Abstract
Although Kir2.1 channels are important in the heart and other excitable cells, there are virtually no specific drugs for this K+ channel. In search of Kir2.1 modulators, we screened a library of 720 naturally occurring compounds using a yeast strain in which mammalian Kir2.1 enables growth at low [K+]. One of the identified compounds, gambogic acid (GA), potently (EC50 ≤ 100 nm) inhibited Kir2.1 channels in mammalian cells when applied chronically for 3 h. This potent and slow inhibition was not seen with Kv2.1, HERG or Kir1.1 channels. However, acutely applied GA acted as a weak (EC50 = ∼10 μm) non-selective K+ channel blocker. Intracellular delivery of GA via a patch pipette did not potentiate the acute effect of GA on Kir2.1, showing that slow uptake is not responsible for the delayed, potent effect. Immunoblots showed that total Kir2.1 protein expression was not altered by GA. Similarly, immunostaining of intact cells expressing Kir2.1 with an extracellular epitope tag demonstrated that GA does not affect Kir2.1 surface expression. However, the 3-h treatment with GA caused redistribution of Kir2.1 and Kv2.1 from the Triton X-100-insoluble to the Triton X-100-soluble membrane fraction. Thus, GA changes the K+ channel membrane microenvironment resulting in potent, specific, and slow acting inhibition of Kir2.1 channels.
K+ channels of the inwardly rectifying family (Kir)4 play key roles in the electric activity of many cell types. Kir2.1 channels are particularly important in the heart where they set the resting potential and contribute to the terminal phase of action potential repolarization. Mutations in the Kir2.1 gene cause Andersen syndrome, a triad of periodic paralysis, arrhythmia, and dysmorphic features (1), as well as short QT syndrome (2). Kir channel activity is regulated by endogenous magnesium and polyamines (3–5), as well as by membrane lipids including phosphoinositides (6, 7), fatty acid acyl-CoA esters (8), and cholesterol (9). Kir channels are blocked nonspecifically by extracellular cations such as cesium and barium (10), but there are no known Kir2.1-specific pharmacologic modulators that could be used in physiological studies or as drugs.
High throughput screening (HTS) methods have been used for identifying novel K+ channel modulators in organic compound libraries (11–14). This approach has led to identification of inhibitors that target the K+ channel pore directly (11–13). Usually, such inhibitors act rapidly and reversibly. However, a few inhibitors inhibit Kir2.1 current with chronic application for hours. Thus far, such inhibitors have been found to interfere with Kir2.1 channel trafficking to the cell surface (14).
Here an assay utilizing Kir2.1-dependent growth of yeast is used to identify gambogic acid (GA) as a Kir2.1 inhibitor. Functional studies in mammalian cells showed that GA acts acutely in the micromolar range as a nonselective K+ channel blocker. However, GA also acts slowly at nanomolar concentrations to abolish Kir2.1, but not Kv2.1, HERG, or Kir1.1 channel activity. The time course of this specific high affinity action does not reflect limited penetration into the cell or a change in Kir2.1 surface expression. Instead, GA causes marked partitioning of Kir2.1 into the Triton X-100-soluble membrane fraction, consistent with a redistribution of Kir2.1 into plasma membrane microdomains whereby its activity is silenced. Thus, GA has revealed a novel regulatory mechanism that specifically abolishes the activity of Kir2.1 inwardly rectifying channels.
EXPERIMENTAL PROCEDURES
Screening for K+ Channel Drugs in Yeast
Yeast strains and culturing conditions are described in Ref. 11. HTS was performed in 96-well plates. The MicroSource Natural Products Library (Discovery Systems, Inc., Gaylordsville, CT) consists of 720 biologically active molecules extracted from microorganisms, plants, and animals. Compounds in master plates were diluted and transferred to test plates using the Biomek 2000 robot (Beckman Coulter, Fullerton, CA). 50-μl aliquots of logarithmic yeast culture with A560 = 0.05 were seeded in wells using a multichannel pipettor, and plates were incubated at 30 °C in a humidified incubator for 48 h. Spaces between wells were filled with water to prevent evaporation. The A560 of yeast cultures was measured at 10 wells/s using Victor plate reader (PerkinElmer Life Sciences). Plates were vortexed immediately before measurements to disperse cells.
86Rb+ Flux Assay
HEK293 cells stably expressing Kir2.1, Kir1.1, or HERG channels (gifts from Dr. Min Li, the Johns Hopkins University, Dr. Jerod Denton, Vanderbilt University, and Dr. Eckhard Ficker, Case Western Reserve University) were grown in polylysine-coated plates to 80–90% confluence. Cells were loaded with 86Rb+ for 4 h by incubation with 1 μCi/ml 86RbCl (PerkinElmer Life Sciences). Prior to 86Rb+ flux measurements cells were washed two times with 86Rb+-free medium. Medium aliquots were collected at 1–40-min time points, and 86Rb+ release was measured by scintillation counter (Beckman Coulter, Fullerton, CA). At 40 min, cells were lysed with 1% SDS and residual radioactivity in cells was determined. GA (Calbiochem, La Jolla, CA) was added after 86Rb+ loading for 1–3 h, or during last wash for chronic and acute application, respectively, and was present during efflux measurement. Kir1.1 expression was induced by adding 1 μg/ml tetracycline 12 h prior to GA treatment. Rb+ efflux via HERG channels was induced by adding 50 mm KCl to the wash solution, and medium aliquots were collected at 1, 3, and 5 min after washing.
Measurement of Kir2.1 Surface Expression in Live Cells
HEK293 cells stably expressing Kir2.1 channel tagged with an extracellular HA epitope were treated with 1 μm GA or vehicle (0.01% DMSO) for 3 h. Cells were washed with Hank's balanced saline solution (HBSS, Invitrogen, Carlsbad, CA) and harvested by incubation with 0.5 mm EDTA in HBSS for 5–10 min at room temperature. Cells were incubated with rat anti-HA monoclonal Ab 3F10 (Roche Applied Science, Indianapolis, IN) diluted 1:500 on ice for 1 h. After washing twice with HBSS cells were incubated with Cy2-conjugated goat antirat Ab diluted 1:1000 (Jackson ImmunoResearch, West Grove, PA) for 15 min on ice. After 2 washes with HBSS, fluorescence was measured by flow cytometry. The percentage of fluorescent cells was analyzed using the CELLQUEST software (Becton Dickinson). Adherent cells growing on polylysine-coated cover slips were blocked with 10% normal goat serum in phosphate-buffered saline (NGS), incubated with 3F10 Ab (1:500 in 10% NGS) followed by Cy2-conjugated secondary Ab (1:1000 in 2% NGS) for 30 min each at room temperature. Images were taken at ×400 magnification using a IX70 inverted Olympus microscope.
Preparation of Triton X-100-soluble and -insoluble Membrane Fractions
Triton X-100 (TX100) solubility assay was adapted from Ref. 9. Total membranes were isolated from HEK293 cells expressing Kir2.1-HA and Kv2.1-Myc channels harvested from 3 Petri dishes (9 cm). Cells were treated with GA (1 μm) or vehicle for 3 h. Cells were suspended in buffer A (150 mm NaCl, 20 mm HEPES, 5 mm EDTA, pH7.4, protease inhibitors) and disrupted in a Dounce homogenizer on ice. After clearing by centrifugation at 1000 rpm for 10 min, membranes were pelleted by centrifugation at 75,000 × g for 1 h (TLA120.2 rotor, Beckman Coulter). Membranes were resuspended in buffer A supplemented with 1% TX100 for 15 min and collected by recentrifugation at 75,000 × g. After removing the supernatant (TX100-soluble fraction), the pellet (TX100-insoluble fraction) was resuspended in equal volume of buffer A with TX100. Equal volumes of each fraction were used for immunoblot analysis.
Electrophysiology
K+ currents were recorded in a whole cell configuration from HEK293 cells expressing Kir2.1 channel or CHO cells expressing Kv2.1 channel. Cells were treated with GA 1–3 h prior to experiment (chronic application); alternatively, GA was added to bath or pipette solution (acute application). The pipette solution for recording Kir2.1 current had the following composition (in mm): 140 KCl, 10 K-HEPES, 2 MgCl2, and 1 K-EGTA (pH 7.2). For recording of Kv2.1 current, this solution was supplemented with 1 mm ATP. Bath solution contained in (mm): 140 NaCl, 5.4 KCl, 10 HEPES, 10 glucose, 2 CaCl2, 0.8 MgCl2, pH 7.4.
RESULTS
Identification of GA as Kir2.1 Inhibitor by Screening in Yeast
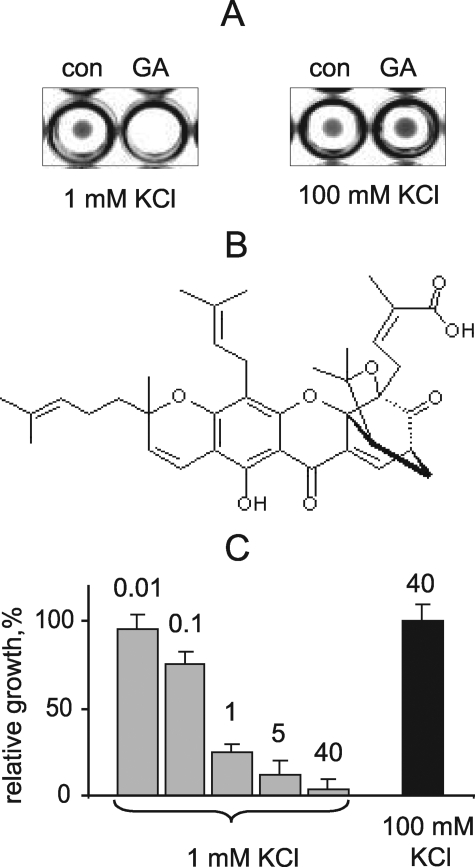
A library of 720 biologically active compounds extracted from microorganisms, plants, and animals was screened with our previously described procedure, which is based on functional complementation of the Δtrk1 trk2 K+ uptake defect in yeast by the mammalian Kir2.1 channel (11). Specifically, growth of yeast expressing the Kir2.1 channel in low [K+] (<2 mm) media depends on Kir2.1 activity and so can be used as a reporter of Kir2.1 pharmacological modulation. Test compounds were added to yeast cultured in 96-well plates in low [K+] medium to the final concentration of 40 μm. Fig. 1A shows that yeast failed to grow in the test well containing GA, a component of Garcinia sp. resin (Fig. 1B). In contrast, at high [K+], which supports yeast growth by nonspecific K+ uptake, GA did not affect culture growth (Fig. 1A). Thus, GA specifically inhibits K+ uptake via Kir2.1 channels. Fig. 1C shows that GA inhibited the Kir2.1-mediated yeast growth in a dose-dependent fashion with an EC50 below 1 μm. Although growth was completely abolished with 40 μm GA, this was not toxic because yeast growth in 100 mm KCl was not affected (Fig. 1C). Thus, a prolonged yeast growth assay shows that GA is a potent inhibitor of Kir2.1 channel function.
FIGURE 1.
Identification of GA as a Kir2.1 channel inhibitor in yeast. A, HTS of the Natural Products library. Kir2.1-expressing yeast was grown in 96-well plates in the presence of test compounds at 40 μm or vehicle for 24 h. GA inhibits yeast growth in media with 1 mm KCl, but not 100 mm KCl. B, structural formula of GA. C, concentration-dependence of GA inhibition of yeast growth. Yeast was grown in 96-well plates with GA at indicated concentrations (μm) or vehicle in media with 1 mm and 100 mm KCl. Bars represent average A600 of cultures in 10 independent wells ± S.E. normalized to control.
GA Is a Potent Inhibitor of Kir2.1 Channels in Mammalian Cells
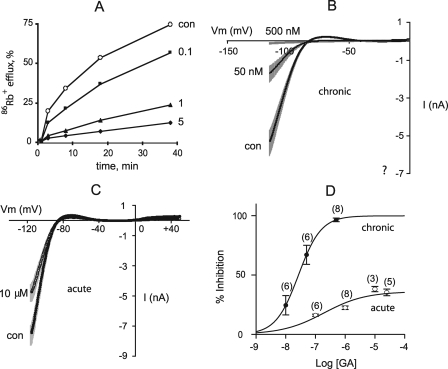
To test whether GA is a Kir2.1 inhibitor in mammalian cells, we measured Kir2.1-mediated flux in a HEK293 cell line stably expressing Kir2.1, using 86Rb+ as a tracer. Because GA was originally identified in yeast as a chronically acting inhibitor, HEK293 cells were exposed to GA for 3 h prior to ion flux measurements. Fig. 2A shows that GA inhibits 86Rb+ efflux via Kir2.1 in a concentration-dependent manner with apparent EC50 in the submicromolar range, as was detected for Kir2.1-mediated yeast growth inhibition.
FIGURE 2.
GA inhibition of Kir2.1 current in HEK293 cells. A, time course of 86Rb+ efflux from cells treated with GA at indicated concentrations (μm) for 3 h. B, Kir2.1 currents evoked by voltage-clamp ramps from −120 mV to 0 mV from holding potential of −40 mV in nontreated cells and in cells treated with 50 nm or 500 nm GA for 3 h. Data represent mean ± S.E., n = 6–10. C, Kir2.1 currents evoked by voltage clamp ramps from −120 mV to 0 mV from holding potential of −40 mV before and after acute application of 10 μm GA. Data represent mean ± S.E., n = 3. D, dose-response relationship for acute and chronic GA inhibition. Inward current was measured at −117 mV. Data points represent mean ± S.E. The number of cells for each data point is indicated in parentheses.
Next, we characterized GA inhibition using the patch-clamp technique. Kir2.1 currents were recorded in whole cell configuration from HEK293 cells stably expressing Kir2.1 channel and treated with GA for 3 h (chronic application). Under these conditions, GA acted in a dose-dependent manner with an EC50 of 27 nm to produce near complete inhibition of Kir2.1 currents at 0.5 μm (Fig. 2, B and D). Thus, GA is a very potent Kir2.1 channel inhibitor in both yeast and mammalian cells.
Potent Inhibition of Kir2.1 by GA Is Delayed
Having established that prolonged exposure of cells to GA inhibits Kir2.1 channels, we set out to determine whether GA is effective with acute application. When GA was added to the bath solution acutely at the time of measurement, inhibition dramatically decreased: 10 μm GA only inhibited Kir2.1 current by 30% of control (Fig. 2, C and D). Thus, GA is dramatically more potent when applied chronically.
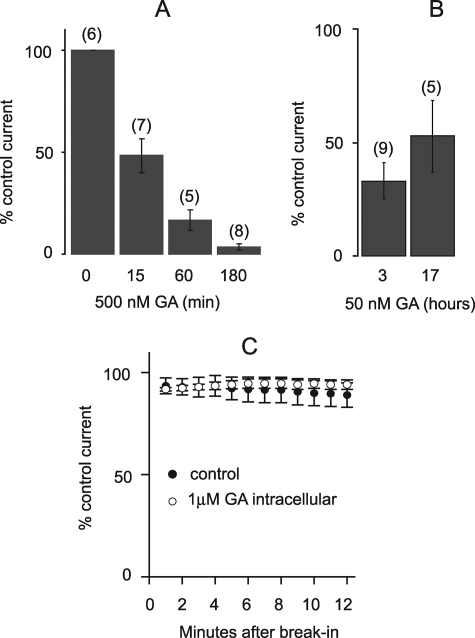
The difference between chronic and acute effects of GA prompted us to examine the time course of the long-term effect. Cells were pretreated with GA at maximum blocking concentration (0.5 μm) for 0–180 min, and Kir2.1 currents were measured by whole cell patch clamp. The effect 0.5 μm GA was time-dependent: 50% inhibition occurred at 15 min, whereas complete inhibition required 3 h (Fig. 3A). Inhibition by a low concentration of GA (50 nm) did not significantly increase upon more prolonged (17 h versus 3 h) incubation (Fig. 3B), indicating that inhibition plateaus at 3 h. To test whether the delayed effect of bath applied GA is caused by slow uptake, GA was added to the pipette solution and Kir2.1 currents were recorded in dialyzed cells at various times following break-in to the whole cell recording configuration. The intracellular delivery of GA at the concentration sufficient for complete inhibition in the chronic application (1 μm) did not cause noticeable Kir2.1 current reduction within 12 min (Fig. 3C). Therefore, even when uptake is not limiting, the potent effect of GA on the Kir2.1 channel is delayed.
FIGURE 3.
Time dependence of GA inhibition of Kir2.1 current. A, maximal inward current at −117 mV from holding potential of −40 mV after exposure to 0.5 μm GA for 0–180 min, normalized to control. Data points represent mean ± S.E., n is indicated in parentheses. B, residual Kir2.1 current after 3 and 17 h of exposure to 50 nm GA normalized to control. Data represent mean ± S.E., n indicated in parentheses. C, amplitude of the inward current measured at −117 mV as a function of time after break-in for cells dialyzed with 1 μm GA, n = 3–5.
Specificity of GA Inhibition
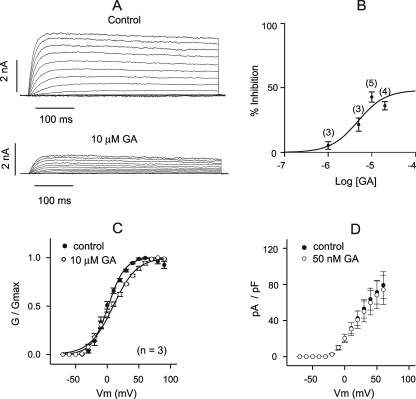
To explore the specificity of GA, we tested other K+ channels. First, voltage-activated currents were recorded in the whole cell configuration from CHO cells stably expressing Kv2.1 channel. At the highest concentration used (10 μm), GA caused 70% inhibition of Kv2.1 currents when added acutely to the bath (Fig. 4A). The effect was dose-dependent, with apparent EC50 of more than 20 μm (Fig. 4B). Moreover, under these conditions, GA slightly altered the voltage-dependence of Kv2.1 activation (Fig. 4C). However, in contrast to Kir2.1, Kv2.1 current density was not noticeably affected by chronic (3 h) application of 50 nm GA (Fig. 4D). Thus, chronic application GA was ineffective against Kv2.1.
FIGURE 4.
GA inhibition of Kv2.1 current. A, representative currents evoked by depolarization steps from holding potential of −70 mV in 10-mV increments in CHO cells transiently expressing Kv2.1 channel before and after acute application of 10 μm GA. B, dose-response relationship for acute GA inhibition. Kv2.1 currents were measured at +50 mV, n for each data point is indicated in parentheses. C, conductance-voltage relationships of Kv2.1 currents before and during acute application of 10 μm GA. Data represent mean ± S.E., n = 5. D, density of Kv2.1 currents versus membrane potential in control cells (n = 4) and cells treated with 0.5 μm GA for 3 h (n = 5).
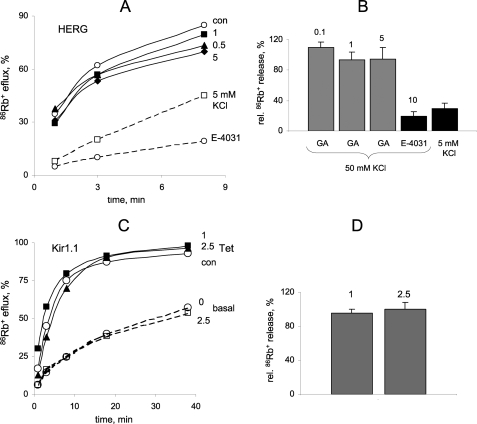
Likewise, Rb+ flux assays showed that GA does not inhibit HERG channel activity in a stable HEK293 cell line evoked by depolarization with 50 mm KCl (Fig. 5A, compare con and 5 mm KCl). HERG channels were largely insensitive to 2 h of exposure of cells to 1–5 μm GA, whereas the specific HERG inhibitor E-4031 caused strong inhibition of 86Rb+ efflux (Fig. 5, A and B). Similarly, Kir1.1 channel activity in a stable HEK293 cell line was completely insensitive to 2.5 μm GA after 2 h of exposure (Fig. 5, C and D). Collectively, these data show that GA is selective for Kir2.1 channel over Kir1.1, Kv2.1, and HERG channels.
FIGURE 5.
GA does not affect HERG and Kir1.1 channels. A, time course of 86Rb+ efflux from HEK293 cells stably expressing HERG channel. Cells were incubated with GA at indicated concentrations (μm) for 2 h. 86Rb+ efflux was induced by 50 mm KCl and repressed by 10 μm E-4031. Baseline efflux was measured at external KCl of 5 mm. B, relative amount of released 86Rb+ in 3 min via HERG channel. Control cells were not treated with GA. Numbers indicate GA and E-4031 concentrations in μm. Bars represent mean ± S.E., n = 3–5. C, time course of 86Rb+ efflux from HEK293 cells with Tet-inducible Kir1.1 channels. Cells were incubated with GA at indicated concentrations (μm) for 2 h. D, relative amount of released 86Rb+ in 3 min via Kir1.1 channel. Numbers indicate GA concentrations in μm. Bars represent mean ± S.E., n = 3–5.
Mechanism of GA Inhibition
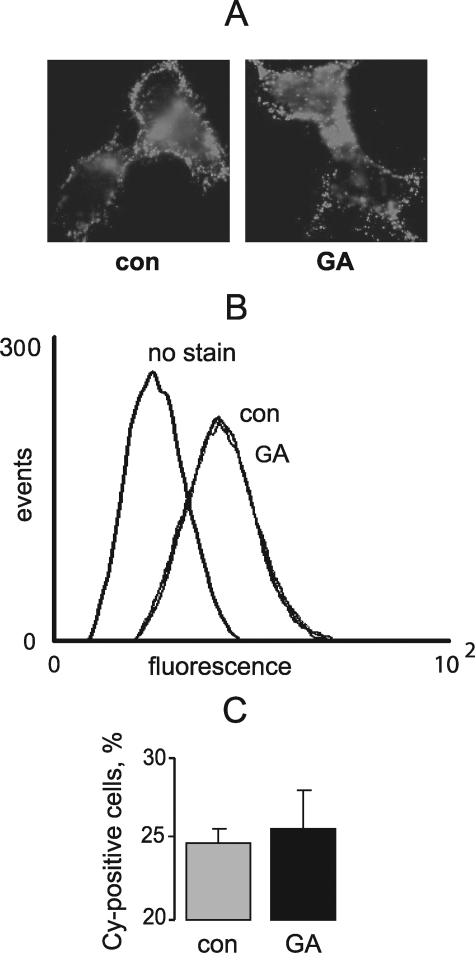
To test whether long-term inhibition of Kir2.1 by GA is due to impaired channel trafficking, we used a stable HEK293 cell line expressing Kir2.1 channels with an HA epitope inserted into the extracellular loop. Specifically, cells were chronically treated with GA and examined for surface expression of Kir2.1 by immunofluorescence. A 3-h exposure to 1 μm GA did not noticeably change surface expression of Kir2.1 measured by epifluorescence microscopy (Fig. 6A) and flow cytometry (Fig. 6, B and C). Thus, inhibition of Kir2.1 by GA is not associated with impaired trafficking of the channel to the cell surface.
FIGURE 6.
GA does not affect Kir2.1 channel surface expression in HEK cells. A, live cells stably expressing Kir2.1 channel with extracellular HA epitope were stained with anti-HA Cy-2 conjugated antibody following treatment with 1 μm GA or vehicle for 3 h. B, flow cytometry analysis of HEK cells treated with GA and stained as in A. C, quantification of B, n = 4.
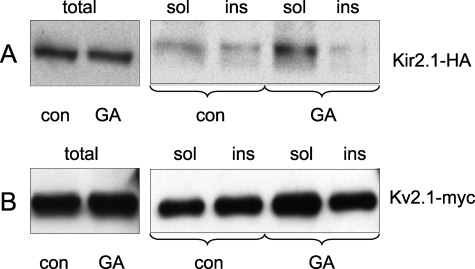
We next hypothesized that GA inhibits Kir2.1 current by changing the plasma membrane microenvironment of the channels. To test this hypothesis, we examined the effect of GA on extraction of Kir2.1 and Kv2.1 with TX100, an assay routinely used for characterization of K+ channel localization to membrane microdomains. HEK293 cells expressing Kir2.1-HA or Kv2.1-Myc channel were incubated with 1 μm GA for 3 h. Following mechanical cell disruption, membranes were isolated and then incubated with 1% TX100 for 15 min on ice. TX100-soluble and TX100-insoluble membrane fractions were separated by centrifugation and distribution of K+ channel protein in the fractions was determined by immunoblotting. Fig. 7A shows that GA shifts Kir2.1 partitioning toward the TX100-soluble fraction. Quantitatively, the ratio of TX100-soluble to TX100-insoluble Kir2.1 in control cells is 2:1 whereas in GA-treated cells this ratio is 12:1. Similarly, GA shifted Kv2.1 partitioning to TX100-soluble fraction (Fig. 7B). Thus, GA may have a global effect on plasma membrane microdomains that results in specific inhibition of Kir2.1 channels.
FIGURE 7.
GA changes Kir2.1 and Kv2.1 solubility in Triton X-100. A and B, HEK cells stably expressing Kir2.1-HA and HEK cells transiently expressing Kv2.1-Myc were incubated with 1 μm GA for 3 h. Total membrane fractions from GA-exposed and control cells were solubilized in cold 1% TX100 for 15 min and separated by centrifugation. Channel protein in total, TX100-soluble and TX-insoluble membrane fractions were analyzed by anti-HA and anti-Myc immunoblotting.
DISCUSSION
Yeast-based Identification of GA as a K+ Channel Inhibitor
The purpose of this study was to find specific inhibitors of the inwardly rectifying K+ channel Kir2.1. To enable HTS for K+ channel drugs, we developed an assay (11) that utilizes Kir2.1-dependent yeast growth (15) as a reporter of K+ current pharmacological modulation. Earlier, using this assay we screened 15,000 small organic molecules from unbiased libraries and identified several K+ channel modulators that act at micromolar concentrations (11). Now, in search of more potent Kir2.1 modulators, we screened a library of 720 compounds extracted from plants, animals, and microorganisms. GA was identified, together with other 36 hits (or over 5% of the library), as a Kir2.1 inhibitor in the primary screen. Sequential rounds of primary hit testing at decreasing concentrations identified GA as the only inhibitor active at submicromolar concentrations. Previously, we validated this assay by finding a rapidly acting blocker of the K+ channel pore (11). The discovery of GA shows that the assay is capable of identifying chronic modulators as well.
Potency and Selectivity of Chronic Inhibition of Kir2.1 Current by GA
K+ current measurements in mammalian cells confirmed that GA specifically inhibits Kir2.1 current. Whole cell recordings demonstrated that chronic inhibition of Kir2.1 current by GA occurs with an EC50 of 27 nm (Fig. 2). Currently, no known drug acts on Kir2.1 channel at nanomolar concentrations. Thus, GA is a relatively potent Kir2.1 inhibitor.
To investigate GA selectivity, we examined its effects on three other K+ channels. Patch clamp data indicated that chronic GA application at nanomolar concentrations does not inhibit the Kv2.1 current (Fig. 4). Moreover, Rb+ flux measurements showed that 2 h of exposure to micromolar concentrations of GA has no effect on K+ flux through the HERG and Kir1.1 channels (Fig. 5). The HERG channel, which produces the cardiac IKr current, is known for its promiscuous sensitivity to drugs. The fact that HERG is insensitive to GA suggests that this drug can be used for pharmacologic separation of IK1 and IKr currents. Furthermore, these results show that GA sensitivity varies among members of the Kir family.
Mechanisms of K+ Channel Inhibition by GA
Our results indicate two distinct mechanisms of K+ channel inhibition by GA: a slow and potent Kir2.1-specific mechanism and a fast, low affinity nonspecific effect. The latter effect suggests a shared target on the conserved pore domain. Because GA is an anionic compound, it might not be considered a traditional pore blocker. However, another compound identified by the yeast screen acts at the extracellular tetraethylammonium site despite being uncharged (11). Thus, an effect on conduction cannot be excluded. Alternatively, the low affinity nonselective effect of GA might be caused by allosteric channel closure.
The high affinity, specific inhibition of Kir2.1 develops slowly. Adding GA to pipette solution, as opposed to bath solution, does not accelerate inhibition of Kir2.1 (Fig. 3). Therefore, the delayed effect of GA is not due to slow entry of the drug into cells. Long-term inhibition of K+ currents may occur at various levels of K+ channel biogenesis including transcription, translation, subunit assembly, trafficking, and recycling. However, immunoblotting showed that GA does not affect synthesis of Kir2.1 protein (Fig. 7). Similarly, GA does not affect surface expression of this channel (Fig. 6). Thus, GA-induced inhibition is not due to changes in biogenesis or trafficking to the plasma membrane.
Recent observations suggest that, in addition to gating and surface expression of K+ channels, K+ conductance can be regulated by localization of K+ channels to specific microdomains of the plasma membrane. Specifically, several K+ channels dynamically associate with membrane microdomains enriched in cholesterol, sphingolipids, and glycosylphosphatidyl inositol (GPI)-anchored proteins, known as lipid rafts (16). Proteins associated with lipid rafts tend to co-fractionate with membranes resistant to solubilization with TX100 (17). Therefore, solubilization with TX100 was used to assess the effect of GA on association of Kir2.1 with distinct membrane microdomains. We found that pretreatment of HEK293 cells with GA shifts Kir2.1 and Kv2.1 channels from the TX100-insoluble to the TX100-soluble fraction (Fig. 7), suggesting a nonspecific redistribution between distinct microdomains of the plasma membrane. Moreover, because GA also decreases Kir2.1 activity, this result is consistent with Kir2.1 being physiologically active in the context of TX100-insoluble microdomains, but not of TX100-soluble microdomains. The GA physico-chemical properties are compatible with this possibility: GA is a lipophilic molecule (LogP = 6.77 by Ghose and Crippen, Ref. 18) that can potentially insert into the lipid bilayer and thereby might perturb association of Kir2.1 with regulatory lipids and proteins that specifically facilitate the function of Kir2.1 channels. Such facilitation might be due to direct binding or by stimulating a modification of the channel.
Depletion of membrane cholesterol by methyl-cyclodextrin and partitioning of K+ channels into TX100-soluble microdomains are associated with decreases in Kv1.5 (19), Kv2.1 (20), BK (21), and Kir4 conductances (22). In contrast, the same treatment correlates with increased Kir2.1 conductance in transfected CHO cells and vascular endothelial cells (9). The opposite relationship between Kir2.1 conductance and partitioning into membrane microdomains found with GA could reflect different mechanisms of action for GA and methyl cyclodextrin. Likewise, GA has been shown to be a novel chronic Kir2.1 inhibitor whose mechanism of action differs from other slow acting compounds that arrest Kir2.1 trafficking, including celastrol (14), helenine, and pomiferin.5
Caveats about GA As an Anticancer Drug
GA attracted attention as a potential anticancer drug after it was identified as a potent inducer of apoptosis in cell- and enzyme-based high-throughput screening programs (23). Selective induction of apoptosis by GA involves binding of the drug to transferrin receptors that are overexpressed in cancer cells (24). GA induces apoptosis in model cancer cells (NL60 lymphocytes) with LD50 = 115 nm. Our data show that at this concentration GA may also block inwardly rectifying currents and thus adversely affect the function of excitable tissues. Therefore, use of this drug as an anticancer drug may depend on delivery methods that minimize GA acid inhibition of nervous system and cardiovascular Kir2.1 channels.
Acknowledgments
We thank Drs. Min Li, Jerod Denton, and Eckhard Ficker for providing channel expressing stable cell lines.
This work was supported, in whole or in part, by National Institutes of Health Grants NSO48089-01 (to E. Z. M.) and HL80632 (to E. S. L.).
E. S. Levitan, manuscript in preparation.
- Kir
- K+ channels of the inwardly rectifying family
- GA
- gambogic acid
- HTS
- high throughput screening
- TX100
- Triton X-100
- HA
- hemagglutinin.
REFERENCES
- 1.Plaster N. M., Tawil R., Tristani-Firouzi M., Canún S., Bendahhou S., Tsunoda A., Donaldson M. R., Iannaccone S. T., Brunt E., Barohn R., Clark J., Deymeer F., George A. L., Jr., Fish F. A., Hahn A., Nitu A., Ozdemir C., Serdaroglu P., Subramony S. H., Wolfe G., Fu Y. H., Ptácek L. J. ( 2001) Cell 105, 511– 519 [DOI] [PubMed] [Google Scholar]
- 2.Priori S. G., Pandit S. V., Rivolta I., Berenfeld O., Ronchetti E., Dhamoon A., Napolitano C., Anumonwo J., di Barletta M. R., Gudapakkam S., Bosi G., Stramba-Badiale M., Jalife J. ( 2005) Circ. Res. 96, 800– 807 [DOI] [PubMed] [Google Scholar]
- 3.Matsuda H., Saigusa A., Irisawa H. ( 1987) Nature 325, 156– 159 [DOI] [PubMed] [Google Scholar]
- 4.Lopatin A. N., Makhina E. N., Nichols C. G. ( 1994) Nature 372, 366– 369 [DOI] [PubMed] [Google Scholar]
- 5.Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. ( 1995) Cell 80, 149– 154 [DOI] [PubMed] [Google Scholar]
- 6.Huang C. L., Feng S., Hilgemann D. W. ( 1998) Nature 391, 803– 806 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., He C., Yan X., Mirshahi T., Logothetis D. E. ( 1999) Nat. Cell Biol. 1, 183– 188 [DOI] [PubMed] [Google Scholar]
- 8.Rapedius M., Soom M., Shumilina E., Schulze D., Schönherr R., Kirsch C., Lang F., Tucker S. J., Baukrowitz T. ( 2005) J. Biol. Chem. 280, 30760– 30767 [DOI] [PubMed] [Google Scholar]
- 9.Romanenko V. G., Fang Y., Byfield F., Travis A. J., Vandenberg C. A., Rothblat G. H., Levitan I. ( 2004) Biophys. J. 87, 3850– 3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabirov R. Z., Tominaga T., Miwa A., Okada Y., Oiki S. ( 1997) J. Gen. Physiol. 110, 665– 677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaks-Makhina E., Kim Y., Aizenman E., Levitan E. S. ( 2004) Mol. Pharmacol. 65, 214– 219 [DOI] [PubMed] [Google Scholar]
- 12.Liu H., Gao Z. B., Yao Z., Zheng S., Li Y., Zhu W., Tan X., Luo X., Shen J., Chen K., Hu G. Y., Jiang H. ( 2007) J. Med. Chem. 50, 83– 93 [DOI] [PubMed] [Google Scholar]
- 13.Slack M., Kirchhoff C., Moller C., Winkler D., Netzer R. ( 2006) J. Biomol. Screen 11, 57– 64 [DOI] [PubMed] [Google Scholar]
- 14.Sun H., Liu X., Xiong Q., Shikano S., Li M. ( 2006) J. Biol. Chem. 281, 5877– 5884 [DOI] [PubMed] [Google Scholar]
- 15.Tang W., Ruknudin A., Yang W. P., Shaw S. Y., Knickerbocker A., Kurtz S. ( 1995) Mol. Biol. Cell 6, 1231– 1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguy A., Hebert T. E., Nattel S. ( 2006) Cardiovasc. Res. 69, 798– 807 [DOI] [PubMed] [Google Scholar]
- 17.Lingwood D., Simons K. ( 2007) Nat. Protoc. 2, 2159– 2165 [DOI] [PubMed] [Google Scholar]
- 18.Ghose A. K., Crippen G. M. ( 1987) J. Chem. Inf. Comput. Sci. 27, 21– 35 [DOI] [PubMed] [Google Scholar]
- 19.Martens J. R., Sakamoto N., Sullivan S. A., Grobaski T. D., Tamkun M. M. ( 2001) J. Biol. Chem. 276, 8409– 8414 [DOI] [PubMed] [Google Scholar]
- 20.Martens J. R., Navarro-Polanco R., Coppock E. A., Nishiyama A., Parshley L., Grobaski T. D., Tamkun M. M. ( 2000) J. Biol. Chem. 275, 7443– 7446 [DOI] [PubMed] [Google Scholar]
- 21.Weaver A. K., Olsen M. L., McFerrin M. B., Sontheimer H. ( 2007) J. Biol. Chem. 282, 31558– 31568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibino H., Kurachi Y. ( 2007) Eur. J. Neurosci. 26, 2539– 2555 [DOI] [PubMed] [Google Scholar]
- 23.Zhang H. Z., Kasibhatla S., Wang Y., Herich J., Guastella J., Tseng B., Drewe J., Cai S. X. ( 2004) Bioorg. Med. Chem. 12, 309– 317 [DOI] [PubMed] [Google Scholar]
- 24.Kasibhatla S., Jessen K. A., Maliartchouk S., Wang J. Y., English N. M., Drewe J., Qiu L., Archer S. P., Ponce A. E., Sirisoma N., Jiang S., Zhang H. Z., Gehlsen K. R., Cai S. X., Green D. R., Tseng B. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 12095– 12100 [DOI] [PMC free article] [PubMed] [Google Scholar]