Abstract
α- and θ-defensin-, magainin-, and cathelicidin-type antimicrobial peptides (AMPs) can kill the pathogenic protozoan Leishmania. Comparative studies of a panel of AMPs have defined two distinct groups: those that induce nonapoptotic (Class I) and apoptotic (Class II) parasite killing based on their differential ability to induce phosphatidyl serine exposure, loss of mitochondrial membrane potential and decreased ATP production, induction of caspase-3/7 and -12 activity, and DNA degradation. Class II AMPs cause rapid influx of the vital stain SYTOX and an increase in intracellular Ca2+, whereas Class I AMPs cause a slow accumulation of SYTOX and do not affect intracellular Ca2+ levels. Inhibitors of cysteine or caspase proteases diminished fast influx of SYTOX through the surface membrane and DNA degradation but do not ablate the annexin V staining or the induction of apoptosis by Class II AMPs. This suggests that the changes in surface permeability in AMP-mediated apoptosis are related to the downstream events of intracellular cysteine/caspase activation or the loss of ATP. The activation of caspase-12-like activity was Ca2+-dependent, and inhibitors of voltage-gated and nonspecific Ca2+ channels diminished this activity. Flufenamic acid, a nonspecific Ca2+ inhibitor, completely ablated AMP-induced mitochondrial dysfunction and cell death, indicating the importance of dysregulation of Ca2+ in antimicrobial peptide-induced apoptosis.
Leishmania are vector-borne protozoa that parasitize host macrophage phagolysosomes. Activated macrophages kill intracellular parasites in part by induction of apoptosis by nitric oxide in a caspase- and cysteine protease-independent manner (1, 2). Limited apoptosis of Leishmania within the sandfly vector, prior to infection of host, may be important to the establishment of infection (3, 4). In vitro Leishmania can undergo apoptosis by exposure to many different compounds that lead to alteration of surface the cell membrane causing enhanced annexin V staining, activation of caspase-like proteases, DNA laddering, and mitochondrial membrane depolarization. Our work has recently shown that antimicrobial peptide exposure of Leishmania can induce apoptosis, which occurs concomitant with activation of both caspase and cysteine protease activities (5).
Antimicrobial peptides (AMPs)2 are structurally diverse highly cationic proteins between 10 and 50 amino acids and are components of the innate immune systems of organisms within all kingdoms. AMPs have a myriad of functions and are known to interact with and disrupt microbial surface membranes leading to cell death. Magainins are α-helical AMPs expressed in the skin of frogs, whereas cathelicidins are mammalian β-pleated sheet peptides, both of which can kill Leishmania at physiological concentrations (5). We have documented that magainins are quite apt at causing AMP-induced apoptosis of surface metalloprotease null mutants of L. major and that these exhibited features of apoptotic Leishmania induced by different stimuli (5). These two different structurally diverse classes of AMPs are known to interact with membranes in distinct ways (6), yet it is unknown whether we they can kill parasites via disparate mechanisms.
Apoptosis of Leishmania induced by antimony, camptothecin, and hydrogen peroxide all induce significant increases in cytosolic calcium, which is toxic to mitochondrial membrane potential (7–9). Calcium-related cell death and mitochondrial toxicity are related to the activity of nonspecific calcium channels (7, 8, 10), which may be attributable to their location in the mitochondrial membrane (11, 12). Apoptosis of Leishmania is also associated with increases in caspase-like and cysteine protease activity (5, 7, 9, 13, 14). Caspase-3/7-like activation can be induced by a number of stimuli despite the absence of genes encoding caspases within the L. major genome (15). In addition, the ancestral metacaspase expressed by Leishmania donovani is a trypsin-like protease not inhibitible with caspase inhibitors (16). We and others have found that cysteine protease activity may be important for certain features of Leishmania apoptosis such as DNA degradation (5). It is quite clear that Leishmania can undergo apoptosis in caspase-dependent and caspase-independent pathways (17), yet we do not know what mode of apoptosis occurs upon AMP exposure.
In the work presented here we show that two structurally diverse AMPs kill Leishmania differentially inducing either nonapoptotic or apoptotic cell death. We have characterized AMP-induced apoptotic cell death and found that although it is associated with the activation of caspase-3/7- and -12-like activities, these activities are not essential to apoptosis. Conversely we find that a dramatic increase in cytosolic Ca2+ correlates with the mitochondrial membrane dysfunction and the decline in ATP production and cell death and that this is completely inhibited by blockade of nonspecific calcium channels. Blockade of caspase and cysteine proteases affect surface membrane permeability changes and DNA degradation but do not prevent cell death, suggesting that AMP-induced apoptosis may be a caspase-independent process. These data provide a framework to understand the critical events in AMP-mediated apoptosis and may be helpful in the rational design of chemotherapeutic strategies for manipulation of cell death pathways in Leishmania.
EXPERIMENTAL PROCEDURES
Parasites and Peptides
The cell line used in this study is the leishmanolysin knock-out derivative (termed KO, in this paper) of Leishmania major (NHOM/SN/74/Seidman) (18) and was routinely cultivated as insect forms in M199 containing 10% heat-inactivated fetal bovine serum. The magainin-type peptide pexiganan (19) and porcine cathelicidin protegrin-1 (20) were synthesized on an Applied Biosystems model 433A synthesizer as described previously (21).
Parasite Survival Assay
A standard parasite survival assay was used as described previously for Leishmania (5). Routinely 107 parasites were incubated in 100 μg of MTT reagent followed by treatment with 10% SDS for 6–8 h followed by reading in spectrophotometer at 570 nm. Treated parasites were compared with parasites incubated in the same conditions in buffer alone. All of the AMP-treated cells were incubated for 2 h with 12.5 μm peptide prior to analysis with the MTT assay.
SYTOX Green Assay for Cell Membrane Permeability
107 parasites were washed thrice in PBS then incubated in the dark with 1 μm SYTOX Green (Promega) in PBS for 15 min essentially as described (22). Fluorescence was measured every 5 min after peptide addition for up to 2 h. Control for maximum fluorescence was shown by the addition of 0.5% Triton X-100. Fluorescence was measured in a microplate reader with excitation and emission wavelengths of 485 and 520 nm, respectively.
Caspase Assays
Caspase-3/7 protease activity was measured using the Apo-1 homogenous caspase-3/7 activity assay kit (Promega, Madison, WI). The assay was done according to the manufacturer's instructions with the following minor modifications. 107 parasites were incubated in 100 μl of PBS with or without pexiganan for 2 h followed by the addition of kit reagents. At the completion of the reactions, the increase in fluorescence, indicative of the cleavage of the Z-DEVD-R110 substrate, was read fluorometrically for 2 h at excitation and emission wavelengths of 485 and 530 nm, respectively. Cysteine protease inhibitors E-64 (Sigma) and 1–3-Boc-aspartyl-fluoromethyl ketone (BAF) (Calbiochem, San Diego, CA) were added in control reactions at 100 μm for 30 min prior to addition of 6.25–12.5 μm pexiganan. Caspase-12 activity was measured fluorometrically using caspase-12 assay kit (Biovision Research Products). The assay is based on detection of cleavage of substrate ATAD-AFC (7-amino-4-trifluoromethyl coumarin) to free AFC, which was measured by a micro plate reader (emission, 400 nm; excitation, 505 nm). Comparison of fluorescence from AMP-treated parasites with untreated parasites slows determination of increase in caspase-12 activity. 10 μl of caspase-12 inhibitor Z-ATAD-FMK was added in control reactions before AMP addition and incubated for 30 min.
TUNEL Assay
In situ detection of DNA fragmentation was measured for parasites treated with an AMP pexiganan by the terminal deoxyribonucleotide transferase-mediated deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay according to the manufacturer's instructions (Titer TACS kit from R & D System). This spectrophotometerically based kit provides direct quantification of apoptosis in treated cells without actual counting of labeled cells. Untreated parasites and cells treated with nuclease provided in kit and known apoptotic drug staurosporine were used as negative and positive controls, respectively.
Flow Cytometric Analysis
Flow cytometry analysis was performed using a FACSCalibur flow cytometer and CellQuestPro software (Becton Dickinson). The cells were prepared as indicated above and stained with annexin V-fluorescein isothiocyanate and propidium iodide using an apoptosis detection kit (BD Pharmingen) according to the manufacturer's instructions. For measurement of mitochondrial membrane potential changes, the cells were treated as indicated above and loaded with for 5 min at 25 °C with 0.3 μg/ml of rhodamine 123 as described previously (23). The cells were washed thrice in PBS prior to flow cytometric analysis using 488- and 525-nm excitation and emission wavelengths, respectively. The cells incubated under the same conditions with 7.5 mm with the mitochondrial poison, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), were used as a positive control.
Ca2+ Analysis
Intracellular Ca2+ concentration measurements were done using with the fluorescent probe Fura 2AM as described previously (8). Parasites treated with AMP and untreated were harvested and washed twice with wash buffer (116 mm NaCl, 5.4 mm KCl, 0.8 mm MgCl2, 5.5 mm glucose, 1 mm CaCl2, and 50 mm MOPS, pH 7.4). The parasites were then suspended in the same buffer containing 15% sucrose and were incubated with Fura 2AM (6 μm) for 1 h with mild shaking. The cells were suspended in the same wash buffer after two washes, and fluorescence measurements were performed for 8 h with plate reader (excitation, 340 nm; emission, 510 nm). Parasites incubated with 10 mm CaCl2 were used as positive control for maximum fluorescence. The measurement of intracellular Ca2+ was carried out in the presence of Ca2+ channel inhibitors. The parasites were incubated with 10 μm verapamil, specific voltage-gated channel blocker and 100 μm flufenamic acid (FFA); the nonspecific calcium channel blocker for 1 h before addition of AMP to the cells.
Microscopic Analysis
The labeling of pexiganan and PG1 with fluorescein isothiocyanate was done using the protein labeling kit (Pierce) according to the protocol from the manufacturer. For fluorescent microscopic analysis, stationary phase leishmanolysin KO promastigotes were incubated with fluorescein isothiocyanate-labeled pexiganan and PG1 for different time points. At every time point the parasites were washed thrice with PBS and fixed with 0.5% glutaradehyde.
RESULTS
Differential Induction of Nonapoptotic and Apoptotic Cell Death by Different Antimicrobial Peptides
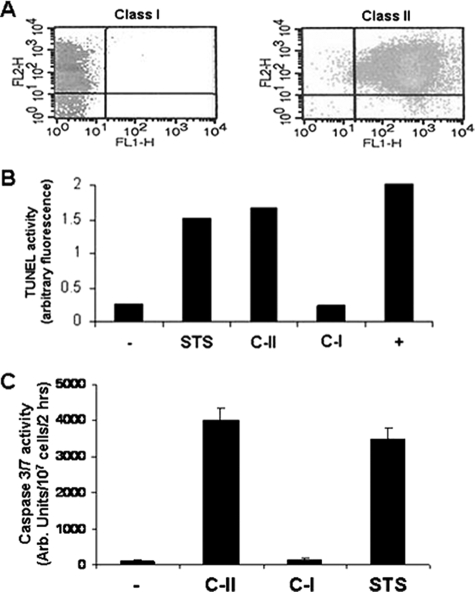
In our previous work we showed that the surface metalloprotease (leishmanolysin) degradation of AMP prevents AMP-induced apoptosis. Mutants devoid of leishmanolysin undergo killing by a variety of AMPs including cathelicidins and magainins. Because the type of cell death parasites undergo has implications with regard to the outcome of mammalian infection, we sought to determine whether there was a difference in the mode of cell death induced by these two AMP subtypes. Parasites incubated with either cathelicidin (protegrin-I, PG-1) and magainin (pexiganan) AMPs were assessed for markers of apoptosis (Fig. 1). PG-1-treated cells stained with propidium iodide (PI), although they had minimal staining with annexin V, were indicative of nonapoptotic-cell death. Pexiganan-treated cells, however, had marked dual staining with both PI and annexin V indicative of apoptosis. TUNEL analysis for DNA degradation of AMP-treated cells showed that only pexiganan induced TUNEL activity equivalent to that of staurosporine, an agent known to induce apoptosis in Leishmania (14). PG-1 treatment of cells did not lead to DNA degradation. We also measured the activation of caspase-3/7-like activity in AMP-treated cells. Pexiganan treatment led to the activation of significant caspase-3/7 activity to the level of that in cells treated with staurosporine, whereas PG-1 treatment did not cause enzyme activation. The induction of TUNEL activity, caspase-3/7-like enzyme activation, and PI-annexin V is selective of apoptotic cell death induced by Class II (C-II). We term AMPs, such as pexiganan, which induce apoptosis as C-II peptides and those that kill by nonapoptotic cell death, as Class I (C-I).
FIGURE 1.
AMP induction of two distinct modes of leishmanial killing: nonapoptotic (Class I) and apoptotic (Class II) cell death. L. major mutants lacking leishmanolysin were treated with either protegrin-1 (Class I AMP, C-I) or pexiganan (Class II AMP, C-II) and then analyzed using propidium iodide/annexin V staining by flow cytometry (A), TUNEL analysis for DNA degradation (B), or caspase-3/7 activation (C). Cells treated with staurosporine (STS) were used as a control for apoptotic cells. Cell lysates treated with DNase (indicated by + in B) were used for a positive control in the TUNEL assay.
Class II AMP Induces Mitochondrial Membrane Depolarization and Decline in ATP Production
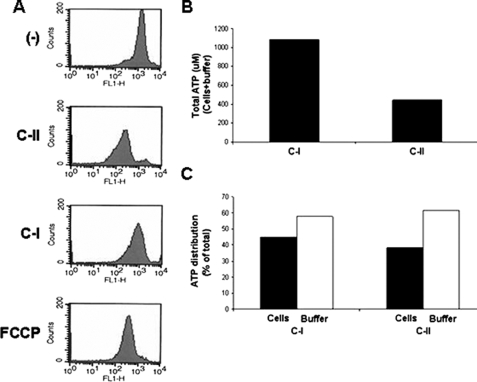
AMP-mediated apoptosis leads to a breakdown in mitochondrial membrane potential (5). We compared the mitochondrial membrane potential changes induced by C-I and C-II AMPs (Fig. 2). Flow cytometric analysis of parasites treated with C-II led to a decrease in fluorescence of rhodamine 123-loaded cells, whereas treatment of cells with C-I did not cause a decrease in fluorescence. Treatment of cells with the mitochondrial poison FCCP led to a breakdown in mitochondrial membrane potential similar to that if the cells were treated with C-II. We measured the ATP content of parasites treated with C-I and -II AMPs to determine whether the breakdown in mitochondrial function correlated with the decline in the production of ATP. Total cellular ATP was ∼2.5-fold lower in parasites treated with C-II than in those treated with C-I (Fig. 2B). Because AMPs are known to disrupt the outer membrane of cells, we determined the relative distribution of ATP in the supernatant and in the cells (Fig. 2C). The relative distribution of ATP did not differ significantly between cells treated with different AMPs.
FIGURE 2.
Differential induction of mitochondrial membrane potential disruption and ATP production. L. major mutants were treated with either protegrin-1 (Class I AMP, C-I) or pexiganan (Class II AMP, C-II) and analyzed for fluorescence in flow cytometry after staining with rhodamine 123 (A). Cells treated with the mitochondrial poison FCCP were used as a positive control. AMP-treated cells were also analyzed for total ATP content (B), showing that C-II-treated cells have an ∼4-fold lower amount of ATP. The distribution of ATP in the cell and buffer fractions of the reactions was also determined (C) and found to be similar in both cases.
Caspase and Cysteine Protease Dependence of Certain Features of AMP-induced Apoptosis
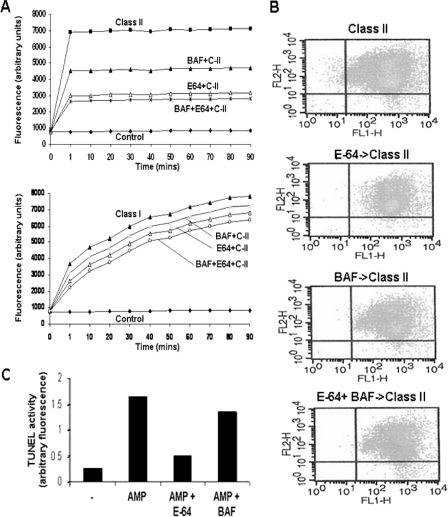
In our previous work we showed that DNA degradation in C-II-treated parasites could be inhibited by pretreatment of cells by E-64 but not by the pancaspase inhibitor BAF (5). We wanted to determine whether other features of AMP-induced apoptosis were affected by these inhibitors. We pretreated cells with E-64, BAF, or both together followed by the addition of C-II and analyzed cells for changes in cell surface permeability and annexin PI staining as well as TUNEL activity (Fig. 3). Pretreatment of cells with either BAF or E-64 caused dramatic decreases in the permeability of SYTOX. The decrease in surface permeability to SYTOX was greater in cells incubated with E-64 than BAF (Fig. 3A). The addition of BAF to E-64 has a marginal effect, further diminishing SYTOX permeability. Neither inhibitor fully reduced the surface permeability to the level of the untreated control even when the concentration was increased up to 5-fold (not shown). We also compared the change in cell permeability by the C-I AMP protegrin in the presence of these inhibitors. C-I caused a slowly progressive increase in SYTOX permeability, which contrasted dramatically with the rapid influx of SYTOX caused by C-II. Pretreatment of cells with E-64+BAF had a minimal (∼20%) effect by reducing cell permeability, and the individual agents alone have even less effect (Fig. 3A, lower panel). To determine whether the changes in annexin V staining at the surface membrane caused by AMPs was related to the activity of cysteine and/or caspase activity, we analyzed cells for annexin V-PI co-staining after preincubation with E-64 or BAF and then with C-II (Fig. 3B). C-II treatment alone caused almost all cells to co-stain with annexin V-PI as seen previously (5). Pretreatment with inhibitors, either alone or in combination, did not appreciably change the profile of annexin V-PI staining caused by C-II. C-II-induced DNA degradation, as demonstrated by increased TUNEL activity (Fig. 3C) was substantially diminished by E-64, but not BAF, as we have demonstrated previously using agarose gel electrophoresis (5).
FIGURE 3.
Caspase and cysteine protease dependence of certain features of AMP-induced apoptosis. L. major mutants were pretreated with E-64 and/or BAF for 30 min prior to treatment with pexiganan (Class II AMP, C-II) and then analyzed for changes surface membrane permeability by the vital dye SYTOX (A), in propidium iodide/annexin V staining (B), and in DNA degradation by TUNEL assay (C).
Class II AMP-mediated Apoptosis Is Associated with Increases in Intracellular Calcium
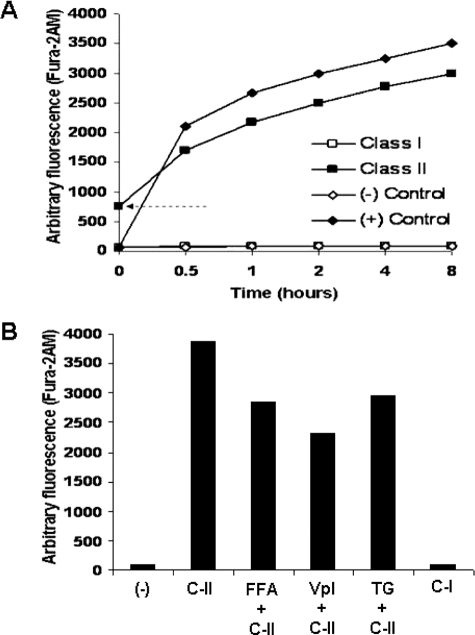
Because apoptosis of Leishmania induced by other stimuli can lead to release of calcium from intracellular stores, we wanted to determine whether AMP-mediated apoptosis was associated with significant delocalization of intracellular calcium. Fura 2AM-loaded cells were incubated with either C-I or -II AMP under our standard conditions, and increasing fluorescence was measured over 8 h (Fig. 4A). Class II AMP treatment caused a rapid rise in the amount of intracellular calcium nearly equivalent to that of the control of cells incubated in buffer alone. We detected elevated calcium even at time 0 in the AMP-treated cells. The processing time of this sample is ∼20 s, so AMP induced calcium flux is detectable within this time frame. Class I AMP-treated cells did not have a demonstrable increase in intracellular calcium. To investigate whether voltage-gated or nonspecific calcium channels are involved in AMP-meditated apoptotic calcium changes, we preincubated cells in FFA and verapamil, nonspecific, and voltage-gated calcium channel inhibitors, respectively. FFA treatment followed by incubation with C-II caused a ∼25% reduction in calcium flow at 8 h (Fig. 4B). Verapamil treatment of cells further reduced calcium delocalization to ∼40% of that caused by AMP alone.
FIGURE 4.
Class II AMP-induced apoptosis leads to dramatic increases in intracellular Ca2+ that are in part due to both nonspecific and voltage-gated Ca2+ channels. Parasites were preloaded with the calcium-dependent fluor Fura 2AM, then treated with PG-1 (C-I) and pexiganan (C-II), and analyzed for increasing fluorescence (A). Controls include cells left untreated (−) and those treated incubated in buffer containing 25 μm CaCl2. The arrow indicates the starting baseline elevated intracellular calcium in the sample just after the addition of and mixing of AMP and placement into the detector. The effects of inhibition of nonspecific and voltage-gated calcium channels (using flufenamic acid (FFA) and verapamil (Vpl), respectively) on calcium delocalization were tested as described in A except that cells were preincubated with the designated inhibitors for 30 min prior to incubation with AMP (B). The Ca2+-ATPase thapsigargin (TG) was also included as a positive control for release of cytosolic calcium.
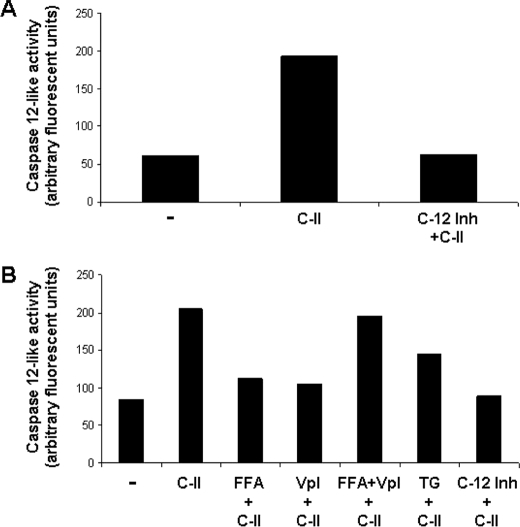
Caspase-12-like Activation Occurs during AMP-mediated Apoptosis
Mammalian caspase-12 undergoes activation in a calcium-dependent manner and is thought to be related to endoplasmic reticulum-related stress (24, 25). Because AMP-mediated apoptosis is associated with the large increases in the cytosolic calcium, we investigated whether cells exposed to C-II AMP led to an increase in caspase-12-like activity. Parasites were exposed to AMP, and lysates were then incubated with the caspase-12-specific substrate. The cells exposed to AMP had ∼4-fold higher levels of activity compared with the background level in untreated cells (Fig. 5A). Preincubation of cells with caspase-12-specific inhibitor prior to analysis had only background levels of activity. We also tested cells exposed to C-II for activation of caspase-8- and 10-like activity in the same manner and have not detected these activities (not shown). We tested whether induction of caspase-12-like activity was dependent on the either nonspecific or voltage-gated Ca2+ channels using FFA and verapamil (Fig. 5B). Preincubation of cells with either inhibitor followed by AMP exposure and analysis of caspase-12-like activity decreased activity nearly to the base-line level. FFA+verapamil preincubation of cells followed by C-II did not appreciably reduce caspase-12-like activity, suggesting that the FFA and verapamil may be antagonistic.
FIGURE 5.
Class II AMP induced increases in Ca2+-dependent caspase-12-like activity. A, parasites were treated for 2 h with pexiganan (C-II) under standard conditions and then assessed for caspase-12-like activity using the fluorescent caspase-12 substrate. The specificity of the activity was tested by preincubation of cells with a caspase-12 specific inhibitor prior to analysis of caspase-12 activity. B, the effects of nonspecific (FFA) and voltage-gated (verapamil, Vpl) calcium channel inhibitors on caspase-12-like activity were tested as described for A, except the cells were preincubated with the designated inhibitors for 30 min prior to incubation with AMP. The Ca2+ ATPase thapsigargin (TG) was also included as a positive control for release of cytosolic calcium.
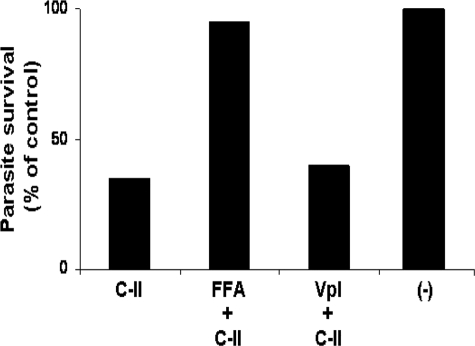
Blockade of Nonspecific Ca2+ Channels Abolishes AMP-mediated Apoptosis of Leishmania
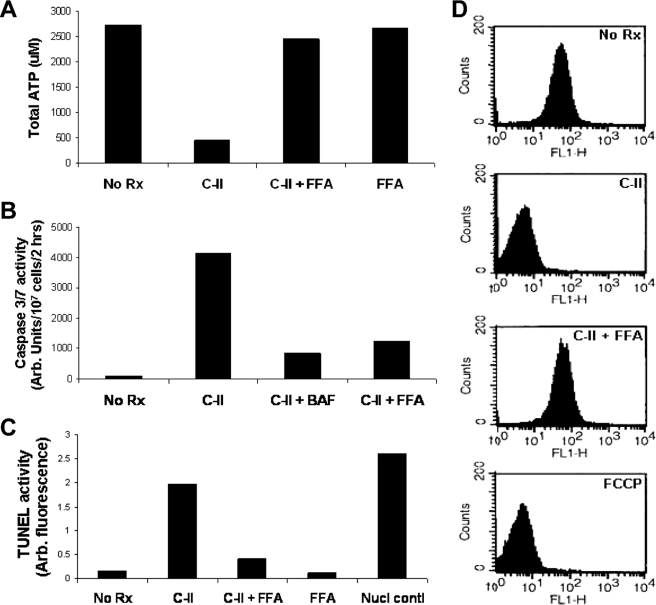
Increased levels of free intracellular calcium can be toxic to mitochondria and cause apoptosis of Leishmania by other agents (7, 8, 10). FFA can diminish Ca2+-mediated mitochondrial toxicity induced by camptothecin and antimony. We tested whether the Ca2+ toxicity mediated through FFA-sensitive channels plays a role in AMP-mediated cell death. Parasites were preincubated in either FFA or verapamil and then exposed to C-II followed by analysis of cell death using metabolism of MTT (Fig. 6). The cells preincubated in FFA were completely protected from AMP killing, surviving just as well as controls cells. The cells preincubated in verapamil were killed by AMP as readily as controls incubated in AMP alone. We analyzed the effect of FFA on various aspects of AMP-induced apoptosis by C-II. FFA preincubation of cells prior to exposure to C-II led to the ability of cells to produce ATP similar to that of untreated cells (Fig. 7A), whereas cells treated with C-II alone lost their ability to produce ATP. Interestingly, FFA-treated cells produced only minimal caspase-3/7-like activity when exposed to C-II, whereas cells treated with C-II alone exhibited substantial amounts of this activity (Fig. 7B), consistent with that we have observed before (Fig. 1C) (5). Similarly, FFA pretreatment of cells substantially diminished AMP-induced TUNEL activity (Fig. 7C). Lastly we tested whether FFA pretreatment could protect mitochondria from Ca2+-dependent effects on mitochondrial membrane potential, contributing to the overall mitochondrial toxicity and diminished ATP production. Indeed, pretreatment of cells with FFA protected cells from C-II-induced effects on mitochondrial membrane depolarization (Fig. 7D).
FIGURE 6.
Selective inhibition of nonspecific Ca2+-channels abolishes Class II AMP-mediated apoptosis. The parasites were preincubated with either FFA or verapamil (Vpl) as indicated and then exposed to C-II AMP under standard conditions. Parasite survival was assessed using the MTT survival assay (30). The percentage of survival was determined relative to untreated control cells (−).
FIGURE 7.
Effect of nonspecific Ca2+ channel inhibition on aspects of Class II AMP-mediated apoptosis. Total ATP (A), caspase-3/7-like activity (B), TUNEL activity (C), and flow cytometry after staining with rhodamine 123 (D) were measured in cells with and without pretreatment with FFA. C-II AMP and FFA treatment was done according to conditions described above. BAF, FCCP, and nuclease treatment were done as described previously (5).
Differential Cellular Localization of Class 1 and II AMPs
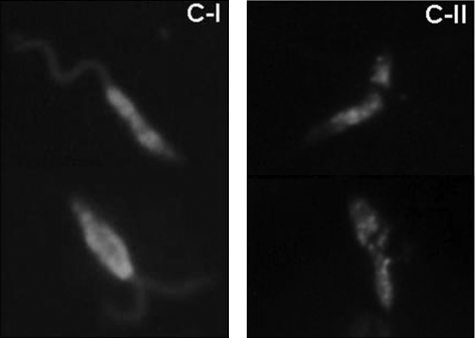
Because C-I and -II AMPs appear to kill Leishmania in different ways, we hypothesized that they may associate with cells differently. To test this we used fluorescently tagged PG-1 and pexiganan to treat stationary phase parasites and then analyzed them using direct fluorescent microscopy (Fig. 8). Class 1 AMP predominantly decorated the surface membrane of the cell, permitting visualization of the entire cell body and flagellum, whereas C-II AMP treatment showed a punctate intracellular distribution localized only to the cell body.
FIGURE 8.
Differential cell association of Class I and II AMPs. Fluoresceinated Class I and II AMPs were used to treat stationary phase L. major promastigotes for 20 min prior to washing and direct fluorescence analysis. Class I AMP mostly decorates the surface membrane of the cell including cell body and flagellum, whereas Class II AMP penetrates the cell becoming predominately intracellular.
DISCUSSION
There is a wide range of structural and functional diversity among antimicrobial peptides. Our work here documents that AMPs of different structure can kill Leishmania by distinct mechanisms. The magainin- type AMPs induce apoptosis (here termed Class II AMPs), and those of the cathelicidin-type kill by nonapoptosis (termed Class I). Class II AMP causes a number of notable features consistent with apoptosis including changes in the surface membrane permitting annexin V staining, DNA degradation, depolarization of mitochondrial membrane potential, and caspase-3/7-like activation. None of these occur in cell death caused by C-I AMPs. Both peptides caused cell membrane permeability changes but with vastly different kinetics. Class II AMP exposure led to a rapid increase in SYTOX permeability to a maximal level at 1 min post-exposure (fast influx), whereas C-I AMP lead to a gradual increase of SYTOX permeability (slow influx). The nature of these differences on SYTOX influx is not entirely clear but probably relate to differences in size, density, and kinetics of pore formation on the membrane. Magainin- and protegrin-type AMPs are known create vastly different patterns of membrane disruption. Magainins adopt α-helical structures, whereas protegrins adopt a β-sheet structure, and these differences translate into differences in interactions with microbial membranes (6). The downstream effects of peptide action at the membrane may influence their differential modes of cell death. This may involve intracellular signaling and/or changes in ion flux. We have analyzed fluorescently labeled AMPs and their penetration into cells and found that C-II but not C-I AMPs appear to enter cells (Fig. 8). Thus C-II may directly interact with intracellular organelles or proteins, whereas C-I interacts predominantly with surface membranes, disrupting them and killing cells by osmotic lysis as we have seen in the African trypanosomes treated with C-I AMPs (21).
Caspase-3/7-like activation occurs in AMP-mediated apoptosis. The trigger for this activation is unclear in our system but may involve the leakage of cytochrome c from mitochondria and activation of a caspase-9-like enzyme, which activates caspase-3/7. Alternatively caspase-8-like enzyme activation, via a death signal equivalent to that which occurs by activation of the Fas/FADD system (26, 27), could trigger caspase-3/7 activation. We have not detected caspase-8-like activity in our system. Activated caspase-12 can, in turn, activate caspase-3/7, so we analyzed AMP -treated parasites for this activity. Indeed we found that AMP treatment leads to an increase in caspase-12-like activity. This was specific because pretreatment of cells with caspase-12 inhibitor ablated the activity in AMP-treated cells. Interestingly preincubation with this inhibitor did not ablate AMP-induced apoptotic death. This suggests that although AMP induced caspase-12-like activity that this, by itself, is not an initiating event in apoptosis in our system. Preincubation of cells with the pancaspase inhibitor BAF followed by AMP also did not stop cell death, suggesting that caspase-like activity alone is not responsible for apoptosis. Inhibition of cysteine proteases (CP) using E-64 stops DNA degradation associated with apoptosis (Fig. 3) (5), yet it does not stop cell death, suggesting that CP-driven DNA degradation is a byproduct of but does not directly contribute to cell death. Inhibition of both the caspase and CP enzyme systems together using BAF and E-64 does not ablate AMP-mediated apoptosis. Thus AMP treatment of Leishmania may trigger both caspase-dependent and caspase-independent events within the apoptotic pathway, and ablation of CP-induced DNA degradation is not sufficient to rescue cells from apoptosis. In the results presented here and in our previous studies, E-64 treatment did not lead to complete inhibition of DNA degradation in AMP-treated cells, so it is possible that other caspase-independent processes, triggering the DNA “degradosome” endonucleases as seen in baicalein-treated L. donovani, may be operative in our system (17).
Caspase-12 activation is a calcium-dependent process. Because we detected increased caspase-12-like activity in AMP-treated parasites, we investigated whether this was related to increases in intracellular calcium. Indeed, we detected increases in intracellular Ca2+ specifically in cells treated with C-II, but not C-I AMPs. These assays were performed in the absence of extracellular calcium in the buffer system, indicating that the increase in cytosolic Ca2+ was derived from intracellular stores within the endoplasmic reticulum, glycosomes, acidocalcisomes, and/or the mitochondria (28). Increases in intracellular Ca2+ were only partially inhibited with FFA or verapamil, suggesting that AMP treatment causes minimal dysfunction to nonspecific or voltage-gated channels. Increases in cytosolic Ca2+ have been found in Leishmania undergoing apoptosis caused by other agents (7, 8, 10). Both antimony and camptothecin-treated L. donovani undergo apoptosis associated with increased cytosolic Ca2+. Camptothecin-induced increase in Ca2+ led to breakdown of mitochondrial membrane depolarization, which may lead to cytochrome c leakage and caspase activation (10). Flufenamic acid can block the rise in antimony-related increases in intracellular Ca2+, and this inhibitor can prevent Ca2+ flux across isolated mitochondria. We hypothesize that increases in cytosolic Ca2+ caused by AMP are directly toxic to mitochondria, causing a breakdown in membrane potential and a loss of ATP production. Because FFA pretreatment can block nonspecific Ca2+ channels of mitochondria (11), we tested whether this inhibitor could affect AMP-mediated apoptotic death of Leishmania. To our surprise, pretreatment of parasites with FFA followed by AMP exposure completely ablated cell death. The mitochondrial membrane potential of these cells was totally preserved, yet the SYTOX permeability was similar to cells treated with AMP alone (data not shown). The ability of these cells to stain with annexin V was similar to that of untreated controls. These data suggest that FFA blocks Ca2+-related mitochondrial toxicity and that some changes at the surface membrane are unrelated to mitochondrial function and availability of ATP. Alternatively FFA may block calcium release from other compartments in the cell indirectly, preventing its toxic effects on mitochondrial function. FFA pretreatment of cells also blocked caspase-3/7-like enzyme activation and increased TUNEL activity. Clearly, C-II AMPs kill parasites through intracellular calcium delocalization, which leads to mitochondrial toxicity and cell death. We hypothesize that C-II causes a release of calcium for intracellular stores. Whether this release is from the endoplasmic reticulum, glycosomes, or acidocalcisomes and by what mechanism (nonspecific organellar disruption or a more discrete process) awaits further investigation. The effects of AMP on caspase-3/7- and -12-like activity probably do not contribute to cell death because preincubation of cells with the corresponding inhibitors prior to exposure to C-II does not prevent apoptosis. Because FFA prevents activation of both of these caspases during death, it appears that their activation is dependent on either maintenance of intracellular calcium regulation and/or ATP levels. FFA alone had no toxic effects on parasites, which is in contrast to what has been reported in isolated mammalian mitochondria where it uncouples mitochondrial oxidative phosphorylation and diminishes ATP production (29). We hypothesize that FFA blocks calcium uptake through nonspecific channels similar to that seen in mammalian mitochondria (11). This may protect mitochondria from changes in membrane depolarization induced by calcium preserving mitochondrial respiration.
Clearly AMPs of different subclasses can kill Leishmania in two distinct ways. Our work here provides some clues as to the pathway in which Leishmania undergo AMP-induced apoptosis and the pivotal role of calcium to this process. Apoptosis in Leishmania may influence the outcome of mammalian infection, so it is important to further understand the pathways leading to cell death. This information may be important for the design of chemotherapeutics against leishmaniasis.
This work was supported, in part, by the funding from the Ohio State University, American Heart Association Beginning Grant in-Aid and Scientist Development Grant awards (to B. S. M.), and Canadian Institutes of Health Grant MOP 7399 (to W. R. M.).
- AMP
- antimicrobial peptide
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
- phosphate-buffered saline
- BAF
- 1-3-Boc-aspartyl-fluoromethyl ketone; Z, benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- TUNEL
- deoxynucleotidyltransferase-mediated dUTP nick end labeling
- FCCP
- carbonyl cyanide p-trifuoromethoxyphenylhydrazone
- MOPS
- 4-morpholinepropanesulfonic acid
- FFA
- flufenamic acid
- PI
- propidium iodide
- C-I
- Class I
- C-II
- Class II.
REFERENCES
- 1.Holzmuller P., Sereno D., Cavaleyra M., Mangot I., Daulouede S., Vincendeau P., Lemesre J. L. ( 2002) Infect. Immun. 70, 3727– 3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zangger H., Mottram J. C., Fasel N. ( 2002) Cell Death Differ. 9, 1126– 1139 [DOI] [PubMed] [Google Scholar]
- 3.van Zandbergen G., Solbach W., Laskay T. ( 2007) Autoimmunity 40, 349– 352 [DOI] [PubMed] [Google Scholar]
- 4.van Zandbergen G., Bollinger A., Wenzel A., Kamhawi S., Voll R., Klinger M., Müller A., Hölscher C., Herrmann M., Sacks D., Solbach W., Laskay T. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 13837– 13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni M. M., McMaster W. R., Kamysz E., Kamysz W., Engman D. M., McGwire B. S. ( 2006) Mol. Microbiol. 62, 1484– 1497 [DOI] [PubMed] [Google Scholar]
- 6.Yang L., Weiss T. M., Lehrer R. I., Huang H. W. ( 2000) Biophys. J. 79, 2002– 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das M., Mukherjee S. B., Shaha C. ( 2001) J. Cell Sci. 114, 2461– 2469 [DOI] [PubMed] [Google Scholar]
- 8.Sudhandiran G., Shaha C. ( 2003) J. Biol. Chem. 278, 25120– 25132 [DOI] [PubMed] [Google Scholar]
- 9.Sen N., Das B. B., Ganguly A., Mukherjee T., Tripathi G., Bandyopadhyay S., Rakshit S., Sen T., Majumder H. K. ( 2004) Cell Death Differ. 11, 924– 936 [DOI] [PubMed] [Google Scholar]
- 10.Sen N., Das B. B., Ganguly A., Mukherjee T., Bandyopadhyay S., Majumder H. K. ( 2004) J. Biol. Chem. 279, 52366– 52375 [DOI] [PubMed] [Google Scholar]
- 11.McDougall P., Markham A., Cameron I., Sweetman A. J. ( 1988) Biochem. Pharmacol. 37, 1327– 1330 [DOI] [PubMed] [Google Scholar]
- 12.Jordani M. C., Santos A. C., Prado I. M., Uyemura S. A., Curti C. ( 2000) Mol. Cell Biochem. 210, 153– 158 [DOI] [PubMed] [Google Scholar]
- 13.Paris C., Loiseau P. M., Bories C., Bréard J. ( 2004) Antimicrob. Agents Chemother. 48, 852– 859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnoult D., Akarid K., Grodet A., Petit P. X., Estaquier J., Ameisen J. C. ( 2002) Cell Death Differ. 9, 65– 81 [DOI] [PubMed] [Google Scholar]
- 15.Ivens A. C., Peacock C. S., Worthey E. A., Murphy L., Aggarwal G., Berriman M., Sisk E., Rajandream M. A., Adlem E., Aert R., Anupama A., Apostolou Z., Attipoe P., Bason N., Bauser C., Beck A., Beverley S. M., Bianchettin G., Borzym K., Bothe G., Bruschi C. V., Collins M., Cadag E., Ciarloni L., Clayton C., Coulson R. M., Cronin A., Cruz A. K., Davies R. M., De Gaudenzi J., Dobson D. E., Duesterhoeft A., Fazelina G., Fosker N., Frasch A. C., Fraser A., Fuchs M., Gabel C., Goble A., Goffeau A., Harris D., Hertz-Fowler C., Hilbert H., Horn D., Huang Y., Klages S., Knights A., Kube M., Larke N., Litvin L., Lord A., Louie T., Marra M., Masuy D., Matthews K., Michaeli S., Mottram J. C., Müller-Auer S., Munden H., Nelson S., Norbertczak H., Oliver K., O'Neil S., Pentony M., Pohl T. M., Price C., Purnelle B., Quail M. A., Rabbinowitsch E., Reinhardt R., Rieger M., Rinta J., Robben J., Robertson L., Ruiz J. C., Rutter S., Saunders D., Schäfer M., Schein J., Schwartz D. C., Seeger K., Seyler A., Sharp S., Shin H., Sivam D., Squares R., Squares S., Tosato V., Vogt C., Volckaert G., Wambutt R., Warren T., Wedler H., Woodward J., Zhou S., Zimmermann W., Smith D. F., Blackwell J. M., Stuart K. D., Barrell B., Myler P. J. ( 2005) Science 309, 436– 442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N., Gannavaram S., Selvapandiyan A., Debrabant A. ( 2007) Eukaryot. Cell 6, 1745– 1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BoseDasgupta S., Das B. B., Sengupta S., Ganguly A., Roy A., Dey S., Tripathi G., Dinda B., Majumder H. K. ( 2008) Cell Death Differ. 15, 1629– 1640 [DOI] [PubMed] [Google Scholar]
- 18.Joshi P. B., Kelly B. L., Kamhawi S., Sacks D. L., McMaster W. R. ( 2002) Mol. Biochem. Parasitol. 120, 33– 40 [DOI] [PubMed] [Google Scholar]
- 19.Ge Y., MacDonald D. L., Holroyd K. J., Thornsberry C., Wexler H., Zasloff M. ( 1999) Antimicrob. Agents Chemother. 43, 782– 788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellm L., Lehrer R. I., Ganz T. ( 2000) Exp. Opin. Investig. Drugs 9, 1731– 1742 [DOI] [PubMed] [Google Scholar]
- 21.McGwire B. S., Olson C. L., Tack B. F., Engman D. M. ( 2003) J. Infect. Dis. 188, 146– 152 [DOI] [PubMed] [Google Scholar]
- 22.Chicharro C., Granata C., Lozano R., Andreu D., Rivas L. ( 2001) Antimicrob. Agents Chemother. 45, 2441– 2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaz-Achirica P., Ubach J., Guinea A., Andreu D., Rivas L. ( 1998) Biochem. J. 330, 453– 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong W. X., Li C., Hatzivassiliou G., Lindsten T., Yu Q. C., Yuan J., Thompson C. B. ( 2003) J. Cell Biol. 162, 59– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. ( 2000) Nature 403, 98– 103 [DOI] [PubMed] [Google Scholar]
- 26.Chen C. Y., Juo P., Liou J. S., Li C. Q., Yu Q., Blenis J., Faller D. V. ( 2001) Cell Growth Differ. 12, 297– 306 [PubMed] [Google Scholar]
- 27.Eichler T., Ma Q., Kelly C., Mishra J., Parikh S., Ransom R. F., Devarajan P., Smoyer W. E. ( 2006) Toxicol. Sci. 90, 392– 399 [DOI] [PubMed] [Google Scholar]
- 28.Moreno S. N., Docampo R. ( 2003) Curr. Opin. Microbiol. 6, 359– 364 [DOI] [PubMed] [Google Scholar]
- 29.McDougall P., Markham A., Cameron I., Sweetman A. J. ( 1983) Biochem. Pharmacol. 32, 2595– 2598 [DOI] [PubMed] [Google Scholar]
- 30.Kiderlen A. F., Kaye P. M. ( 1990) J. Immunol. Methods 127, 11– 18 [DOI] [PubMed] [Google Scholar]