Abstract
Over the past 100 years, changes in the food supply in Western nations have resulted in alterations in dietary fatty acid consumption, leading to a dramatic increase in the ratio of omega-6 (ω6) to ω3 polyunsaturated fatty acids (PUFA) in circulation and in tissues. Increased ω6/ω3 ratios are hypothesized to increase inflammatory mediator production, leading to higher incidence of inflammatory diseases, and may impact inflammatory gene expression. To determine the effect of reducing the ω6/ω3 ratio on expression of inflammatory pathway genes in mononuclear cells, healthy humans were placed on a controlled diet for 1 week, then given fish oil and borage oil for an additional 4 weeks. Serum and neutrophil fatty acid composition and ex vivo leukotriene B4 production from stimulated neutrophils were measured at the start and end of the supplementation period and after a 2-week washout. RNA was isolated from mononuclear cells and expression of PI3K, Akt, NFκB, and inflammatory cytokines was measured by real-time PCR. A marked increase was seen in serum and neutrophil levels of long-chain ω3 PUFA concomitant with a reduction in the ω6/ω3 PUFA ratio (40%). The ex vivo capacity of stimulated neutrophils to produce leukotriene B4 was decreased by 31%. Expression of PI3Kα and PI3Kγ and the quantity of PI3Kα protein in mononuclear cells was reduced after supplementation, as was the expression of several proinflammatory cytokines. These data reveal that PUFA may exert their clinical effects via their capacity to regulate the expression of signal transduction genes and genes for proinflammatory cytokines.
Since the beginning of the 20th century, the fatty acid composition of complex lipids (such as triglycerides) in Western diets has changed dramatically, largely due to a marked increase in the consumption of omega-6 (ω6) polyunsaturated fatty acids (PUFA)2 and a concomitant decrease in the consumption of omega-3 (ω3) PUFA (1, 2). The increased ingestion of ω6 PUFA is due in large part to growth in the production and consumption of vegetable oils, beef, pork, and poultry and has been exacerbated by changes in livestock husbandry and feeding practices (3). This together with a reduced consumption of wild, fatty fish containing high concentrations of ω3 PUFA has resulted in lower ω3 intake and in ω6/ω3 ratios in the United States diet of greater than 10:1. Anthropological evidence suggests that our hunter-gatherer ancestors maintained a ratio closer to 2:1 for ∼100,000 generations (3, 4). This dietary change is postulated to enhance circulating and cellular pro-inflammatory mediators (eicosanoids and cytokines) and reduce anti-inflammatory mediators, resulting in an overall increase in systemic inflammation and a higher incidence of allergic and inflammatory disease including asthma, allergies, diabetes, cardiovascular disease, and arthritis.
However, despite 50 years of research supporting the efficacy of long chain PUFA, such as eicosapentaenoic acid (EPA, 20:5, ω3) and docosahexaenoic acid (DHA, 20:6, ω3) in treating inflammatory diseases, a great deal remains unknown regarding the underlying molecular mechanisms responsible for their potent biological effects. The most studied mechanisms center around the observation that shifts in dietary consumption of ω6 and ω3 PUFA lead to alterations in the quantities of ω6 and ω3-derived eicosanoids produced in animals and humans, thereby disturbing the balance of lipid-based pro- and anti-inflammatory mediators produced at sites of inflammation. For example, the 4-series leukotrienes (LT) (e.g. LTB4) produced by the action of 5-lipoxygenase (5-LO) on the ω6 PUFA arachidonic acid (AA, 20:4) are highly inflammatory, while the 5-series LT (e.g. LTB5) produced from the ω3 PUFA EPA are 10–100-fold less active (5). Also, Serhan et al. (6) have demonstrated that increasing cellular uptake of ω3 fatty acids causes an enhancement in the production of resolvins and protectins, which are proposed to dampen and resolve inflammatory responses.
Alternatively, the presence of high concentrations of ω3 PUFA, or shifts in ω6/ω3 ratios may modulate the expression of genes known to be critical to inflammatory processes. Curtis et al. (7) have shown that α-linolenic acid (LNA, 18:3, ω3), EPA, or DHA reduce the expression of genes for TNFα and IL-1β in bovine chondrocytes. Similarly, mice fed fish oil have decreased mRNA levels for numerous inflammatory cytokines including IL-1β, IL-6, and TNFα in kidney, spleen, and peritoneal macrophages (8, 9). In vitro studies from our laboratory suggest that AA alters cell cycle progression and apoptosis via its capacity to regulate several members of the AP1 family of transcription factors (10).
To date, the majority of studies examining PUFA and gene expression have been carried out in isolated cell systems and a few animal studies. This raises the question of whether the observed alterations in gene expression apply to humans. The current study has utilized a dietary intervention strategy in which healthy humans were fed controlled diets including dietary supplements containing specific dosages of short chain (18 carbon) PUFA and long chain (>20 carbon) PUFA in a manner, which is consistent with our understanding of early human diets. These experiments suggest that altering circulating levels of ω6 and ω3 PUFA likely influences inflammatory responses in part by the capacity of these fatty acids or their metabolites to regulate the expression of early signal transduction genes and to block the expression of pivotal cytokines and chemokines at a transcriptional level.
EXPERIMENTAL PROCEDURES
Materials
Triheptadecanoin internal standard was purchased from Nu-Check Prep (Elsyian, MN). Isolymph was purchased from Gallard-Schlesinger (Plainview, NY). Ionophore A23187 was purchased from Calbiochem. TRIzol reagent was purchased from Invitrogen. All reverse transcription reagents and Sybr Green PCR Master Mix were purchased from Applied Biosystems (Foster City, CA). All forward and reverse primer pairs were purchased from SuperArray (Frederick, MD). Protran nitrocellulose membranes were purchased from Schleicher and Schuell (Keene, NH). Anti-β-actin and anti-Akt antibodies were purchased from Cell Signaling Technology (Danvers, MA) and anti-PI3K p110α antibody was purchased from US Biological (Swampscott, MA). All other chemicals and solvents were purchased from Sigma or Fisher Scientific.
Study Design
Healthy human volunteers (n = 27) were screened for exclusion criteria and enrolled in the study in groups of three to five. The volunteers were provided with a background diet for 5 weeks. After 1 week on the diet they were given dietary supplements containing fish oil (775 mg EPA/day) and borage oil (831 mg GLA/day) for 4 weeks. The study period was followed by a two-week washout period during which the volunteers resumed their normal diets. Fasting blood draws were performed once per week, and urine samples were collected.
Isolation of Mononuclear Cells and Neutrophils from Whole Blood
Approximately 30 ml of whole blood was mixed with 6 ml of Isolymph and allowed to settle at room temperature for 1 h. The plasma layer was spun, and the cell pellet was resuspended in phosphate-buffered saline (PBS) to separate red blood cells, neutrophils, and peripheral blood mononuclear cells (PBMCs). The PBMCs were removed, washed several times in cold PBS to remove all traces of Isolymph, then counted and resuspended in PBS. Contaminating red blood cells were removed from the neutrophil pellet via hypotonic lysis with 0.2% NaCl, and neutrophils were resuspended in Hank's buffer plus Ca2+ and Mg2+ at a concentration of 1 × 107 cells/ml.
Ionophore-stimulated Leukotriene Production
1-ml aliquots of neutrophils were preincubated in a 37 °C water bath, then stimulated for 5 min with 2 μm or 5 μm Ca2+ ionophore A23187 (2.5 mm in DMSO). The stimulation was stopped by adding 1 ml of ice cold Hank's buffer followed by centrifugation at 200 × g at 4 °C for 10 min. The neutrophil pellet was used for fatty acid analysis, and the supernatant was combined with 250 ng of PGB2 internal standard, acidified with two drops of 9% formic acid and extracted twice with ethyl acetate. The extracts were dried under N2 and resuspended in methanol/water (0.45:0.55) for HPLC analysis. Leukotrienes were separated on an Agilent Zorbax ODS analytical column (4.6 × 250 mm) using a Hewlett Packard Series 1050 instrument equipped with a Hewlett Packard 1040M diode array detector and an Agilent 1200 series autosampler. The solvent system was as described previously (11). Data were processed using a Hewlett Packard Compac computer and Agilent Chemstation software. Quantification of the leukotrienes was by reference to the internal standard.
GC Analysis of Serum Fatty Acids
Fatty acid methyl esters (FAME) were prepared following a modification of the protocol by Metcalfe et al. (12). Serum samples (100 μl) were added to tubes containing 25 μg of triheptadecanoin (C17:0) as internal standard, and saponified with EtOH and 50% KOH at 60 °C for 30 min. Neutral lipids were then extracted and discarded. The samples were acidified, and the fatty acids were extracted into hexane. Samples were brought to dryness under nitrogen, incubated for 5 min at 100 °C with 0.5 n NaOH in methanol and FAME were prepared by incubation with 12% boron trifluoride for an additional 5 min at 100 °C. The FAME were extracted into hexane, blown to dryness under nitrogen, and resuspended in a small volume of isooctane for the GC analysis. The FAME were separated using a CP Select CB for FAME capillary GC column (100 m × 0.25 mm ID, film thickness 0.25 μm) and a HP 5890 temperature-programmed GC with H2 as the carrier gas and helium as the make-up gas. The fatty acid distribution was determined via reference to the internal standard.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
RNA was isolated from peripheral blood mononuclear cells using TRIzol reagent following the manufacturer's directions. The RNA concentrations were assessed by measuring the absorbance at 260 nm. Approximately 1 μg of total RNA was reverse-transcribed using 2.5 units/μl MuLV reverse transcriptase in a 20-μl reaction mixture containing 1 mm of each deoxyribonucleotide, 5 mm MgCl2, 1 unit/μl RNase inhibitor, and 2.5 μm random hexamers in PCR Buffer II. The reaction mixture was heated to 42 °C for 15 min, then 99 °C for 5 min, then held at 5 °C for at least 5 min.
Quantitative Real-time Polymerase Chain Reaction
Real-time quantitative PCR of the cDNA template was performed in an ABI Prism 7000 SDS (Applied Biosystems). The PCR reaction contained 150 ng of cDNA, 10 μm forward and reverse primers, and 12.5 μl of 2× RT2 Real-Time Sybr Green PCR Master Mix in a total volume of 25 μl. The PCR cycling conditions were: hold for 10 min at 95 °C; 40 cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. Results were calculated as expression of the target gene relative to expression of the reference gene (GAPDH).
Western Blot
PBMCs were pelleted and lysed by addition of 1 ml of TRIzol reagent per 106 cells. Protein was extracted following the manufacturer's protocol. 10 μg of protein was electrophoresed on 8% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with primary antibodies that recognize Akt, PI3K, and β-actin, followed by infrared fluorophore-conjugated secondary antibodies for detection and quantification using the Odyssey Infared Imaging System (Li-Cor, Lincoln, NE).
Statistical Analysis
All data were analyzed using two-tailed paired Student's t-tests to compare the week 1 value to the week 5 value, or the week 5 value to the week 7 value, with a significance level of 0.05.
RESULTS
Serum Fatty Acid Distribution
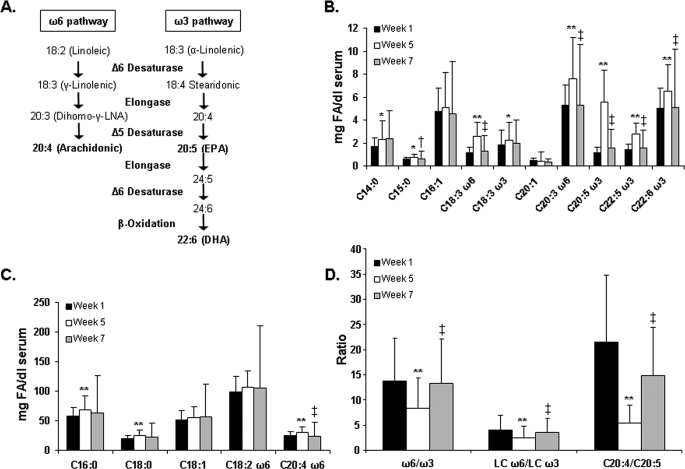
Healthy human volunteers were placed on a background control diet for 5 weeks, followed by a 2-week washout period. This diet contained 27% fat, 18% protein, and 55% carbohydrate and maintained dietary AA, EPA, and DHA levels below 100 mg/day. After 1 week on the diet, study participants were placed on two supplements: borage oil (containing the short [18 carbon], ω6 fatty acid, γ-linolenic acid [GLA], and other 18 carbon ω6 fatty acids) and fish oil (containing the long [≥20 carbon] chain ω3 fatty acids, EPA and DHA). Serum was collected and circulating fatty acids were measured at week 1 and after 4 weeks (week 5) of supplementation with fish oil and borage oil. Fig. 1 shows the change in the levels of key serum fatty acids from week 1 to week 5 and following the 2-week washout (week 7). Fig. 1A shows the biochemical pathway responsible for the conversion of short chain ω6 and ω3 PUFA to long chain PUFA. There was a significant increase in GLA and its elongation metabolite dihomo-γ-linolenic acid (DGLA, 20:3, ω6) after supplementation; both decreased significantly during the washout (Fig. 1B). Similarly, EPA and its elongation metabolites DPA (22:5, ω3) and DHA increased significantly after dietary supplementation with borage and fish oil. All three ω3 PUFA decreased to essentially starting values during the washout. Fig. 1C shows a small but statistically significant increase in AA, possibly due to a portion of GLA in the borage oil supplement being converted to AA via elongation and desaturation by the Δ5 desaturase; however, AA levels also returned to baseline following the washout. The ratios of all ω6 to ω3 PUFA, long chain ω6 to ω3 PUFA, and AA to EPA are shown in Fig. 1D. From week 1 to week 5, the overall ω6 to ω3 PUFA ratio decreased by 40%, from a ratio of ∼15:1 typical of a Western diet to less than 9:1, followed by a return to the starting level after the washout. The ratio of long-chain ω6 to long-chain ω3 PUFA decreased by 39%, while the ratio of AA to EPA decreased by 75%, and both increased from week 5 to week 7.
FIGURE 1.
Total serum fatty acids. Fatty acid methyl esters were derived from serum of study participants at the start of supplementation (week 1), at the end of supplementation (week 5), and after a 2-week washout period (week 7), and total fatty acids were measured by GC (B and C). Ratios of ω6 and ω3 PUFA were calculated based on the total serum values (D). A shows the ω6 and ω3 fatty acid metabolic pathways. Data are expressed as mean ± S.D. *, p < 0.05; **, p < 0.01 week 1 versus week 5; †, p < 0.05; ‡, p < 0.01 week 5 versus week 7, n = 27.
Neutrophil Fatty Acid Distribution and Leukotriene Production
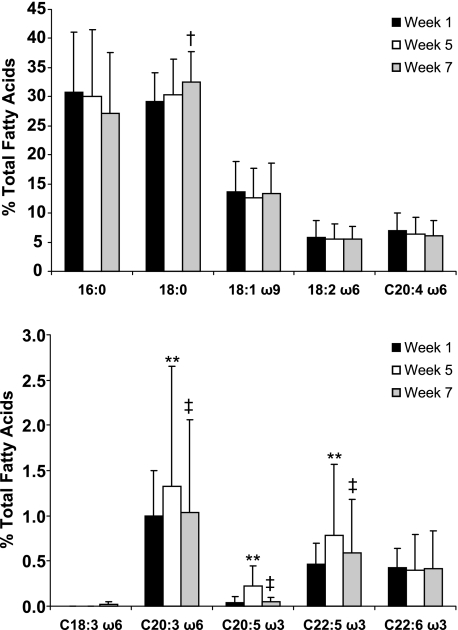
Similar to serum, the fatty acid composition of circulating neutrophils reflected the changes in the diet (Fig. 2). Both EPA and its elongation metabolite DPA increased significantly from week 1 to week 5, followed by a decrease to near baseline values 2 weeks after completing the supplementation (washout). Additionally, there was a statistically significant increase in DGLA, the elongation product of GLA from the borage oil, from week 1 to week 5. Levels of DGLA were returning to baseline values 2 weeks after completion of the supplementation. There was no difference (from baseline) in AA levels within neutrophil glycerolipids after the supplementation.
FIGURE 2.
Fatty acid composition of neutrophil glycerolipids. Fatty acid methyl esters were derived from neutrophils isolated from study participants at the start of supplementation (week 1), at the end of supplementation (week 5), and after a 2-week washout period (week 7) and total fatty acids were measured by GC. Data are expressed as mean ± S.D. *, p < 0.05; **, p < 0.01 week 1 versus week 5; †, p < 0.05; ‡, p < 0.01 week 5 versus week 7, n = 27.
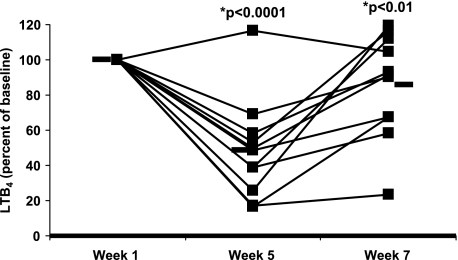
Previous experiments from our laboratory and others have demonstrated that the ex vivo capacity of neutrophils to produce leukotrienes is a reliable pharmacodynamic marker to gauge the effect of specific diets on systemic eicosanoid production (11, 13, 14). Neutrophils were isolated from whole blood and stimulated before supplementation (week 1), after supplementation (week 5), and again after a 2-week washout period (week 7). LTB4, 20-hydroxy and 20-carboxyl LTB4, as well as the two non-enzymatic isomers of LTB4 were measured by reverse phase HPLC from 11 of the initial study subjects (Fig. 3). LTB4 production decreased by 31% after 4 weeks of supplementation with fish oil and borage oil, and ten out of eleven subjects (the responders) responded to the diet with an average decrease of 55%. Two weeks after returning to individual diets, leukotriene levels returned to baseline values, on average. These data indicate that altering the ω6 and ω3 PUFA intake in healthy subjects with fish oil and borage oil supplementation markedly alters the composition of supplementation fatty acids in neutrophil glycerolipids and inhibits the capacity of neutrophils to produce leukotrienes, a putative marker of inflammation.
FIGURE 3.
LTB4 production from stimulated neutrophils. Neutrophils were isolated from whole blood of study participants at the start of supplementation (week 1), at the end of supplementation (week 5), and after a 2 week washout period (week 7). Cells were stimulated ex vivo with A23187 for 5 min and LTB4 production was measured by HPLC. Week 1 data were normalized to 100%. Black bars represent the mean LTB4 production. n = 11.
Expression of Genes That Participate in Eicosanoid Generation
The next series of experiments were designed to determine whether leukotrienes may be inhibited at least in part by the effects of dietary fatty acids on the expression of enzymes that participate in leukotriene generation. Studies to date indicate that it is difficult to monitor global gene expression in terminal-differentiated, circulating neutrophils; therefore, to test this hypothesis, mRNA was isolated from a second type of circulating inflammatory cell, peripheral blood mononuclear cells. White blood cell differentials measured for each subject showed only a small, though significant, increase in the number of lymphocytes and neutrophils after supplementation (Table 1). Initial experiments utilizing an inflammation-specific microarray demonstrated that supplementation caused no effect on message levels of enzymes that participate in leukotriene generation, including cytosolic group IV PLA2, 5-lipoygenase (5-LO), LTA4 hydrolase (also known as LTB4 synthase), and LTC4 synthase (data not shown).
TABLE 1.
White blood cell differential
White blood cell differential was measured in study participants at the start of the study (week 1), after 4 weeks of supplementation (week 5), and after a 2-week washout period (week 7). Values are expressed as mean ± S.E.n = 27.
| Week 1 | Week 5 | Week 7 | |
|---|---|---|---|
| Lymphocytes (per mm3) | 2.06 ± 0.08 | 2.21 ± 0.01a | 2.19 ± 0.12 |
| Monocytes (per mm3) | 0.54 ± 0.03 | 0.52 ± 0.03 | 0.53 ± 0.04 |
| Eosinophils (per mm3) | 0.18 ± 0.04 | 0.2 ± 0.03 | 0.2 ± 0.03 |
| Basophils (per mm3) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| Neutrophils (×108/dL serum) | 3.69 ± 0.39 | 4.43 ± 0.49a | 4.39 ± 0.38 |
ap< 0.05 Week 1 vs. Week 5.
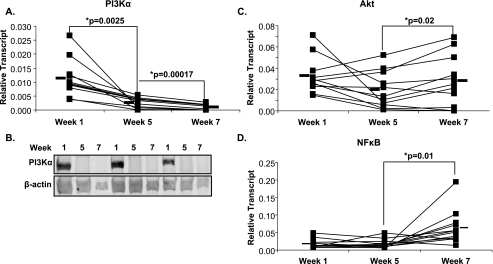
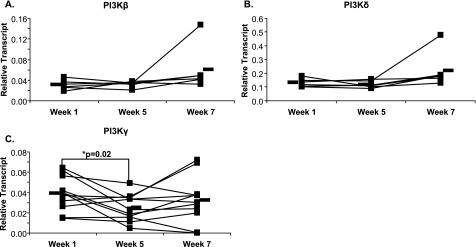
Numerous studies have implicated the PI 3-kinase (PI3K)/Akt pathway as having an important role in initiating early eicosanoid generation in response to several immunologic stimuli. Accordingly, the mRNA expression of the proximal signaling genes PI3Kα, Akt, and NFκB was measured by quantitative real-time RT-PCR (Fig. 4). Fig. 4A shows that supplementation induced a large decrease in the expression of PI3Kα, which was maintained after the washout. Immunoblot analysis of protein from mononuclear cells supported the PCR results, with PI3Kα abundantly present at week 1 and present at much lower amounts at week 5 and week 7 (Fig. 4B). Downstream of PI3Kα, the expression of Akt (Fig. 4C) and NFκB (Fig. 4D) in circulating mononuclear cells was not significantly altered during supplementation, although expression of both increased upon washout. Akt protein remained constant at all time points (data not shown). The aforementioned experiments raised the question of whether the effects of supplementation observed with PI3Kα were observed with other PI3K isoforms. To address this, PI3Kβ, PI3Kδ, and PI3Kγ were measured by real-time PCR. PI3Kγ mRNA was significantly decreased following supplementation while PI3Kβ and PI3Kδ remained unchanged following supplementation and washout (Fig. 5).
FIGURE 4.
Gene expression in mononuclear cells. Mononuclear cells were isolated from whole blood before supplementation (week 1), after supplementation (week 5), and after a 2-week washout (week 7). Gene expression was measured by real-time PCR for (A) PI3Kα, (C) Akt, and (D) NFκB. Black bars represent mean expression. Protein was isolated from mononuclear cells and immunoblot analysis was performed using an antibody to PI3Kα (B). n = 12 for real-time analysis, n = 3 for immunoblot analysis.
FIGURE 5.
Gene expression in mononuclear cells. Mononuclear cells were isolated from whole blood before supplementation (week 1), after supplementation (week 5), and after a 2-week washout (week 7). Gene expression was measured by real-time PCR for PI3Kβ (A), PI3Kδ (B), and PI3Kγ (C). Black bars represent mean expression. n = 6 for A and B, n = 12 for C.
Inflammatory Cytokine Expression
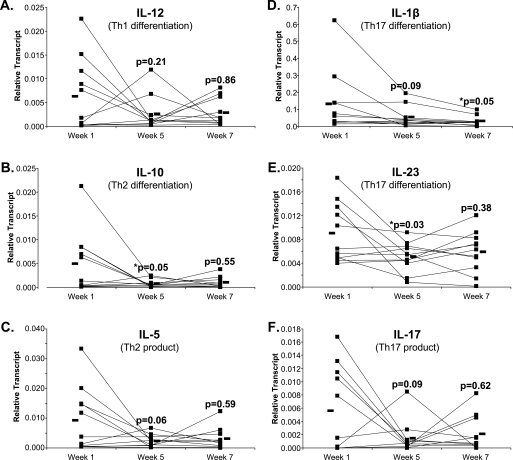
PI3K not only plays an important role in eicosanoid formation, but also in cell growth, survival, and inflammation by utilizing downstream effectors including Akt and NFκB, which in turn influence the production of a variety of signaling molecules such as cytokines. To better understand the potential overall effect of these dietary PUFA, the expression of eight cytokines known to play an important role in the inflammatory response was measured by real-time PCR. The expression of six cytokines involved in the immune response is shown in Fig. 6. A significant decrease in expression was observed for IL-1β, IL-10, and IL-23. Additionally, IL-5 and IL-17 showed strong trends toward decreased expression after supplementation. Changes were not seen for TNFα (p = 0.11) and IL-6 (p = 0.27) (data not shown). Only IL-1β showed a significant continued decrease from week 5 to week 7, while expression of the other cytokines showed trends toward increasing back to the baseline week 1 values during the washout. These washout results were not statistically significant, possibly due to the shorter duration of the washout period (2 weeks) compared with the treatment period (4 weeks), or due to metabolic differences between mononuclear cells and neutrophils.
FIGURE 6.
Expression of inflammatory cytokines in mononuclear cells. Mononuclear cells were isolated from whole blood before supplementation (week 1), after supplementation (week 5), and after a 2-week washout period (week 7) and real-time PCR was used to measure cytokine expression. A, IL-12, a Th1 differentiation cytokine; B, IL-10, a Th2 differentiation cytokine; C, IL-5, a Th2 product; D, IL-1β, a Th17 differentiation cytokine; E, IL-23, a Th17 differentiation cytokine; F, IL-17, a Th17 product. Black bars represent mean expression. The p value above the week 5 values represents the difference between the week 1 values and the week 5 values; the p value above the week 7 values represents the difference between the week 5 values and the week 7 values, n = 11.
DISCUSSION
Research over the past 50 years has shown that ω3 PUFA have potent therapeutic effects in a wide variety of inflammatory diseases, including cardiovascular disease (15), rheumatoid arthritis (16, 17), allergic disorders, and depression (18). It is not clear at this time whether it is the ratio of total ω6 to ω3 PUFAs, the ratio of long chain ω6 to ω3 PUFAs, or merely the presence of high concentrations of ω3 PUFAs (long or short chain) that is most important in determining the clinical effectiveness of these dietary oils. It is also uncertain how the PUFA and their metabolites exert their effects to alter the course of inflammation in such diseases. The aim of this study was to provide both long-chain ω3 fatty acids (EPA and DHA) in fish oil and a short-chain ω6 fatty acid (GLA) in borage oil to identify the potential anti-inflammatory mechanisms by which these fatty acids exert their effects. Two recent studies suggest that EPA/GLA combinations are especially effective in critical care patients suffering from sepsis (19, 20). Specifically, addition of relatively high concentrations of these oils decreased the amount of time patients were on the ventilator, decreased the number of days patients were in the ICU, and increased overall survival.
An important aspect of the current study was that all participants were fed a controlled diet. This diet was provided at a caloric content necessary to maintain the body weight of each study subject while keeping the fat content equal for all individuals. A previous study from our laboratory which measured changes in serum fatty acid content with age determined that a controlled diet which normalizes background ingestion of fatty acids is necessary to observe consistent changes in circulating fatty acids in relatively small numbers of people (21).
Our studies as well as others have demonstrated that ex vivo leukotriene production by neutrophils can serve as an important pharmacodynamic marker to determine the effects of leukotriene blockers in humans (11, 13, 14). Consequently leukotriene production was measured in eleven initial subjects, and we found that the fish oil/borage oil combination was sufficient to decrease leukotriene production. Moreover, this effect was reversed after the 2-week washout period (Fig. 3), similar to the reversal of ω3 and ω6 PUFA levels in serum and PMN (Figs. 1 and 2). We next examined the influence of the supplements on the expression of enzymes directly responsible for leukotriene generation in circulating mononuclear cells, including group IV cPLA2, 5-LO, LTA4 hydrolase, and LTC4 synthase utilizing a combination of microarray technology and real-time PCR. These studies revealed no changes in the expression of any of the examined enzymes associated with leukotriene generation. This result suggests that the observed leukotriene inhibition was a consequence of the well-described effects of this type of supplementation on AA substrate availability and/or the production of alternative fatty acid substrates such as those from DGLA or EPA and not due to expression of key enzymes of leukotriene synthesis, consistent with the 2-week metabolic washout.
Next, we evaluated whether the in vivo changes in eicosanoids could be explained by supplement-induced alterations of key signaling enzymes or transcription factors in the signal transduction pathways that lead to their biosynthesis. Surprisingly, altering PUFA ratios caused a dramatic decrease in PI3K expression and, in particular, the expression of PI3Kα and PI3Kγ. In contrast, there was no change in expression of the other PI3K isoforms, PI3Kβ or PI3Kδ, nor were there changes in message levels of the downstream effectors, Akt and NFκB. Although there was an increase in the number of lymphocytes from week 1 to week 5, this change was very small (<8%), suggesting that the changes we saw in gene expression were not due to a change in the composition of the white blood cells.
PI3K is the initial step in a wide array of signaling pathways, controlling cellular processes from apoptosis to cell growth and differentiation, glucose transport to cell migration, and from eicosanoid and cytokine production to the leukocyte oxidative burst. Four distinct PI3K isoforms appear to be responsible for these activities. PI3Kδ and PI3Kγ are thought to play important roles in the inflammatory response (22–24). The specific functions of PI3Kα and PI3Kβ are just beginning to be understood, with studies to date showing they are involved in control of glucose uptake and metabolism in muscle, liver, and adipose tissue (25).
Only a few studies have examined the regulation of PI3K expression. PUFA such as AA have been demonstrated to affect PI3K activity and phosphorylation. Addition of AA to PC-3 prostate cancer cells has been shown to induce at least ten NFκB-regulated genes, including IL-1β, IL-6, and IL-8. Also, PI3K and Akt, both upstream regulators of the NFκB pathway, have been shown to be activated after AA addition (26). To our knowledge, this is the first paper to demonstrate that altering either concentrations or ratios of circulating ω6 and ω3 PUFA directly affects the expression of specific PI3K isoforms.
To better understand the effect of this combination of long-chain ω3 PUFA and short-chain ω6 PUFA on the immune response, message levels of several T helper 1 (Th1), Th2, and Th17 cytokines were measured from circulating mononuclear cells before and after supplementation. This area of immune regulation was examined because several studies have highlighted the connection between T cell phenotypes and PI3K expression/activation. Class IA PI3K has been shown to control the balance of the Th response by inducing the Th2 response (i.e. humoral immunity) and/or repressing the Th1 response (27, 28). The Th1 response is thought to be supressed because PI3K inhibits IL-12 production from dendritic cells. Agonists that induce the production of IL-12 (e.g. TLR ligands such as LPS) typically activate PI3K, creating a negative feedback loop to encourage the Th2 antibody-mediated response (29, 30). In addition, PI3K negatively regulates TLR signaling at the first encounter with a pathogen, thereby turning off the Th1 response that is initiated by TLRs upon endotoxin binding (31, 32).
The current study revealed that dietary supplementation had no effect on the Th1 cytokine IL-12 (Fig. 6A) but reduced the Th2 cytokines IL-5 and IL-10 (Fig. 6B). It has been shown that allergy is characterized by an imbalance toward the Th2 response (33); therefore, the current results suggest that altering cytokine profiles is a potential mechanism by which this fish oil/borage oil combination may influence allergic responses.
The Th17 axis was also examined in this study. The current literature suggests Th17 immunity plays an important role in autoimmune diseases including irritable bowel disease, multiple sclerosis, and psoriasis and blocking this cytokine network protects against autoimmune disease. Fig. 6, D and E show that supplementation blocks the expression of IL-1β and IL-23, both cytokines shown to enhance Th17 differentiation. Additionally, supplementation inhibits IL-17, a product of Th17 cells (Fig. 6F). These data suggest that in healthy populations, supplementation alters the profile of cytokines known to participate in inflammatory and autoimmune responses. In combination, changes in signaling molecule expression and cytokine production may provide prolonged changes in inflammatory responses relative to the rapidly re-equilibrating levels of PUFA and their metabolites. This suggests that effects of dietary PUFA differentially impact inflammatory leukocyte functions, as LTB4 production in neutrophils was more rapidly reversed than kinase levels and cytokine production from mononuclear cells during the washout.
It has been hypothesized that the marked shift in ω6/ω3 fatty acids in the Western diet over the past three generations may be responsible, in part, for the increase in the risk and incidence of numerous inflammatory diseases including asthma, allergic rhinitis, diabetes, and inflammatory joint diseases. Further it has been suggested that returning PUFA concentrations and ratios in human diets to those that were found during early human development or those found in less developed populations would have anti-inflammatory effects and reduce the incidence of inflammatory diseases in the population. Several mechanisms have been postulated to explain the apparent relationship between altered PUFA ratios and increased inflammation. This report demonstrates, for the first time in humans, that the expression of an early step (PI3K) in signal transduction, as well as several important downstream effectors, are significantly reduced by altering ingestion of PUFA to shift circulating ω6 to ω3 ratios. Whether this effect is due to higher overall levels of long chain ω3 PUFA or short chain ω6 PUFA or the ratio of ω6 to ω3 PUFA is beyond the scope of this in vivo study and is presently being examined in our laboratory. However, these data provide evidence that large changes in gene expression are likely an important mechanism by which PUFA exert their potent effects in clinical conditions.
Acknowledgments
We thank B. Undem for critical editing of the manuscript, M. Wilson for technical support with FAME analysis, and M. Pace for administrative assistance. All authors discussed the results and commented on the manuscript. K. L. W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The GCRC at Wake Forest University Baptist Medical Center provided the diets and nutritional counseling to study participants and is supported by Grant Number M01-RR07122. Potential Conflicts of Interest: F. H. C. has published books Inflammation Nation, Win the War Within, and The Gene Smart Diet and is a founder and a consultant to Pilot Therapeutics Holdings, which may be partially related to his research. His conflict of interest has been disclosed to Wake Forest University Health Sciences and outside sponsors and is institutionally managed.
This work was supported by National Institutes of Health NCCAM Grant 1P50AT0027820.
- PUFA
- polyunsaturated fatty acid
- PBMC
- peripheral blood mononuclear cell
- FAME
- fatty acid methyl ester
- LT
- leukotriene
- EPA
- eicosapentaenoic acid
- DPA
- docosapentaenoic acid
- IL
- interleukin
- TNF
- tumor necrosis factor
- AA
- arachidonic acid
- DGLA
- dihomo-γ-linolenic acid
- PI3K
- phosphatidylinositol 3-kinase.
REFERENCES
- 1.Kris-Etherton P. M., Harris W. S., Appel L. J. ( 2002) Circulation 106, 2747– 2757 [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A. P. ( 2002) J. Am. Coll. Nutr. 21, 495– 505 [DOI] [PubMed] [Google Scholar]
- 3.Cordain L., Watkins B. A., Florant G. L., Kelher M., Rogers L., Li Y. ( 2002) Eur. J. Clin. Nutr. 56, 181– 191 [DOI] [PubMed] [Google Scholar]
- 4.Cordain L. ( 2002) The Paleo Diet, Wiley, Inc., New York, NY [Google Scholar]
- 5.Miller A. M., van Bekkum D. W., Kobb S. M., McCrohan M. B., Knaan-Shanzer S. ( 1993) Prostaglandins Leukot. Essent. Fatty Acids 49, 561– 568 [DOI] [PubMed] [Google Scholar]
- 6.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. ( 2002) J. Exp. Med. 196, 1025– 1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis C. L., Hughes C. E., Flannery C. R., Little C. B., Harwood J. L., Caterson B. ( 2000) J. Biol. Chem. 275, 721– 724 [DOI] [PubMed] [Google Scholar]
- 8.Calder P. C. ( 2001) Lipids 36, 1007– 1024 [DOI] [PubMed] [Google Scholar]
- 9.Calder P. C. ( 2002) Proc. Nutr. Soc. 61, 345– 358 [DOI] [PubMed] [Google Scholar]
- 10.Monjazeb A. M., High K. P., Connoy A., Hart L. S., Koumenis C., Chilton F. H. ( 2006) Carcinogenesis 27, 1950– 1960 [DOI] [PubMed] [Google Scholar]
- 11.Chilton F. H., Patel M., Fonteh A. N., Hubbard W. C., Triggiani M. ( 1993) J. Clin. Investig. 91, 115– 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe L. D., Schmitz A. A., Pelka J. R. ( 1966) Anal. Chem. 38, 514– 515 [Google Scholar]
- 13.Barham J. B., Edens M. B., Fonteh A. N., Johnson M. M., Easter L., Chilton F. H. ( 2000) J. Nutr. 130, 1925– 1931 [DOI] [PubMed] [Google Scholar]
- 14.Johnson M. M., Swan D. D., Surette M. E., Stegner J., Chilton T., Fonteh A. N., Chilton F. H. ( 1997) J. Nutr. 127, 1435– 1444 [DOI] [PubMed] [Google Scholar]
- 15.Christensen J. H., Gustenhoff P., Korup E., Aarøe J., Toft E., Møller J., Rasmussen K., Dyerberg J., Schmidt E. B. ( 1996) BMJ 312, 677– 678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin P. R., Lew R. A., Liang M. H., Wright E. A., Beckett L. A., Chalmers T. C., Sperling R. I. ( 1995) J. Clin. Epidemiol. 48, 1379– 1390 [DOI] [PubMed] [Google Scholar]
- 17.Kremer J. M., Bigauoette J., Michalek A. V., Timchalk M. A., Lininger L., Rynes R. I., Huyck C., Zieminski J., Bartholomew L. E. ( 1985) Lancet 1, 184– 187 [DOI] [PubMed] [Google Scholar]
- 18.Su K. P., Huang S. Y., Chiu C. C., Shen W. W. ( 2003) Eur. Neuropsychopharmacol. 13, 267– 271 [DOI] [PubMed] [Google Scholar]
- 19.Pontes-Arruda A., Aragão A. M., Albuquerque J. D. ( 2006) Crit. Care Med. 34, 2325– 2333 [DOI] [PubMed] [Google Scholar]
- 20.Singer P., Theilla M., Fisher H., Gibstein L., Grozovski E., Cohen J. ( 2006) Crit. Care Med. 34, 1033– 1038 [DOI] [PubMed] [Google Scholar]
- 21.High K. P., Sinclair J., Easter L. H., Case D., Chilton F. H. ( 2003) J. Nutr. Health Aging 7, 378– 384 [PubMed] [Google Scholar]
- 22.Hirsch E., Katanaev V. L., Garlanda C., Azzolino O., Pirola L., Silengo L., Sozzani S., Mantovani A., Altruda F., Wymann M. P. ( 2000) Science 287, 1049– 1053 [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Jiang H., Xie W., Zhang Z., Smrcka A. V., Wu D. ( 2000) Science 287, 1046– 1049 [DOI] [PubMed] [Google Scholar]
- 24.Sasaki T., Irie-Sasaki J., Jones R. G., Oliveira-dos-Santos A. J., Stanford W. L., Bolon B., Wakeham A., Itie A., Bouchard D., Kozieradzki I., Joza N., Mak T. W., Ohashi P. S., Suzuki A., Penninger J. M. ( 2000) Science 287, 1040– 1046 [DOI] [PubMed] [Google Scholar]
- 25.Vivanco I., Sawyers C. L. ( 2002) Nat. Rev. Cancer 2, 489– 501 [DOI] [PubMed] [Google Scholar]
- 26.Hughes-Fulford M., Li C. F., Boonyaratanakornkit J., Sayyah S. ( 2006) Cancer Res. 66, 1427– 1433 [DOI] [PubMed] [Google Scholar]
- 27.Fukao T., Tanabe M., Terauchi Y., Ota T., Matsuda S., Asano T., Kadowaki T., Takeuchi T., Koyasu S. ( 2002) Nat. Immunol. 3, 875– 881 [DOI] [PubMed] [Google Scholar]
- 28.Fukao T., Yamada T., Tanabe M., Terauchi Y., Ota T., Takayama T., Asano T., Takeuchi T., Kadowaki T., Hata, Ji J., Koyasu S. ( 2002) Nat. Immunol. 3, 295– 304 [DOI] [PubMed] [Google Scholar]
- 29.Guha M., Mackman N. ( 2002) J. Biol. Chem. 277, 32124– 32132 [DOI] [PubMed] [Google Scholar]
- 30.Herrera-Velit P., Knutson K. L., Reiner N. E. ( 1997) J. Biol. Chem. 272, 16445– 16452 [DOI] [PubMed] [Google Scholar]
- 31.Moser M., Murphy K. M. ( 2000) Nat. Immunol. 1, 199– 205 [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri G. ( 1995) Annu. Rev. Immunol. 13, 251– 276 [DOI] [PubMed] [Google Scholar]
- 33.Bach J. F. ( 2002) N. Engl. J. Med. 347, 911– 920 [DOI] [PubMed] [Google Scholar]