Abstract
p38 MAPKs are typically activated by upstream MAPK kinases that phosphorylate a Thr-X-Tyr motif in the activation loop. An exception is the T cell antigen receptor signaling pathway, which bypasses the MAPK cascade and activates p38α and p38β by phosphorylation of Tyr-323 and subsequent autophosphorylation of the activation loop. Here we show that, unlike the classic MAPK cascade, the alternative pathway results primarily in mono-phosphorylation of the activation loop residue Thr-180. Recombinant mono-phosphorylated and dual phosphorylated p38α differed widely with regard to activity and substrate preference. Altered substrate specificity was reproduced in T cells in which p38 was activated by the alternative or classical MAPK pathways. These findings suggest that T cells have evolved a mechanism to utilize p38 in a specialized manner independent of and distinct from the classical p38 MAPK signaling cascade.
MAPK2 are expressed in all eukaryotic cells and participate in responses to stimuli involved in cell activation, proliferation, differentiation, and death (1, 2). Of the three major MAPK families, the ERKs play a part in mitogenic signal propagation, whereas the JNKs (c-Jun N-terminal kinases) and p38 isoforms participate in responses to pro-inflammatory cytokines and stress. MAPK activity is closely coupled to changes in gene expression, consistent with the fact that their targets include numerous transcription factors and kinases that themselves regulate transcription factors. Aberrant p38 activity has been implicated in inflammation and cancer, for which p38 inhibitors are being explored as therapeutic agents (3, 4).
MAPK are activated by a three-tiered kinase signaling cascade. At the most membrane-proximal level are the MAPK kinase kinases (MAPKKK), serine/threonine kinases that phosphorylate and activate the second level, the MAPK kinases (MAPKK). MAPKK are dual specificity kinases that phosphorylate a MAPK Thr-X-Tyr motif (X being glycine for p38 family members) located on the flexible activation loop that borders the catalytic site and regulates access to substrate. The p38 MAPK family has four members as follows: α, β, γ, and δ. p38γ expression is limited primarily to skeletal muscle (5), whereas the others are more widely distributed. p38α is the major isoform in T cells, which also express lesser amounts of p38 β and δ (6). p38α and -β are the most closely related by amino acid sequence (74% homology), are subject to inhibition by the widely used inhibitor SB203580, and share a tripeptide motif, Pro-Tyr-Asp, involved in the T cell antigen receptor (TCR)-initiated alternative activation pathway (7).
TCRs recognize peptide antigen presented by major histocompatibility molecules on the surface of antigen-presenting cells. Although it had generally been assumed that TCR-induced p38 activation utilized the canonical MAPK cascade (8), we found that this is not the case (7). Rather, stimulation via the TCR results in Lck-dependent activation of ZAP70, which in turn phosphorylates p38α and -β on Tyr-323. Phosphorylation of Tyr-323 leads to p38α and p38β autophosphorylation on the activation loop and increased activity toward third party substrates. Recent evidence suggests that the scaffold protein Dlgh1 bridges Lck/ZAP70 and p38, and in its absence Tyr-323 is not phosphorylated, and p38 is not activated in response to TCR signaling (9). Interestingly, despite the fact that B cells express the ZAP70 relative Syk, the alternative pathway does not appear to exist in antigen receptor-stimulated B cells (7, 10). The importance of the alternative pathway has been demonstrated in mice lacking Gadd45α, in which the alternative pathway is constitutively active, resulting in spontaneous T cell p38 activity and autoimmunity (11). More recently, we have found that p38α cannot be activated by TCR signaling in primary T cells from gene-targeted mice in which Tyr-323 has been replaced with Phe (10). An intriguing question is why T cells acquired a seemingly unique mechanism for p38 activation rather than utilizing the common signaling cascade. Here we find that the two activation pathways are qualitatively different, because unlike MAPKKs, p38α predominantly autophosphorylates a single residue in the activation loop, Thr-180, resulting in altered substrate selectivity.
EXPERIMENTAL PROCEDURES
Cells
Primary T cells were purified from lymph nodes and spleens of 6–8-week-old C57/BL6 mice using negatively selecting T cell purification columns (CL101) from CedarLane. T cells and the Jurkat T lymphoma (ATCC) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Invitrogen), 2 mm glutamine, 50 μm β-mercaptoethanol, and 100 μm gentamicin.
Reagents
Antibodies against p38 (9217), phospho-p38 (9211), and phospho-Thr-71-ATF-2 (9221) were purchased from Cell Signaling. Anti-CD3 (clone 2C11) was from Pharmingen. Rabbit polyclonal antibodies against phospho-Tyr-182-p38 were generated as described (7). Jurkat TCR clonotype-specific antibodies were obtained from the supernatants of the hybridoma C305 (from ATCC) grown to high density (<106 cells/ml) in complete medium. PMA was purchased from Sigma and SB203508 from Calbiochem.
Protein Expression and Purification
p38α and other proteins were expressed in the bacterial strain BL21(DE3) using the vectors pGEX-4T-2 or pET15b. Point mutations for p38α (K53M, Y323F, T180A, and Y182F) and ATF2 (aa 1–109; T69A and T71A) were generated by site-directed mutagenesis using the QuikChange kit (Stratagene). Human MAPKAP kinase 2 (aa 46–400) (12) and STAT4 (aa 538–749) (13) were amplified from cDNA and cloned into pGEX-4T-2. Plasmids encoding GST-Bcl-2, Bcl-XL (14), and GST-MEF2A (aa 151–411) (15) were generously provided by Mercedes Rincon and Silvio Gutkind, respectively. After cultures reached an A600 of 0.5–1.0, protein expression was induced with 0.3 mm isopropyl β-d-thiogalactopyranoside. His-p38α, His-MKK6 (S207E/T211E), and GST-ATF2 were incubated for a further 3 h at 25 °C and GST-MAPKAP kinase 2, GST-MEF2A, GST-STAT4, GST-Bcl-2, and GST-Bcl-XL for 12 h at 15 °C. Cells were resuspended in PBS (including 0.5 m NaCl if His-tagged), 0.1% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride, sonicated, and centrifuged at 20,000 × g for 20 min at 4 °C. His-tagged proteins were purified with cobalt-charged chelating-Sepharose Fast Flow beads (Amersham Biosciences) and eluted with 0.3 m imidazole (in PBS with 0.5 m NaCl). GST-tagged proteins were purified with glutathione-Sepharose Fast Flow beads (Amersham Biosciences) and eluted with 50 mm Tris, pH 8, containing 20 mm glutathione. Proteins were concentrated and washed into PBS using Microcon YM-30 spin columns (Millipore).
In Vitro Kinase Assays
Substrate proteins (3–10 μg) were prewarmed at 30 °C in 30 μl of kinase buffer (20 mm Tris, pH 7.5, 10 mm MgCl2, 2 mm dithiothreitol, 5 mm β-glycerophosphate, 5 mm NaF, and 0.2 mm Na3VO4) with 1–5 μCi [32P]ATP and the indicated amounts of ATP. Reactions were initiated by addition of 100 ng of semisynthesized p38α or MKK6-activated p38, as indicated. At the indicated times aliquots were removed and mixed with sample buffer. Phosphorylated products were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and visualized with a Storm PhosphorImager (GE Healthcare).
Protein Semisynthesis
To permit native chemical ligation, the natural histidine of the N-terminal residue of the synthetic peptide (aa 310–360) was mutated to cysteine. This mutation had no effect on the spontaneous activity of bacterially expressed full-length p38 (data not shown). Residues 1–309 of mouse p38α were cloned into the vector pTYB1 (New England Biolabs) using the NdeI and SapI restriction sites. To overcome insolubility, the resulting fusion protein of p38α (aa 1–309) with the VMA1 intein and the chitin-binding domain was grown in BL21(DE3) cells harboring the plasmid pREP4-GROEL/S, which encodes the chaperone complex GROEL/S. Cultures grown in LB with 100 μg/ml ampicillin and 25 μg/ml kanamycin to an A600 of 0.6 were induced with 0.1 mm isopropyl β-d-thiogalactopyranoside for 15 h at 12 °C, and proteins were extracted by sonication in binding buffer (PBS, 0.5 m NaCl, 0.1% Triton X-100). Fusion proteins were immobilized on chitin beads, and p38α (aa 1–309) having a C-terminal thioester was released by overnight treatment at room temperature with 0.2 m mercaptoethanesulfonate. After elution with mercaptoethanesulfonate/binding buffer and concentrating to ∼10 mg/ml with YM-30 columns, p38α (aa 1–309) was ligated to a 5-fold excess of the N-terminal cysteine of the C-terminal (aa 310–360) peptides with Tyr-323 either unphosphorylated or fully phosphorylated. The ligation reaction mixtures were washed with YM-30 columns into PBS before use in in vitro kinase reactions.
Cell Stimulation
Following incubation overnight in complete medium supplemented with HL-1 (Lonza), T cells were stimulated through the TCR by transferring to wells coated with 5 μg/ml anti-CD3 in PBS.
Immunoblotting and Immunoprecipitation
Cells were lysed in lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 10 mm NaF, 10 mm β-glycerophosphate, 5 mm sodium pyrophosphate, and 1 mm sodium orthovanadate supplemented with protease inhibitor mixture (Roche Applied Science)) and 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride). Lysates were normalized to protein concentration, denatured in sample buffer, resolved by SDS-PAGE, and immunoblotted with the indicated antibodies. Immunoblots were visualized with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce) or with fluorescently conjugated secondary antibodies and the Odyssey imaging system (Li-Cor).
RESULTS
Phosphorylation of Tyr-323 Is Sufficient to Activate p38α
Upstream MAPKKs activate p38α by phosphorylating the activation loop residues Thr-180 and Tyr-182 (16). Bacterially expressed p38α becomes activated when phosphorylated in vitro on Tyr-323 (7). However, p38α acquires a variable degree of activation loop phosphorylation during growth in bacteria (7)3 due to autophosphorylation, because a kinase-inactive (K53M substitution) mutant of p38α lacks any detectable activation loop phosphorylation or kinase activity (data not shown). To determine whether Tyr-323 phosphorylation in the absence of prior Thr-180/Tyr-182 phosphorylation is sufficient to up-regulate kinase activity, we prepared synthetic p38α phosphorylated on only one site, Tyr-323 (Tyr(P)-323), using protein semisynthesis (17). Recombinant p38α residues 1–309 were ligated to synthesized C-terminal (310–360) fragments, creating full-length p38α with Tyr-323 either phosphorylated or unphosphorylated. The effect of Tyr-323 phosphorylation on p38α activity was assessed with an in vitro kinase assay using the physiological substrate ATF2. Whereas semisynthesized unphosphorylated p38α Tyr-323 was inactive, the introduction of a phosphorylated Tyr-323 resulted in an active kinase that phosphorylated ATF2 (Fig. 1, left). Importantly, both semisynthesized molecules were successfully refolded and capable of kinase activity, because after incubation with MKK6, which phosphorylates Thr-180/Tyr-182 in the activation loop, they phosphorylated ATF2 equally well (Fig. 1, right). Thus, Tyr-323 phosphorylation in the absence of activation loop phosphorylation is sufficient to activate p38.
FIGURE 1.
Tyr-323 phosphorylation activates p38α. Left, semisynthesized unphosphorylated or Tyr-323-phosphorylated p38 was incubated with GST-ATF2 in an IVK reaction in the presence of 1 μm ATP. [32P]ATP incorporation was detected by SDS-PAGE and autoradiography. Right, semisynthesized p38 proteins were phosphorylated (P) on the activation loop by MKK6 and then incubated with GST-ATF2 an in vitro kinase reaction.
Characterization of Antibodies Recognizing Individually Phosphorylated p38α Activation Loop Residues
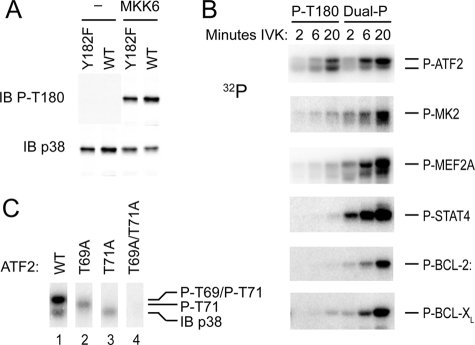
The site of p38α autophosphorylation by the TCR-mediated alternative pathway was previously mapped to the activation loop using an antiserum thought to recognize dual phosphorylated p38 (7). However, the report that a similar antibody against the phosphorylated activation loop could also recognize mono-phosphorylated Thr-180 (18) prompted us to revisit the question of what residues were involved in p38α autophosphorylation. We developed a rabbit polyclonal antibody against a peptide containing the mouse p38α activation loop in which only Tyr-182 was phosphorylated (anti-Tyr(P)-182). A dot blot of peptides comprising p38α residues 176–186 with Thr-180 and Tyr-182 unphosphorylated, phosphorylated singly (Thr(P) or Tyr(P)), or phosphorylated together (Thr(P)/Tyr(P)) was immunoblotted with the putative dual phosphorylation-specific antibody previously used to evaluate p38 phosphorylation in T cells (9211) (7) or anti-P-Tyr-182. 9211 recognized Thr(P)-180 with or without concomitant Tyr(P)-182 (Fig. 2A) but had little activity toward Tyr(P)-182. Anti-Tyr(P)-182 recognized Tyr(P)-182 but not Thr(P)-180. The specificity of these antibodies for the phosphorylated native protein was determined by immunoblotting kinase-inactive p38α that contained mutations of one or both phosphoacceptor sites and that had been phosphorylated by MKK6. Once again, 9211 recognized primarily p38α mono-phosphorylated on Thr-180 (Fig. 2B). Therefore, the 9211 antibody is hereafter referred to as anti-Thr(P)-180.
FIGURE 2.
Specificity of anti-phospho-p38 antibodies. A, dot blots of immobilized p38 peptides were immunoblotted (IB) with the antibodies recognizing p38 phosphorylated (P) on Thr-180 (9211) or Tyr-182 (P-Tyr). B, immunoblots of phosphorylated activation loop mutants of p38α. Kinase-dead GST-p38 (K53M) and the indicated activation loop mutants, either left untreated or phosphorylated on available activation loop phosphoacceptor sites by MKK6, were immunoblotted as in A (upper two panels) and with antibodies recognizing total p38 (lowest panel).
p38α Autophosphorylation Occurs on Thr-180
TCR-induced p38α autophosphorylation follows phosphorylation of the nonactivation loop residue Tyr-323 (7). We observed that p38α expressed in Escherichia coli grown at 37 °C is phosphorylated on both Tyr-323 and the activation loop because of autophosphorylation, because these residues are not phosphorylated on the kinase-inactive p38α mutant (data not shown). Mutation of Tyr-323 to Phe also abolished activation loop autophosphorylation and activation of p38α in bacteria (data not shown), indicating that autophosphorylation requires phosphorylation of Tyr-323. This suggested that E. coli-expressed p38α can serve as a model for p38α activated by the alternative pathway. We asked if active p38α can phosphorylate kinase-inactive p38α and if so on which site(s). GST-tagged kinase-inactive p38α was offered as a substrate for active p38α in an in vitro kinase (IVK) assay. After incubation with active p38α, GST-p38α was recognized by anti-Thr(P)-180 but not anti-Tyr(P)-18 (Fig. 3). Thus, p38α can phosphorylate itself in trans, which is perhaps the mechanism of its autophosphorylation in TCR-activated T cells, on the activation loop residue Thr-180 but not Tyr-182.
FIGURE 3.
Trans-autophosphorylation of p38α takes place on Thr-180. Kinase-dead GST-p38 (Lys-53-Met) was left unphosphorylated or phosphorylated by active His-p38 in an IVK reaction. Reactions were immunoblotted (IB) with the indicated anti-phospho-p38α antibodies (upper panels) and with antibodies recognizing total p38 (lower panels).
To determine whether the recombinant p38α reflects the behavior of the endogenous protein when activated in cells, we examined the phosphorylation status of p38 in TCR-stimulated T cells. Anti-Thr(P)-180 detected Thr-180 phosphorylation in cells stimulated through the TCR (the alternative pathway) or with PMA (which initiates the MAPK cascade) (Fig. 4). In contrast, anti-Tyr(P)-182 gave a strong signal with p38α from T cells stimulated with PMA but only a faint signal with TCR-stimulated cells. Therefore, unlike the MAPK cascade, TCR-triggered alternative p38 activation causes primarily mono-phosphorylation of Thr-180.
FIGURE 4.
In vivo alternatively activated p38 becomes phosphorylated on Thr-180 and not on Tyr-182. Purified T cells were stimulated with PMA or plate-bound anti-CD3 for 15 min. Lysates were immunoblotted with antibodies recognizing p38 Thr(P)-180 or Tyr(P)-182. All samples were from the same experiment, and some intervening lanes were removed from the figure for clarity.
Mono-phosphorylated and Dual Phosphorylated p38α Have Different Substrate Specificities
Dual phosphorylated p38 is the only form of the activated enzyme that has thus far been implicated in cellular events. That TCR signaling causes p38α mono-phosphorylation raises the possibility that the enzyme functions differently in cells stimulated via the TCR compared with stimuli that set off the MAPK cascade. As a first approach, we asked if dual and mono-phosphorylated p38α differ in their ability to phosphorylate known p38α substrates. Dual and mono-phosphorylated p38α were generated by phosphorylating recombinant wild type and Y182F p38α with MKK6. To exclude potential confounding effects of Tyr-323 phosphorylation, Tyr-323 in both proteins was replaced with Phe. MKK6 phosphorylated both proteins on Thr-180 to yield mono- and dual phosphorylated p38α (Fig. 5A) that were then tested in an IVK assay with the substrate GST-ATF2-(1–109) (19). ATF2 contains two p38 target residues, Thr-69 and Thr-71 (20). Thr-69 and Thr-71 are equivalent targets for p38α, but initial phosphorylation of Thr-71 favors the subsequent phosphorylation of Thr-69 to yield the doubly phosphorylated substrate (21). Although the mono-phosphorylated p38α was somewhat less efficient that the dual phosphorylated form, both generated phospho-ATF2 that resolved as two bands on SDS-PAGE (Fig. 5B, top row). Interestingly, whereas dual phosphorylated p38α generated predominantly the slower migrating ATF2 band over time, mono-phosphorylated p38α reproducibly yielded similar amounts of the slower and faster migrating species. Quantitation of incorporated 32P in the experiment shown, for example, revealed that the upper to lower band ratio was ∼1:1 at all time points using mono-phosphorylated p38α but increased from 1:1 at 3 min to 5:1 at 30 min using dual phosphorylated p38α. To identify the different ATF2 species, we mutated the known sites of phosphorylation and determined how these proteins migrated when phosphorylated by dual phosphorylated p38α (Fig. 5C). The upper band was not found in any of the mutants, demonstrating that it represents dual phosphorylation of Thr-69 and Thr-71. Phosphorylation of Thr-69 alone (Fig. 5C, lane 3) yielded the faster migrating species, and phosphorylation of Thr-71 alone (Fig. 5C, lane 2) resulted in a band with intermediate migration. Thus, three phosphorylated ATF2 species can be resolved; the lower band is phospho-Thr-69, and the upper is doubly phosphorylated ATF2, and the intermediate band (a transient species that cannot be distinguished in phosphorylated wild type ATF2, Fig. 5C, lane 1) is phospho-Thr-71. We therefore conclude that whereas both forms of p38α can phosphorylate ATF2, the mono- and dual phosphorylated p38α differ in their fine specificity, the latter being better able to convert mono-phosphorylated ATF2 to the doubly phosphorylated and slower migrating species.
FIGURE 5.
Thr-180 mono-phosphorylated p38α displays altered substrate specificity. A, Thr-180 phosphorylation status of the Thr-180 mono-phosphorylated or dual phosphorylated p38α (Y323F) proteins phosphorylated by MKK6 was evaluated by immunoblotting (IB). B, indicated substrate proteins were incubated with either Thr-180 mono-phosphorylated or dual phosphorylated p38α in an in vitro kinase reaction with 3 mm ATP, and [32P]ATP incorporation was detected as in Fig. 1. C, determination of the phosphorylation status of migratory variants of wild type (WT) ATF2. GST-ATF2 and the phosphoacceptor site mutants T69A, T71A, and T69A/T71A were incubated with dual phosphorylated p38α in an IVK reaction for 10 min.
We compared the activity of mono- and dual phosphorylated p38α on other p38α substrates (Fig. 5B). MAPKAP kinase 2 (2, 22) and the transcription factor MEF2A (23, 24) were each phosphorylated by mono-phosphorylated p38 but much less well than by the dual phosphorylated form. An even greater dichotomy was observed with the substrate STAT4 (Fig. 5B) (25, 26). Bcl-2 and Bcl-XL were also examined, because it has been suggested that their phosphorylation by p38 can disable their anti-apoptotic function (14, 27). Bcl-XL was a very poor substrate for mono-phosphorylated p38α, and Bcl-2 was not phosphorylated at all. Thus, the activity of mono-phosphorylated compared with dual phosphorylated p38α was very substrate-dependent, being almost equivalent for ATF2 (although with a difference in fine specificity) and being virtually nil for STAT4 and Bcl-2.
Alternatively Activated p38α Has Altered Substrate Specificity in Vivo
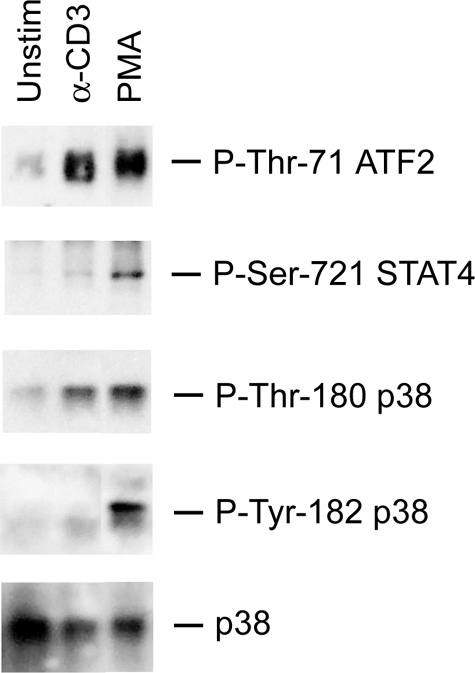
The question of whether the altered substrate specificity of recombinant mono-phosphorylated p38 is reflected in vivo was addressed by examining the phosphorylation of several substrates in TCR-activated cells. Lysates of primary mouse T cells activated with either anti-CD3 (the alternative pathway) or with PMA (the MAPK cascade) were immunoblotted with the antibodies that recognize the p38 phosphorylation sites on ATF2 and STAT4. Phosphorylation of p38α Thr-180 in response to both stimuli was similar (Fig. 6, 3rd row), but only PMA caused Tyr-182 phosphorylation (Fig. 6, 4th row). Consistent with the in vitro results, TCR and PMA stimulation induced similar phosphorylation of ATF2. Note that the antibody used to detect phospho-ATF2 recognizes Thr-71, and therefore one cannot detect Thr-69 (mono-phosphorylated) ATF2. STAT4, one of the substrates least favored by mono-phosphorylated p38 in vitro, was phosphorylated well after PMA but not TCR-mediated activation. Therefore, as for recombinant p38α, the substrate specificity of endogenous TCR-activated (mono-phosphorylated) p38 differs from MAPK cascade-activated (dual phosphorylated) p38.
FIGURE 6.
In vivo TCR-activated p38α displays altered substrate specificity. T cells were stimulated as in Fig. 4, and lysates were immunoblotted with antibodies recognizing the indicated phosphorylated proteins.
DISCUSSION
MAPKs are unusual among kinases in having two phosphoacceptor sites on their activation loops, and the role of the second (tyrosine) site is not well understood. The existence of phosphatases specific for one or the other of the sites has prompted the hypothesis that mono-phosphorylated species exist, but biological examples have not been described, and Thr-180 mono-phosphorylated p38α has been observed only in nonphysiological contexts (28, 29). Constitutively active point mutants of the p38α homolog Hog1 were isolated in a genetic screen in yeast (30). When introduced into p38α, some of these mutations induced kinase activity and autophosphorylation in trans on Thr-180 (18). Notably, these constitutively active mutants bearing an additional Y182F substitution retained activity, albeit at a reduced level (31, 32). The majority of the Hog1-activating mutations were located along the C-terminal extension known as L16, a flexible loop that wraps around the N-terminal domain of all MAPK and which contains Tyr-323 (30). The many similar features between the Hog1-activating mutations (18, 32) and Tyr(P)-323-activated p38α reported here strongly suggest that they activate kinase activity by a common mechanism. Such a mechanism could involve disruption of a hydrophobic core made up of the aromatic side chains of Tyr-69, Phe-327, and Trp-337 (31). The side chain of unphosphorylated Tyr-323 is oriented inward toward this core, and addition of a negative charge could perturb the pocket, twist L16, and, via an interaction between L16 and the activation loop analogous to that seen in dual phosphorylated ERK2, induce a rotation between the C- and N-terminal domains that remodels the catalytic site of p38 (18, 32–34).
The individual contributions of Thr-180 and Tyr-182 phosphorylation to p38 function have recently been addressed (29, 35). Using ATF2 as a substrate, it was found that Thr-180 mono-phosphorylated p38α was an order of magnitude less active than dual phosphorylated p38α in vitro. We found that the difference between mono- and dual phosphorylated p38α became smaller as the concentration of ATP was increased. 3 mm ATP was used in this study because it approximates physiological intracellular levels (36), and under these conditions ATF2 was phosphorylated almost as well by mono-phosphorylated p38α as by the dual phosphorylated species. Deuterium exchange mass spectroscopy revealed that dual phosphorylation induces significant alterations in solvent accessibility to the activation loop, active site, and substrate docking domains, whereas Tyr-182 mono-phosphorylation has little effect (37). Therefore, and consistent with our data, it seems likely that Thr-180 phosphorylation has the major role in formation of the active site, and Tyr-182 phosphorylation enhances the activity and plays a role in determining specificity.
An important question is why have T cells acquired an alternative p38α and -β activation pathway that is not shared even by closely related B cells. The finding in this report that activation of p38α by TCR stimulation is qualitatively different raises several possibilities. T cells are equipped to participate in inflammatory responses and receive stimulation through receptors such as interleukin-12 and -18, which activate p38 by the classical MAPK cascade and thus increase the production of downstream pro-inflammatory molecules such as interferon-γ (IFN-γ) (13, 38–41). The responses a T cell makes to antigen via the TCR, such as clonal expansion and providing help to B cells, can occur independently of inflammatory conditions, and induction of pro-inflammatory downstream events might be deleterious. Therefore, the alternative pathway may be a means of allowing p38 activation and some limited gene up-regulation without inflammatory sequelae. Another possibility is that MAPK cascade-activated p38α is itself harmful to T cell development and/or cell function. Phosphorylation of Bcl-2 by p38 has been shown to induce its release from mitochondria, thereby disrupting its ability to inhibit apoptosis (27, 42). Mice expressing a constitutively active form of MKK6 in T cells, and therefore having constitutively active dual phosphorylated p38, have a reduction in the number of CD8+ T cells because of their apoptosis, and the cells that survive proliferate less well in response to mitogen (43). It was suggested that the spontaneous apoptosis of CD8+ cells was because of p38-mediated phosphorylation of the anti-apoptotic protein Bcl-2, a mechanism that has also been implicated in Fas-induced CD8+ T cell death (14). Our finding that Bcl-2 is a very poor substrate for mono-phosphorylated, in contrast to dual phosphorylated, p38α is consistent with the notion that activation of p38 by the alternative pathway helps to maintain cell viability in TCR-signaled cells. IFN-γ is a target for TCR and cytokine signaling pathways, either separately or in combination, in T cells. ATF2, which we found to be a reasonably good substrate for mono-phosphorylated p38α, is implicated in TCR-mediated induction of IFN-γ (44), whereas STAT4, which is not a target of monophosphorylated p38α, is important for interleukin-12-induced (25) but not TCR-induced (26) IFN-γ (via the MAPK cascade). Thus, the evolutionarily conserved loss of TCR-coupled MAPK cascade initiation and the acquisition of the alternative p38 activation pathway may be driven by the need of T cells to tightly control p38 activation and limit its substrates to those useful in immune responses.
Acknowledgments
We thank Bei Dong for subcloning and mutagenesis.
This work was supported, in whole or in part, by the National Institutes of Health NCI Intramural Research Program, Center for Cancer Research.
P. R. Mittelstadt and J. D. Ashwell, unpublished results.
- MAPK
- mitogen-activated protein kinase
- MAPKK
- MAPK kinase
- TCR
- T cell antigen receptor
- ERK
- extracellular signal-regulated kinase
- aa
- amino acid
- PBS
- phosphate-buffered saline
- IVK
- in vitro kinase
- PMA
- phorbol 12-myristate 13-acetate
- IFN
- interferon.
REFERENCES
- 1.Kyriakis J. M., Avruch J. ( 2001) Physiol. Rev. 81, 807– 869 [DOI] [PubMed] [Google Scholar]
- 2.Roux P. P., Blenis J. ( 2004) Microbiol. Mol. Biol. Rev. 68, 320– 344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelberg D. ( 2004) Semin. Cancer Biol. 14, 271– 282 [DOI] [PubMed] [Google Scholar]
- 4.Lee J. C., Kumar S., Griswold D. E., Underwood D. C., Votta B. J., Adams J. L. ( 2000) Immunopharmacology 47, 185– 201 [DOI] [PubMed] [Google Scholar]
- 5.Li Z., Jiang Y., Ulevitch R. J., Han J. ( 1996) Biochem. Biophys. Res. Commun. 228, 334– 340 [DOI] [PubMed] [Google Scholar]
- 6.Hale K. K., Trollinger D., Rihanek M., Manthey C. L. ( 1999) J. Immunol. 162, 4246– 4252 [PubMed] [Google Scholar]
- 7.Salvador J. M., Mittelstadt P. R., Guszczynski T., Copeland T. D., Yamaguchi H., Appella E., Fornace A. J. J., Ashwell J. D. ( 2005) Nat. Immunol. 6, 390– 395 [DOI] [PubMed] [Google Scholar]
- 8.Rincon M., Flavell R. A., Davis R. A. ( 2000) Free Radic. Biol. Med. 28, 1328– 1337 [DOI] [PubMed] [Google Scholar]
- 9.Round J. L., Humphries L. A., Tomassian T., Mittelstadt P., Zhang M., Miceli M. C. ( 2007) Nat. Immunol. 8, 154– 161 [DOI] [PubMed] [Google Scholar]
- 10.Jirmanova L., Sarma D. N., Jankovic D., Mittelstadt P. R., Ashwell J. D. ( 2008) Blood [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvador J. M., Mittelstadt P. R., Belova G. I., Fornace A. J. J., Ashwell J. D. ( 2005) Nat. Immunol. 6, 396– 402 [DOI] [PubMed] [Google Scholar]
- 12.Lukas S. M., Kroe R. R., Wildeson J., Peet G. W., Frego L., Davidson W., Ingraham R. H., Pargellis C. A., Labadia M. E., Werneburg B. G. ( 2004) Biochemistry 43, 9950– 9960 [DOI] [PubMed] [Google Scholar]
- 13.Visconti R., Gadina M., Chiariello M., Chen E. H., Stancato L. F., Gutkind J. S., O'Shea J. J. ( 2000) Blood 96, 1844– 1852 [PubMed] [Google Scholar]
- 14.Farley N., Pedraza-Alva G., Serrano-Gomez D., Nagaleekar V., Aronshtam A., Krahl T., Thornton T., Rincon M. ( 2006) Mol. Cell. Biol. 26, 2118– 2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinissen M. J., Chiariello M., Pallante M., Gutkind J. S. ( 1999) Mol. Cell. Biol. 19, 4289– 4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson G., Robinson F., Beers G. T., Xu B. E., Karandikar M., Berman K., Cobb M. H. ( 2001) Endocr. Rev. 22, 153– 183 [DOI] [PubMed] [Google Scholar]
- 17.Muir T. W. ( 2003) Annu. Rev. Biochem. 72, 249– 289 [DOI] [PubMed] [Google Scholar]
- 18.Diskin R., Lebendiker M., Engelberg D., Livnah O. ( 2007) J. Mol. Biol. 365, 66– 76 [DOI] [PubMed] [Google Scholar]
- 19.Raingeaud J., Gupta S., Rogers J. S., Dickens M., Han J., Ulevitch R. J., Davis R. J. ( 1995) J. Biol. Chem. 270, 7420– 7426 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs S. Y., Tappin I., Ronai Z. ( 2000) J. Biol. Chem. 275, 12560– 12564 [DOI] [PubMed] [Google Scholar]
- 21.Waas W. F., Lo H. H., Dalby K. N. ( 2001) J. Biol. Chem. 276, 5676– 5684 [DOI] [PubMed] [Google Scholar]
- 22.Salmon R. A., Foltz I. N., Young P. R., Schrader J. W. ( 1997) J. Immunol. 159, 5309– 5317 [PubMed] [Google Scholar]
- 23.Zhao M., New L., Kravchenko V. V., Kato Y., Gram H., di Padova F., Olson E. N., Ulevitch R. J., Han J. ( 1999) Mol. Cell. Biol. 19, 21– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaeser F., Ho N., Prywes R., Chatila T. A. ( 2000) J. Biol. Chem. 275, 197– 209 [DOI] [PubMed] [Google Scholar]
- 25.Morinobu A., Gadina M., Strober W., Visconti R., Fornace A., Montagna C., Feldman G. M., Nishikomori R., O'Shea J. J. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 12281– 12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park W. R., Nakahira M., Sugimoto N., Bian Y., Yashiro-Ohtani Y., Zhou X. Y., Yang Y. F., Hamaoka T., Fujiwara H. ( 2004) Int. Immunol. 16, 295– 302 [DOI] [PubMed] [Google Scholar]
- 27.De Chiara G., Marcocci M. E., Torcia M., Lucibello M., Rosini P., Bonini P., Higashimoto Y., Damonte G., Armirotti A., Amodei S., Palamara A. T., Russo T., Garaci E., Cozzolino F. ( 2006) J. Biol. Chem. 281, 21353– 21361 [DOI] [PubMed] [Google Scholar]
- 28.Zhou B., Zhang Z. Y. ( 2002) J. Biol. Chem. 277, 13889– 13899 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y. Y., Mei Z. Q., Wu J. W., Wang Z. X. ( 2008) J. Biol. Chem. 283, 26591– 26601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell M., Capone R., Pashtan I., Levitzki A., Engelberg D. ( 2001) J. Biol. Chem. 276, 25351– 25358 [DOI] [PubMed] [Google Scholar]
- 31.Diskin R., Askari N., Capone R., Engelberg D., Livnah O. ( 2004) J. Biol. Chem. 279, 47040– 47049 [DOI] [PubMed] [Google Scholar]
- 32.Maayan I., Engelberg D. ( 2008) in Stress-activated Protein Kinases ( Posas F., Nebreda A. R. eds) pp. 171– 186, Springer-Verlag, Berlin [Google Scholar]
- 33.Canagarajah B. J., Khokhlatchev A., Cobb M. H., Goldsmith E. J. ( 1997) Cell 90, 859– 869 [DOI] [PubMed] [Google Scholar]
- 34.Bellon S., Fitzgibbon M. J., Fox T., Hsiao H. M., Wilson K. P. ( 1999) Struct. Fold. Des. 7, 1057– 1065 [DOI] [PubMed] [Google Scholar]
- 35.Askari N., Beenstock J., Livnah O., Engelberg D. ( 2009) Biochemistry 48, 2497– 2504 [DOI] [PubMed] [Google Scholar]
- 36.Gronostajski R. M., Pardee A. B., Goldberg A. L. ( 1985) J. Biol. Chem. 260, 3344– 3349 [PubMed] [Google Scholar]
- 37.Sours K. M., Kwok S. C., Rachidi T., Lee T., Ring A., Hoofnagle A. N., Resing K. A., Ahn N. G. ( 2008) J. Mol. Biol. 379, 1075– 1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd C. E. ( 2005) Nat. Immunol. 6, 368– 370 [DOI] [PubMed] [Google Scholar]
- 39.Lu B., Ferrandino A. F., Flavell R. A. ( 2004) Nat. Immunol. 5, 38– 44 [DOI] [PubMed] [Google Scholar]
- 40.Berenson L. S., Yang J., Sleckman B. P., Murphy T. L., Murphy K. M. ( 2006) J. Immunol. 176, 4616– 4621 [DOI] [PubMed] [Google Scholar]
- 41.Yang J., Zhu H., Murphy T. L., Ouyang W., Murphy K. M. ( 2001) Nat. Immunol. 2, 157– 164 [DOI] [PubMed] [Google Scholar]
- 42.Blagosklonny M. V. ( 2001) Leukemia (Basingstoke) 15, 869– 874 [DOI] [PubMed] [Google Scholar]
- 43.Merritt C., Enslen H., Diehl N., Conze D., Davis R. J., Rincon M. ( 2000) Mol. Cell. Biol. 20, 936– 946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penix L. A., Sweetser M. T., Weaver W. M., Hoeffler J. P., Kerppola T. K., Wilson C. B. ( 1996) J. Biol. Chem. 271, 31964– 31972 [DOI] [PubMed] [Google Scholar]