride and cholesterol assays were obtained from Wako Chemicals USA (Richmond, VA). The recombinant apoE3 protein was obtained from Abcam Inc. (Cambridge, MA). Unless otherwise stated, compounds were of analytical grade.
Study Population
The study was approved by the Human Research Ethics Board of the University of Ottawa Heart Institute. The normolipidemic cohort consisted of healthy men (n = 9) and women (n = 10) with fasted cholesterol levels between 3.5 mmol/L and 5.2 mmol/L and TG levels below 2.0 mmol/L. The combined hyperlipidemic cohort (n = 8) consisted of patients with fasted cholesterol levels above 5.2 mmol/L and TG levels above 2.0 mmol/L. The hypercholesterolemic cohort (n = 14) consisted of patients with fasted cholesterol levels above 5.2 mmol/L and TG levels below 2.0 mmol/L. No patients were on any medication that would alter their lipid profiles at the time of the study.
Lipoprotein Isolation Using Sequential Ultracentrifugation
The blood samples were obtained from patients after 12 hours of fasting. The postprandial blood samples were obtained 4 hours after the subjects had consumed an 1800 calorie meal. The blood samples were obtained in Vacuette EDTA-coated tubes, for lipoprotein isolation. Blood samples obtained for the isolation of serum were collected in Vacuette SST tubes. Very low density lipoprotein (VLDL)/low density lipoprotein (LDL), and HDL were isolated by sequential ultracentrifugation according to the procedure described by Havel et al from fasting and postprandial human plasma samples using density ranges ρ = 1.006 to 1.065 g/ml and ρ = 1.065 to 1.25 g/ml respectively.21 Briefly, the plasma was isolated by centrifuging the EDTA coated tubes for 15 minutes at 3000 rpm. The plasma was removed and its density was adjusted to 1.065 g/ml using dry KBr. The plasma solution was sealed in Quick-Seal centrifuge tubes (25 mm × 89 mm) (Beckman, Palo Alto, CA). The tubes were spun using the L8-70 M Beckman ultracentrifuge for 20 hours at 40,000 rpm in a 70Ti rotor. The VLDL and LDL were in the top fraction of the solution and the bottom fraction was collected to spin for HDL. The density of the bottom fraction was increased to 1.25 g/ml using dry KBr and was spun for 40 hours at 40,000 rpm. Again the top fraction, containing the HDL, was isolated. All of the lipoprotein samples were dialyzed extensively against PBS(50 mmol/L sodium phosphate, 150 mmol/L NaCl, pH 7.2). The heterogeneity of the lipoprotein samples was determined using agarose gel electrophoresis on Lipogels (Beckman-Coulter, Mississauga, ON) using neutral lipid staining. The protein concentrations were determined using the Lowry method as modified by Markwell et al.22 Cholesterol and triglyceride concentrations were determined using commercial diagnostic assays from Wako Chemicals USA (Richmond, VA).
Triglyceride Enrichment of HDL Samples
The blood samples were obtained from patients after 12 hours of fasting. The blood samples were collected in Vacuette EDTA-coated tubes, for lipoprotein isolation. VLDL/LDL were isolated by sequential ultracentrifugation according to the procedure described by Havel et al from fasting plasma samples using the density range ρ = 1.006 to 1.065 g/ml.21 Once the VLDL and LDL had been removed, half the remaining fraction with ρ = 1.065 g/ml was incubated with Intralipid, a 20% TG emulsion (Sigma- Aldrich, St. Louis, MO) for 3 hours at 37°C. The non-absorbed Intralipid was then removed using sequential ultracentrifugation using a density of ρ = 1.065 g/ml. HDL from both the fasted and TG enriched samples was then isolated at ρ = 1.065 to 1.25 g/ml.
Purification of apoA-I
ApoA-I was isolated from human HDL that had been delipidated in chloroform:methanol as previously described.23 ApoA-I was isolated using size exclusion chromatography on a Sephacryl S-200 HR column.24 Purified protein samples were lyophilized and stored at −80°C until needed. For use, apoA-I was re-solubilized in 6 mol/L guanidine hydrochloride (GdnHCl), 10 mmol/L Tris (pH 7.2), and dialyzed extensively against PBS.
Cell Culture
Chinese hamster ovary cells overexpressing human HL (CHO-hHL) were obtained from Drs. Robert Brown (University of Ottawa) and Zemin Yao (University of Ottawa). CHO-hHL cells were maintained in Ham’s F12 medium containing 10% fetal bovine serum and 500 μg/ml geneticin selective antibiotic (G418 sulfate) in a 37°C incubator with 5% CO2. Cells were seeded in 6-well plates and allowed to reach ∼85% confluency before treatment. The cells were pre-incubated in serum-free medium for 3 hours before use in an experiment. The cell surface HL displacement ability of HDL was determined by incubating the CHO-hHL cells with 150 μg/ml HDL or apoA-I in FBS-free medium for 45 minutes at 37°C. After the incubation the medium was collected and the amount of HL released was measured using immunochemical analysis.
Preparation of Reconstituted HDL Particles
Reconstituted HDL (rHDL) complexes were prepared by sonicating 1-palmitoyl-2-oleyl-phosphatidylcholine with a mixture of apolipoproteins (apoA-I and apoE) at a molar ratio of one mole protein to 60 moles lipids as previously described by Boucher et al.25 Briefly 1-palmitoyl-2-oleyl-phosphatidylcholine (1 mg) in chloroform was dried under nitrogen in a 12 × 75 mm test tube and 800 μl of PBS, pH 7.4 was added. The lipid-buffer solution was initially sonicated for 1 minute at a 100% duty cycle. The suspension was then incubated in a sealed tube for 30 minutes at 37°C and sonicated again for 5 minutes using a 95% duty cycle. ApoA-I and apoE were added to the lipid suspension and the protein-lipid mixture was sonicated for 4 × 1 minute punctuated by 1-minute cooling periods. The size and homogeneity of rHDL complexes were estimated by non-denaturing gel electrophoresis on a 4% to 20% gel (Invitrogen, Carlsbad, CA).
Quantification of Cell-Surface HL Displacement by HDL
The media samples were combined in a 1:1 ratio with Laemmli sample buffer (Biorad, Mississauga, ON) containing 5% β-mercaptoethanol. The samples were electrophoresed on an 8% polyacrylamide gel under denaturing conditions. The proteins were immunoblotted onto PVDF membrane (Biorad) and probed using a 1:5000 dilution of the mouse monoclonal anti-human HL antibody and a 1:20,000 dilution of the HRP linked goat anti-mouse IgG secondary antibody (KPL, Gaithersburg, MD) in 1% bovine serum albumin/Tris buffered saline Tween-20. The blots were developed using the West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) on the Fluorchem AlphaImager instrument. The band intensities were analyzed with spot-densitometry using the AlphaEase software provided with the AlphaImager. The band intensities of the blots were normalized to total cell protein (from bicinchoninic acid assay) and expressed as a percent relative to apoA-I control displacement values, derived from the same experiment.
Non-Denaturing Electrophoresis and HL Immunoblotting
Isolated HDL samples were diluted with Ham’s F12 media to a concentration of 150 μg/ml. The samples were then diluted 1:1 with non-denaturing gradient gel Tris/glycine sample buffer. The samples were electrophoresed on a 4% to 20% non-denaturing gradient gel (Invitrogen) under non-denaturing conditions for 19 hours at 100V. The gel was incubated in 0.1% SDS solution for 15 minutes to ensure the proteins had enough negative charge to transfer unidirectionally toward the anode. The proteins were transferred onto a PVDF membrane (Biorad) at 125 V for 4 hours and immunoblotted using the monoclonal anti-human HL XHL3-6a antibody (1:5000). The molecular weight and the densitometry profiles were generated using AlphaEase software.
Quantification of apoE in HDL Samples Using Immunochemical Analysis
The HDL samples were diluted in serum free F12 media to a protein concentration of 150 μg/ml. The diluted HDL samples were combined in a 1:1 ratio with Laemmli sample buffer (Biorad) containing 5% β-mercaptoethanol. The samples were electrophoresed on a 12% polyacrylamide gel under denaturing conditions. The proteins were transferred onto PVDF membrane (Biorad) and probed using a 1:2000 dilution of both mouse anti apoE monoclonal primary antibodies 3H1 and 6C5 and a 1:20,000 dilution of the HRP linked goat anti-mouse IgG secondary antibody (KPL) in 1% bovine serum albumin/Tris buffered saline Tween-20. The blots were developed using the West Dura Sensitivity Substrate (Pierce) on the Fluorchem AlphaImager. The band intensities were analyzed with spot-densitometry using the AlphaEase software.
Quantification of apoE in HDL Samples using Enzyme-Linked Immunosorbent Assay
The apoE protein content from each isolated HDL sample was analyzed by enzyme-linked immunosorbent assay on a 96-well plate. Briefly, the Nunc Immuno-maxisorp 96-well plates (Nalge Nunc International, Rochester, NY) were coated with 10 μg/ml mouse anti-human apoE monoclonal antibody 6C5 in PBS and incubated overnight at 4°C. The wells were then blocked for 1 hour with a 0.5% solution of BSA. Samples and standards were incubated in the wells for 2 hours at 37°C. A biotinylated polyclonal anti-apoE was then added and incubated for 1 hour at 37°C. The plate was then incubated for 1 hour with avidin alkaline phosphatase (Sigma-Aldrich). Alkaline phosphatase yellow liquid substrate (Sigma-Aldrich) was added to the plate and the absorbance was recorded at 415 nm. All of the apoE immunoblot results were validated by enzyme-linked immunosorbent assay.
Statistical Analysis
Values are shown as mean ± SD and P < 0.05 was considered significant. Differences between mean values were evaluated using a one-way analysis of variance and the Student-Newman-Keuls Method (SigmaStat; Systat Software, Inc., SanJose, CA).
Results
Postprandial Lipemia Increases HL Liberation by HDL
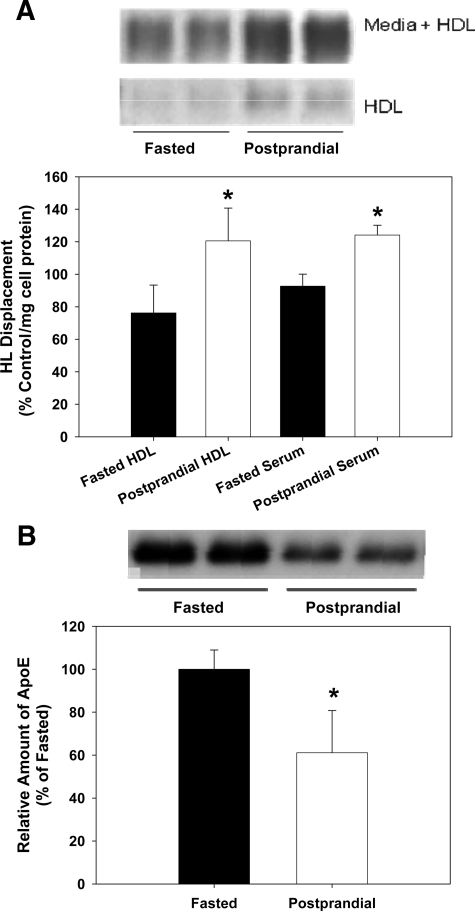
To determine the effect of a postprandial response on HL displacement, subjects (n = 3) were given a high-fat meal (1800 calories) and blood samples were drawn. Displacement experiments were performed on fasted blood samples and on samples drawn 4 hours after the meal. CHO-hHL cells were then treated with serum or ultracentrifugally isolated HDL (150 μg/ml). HDL isolates contained small amounts of endogenous HL and HDL isolated from the postprandial blood samples contained higher levels of endogenous, HDL-bound HL (∼twofold), than fasted HDL (Figure 1A). The amount of HL present in the HDL isolates was generally ∼10% of the amount in the media after incubation of HDL with the CHO-hHL cells. In Figure 1A, displacement is presented relative to control apoA-I displacement values. HDL isolated at the peak of a postprandial response displaces ∼60% more HL than fasting HDL samples. Serum samples showed a similar magnitude of displacement and postprandial stimulation of HL displacement. The amount of apoE in fasted and postprandial HDL samples was quantified immunochemically and is shown in Figure 1B. Similar to that previously reported,26,27 HDL-apoE content decreases during a postprandial response, while a slight increase in relative apoA-I and apoA-II levels was observed in the postprandial HDL samples. As expected, an increase in TG content of the postprandial HDL samples was also seen.
Figure 1.
Postprandial lipemia affects HL displacement by HDL. CHO-hHL cells were seeded in 6-well plates and treated with serum and HDL isolated from normolipidemic subjects for 45 minutes. The blood samples were taken after fasting, as well as 4 hours postprandial. The amount of HL present in HDL and media samples was measured using an immunochemical analysis. A: HL Western blot images are shown for fasted and postprandial HDL samples and also CHO-hHL media samples, after incubation with HDL (upper panel). HL displacement from the cell surface by HDL and serum was analyzed immunochemically and quantified by densitometry (lower panel). Averaged HL values are shown for serum and HDL samples obtained from three subjects after fasting and 4 hours postprandial, following an isocaloric meal. All values were calculated as a percentage of the HL displaced by the apoA-I control and were normalized to total cell protein. Values are the mean ± SD of triplicate determinations from one assay and represent three displacement experiments. Significance of difference relative to fasted sample *P < 0.01. B: The amount of apoE present in fasted and postprandial HDL samples was measured immunochemically. Western blot images are shown for the apoE in fasted and postprandial HDL samples (upper panel). HDL-apoE was quantified by densitometry and is shown (lower panel) relative to fasted values. Significant difference relative to fasted HDL, *P < 0.01.
ApoE Inhibits HL Displacement
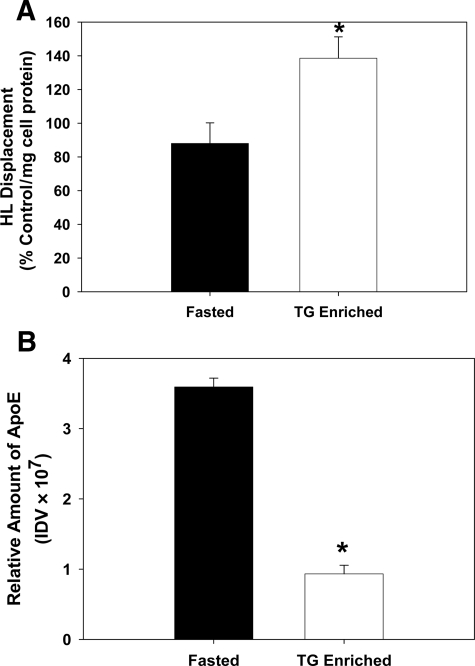
To determine whether the apoE concentration of HDL influences HL displacement, experiments were performed with both native and synthetic HDL preparations containing variable apoE levels. For native HDL studies, the ρ = 1.065 to 1.25 g/ml fraction was obtained from fasted normolipidemic subjects (n = 3) and then dialyzed. HDL was enriched in TG and depleted in apoE by incubating the ρ > 1.065 with Intralipid (20% TG emulsion, Sigma), at a ratio of 1:2 by volume (ρ > 1.065:Intralipid), for 3 hours at 37°C. HDL was then isolated by ultracentrifugation. HDL-TG content was increased by fivefold. There was no significant change in the apoA-I or apoA-II content of the TG-enriched HDL compared with the native HDL. TG enrichment of synthetic HDL has been previously shown to inhibit HL displacement.14 In contrast, TG enrichment of native HDL stimulates HL displacement (Figure 2A). This stimulation is associated with a significant reduction in HDL-apoE content in the TG-enriched HDL (Figure 2B).
Figure 2.
Effect of enriching HDL with TG on HL displacement. CHO-hHL cells were seeded in 6-well plates and treated with HDL for 45 minutes. Values are the mean ± SD of triplicate determinations from two different subjects. Significant difference relative to fasted HDL, *P < 0.01. A: HDL samples were obtained from fasted normolipidemic subjects and then enriched in TG as described. HL displacement from the cell surface by the control and TG-enriched HDL sample was analyzed immunochemically and is shown relative to apoA-I displacement, normalized to total cell protein. B: The relative amount of apoE present in control and TG-enriched HDL samples was measured immunochemically.
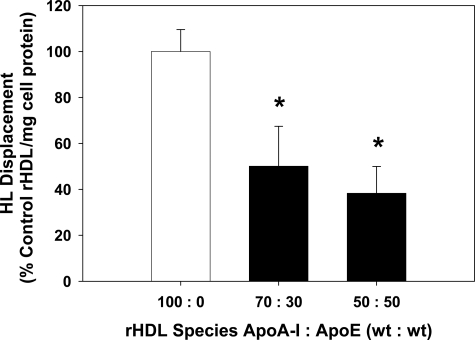
rHDL particles were synthesized using 1-palmitoyl-2-oleyl-phosphatidylcholine and varying amounts of apoA-I and apoE. These particles were then used to treat CHO-hHL cells and the amount of HL displaced was measured. Inclusion of apoE into the rHDL complexes decreased HL displacement (Figure 3). Particles with 70:30 and 50:50 apoA-I:apoE (wt:wt) displaced 50% and 60% less HL than the control rHDL containing only apoA-I (Figure 3).
Figure 3.
ApoE inhibits HL displacement by rHDL. Reconstituted HDL particles were prepared using 1-palmitoyl-2-oleyl-phosphatidylcholine and differing amounts of apoA-I and apoE. CHO-hHL cells were cultured in 12-well plates and treated with the rHDL for 45 minutes. The HL displacement from the cell surface caused by each rHDL species was measured using an immunochemical analysis and is shown relative to apoA-I displacement, normalized to total cell protein. Values are the mean ± SD of triplicate determinations from one assay and represent three displacement experiments. Significant difference, relative to control apoA-I ,rHDL *P < 0.005.
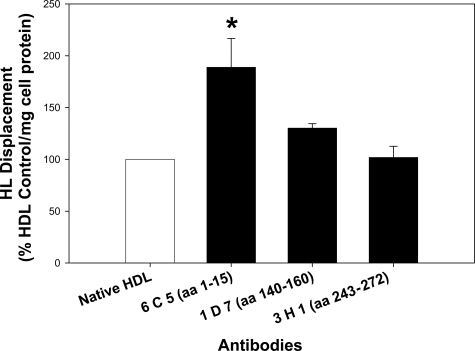
Antibodies to apoE also affected the ability of HDL to displace HL. HDL was incubated with an anti-apoE antibody (450 μg/ml) for 2 hours before its use in a displacement experiment. Three different antibodies were tested, which were specific for three distinct apoE epitopes; 6C5 (aa 1 to 13), 1D7 (aa 140 to 160), and 3H1 (aa 243 to 272). 1D7 and 3H1 had no significant affect on HL displacement; however, when HDL was incubated with 6C5, HL displacement increased by ∼85% (Figure 4).
Figure 4.
ApoE antibodies stimulate HL displacement. HDL from three different subjects was pooled and incubated with an anti-apoE antibody (6C5, 1D7, or 3H1) for 2 hours in serum-free media. CHO-hHL cells were cultured in 6-well plates and treated with HDL ± anti-apoE antibody for 45 minutes. HL displacement from the cell surface was measured by immunochemical analysis and is shown relative to control HDL displacement, normalized to total cell protein. Values are the mean ± SD of triplicate determinations from three displacement experiments. Significant difference relative to control HDL, *P < 0.01.
The Effect of Gender on HL Displacement
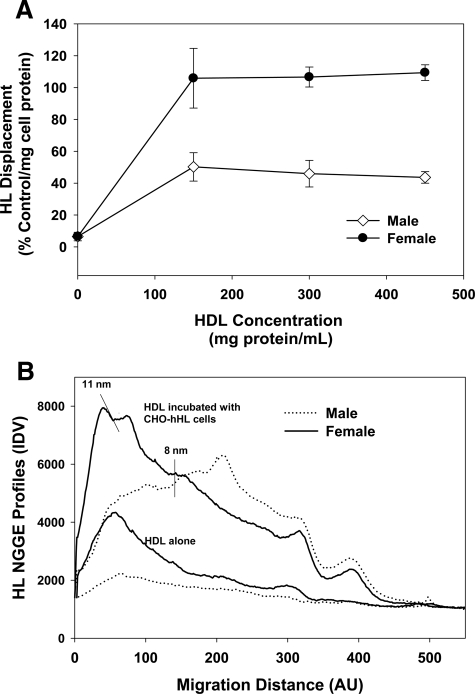
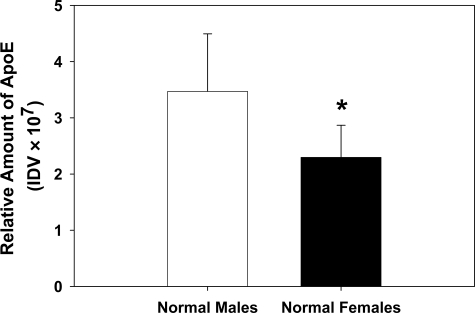
CHO-hHL cells were treated with increasing concentrations of HDL isolated from male and female normolipidemic subjects and the media was collected and probed for HL. HDL from both groups reached their maximum displacement at 150 μg protein/ml; however, HDL from women consistently displaced more HL from the CHO-hHL cell surface than male HDL (Figure 5A). Not only does female HDL displace more HL from the surface of CHO-hHL cells, it also contains more endogenous HL than male HDL, as measured by NGGE (Figure 5B). Consistent with our previous report, HL displaced to the media associated with HDL and showed a similar profile to that observed for apoA-I.14 HDL from female subjects had a higher HDL2:HDL3 ratio than HDL from male subjects. ApoE levels in HDL from male and female normolipidemic subjects was quantified immunochemically and shown to be significantly decreased in the female HDL (Figure 6). This was confirmed using an apoE enzyme-linked immunosorbent assay. HDL from normolipidemic females had approximately 3 mg/dL apoE (/mg HDL protein), whereas normolipidemic male HDL had 4 mg/dL apoE. Previous work has reported the amount of apoE in normolipidemic HDL to be approximately 2.5 mg/dL.28,29 A lower concentration of apoE in females compared with males is also consistent with previous reports.15,16 While HDL from women did have a slight, but not significant, increase in apoA-I content than HDL from men, no difference was observed between male and female HDL for apoA-II, apoC-III, TG or cholesterol content.
Figure 5.
Gender affects HL displacement. CHO-hHL cells were cultured to confluency and treated with increasing concentrations of HDL from female (n = 3) and male (n = 3) normolipidemic subjects for 45 minutes. A: The displacement of HL from the cell surface was measured using an immunochemical analysis and is shown relative to apoA-I displacement, normalized to total cell protein. Values are the mean of triplicate determinations ± SD. B: The amount of HL present in media samples from displacement experiments was compared with the amount of HL present in the isolated HDL samples using non-denaturing gradient gel electrophoresis and probed for HL. The HL profiles shown are from one normolipidemic female and one normolipidemic male and are representative of HL profiles seen for other subjects (n = 6) and are representative of HL profiles seen for other subjects.
Figure 6.
HDL-apoE levels are elevated in normolipidemic males. The relative amount of apoE present in HDL from fasted normolipidemic males (n = 6) and females (n = 9) was measured using an immunochemical analysis. Values are the mean ± SD Significant difference, *P < 0.05.
Hypercholesterolemia Decreases HL Displacement in Women
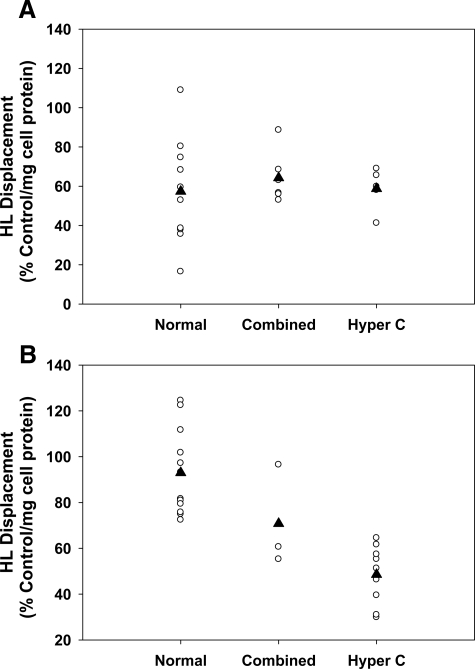
HDL was obtained from fasted normolipidemic and dyslipidemic patients. The normolipidemic subjects had fasted cholesterol levels between 3.5 mmol/L and 5.2 mmol/L and TG levels below 2.0 mmol/L. The dyslipidemic patients had cholesterol levels above 5.2 mmol/L. The HDL obtained from fasted normolipidemic and dyslipidemic patients was used to displace HL from CHO-hHL cells. These experiments revealed that hyperlipidemia affected the ability of female HDL to displace HL, whereas hyperlipidemia had little effect on HL displacement by HDL from men (Figure 7). HDL from normolipidemic and hyperlipidemic men displaced, on average, ∼60% that of control incubations (Figure 7A). HDL from normolipidemic females displaced HL by ∼100% that of control, while HDL from female combined hyperlipidemic and hypercholesterolemic patients had an HL displacement that was ∼70% and 50% that of the control, respectively (Figure 7B). HDL composition analysis showed that there was no significant difference in HDL-apoA-I, apoA-II, apoC-III, TG, or cholesterol between the HDL from normolipidemic females, males, and hyperlipidemic patients. Normolipidemic females had a slightly lower HDL-apoE content than their hyperlipidemic counterparts, but the difference was not significant. In other studies, hyperlipidemic patients have been shown to have higher plasma apoE levels than normolipidemic subjects.17,18,19
Figure 7.
Hyperlipidemia affects HL displacement by HDL. CHO-hHL cells were cultured in 6-well plates and treated with HDL from fasted female and male normolipidemic subjects as well as HDL from fasted hyperlipidemic patients for 45 minutes. The displacement of HL from the cell surface was measured using an immunochemical analysis. A: HL displacement by HDL from male normolipidemic (normal), combined hyperlipidemic (combined), and hypercholesterolemic (hyper C) patients. B: HL displacement by HDL from female subjects. All values were calculated as a percentage of the HL displaced by the apoA-I control and were normalized to total cell protein. The black triangle represents the group mean value.
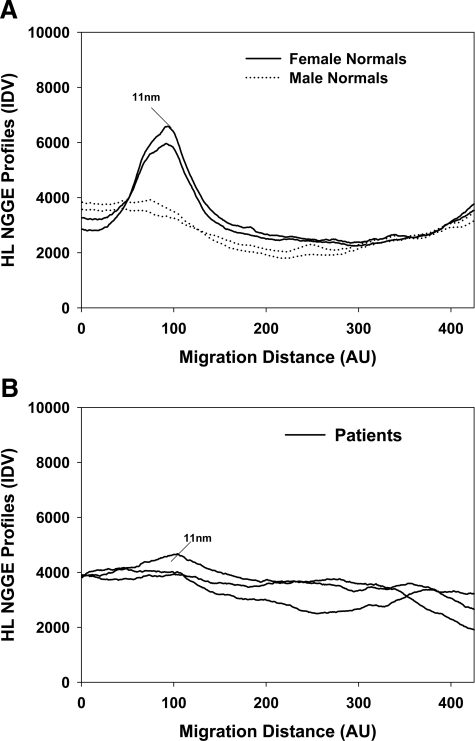
Immunoblots of nondenaturing gradient gels were used to determine whether HL was associated with isolated HDL samples from normolipidemic and hyperlipidemic patients. As shown in Figure 8A, female normolipidemic HDL samples contained endogenous HL, which was associated with an 11 nm, HDL2 class of HDL (Figure 8A). Only two female profiles are illustrated, but similar profiles were observed for HDL preparations from all other normolipidemic female subjects. This appears to agree with our previous reports, which have shown that HDL2 is more effective at displacing HL from the surface of liver cells.13,14 HL was shown to associate with HDL2 after it is displaced from CHO-hHL cells by human HDL.14 Very little HL was detectable in HDL isolated from male or hyperlipidemic subjects (Figure 8, A and B). Figure 8B shows representative profiles for three female hypercholesterolemic patients. Similar profiles were evident for all other hyperlipidemic patients.
Figure 8.
Hyperlipidemic patients have reduced circulating HDL bound HL levels. HDL was isolated from blood samples obtained from female normolipidemic, male normolipidemic and female hypercholesterolemic subjects. The HDL samples, isolated using sequential density gradient ultracentrifugation, were analyzed using non-denaturing gradient gel electrophoresis and probed for HL. A: HL densitometry profiles of normolipidemic subjects. Profiles are similar to that observed from experiments with HDL isolated from other normolipidemic subjects. B: HL densitometry profiles of female hypercholesterolemic patients. The profiles are from three female patients and are similar to that observed from separate experiments with HDL isolated from other hyperlipidemic male and female patients.
Discussion
HL activity is essentially undetectable in fasted plasma and can only be measured after an intravenous injection of heparin. An increased postheparin HL activity is associated with a pro-atherogenic lipid profile including low HDL levels30,31,32 and small, dense LDL particles.33,34,35 In vitro experiments have shown that proteoglycan-bound HL is inactive and that the displacement of HL from the cell surface stimulates TG hydrolysis.12 An elevated postheparin activity, therefore, may reflect an increased amount of inactive HL bound to the surface of cells in the liver. Postheparin HL activity in familial low HDL patients is higher than in normal subjects.36 Subjects with high postheparin HL activity would be expected to exhibit decreased vascular lipolysis and increased plasma TG levels.2
Both the lipid and apoprotein components of HDL have been shown to affect its HL displacement ability14 and HDL has been shown to displace HL similarly in both HepG2 and CHO-hHL cells.13,14 To determine the effect of lipid enrichment on HL displacement, Rouhani et al enriched native and rHDL particles with various lipids and characterized HL displacement. Enrichment with free fatty acids and cholesteryl esters had minimal effects on HL displacement; however, phospholipid and TG enrichment of reconstituted HDL blocked HL displacement.14 TG enrichment of native HDL, however, did not show similar results. When native HDL was enriched with TG, through incubation with Intralipid, an increase in HL displacement was observed (Figure 2). Since TG itself has an opposite effect on HL displacement, increased displacement must have been due to some other factor. The present work suggests that a decrease in HDL-apoE, which occurs when the native HDL is enriched with TG, overcomes the inhibitory effects of TG and stimulates HL displacement. Previous work has shown that HDL apolipoproteins directly affect HL displacement. ApoA-II has been shown to directly increase the affinity of HL for HDL25 and increase HL displacement.14 ApoA-II has also been shown to inhibit HL activity and to alter the conformation of apoA-I, which may increase the affinity of HL for apoA-I.25 ApoC-I can also increase HL displacement by HDL.14 Some component in isolated HDL apolipoproteins was shown to inhibit HL displacement, but the apolipoprotein was not identified.14 Evidence from the present study suggests that this inhibitor is apoE.
ApoE is an exchangeable apolipoprotein that is found on HDL as well as triglyceride-rich lipoproteins. ApoE mediates the uptake of lipoproteins through its ability to bind the LDL receptor37 and the LDL-receptor related protein (LRP).38,39 HDL acts as a storage depot for apoE and during a postprandial response, apoE is shuttled to VLDL and chylomicron remnants to act as a ligand for LRP and LDL-receptor.26 Once the response is over, the apoE is returned to the HDL. Following an 1800-calorie meal, a 40% decrease in HDL-apoE content was observed in normolipidemic subjects (Figure 1B). This decrease corresponded to an almost 50% increase in HL displacement from the surface of CHO-hHL cells (Figure 1A). Increased HL displacement during a postprandial response therefore may be due to a change in HDL-apoE composition. Postprandial HDL samples also appeared to contain more endogenous HDL-associated HL (Figure 1A). TG-enrichment of native HDL samples appears to have exactly the same effect as a postprandial response. Native HDL enriched fivefold with TG showed a 3.5-fold decrease in the amount of HDL-apoE. This compositional change stimulated HL displacement 1.5-fold (Figure 2). To directly evaluate the effect of apoE content on HL displacement, reconstituted particles were created to contain differing amounts of apoE and apoA-I. Increasing apoE content in the synthetic HDL particles significantly decreased HL displacement, by >50% in the complex that was half apoE and half apoA-I (Figure 3). This data shows that HDL-apoE content directly inhibits HL displacement.
Female normolipidemic subjects in this study were also found to have significantly lower HDL-apoE levels than normolipidemic males. This is consistent with previous reports, showing that females over the age of 25 have lower plasma apoE levels than males.15,16 No other significant differences were found in HDL composition between male and female normolipidemic subjects. Reduced HDL-apoE may therefore contribute to the enhanced HL displacement observed for female HDL samples. ApoA-II has previously been shown to increase HL displacement,14 however, no difference was observed in the amount of apoA-II or apolipoprotein C-III (apoC-III) in the male and female HDL samples (data not shown). HDL from normolipidemic females displaced approximately 50% more HL from the cell surface than HDL from male subjects (Figures 5 and 7). HDL from female subjects also contained more endogenous HDL-bound HL than HDL from all other subjects (Figure 8). Western blots of ultracentrifugally isolated HDL show that HL associates with HDL in the bloodstream in subjects, even without heparin administration. HL has been shown to primarily associate with the larger, 11 nm HDL2 particles when it is displaced from the surface of CHO-hHL cells by HDL14 (Figures 5 and 8). It also had previously been shown that HDL2 has a greater ability to displace cell surface HL, while HDL3 is poor at liberating HL.13,14 HDL from normolipidemic females had the highest endogenous HDL-bound HL levels, compared with normolipidemic males and hypercholesterolemic patients (Figures 5B and 8) and was the best at displacing HL from CHO-hHL cells. Increased levels of circulating HDL-bound HL in women may partly explain why they usually have lower postheparin HL activity than men.11 Small amounts of HL were shown to be associated with HDL from all patients; however, the lack of HL activity in fasted plasma samples shows that this endogenous HDL-bound HL has no activity in the circulation.
When comparing HL displacement by HDL from male donors, no difference was observed between normolipidemic and hyperlipidemic subjects. HDL obtained from hypercholesterolemic women, however, showed a 50% decrease in HL displacement ability, when compared with normolipidemic women (Figure 7A). Hypercholesterolemic females also had less circulating HDL-bound HL in their bloodstream (Figure 8B). This may suggest that less HL is liberated from the liver by the HDL of hypercholesterolemic patients. Hypercholesterolemia is also commonly associated with elevated postheparin HL activity.2 In contrast to that seen in normolipidemic subjects, there was no significant difference evident in HDL-apoE levels in hyperlipidemic patients. There was also no difference observed in the HDL-apoA-II or apoC-III levels between the normolipidemic and hyperlipidemic subjects. HDL from female hypercholesterolemic patients showed a slight but not significant increase in apoE, compared with the normolipidemic female subjects, which appears consistent to earlier studies that showed a significant increase in plasma apoE levels in hyperlipidemic patients, as compared with normal subjects.17,18,19
HDL-apoE concentration appears to regulate HL displacement by HDL. Decreases in HDL-apoE content are associated with an increased ability of HDL to displace HL from the cell surface. ApoE may affect HL displacement by regulating the association of HL with HDL, in an opposite manner to that observed with apoA-II.25 Incubating HDL with the anti-apoE 6C5 antibody resulted in an 85% increase in HL displacement. The antibody 6C5 recognizes an epitope in the N-terminus of apoE consisting of the first 15 amino acids. This epitope is enriched in anionic residues, ie, glutamic acid. Blocking this negatively charged region of apoE appears to inhibit the ability of apoE to block the displacement of HL by HDL. This may suggest that HDL charge can affect the association of HL with the lipoprotein and is consistent with earlier work by Boucher et al, which showed that anionic lipids can decrease HL association with HDL and activate the enzyme.40 In contrast, apoE did not appear to affect HL displacement through its impact on apoE receptor pathways. The monoclonal apoE antibody 1D7 interacts with an epitope specific to amino acids 140 to 160. This amino acid region is rich in arginine and is known to be responsible for the binding of apoE to LDL-R, LRP, and HSPG.41,42 Incubation of HDL with 1D7 had no significant effect on HL displacement.
Both postprandial and female HDL fractions were shown to have reduced apoE levels and also increased endogenous HL (Figures 1A and 8). This appears to confirm that apoE modulates the association of HL with HDL and thereby its ability to bind and displace HL. ApoE is thought to bind HDL through hydrophobic interactions and therefore may block HL binding by occupying space on the HDL interface. ApoA-II, however, would also be expected to occupy interfacial regions and yet this apolipoprotein stimulates HL binding to HDL.25 Both HL and apoE contain heparin-binding sites and these domains may play a role in protein-protein interactions on HDL. ApoE has been shown to directly interact with apoA-II43,44 and as such may block apoA-II dependent association of HL with HDL.25
Alternately, apoE may reduce HL release by stimulating an HSPG or receptor-mediated membrane internalization and reducing cell surface HL levels. This may partly explain the lower plateau observed for the male HDL displacement (Figure 5A), which may represent a reduced cellular pool of HL available for displacement, resulting from incubations with apoE-rich HDL. ApoE and HL are known to facilitate the binding and uptake of HDL through HSPG- and LRP-dependent pathways.45 ApoE may bind to cell surface HSPG and promote the internalization of HL-enriched membrane domains, lowering the membrane HL available for displacement. This process does not appear to involve the association/uptake of HDL with the CHO-hHL cells, as there was no detectable difference in media HDL levels after incubations with apoE-rich or apoE-poor HDL particles (data not shown). ApoE may instead be lost from HDL to membrane HSPG and/or LRP and act to promote HL internalization and reduce membrane HL displacement. ApoE isoforms would be expected to impact these membrane events.46 While subjects were not all genotyped in this study, HDL from one male subject, with a known apoE 3/2 phenotype, showed an elevated HL displacement, comparable with that seen for females. ApoE2 binds HSPG to a lesser extent than apoE3 and apoE446 and as such, may have a lesser effect on the membrane internalization of HL. Previous studies have shown that a high level of apoE2 in both rabbits and mice impairs triglyceride lipolysis and VLDL clearance resulting in hypertriglyceridemia.47,48
The displacement and activation of HL appears to be regulated by a three step process.13,14 The first step involves both lipid and apolipoprotein exchanges between HDL and the apoB containing lipoproteins. ApoE depletion of HDL promotes the second step of this process, where HL is displaced from the liver cell surface.14 High affinity association of HL with HDL has been shown to inhibit its lipolytic activity25,40 and therefore, once displaced from the surface of the liver, HL remains bound to the HDL in an inactive form. The final step in HL activation is dissociation from HDL.40 This step may be due to altered electrostatic interactions resulting from apoE exchange or anionic lipids. An increase in vascular fatty acid levels result in an increased negative charge in the interfacial environment around the HDL, which could also result in the release and the activation of HL.40,49 It has previously been shown that an increase in the net negative charge on HDL results in a decreased affinity of HL for HDL and an increase in TG lipolysis.40
Footnotes
Address reprint requests to Daniel L. Sparks, Ph.D., University of Ottawa Heart Institute, 40 Ruskin Street, Ottawa, Ontario, Canada, K1Y 4W7. E-mail: dsparks@ottawaheart.ca.
Supported by the Heart and Stroke Foundation of Ontario grant # T5593 (to D.L.S.), by graduate scholarships from the Ontario Government Scholarship (OGS) Program (E.K.Y.), and the National Sciences and Engineering Research Council of Canada (NSERC).
References
- Connelly PW. The role of hepatic lipase in lipoprotein metabolism. Clin Chim Acta. 1999;286:243–255. doi: 10.1016/s0009-8981(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Seed M, Mailly F, Vallance D, Doherty E, Winder A, Talmud P, Humphries SE. Lipoprotein lipase activity in patients with combined hyperlipidaemia. Clin Investig. 1994;72:100–106. doi: 10.1007/BF00184584. [DOI] [PubMed] [Google Scholar]
- Syvanne M, Ahola M, Lahdenpera S, Kahri J, Kuusi T, Virtanen KS, Taskinen MR. High density lipoprotein subfractions in non-insulin-dependent diabetes mellitus and coronary artery disease. J Lipid Res. 1995;36:573–582. [PubMed] [Google Scholar]
- Eliasson B, Mero N, Taskinen MR, Smith U. The insulin resistance syndrome and postprandial lipid intolerance in smokers. Atherosclerosis. 1997;129:79–88. doi: 10.1016/s0021-9150(96)06028-5. [DOI] [PubMed] [Google Scholar]
- Despres JP, Ferland M, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, Theriault G, Bouchard C. Role of hepatic-triglyceride lipase activity in the association between intra-abdominal fat and plasma HDL cholesterol in obese women. Arteriosclerosis. 1989;9:485–492. doi: 10.1161/01.atv.9.4.485. [DOI] [PubMed] [Google Scholar]
- Marniemi J, Peltonen P, Vuori I, Hietanen E. Lipoprotein lipase of human postheparin plasma and adipose tissue in relation to physical training. Acta Physiol Scand. 1980;110:131–135. doi: 10.1111/j.1748-1716.1980.tb06642.x. [DOI] [PubMed] [Google Scholar]
- Kantor MA, Bianchini A, Bernier D, Sady SP, Thompson PD. Androgens reduce HDL2-cholesterol and increase hepatic triglyceride lipase activity. Med Sci Sports Exerc. 1985;17:462–465. doi: 10.1249/00005768-198508000-00010. [DOI] [PubMed] [Google Scholar]
- Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am J Physiol Endocrinol Metab. 2003;284:E1112–E1118. doi: 10.1152/ajpendo.00524.2002. [DOI] [PubMed] [Google Scholar]
- Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- Tikkanen MJ, Nikkila EA, Kuusi T, Sipinen SU. High density lipoprotein-2 and hepatic lipase: reciprocal changes produced by estrogen and norgestrel. J Clin Endocrinol Metab. 1982;54:1113–1117. doi: 10.1210/jcem-54-6-1113. [DOI] [PubMed] [Google Scholar]
- Despres JP, Gagnon J, Bergeron J, Couillard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Plasma post-heparin lipase activities in the HERITAGE Family Study: the reproducibility, gender differences, and associations with lipoprotein levels. Health, risk factors, exercise training and genetics. Clin Biochem. 1999;32:157–165. doi: 10.1016/s0009-9120(98)00106-4. [DOI] [PubMed] [Google Scholar]
- Ramsamy TA, Neville TA, Chauhan BM, Aggarwal D, Sparks DL. Apolipoprotein A-I regulates lipid hydrolysis by hepatic lipase. J Biol Chem. 2000;275:33480–33486. doi: 10.1074/jbc.M005436200. [DOI] [PubMed] [Google Scholar]
- Ramsamy TA, Boucher J, Brown RJ, Yao Z, Sparks DL. HDL regulates the displacement of hepatic lipase from cell surface proteoglycans and the hydrolysis of VLDL triacylglycerol. J Lipid Res. 2003;44:733–741. doi: 10.1194/jlr.M200339-JLR200. [DOI] [PubMed] [Google Scholar]
- Rouhani N, Young E, Chatterjee C, Sparks DL. HDL composition regulates displacement of cell surface-bound hepatic lipase. Lipids. 2008;43:793–804. doi: 10.1007/s11745-008-3214-1. [DOI] [PubMed] [Google Scholar]
- Vincent-Viry M, Schiele F, Gueguen R, Bohnet K, Visvikis S, Siest G. Biological variations and genetic reference values for apolipoprotein E serum concentrations: results from the STANISLAS cohort study. Clin Chem. 1998;44:957–965. [PubMed] [Google Scholar]
- Schiele F, De BD, Vincent-Viry M, Beisiegel U, Ehnholm C, Evans A, Kafatos A, Martins MC, Sans S, Sass C, Visvikis S, De BG, Siest G. Apolipoprotein E serum concentration and polymorphism in six European countries: the ApoEurope Project. Atherosclerosis. 2000;152:475–488. doi: 10.1016/s0021-9150(99)00501-8. [DOI] [PubMed] [Google Scholar]
- Blum CB, Aron L, Sciacca R. Radioimmunoassay studies of human apolipoprotein E. J Clin Invest. 1980;66:1240–1250. doi: 10.1172/JCI109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal R, Tremblay M, Barrett PH, Jacques H, Fredenrich A, Mamer O, Davignon J, Cohn JS. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res. 2000;41:706–718. [PubMed] [Google Scholar]
- Onat A, Dursunoglu D, Bulur S, Kucukdurmaz Z, Kaya Z, Ordu S, Ugur M. [Turkish Adult Risk Factor Survey 2007: decline in all-cause and coronary mortality continues]. Turk Kardiyol Dern Ars. 2008;36:77–81. [PubMed] [Google Scholar]
- Mooijaart SP, Berbee JF, van HD, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3:e176. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Anantharamaiah GM, Garber DW. Chromatographic methods for quantitation of apolipoprotein A-I. Methods Enzymol. 1996;263:267–282. doi: 10.1016/s0076-6879(96)63019-5. [DOI] [PubMed] [Google Scholar]
- Brewer HB, Jr, Ronan R, Meng M, Bishop C. Isolation and characterization of apolipoproteins A-I. A-II, and A-IV. Methods Enzymol. 1986;128:223–235. doi: 10.1016/0076-6879(86)28070-2. [DOI] [PubMed] [Google Scholar]
- Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45:849–858. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- Blum CB. Dynamics of apolipoprotein E metabolism in humans. J Lipid Res. 1982;23:1308–1316. [PubMed] [Google Scholar]
- Annuzzi G, Holmquist L, Carlson LA. Concentrations of apolipoproteins B. C-I, C-II, C-III, E and lipids in serum and serum lipoproteins of normal subjects during alimentary lipaemia. Scand J Clin Lab Invest. 1989;49:73–81. doi: 10.3109/00365518909089080. [DOI] [PubMed] [Google Scholar]
- Cohn JS, Batal R, Tremblay M, Jacques H, Veilleux L, Rodriguez C, Mamer O, Davignon J. Plasma turnover of HDL apoC-I, apoC-III, and apoE in humans: in vivo evidence for a link between HDL apoC-III and apoA-I metabolism. J Lipid Res. 2003;44:1976–1983. doi: 10.1194/jlr.M300209-JLR200. [DOI] [PubMed] [Google Scholar]
- Fredenrich A, Giroux LM, Tremblay M, Krimbou L, Davignon J, Cohn JS. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J Lipid Res. 1997;38:1421–1432. [PubMed] [Google Scholar]
- Patsch JR, Prasad S, Gotto AMJ, Patsch W. High density lipoprotein2. Relationship of the plasma levels of this lipoprotein species to its composition, to the magnitude of postprandial lipemia, and to the activities of lipoprotein lipase and hepatic lipase. J Clin Invest. 1987;80:341–347. doi: 10.1172/JCI113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi T, Kinnunen PK, Nikkila EA. Hepatic endothelial lipase antiserum influences rat plasma low and high density lipoproteins in vivo. FEBS Lett. 1979;104:384–388. doi: 10.1016/0014-5793(79)80858-3. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Applebaum-Bowden D, Wahl PW, Hoover JJ, Warnick GR, Albers JJ, Hazzard WR. Epidemiological correlates of high density lipoprotein subfractions, apolipoproteins A-I. A-II, and D, and lecithin cholesterol acyltransferase. Effects of smoking, alcohol, and adiposity. Arteriosclerosis. 1985;5:169–177. doi: 10.1161/01.atv.5.2.169. [DOI] [PubMed] [Google Scholar]
- Auwerx JH, Marzetta CA, Hokanson JE, Brunzell JD. Large buoyant LDL-like particles in hepatic lipase deficiency. Arteriosclerosis. 1989;9:319–325. doi: 10.1161/01.atv.9.3.319. [DOI] [PubMed] [Google Scholar]
- Zambon A, Austin MA, Brown BG, Hokanson JE, Brunzell JD. Effect of hepatic lipase on LDL in normal men and those with coronary artery disease. Arterioscler Thromb. 1993;13:147–153. doi: 10.1161/01.atv.13.2.147. [DOI] [PubMed] [Google Scholar]
- Campos H, Dreon DM, Krauss RM. Associations of hepatic and lipoprotein lipase activities with changes in dietary composition and low density lipoprotein subclasses. J Lipid Res. 1995;36:462–472. [PubMed] [Google Scholar]
- Soderlund S, Soro-Paavonen A, Ehnholm C, Jauhiainen M, Taskinen MR. Hypertriglyceridemia is associated with prebeta-HDL concentrations in subjects with familial low HDL. J Lipid Res. 2005;46:1643–1651. doi: 10.1194/jlr.M400480-JLR200. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- Linton MF, Hasty AH, Babaev VR, Fazio S. Hepatic apo E expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest. 1998;101:1726–1736. doi: 10.1172/JCI2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, Nguyen T, Sparks DL. Lipoprotein electrostatic properties regulate hepatic lipase association and activity. Biochem Cell Biol. 2007;85:696–708. doi: 10.1139/o07-137. [DOI] [PubMed] [Google Scholar]
- Maurice R, Marcel YL, Innerarity TL, Milne RW. A potential complication in the use of monoclonal antibodies: inhibition of apoB-mediated receptor binding by an anti-apoE antibody. J Lipid Res. 1989;30:587–596. [PubMed] [Google Scholar]
- Weisgraber KH, Rall SC, Jr, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein E: determination of the heparin binding sites of apolipoprotein E3. J Biol Chem. 1986;261:2068–2076. [PubMed] [Google Scholar]
- Innerarity TL, Mahley RW, Weisgraber KH, Bersot TP. Apoprotein (E–A-II) complex of human plasma lipoproteins. II Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E–A-II) complex. J Biol Chem. 1978;253:6289–6295. [PubMed] [Google Scholar]
- Weisgraber KH, Mahley RW. Apoprotein (E–A-II) complex of human plasma lipoproteins. I Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978;253:6281–6288. [PubMed] [Google Scholar]
- Ji ZS, Dichek HL, Miranda RD, Mahley RW. Heparan sulfate proteoglycans participate in hepatic lipase and apolipoprotein E-mediated binding and uptake of plasma lipoproteins, including high density lipoproteins. J Biol Chem. 1997;272:31285–31292. doi: 10.1074/jbc.272.50.31285. [DOI] [PubMed] [Google Scholar]
- Ji ZS, Fazio S, Mahley RW. Variable heparan sulfate proteoglycan binding of apolipoprotein E variants may modulate the expression of type III hyperlipoproteinemia. J Biol Chem. 1994;269:13421–13428. [PubMed] [Google Scholar]
- Huang Y, Schwendner SW, Rall SC, Jr, Sanan DA, Mahley RW. Apolipoprotein E2 transgenic rabbits—Modulation of the type III hyperlipoproteinemic phenotype by estrogen and occurrence of spontaneous atherosclerosis. J Biol Chem. 1997;272:22685–22694. doi: 10.1074/jbc.272.36.22685. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Rall SC, Jr, Mahley RW. Apolipoprotein E2 reduces the low density lipoprotein level in transgenic mice by impairing lipoprotein lipase-mediated lipolysis of triglyceride-rich lipoproteins. J Biol Chem. 1998;273:17483–17490. doi: 10.1074/jbc.273.28.17483. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Chatterjee C, Young E, Renwick J, Pandey NR. Lipoprotein charge and vascular lipid metabolism. Chem Phys Lipids. 2008;154:1–6. doi: 10.1016/j.chemphyslip.2008.04.006. [DOI] [PubMed] [Google Scholar]