Abstract
Although all established functions of dengue virus NS5 (nonstructural protein 5) occur in the cytoplasm, its nuclear localization, mediated by dual nuclear localization sequences, is essential for virus replication. Here, we have determined the mechanism by which NS5 can localize in the cytoplasm to perform its role in replication, establishing for the first time that it is able to be exported from the nucleus by the exportin CRM1 and hence can shuttle between the nucleus and cytoplasm. We define the nuclear export sequence responsible to be residues 327–343 and confirm interaction of NS5 and CRM1 by pulldown assay. Significantly, greater nuclear accumulation of NS5 during infection due to CRM1 inhibition coincided with altered kinetics of virus production and decreased induction of the antiviral chemokine interleukin-8. This is the first report of a nuclear export sequence within NS5 for any member of the Flavivirus genus; because of its high conservation within the genus, it may represent a target for the treatment of diseases caused by several medically important flaviviruses.
The four serotypes of dengue virus (DENV-1–4)2 are the causative agents of the most common arthropod-borne viral disease, dengue fever, and its more severe and potentially deadly dengue hemorrhagic fever form (1). DENV is a member of the genus Flavivirus within the family Flaviviridae. Like all flaviviruses, DENV possesses an ∼11-kb, positive-sense, single-stranded RNA genome that is translated as one long polyprotein and cleaved into 10 viral proteins: three structural (capsid, pre-membrane/membrane, and envelope) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins (2). Flavivirus replication takes place in the cytoplasm, whereby several viral NS and host proteins are believed to constitute the replication complex, the proposed replication machinery of flaviviruses (3). Two key enzymes in replication, NS3 and NS5, the RNA helicase and RNA-dependent RNA polymerase, respectively, interact within the cytoplasm of infected cells (4).
The multifunctional NS5 protein is the largest (900 amino acids, 105 kDa) and most highly conserved of the dengue NS proteins (5–7). NS5 contains an N-terminal S-adenosylmethyltransferase domain (5) and a C-terminal RNA-dependent RNA polymerase domain (8–10) separated by an “interdomain linker region” (see Fig. 1). Despite all well established functions of NS5 occurring within the cytoplasm (2), NS5 is predominantly nuclear in DENV-2-infected cells (4, 11).
FIGURE 1.
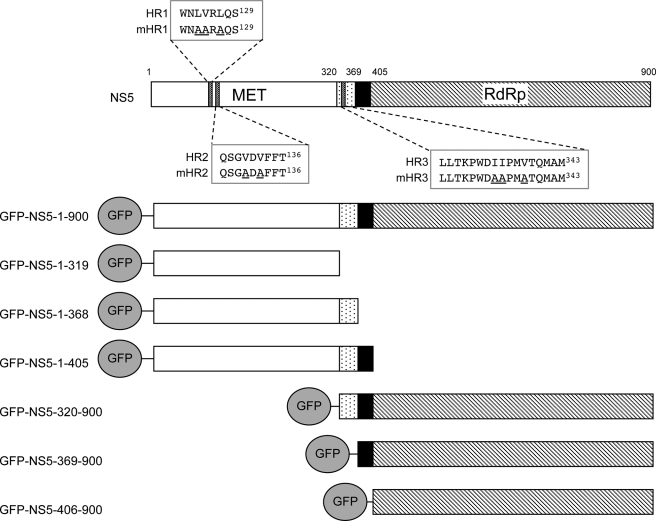
Top, DENV-2 NS5 possesses two domains, the N-terminal methyltransferase (MET) domain and C-terminal RNA-dependent RNA polymerase (RdRp) domain. The interdomain linker region (residues 320–405, containing two adjacent NLSs) is highlighted. bNLS (residues 320–368) and aNLS (residues 369–405) bind Imp-β/NS3 and Imp-α/β, respectively. Bottom, shown are the GFP-NS5 constructs made in this study. Encoded proteins are shown with the N terminus (residues 1–319; white boxes), bNLS (dotted boxes), aNLS (black boxes), and C terminus (residues 406–900; striped boxes) marked. Hydrophobic regions (termed HR1–HR3) identified as putative NESs are highlighted, and the corresponding alanine substitutions (underlined) generated in this study are indicated (mHR1–mHR3).
Proteins >45 kDa require a nuclear localization sequence (NLS) for transport into the nucleus (12, 13). NLSs confer interaction with members of the importin (Imp) superfamily of transporters (either an Imp-α/β heterodimer or Imp-β or a homolog thereof), which mediate the translocation of a cargo into the nucleus. Within the nucleus, the cargo-NLS-Imp complex is dissociated through binding of Ran-GTP to Imp-β, releasing the cargo into the nucleoplasm. Analogously, proteins containing nuclear export sequences (NESs) interact with Imp-β homologs termed exportins, which, when complexed with Ran-GTP, mediate translocation out of the nucleus and into the cytoplasm (14–16). The best characterized of these is CRM1 (exportin 1), which typically binds hydrophobic/leucine-rich NESs (17, 18), such as those of the human immunodeficiency virus Rev protein (19) or the protein kinase A inhibitor PKI (20). The antibiotic leptomycin B (LMB), able to bind CRM1 specifically and to prevent CRM1-NES interaction (21–23), has been widely used to demonstrate the involvement of CRM1 in biological processes (24–28).
Previously, we showed that DENV-2 NS5 possesses two NLSs within the interdomain region (see Fig. 1) (29). The C-terminal NLS (amino acids 369–405) or “aNLS” is recognized by Imp-α/β with high affinity and is able to target β-galactosidase to the nucleus in either microinjected or mechanically perforated rat hepatoma cells (29). In contrast, the N-terminal NLS (amino acids 320–368) or “bNLS” is able to bind either Imp-β or NS3 directly in a competitive fashion (30, 31).
We demonstrated recently that when NS5 nuclear import is impaired by mutation of the viral genome, the virus is no longer viable, indicating that NS5 nuclear import is essential for virus replication (11). This is attributable, at least in part, to the role of nuclear NS5 in inhibiting induction of the antiviral chemokine interleukin-8 (IL-8) during DENV infection; reduced NS5 nuclear accumulation correlates with increased virus production (11, 32). At least one of the roles of nuclear NS5 is thus to reduce the magnitude of the antiviral response in terms of IL-8 induction.
The undisputed role of NS5 is in replication in the cytoplasm. Here, we shed light for the first time on how despite its efficient nuclear localization ability, NS5 is able to fulfill this role. We report the ability of NS5 to be exported from the nucleus in a CRM1-dependent fashion in both transfected and DENV-2-infected cells and identify the NES responsible. Importantly, we show that inhibition of CRM1 during DENV-2 infection results in increased nuclear NS5, reduced IL-8 induction, and increased virus production, underlining the importance of NS5 nuclear export to DENV infection. Our results thus establish for the first time the ability of NS5 to shuttle between the nucleus and cytoplasm and its importance to modulation of the host antiviral response and virus replication.
EXPERIMENTAL PROCEDURES
Cell Lines and Virus Infection
Vero, 293, and HEK-293T cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum. C6/36 mosquito (Aedes albopictus) cells used for infection were cultured in basal Eagle's medium containing 10% fetal calf serum. DENV-2 New Guinea C strain-infected Vero cells were maintained in Dulbecco's modified Eagle's medium containing 2% fetal calf serum. Where indicated, 5 ng/ml LMB (provided by M. Yoshida) was added to the culture medium at 0 h post-infection. At specific time points post-infection, the culture medium was collected for virus titration, and coverslips were seeded with Vero cells fixed for indirect immunofluorescence (see below). Virus titers, calculated as plaque-forming units/ml, were determined in plaque assays using C6/36 cells (33).
Cell Culture and Transfection
DNA transfection of Vero cells was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Where indicated, 2.8 ng/ml LMB was added to the culture medium 15–19 h post-transfection, and live cell imaging was performed (see below) at 20–24 h post-transfection.
Construction of GFP-NS5 and GST-NS5 Mammalian Expression Vectors
Constructs expressing full-length and truncated NS5 forms fused in-frame with GFP at the N terminus (see Fig. 1) were generated using GatewayTM cloning technology (Invitrogen) according to the manufacturer's instructions. Briefly, NS5 PCR fragments from the DENV-2 Townsville strain (TSV01) were generated using primers designed to include attB1 or attB2 sites to allow integration into the Gateway system. These truncations were inserted into the pDONR207 vector by the BP-Reaction (Invitrogen) and subsequently into the mammalian GFP fusion protein construct pEPI-GFP (34) or the pDEST27 mammalian GST fusion protein expression construct using the LR-Reaction (Invitrogen).
Site-directed Mutagenesis
Hydrophobic regions (HR1–HR3) (Fig. 1) were selected as putative NES regions based on their similarity to known NESs (35). Leucine/isoleucine/valine residues within these regions were mutated to alanine to generate mHR1–mHR3, whereby primers containing the appropriate mutations were designed and used in overlap extension PCR as described previously (36). Overlap extension PCR products encoding either full-length NS5 or NS5-(1–368) containing the appropriate mutations were inserted into pDONR207 and then pEPI-GFP using the BP-Reaction and LR-Reaction, respectively.
Introduction of NS5 mHR3 into pDVWS601
Mutations were inserted into the genomic length DENV-2 (New Guinea C) cDNA clone pDVWS601 as described previously (33, 37). Initially, DNA fragments containing the mHR3 mutations were generated by overlap extension PCR using plasmid pDVWS601 as the PCR template. The overlap extension PCR fragments were then transferred into plasmid pDVWS601 using the unique restriction sites AatII8570 and MluI9732. Experiments to generate virus from the genomic length cDNA clones was performed as described previously (11).
GST Pulldown Assay and Western Analysis
HEK-293T cells grown in 10-cm culture dishes were transfected using Lipofectamine 2000 (as per the manufacturer's instructions) to express GST-NS5 fusion protein constructs. Cell lysates were collected 48 h post-transfection using cell lysis buffer (50 mm Tris (pH 7.5), 250 mm NaCl, 5 mm EDTA, 0.1% (v/v) Triton X-100, 0.1 mm Na3VO4, 0.1 mm phenylmethylsulfonyl fluoride, and one protease inhibitor mixture tablet (Sigma)/50 ml) and centrifuged to remove cellular debris. Pulldown assays were performed by adding 50 μl of glutathione-Sepharose 4B (Amersham Biosciences) bead slurry to lysates and rotating at 4 °C for ∼16 h. Beads were gently centrifuged and washed four times with 1 ml of cell lysis buffer. Bound proteins were removed by adding 40 μl of 2× sample buffer and heating at 95 °C for 5 min. 20 μl of sample was separated by 13% SDS-PAGE (38) and subsequently transferred to a nitrocellulose membrane for Western analysis. Membranes were blocked in phosphate-buffered saline, 0.1% Tween 20, and 5% skim milk for 1 h at room temperature; probed using antibodies to CRM1 (1:1500; BD Transduction Laboratories) or anti-GST (1:1000; Santa Cruz Biotechnology), followed by detection using horseradish peroxidase-conjugated secondary antibodies (Chemicon) according to the manufacturers' instructions; and visualized using enhanced chemiluminescence.
Indirect Immunofluorescence Staining
Vero cells were either mock- or DENV-2-infected at a multiplicity of infection of ∼10. At several time points post-infection, cells were fixed with cold methanol/acetone (1:1) for 2 min. Immunostaining was performed using anti-NS5 antibody raised in rabbits against a fusion protein containing residues 397–772 of DENV-2 NS5 (a gift from Dr. Keng Teo) or using anti-capsid protein-specific monoclonal antibodies (39) together with Alexa Fluor® 488 green fluorescent dye-conjugated goat anti-rabbit secondary antibody. Cells were then mounted in glycerol and 2% propyl gallate for imaging using confocal laser scanning microscopy (CLSM).
CLSM Analysis
A Bio-Rad MRC 500 confocal laser scanning microscope with 40× water immersion and 60× oil immersion lens was used for live and fixed cells, respectively, using the Kalman filter mode. CLSM images were analyzed using Image J Version 1.33 software (40, 41), whereby the fluorescence intensity in the nucleus (Fn) or nucleolus (Fnu) and the cytoplasm (Fc) was determined, and the background fluorescence (untransfected cells) was subtracted to enable the nuclear/cytoplasmic ratio (Fn/c) or the nucleolar/cytoplasmic ratio (Fnu/c) to be calculated.
Measurement of IL-8 Production
The concentration of IL-8 in mock- or DENV-2-infected 293 cell culture supernatants at 48 h post-infection was measured using a commercially available enzyme-linked immunosorbent assay kit (Quantikine human IL-8 kit, R&D Systems) as described previously (11). Where indicated, cells were treated with 5 ng/ml LMB for the indicated times post-infection.
RESULTS
The NS5 N Terminus Contains a CRM1-recognized NES
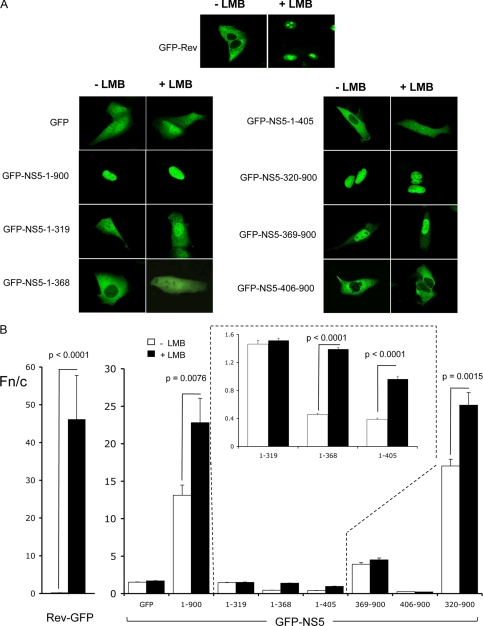
Although the accepted role of NS5 during DENV infection is to mediate replication of the viral genome in the cytoplasm of infected cells, it is predominantly nuclear throughout infection (11). We hypothesized that NS5, in addition to its NLSs, must possess a nuclear export mechanism to enable it to shuttle between the nucleus and cytoplasm. To test this, Vero cells transfected to express various GFP-NS5 derivative proteins were treated with or without the specific inhibitor of nuclear export, LMB, and imaged live by CLSM (Fig. 2A) prior to image analysis to determine the nuclear/cytoplasmic fluorescence ratio (Fn/c) (Fig. 2B). Full-length NS5 (GFP-NS5-(1–900)) showed strong nuclear accumulation (Fn/c ∼ 13), which was increased >70% (Fn/c ∼ 23) upon LMB treatment (p < 0.0076), implying that NS5 contains a CRM1-mediated NES. Similarly, the previously described GFP-NS5-A1+A2 protein impaired in nuclear import through mutation of the aNLS (11) was predominantly nuclear upon LMB treatment, confirming the activity of the NES in the context of full-length NS5 (Fig. 3C). GFP-NS5-(1–368), GFP-NS5-(1–405), and GFP-NS5-(320–900) similarly all showed significant increases in nuclear accumulation upon LMB treatment. That GFP-NS5-(1–368) was the smallest construct showing a change in subcellular localization implied the presence of a CRM1-mediated NES within NS5 residues 1–368.
FIGURE 2.
NS5 is exported from the nucleus via a CRM1-dependent nuclear export pathway dependent on residues 1–368. A, shown are CLSM images of Vero cells transfected to express the indicated GFP-NS5 constructs in the presence or absence of LMB and imaged at 20–24 h post-transfection. Human immunodeficiency virus Rev (GFP-Rev) and GFP were used as positive and negative controls, respectively. B, quantitative analysis of CLSM images such as those in A was performed to determine the Fn/c values (see “Experimental Procedures”). The results represent the means ± S.E., where significant differences (p values) are indicated between LMB-treated and untreated cells (n ≥ 25).
FIGURE 3.
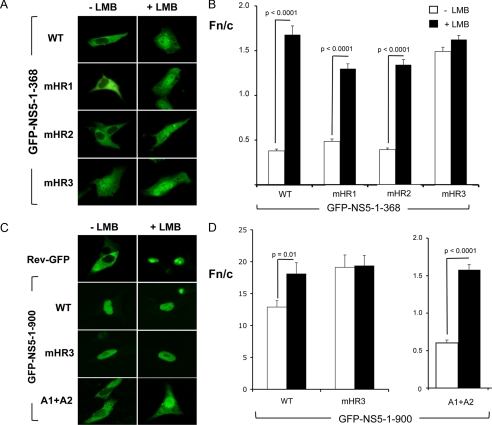
Mapping of the NS5 NES. A, shown are CLSM images of Vero cells transfected to express wild-type (WT) GFP-NS5-(1–368) and mutant derivatives thereof treated with or without LMB. B, quantitative analysis of CLSM images such as those in A was performed to determine the Fn/c values. C, shown are CLSM images of Vero cells transfected to express wild-type GFP-NS5-(1–900), GFP-NS5-(1–900)-mHR3, and GFP-NS5-(1–900)-aNLS-A1+A2 treated with or without LMB. D, quantitative analysis was performed as described for B. Significant differences (p values) are indicated between LMB-treated and untreated cells (n ≥ 27).
The CRM1-recognized NES of NS5 Is Located within the bNLS Region
CRM1-recognized NESs typically comprise four to five hydrophobic residues within ∼10–12 amino acids (35). HR1–HR3 within NS5 residues 1–368 were selected for site-directed mutagenesis to generate the mHR1–mHR3 mutant derivatives (Fig. 1) and tested for functionality by CLSM analysis in the absence and presence of LMB.
The mutant derivatives GFP-NS5-(1–368)-mHR1 and GFP-NS5-(1–368)-mHR2 were both found to resemble wild-type GFP-NS5-(1–368) in terms of nuclear localization ability in either the absence or presence of LMB treatment (Fig. 3, A and B). In contrast, GFP-NS5-(1–368)-mHR3 showed nuclear accumulation in the absence of LMB to an extent similar to that of LMB-treated wild-type GFP-NS5-(1–368). Furthermore, LMB did not markedly increase nuclear localization of the mutant in contrast to wild-type GFP-NS5-(1–368) (p > 0.0001), strongly implicating HR3 as the NS5 CRM1-recognized NES.
To confirm the functionality of HR3 in the full-length NS5 context, mutations were engineered into the HR3 sequence within GFP-NS5-(1–900). Consistent with HR3 being the CRM1-recognized NES of NS5, GFP-NS5-(1–900)-mHR3 was insensitive to LMB and displayed significantly greater nuclear accumulation compared with wild-type GFP-NS5-(1–900) (Fig. 3, C and D).
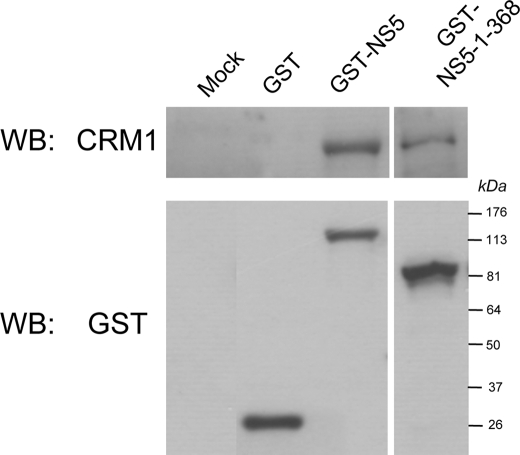
To confirm NS5 interaction with CRM1, HEK-293T cells transiently transfected to express full-length GST-NS5 or GST-NS5-(1–368) were lysed 48 h post-transfection and used in GST pulldown assays. Western analysis of proteins bound to the glutathione-Sepharose beads demonstrated that endogenous CRM1 could be co-precipitated with GST-NS5 or GST-NS5-(1–368), but not with GST alone (Fig. 4).
FIGURE 4.
NS5 complexes with CRM1 in vivo. Non-expressing and GST-, GST-NS5-, and GST-NS5-(1–368)-expressing HEK-293T cells were lysed, and GST co-precipitation assays were performed (see “Experimental Procedures”). Immunoblotting with antibodies to CRM1 and GST was used to detect co-precipitated proteins in combination with horseradish peroxidase-conjugated secondary antibodies for visualization by enhanced chemiluminescence. Molecular mass markers are indicated. WB, Western blot.
NS5 Nuclear Export Occurs Throughout DENV-2 Infection
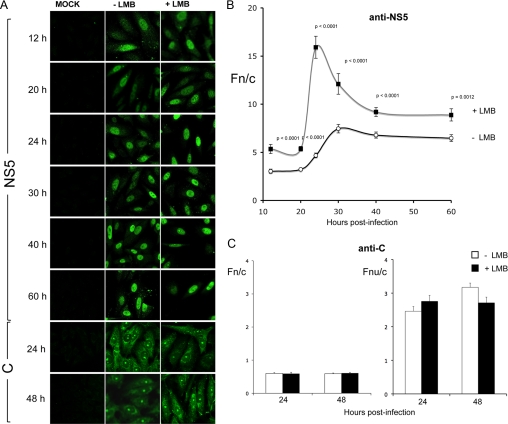
To establish whether the NS5 NES is active during infection, we infected Vero cells with DENV-2 and analyzed NS5 subcellular localization by indirect immunofluorescence/image analysis at various time points throughout infection (Fig. 5, A and B). NS5 was detected within the nucleus as early as 12 h post-infection within ∼42% of cells, and by 20 h post-infection and at all subsequent time points, all cells displayed predominantly nuclear localization. Treatment with LMB increased NS5 nuclear accumulation significantly (p ≤ 0.0012) at all time points (Fig. 5B), indicating that CRM1 actively modulates NS5 localization in infected cells. This was most dramatic at 24 h post-infection, where an Fn/c of 15 was observed, over 3-fold higher than in the absence of LMB. These results show that the NS5 NES is a target of CRM1 action throughout DENV infection.
FIGURE 5.
Inhibition of CRM1 during DENV-2 infection of Vero cells increases nuclear accumulation of NS5, but not capsid protein. A, CLSM images of fixed mock- and DENV-2-infected Vero cells immunostained for NS5 and capsid protein (C) at the indicated time points post-infection and treated with or without LMB. Quantitative analysis to determine Fn/c values for NS5 (B) and Fn/c and Fnu/c values for capsid protein (C) was performed as described in the legend to Fig. 2. Results represent the means ± S.E., where significant differences (p values) are indicated between LMB-treated and untreated cells (n ≥ 48).
CRM1 Activity Dependent on the NS5 NES Modulates Virus Production and IL-8 Induction in DENV-infected Cells
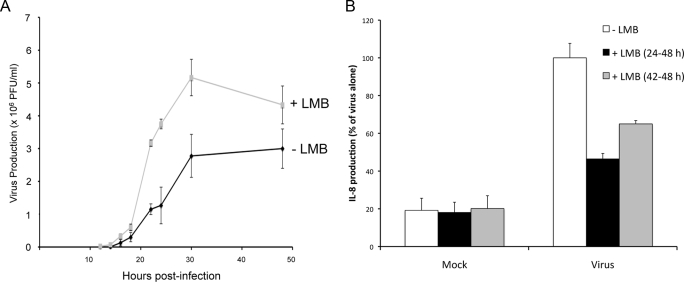
In parallel with our NS5 localization studies during infection (see above), we also examined virus production and IL-8 induction when CRM1 activity was inhibited by LMB. Intriguingly, virus production was higher at all time points post-infection when cells were treated with LMB (Fig. 6A). That the kinetics of virus production were affected by CRM1 inhibition underlines the physiological role of NS5 nuclear export in the infected cell.
FIGURE 6.
Virus production and suppression of IL-8 induction. Virus production (A) and IL-8 induction (B) throughout DENV-2 infection as determined by plaque assay and enzyme-linked immunosorbent assay, respectively, were investigated in the presence or absence of LMB. Results for virus production represent the means ± S.D. of three independent experiments performed in triplicate, whereas those for IL-8 production represent the means ± S.E. of two independent experiments performed in duplicate. PFU, plaque-forming units.
To test the absolute requirement for NS5 nuclear export activity in DENV infection, we used the DENV reverse genetics system as described previously (11) to generate a mutant DENV with a nonfunctional NES (mHR3) (see Fig. 1). Despite repeated attempts, we were unable to isolate recombinant virus containing the NES mutation, consistent with the idea that NS5 nuclear export is critical to DENV infection. However, because the NES-mutated virus was unable to replicate, it was not formally possible to conclude that this effect was due to altered NS5 trafficking as opposed to possible effects on other functions of NS5, such as NS3 interaction, etc.
We have shown previously that one of the roles of nuclear NS5 in infected cells is to reduce the production of IL-8 and thereby dampen host antiviral responses (11). We accordingly analyzed IL-8 production in 293 cells infected with DENV-2 and treated with and without LMB. As expected, we observed a significant reduction of IL-8 induction in cells infected with DENV-2 and treated with LMB compared with untreated cells (Fig. 6B). This effect was more pronounced with longer LMB treatment.
To eliminate the possibility that the effect of LMB on virus production and IL-8 induction may be due to a DENV protein other than NS5, we examined the only other established nuclear protein of DENV, the capsid protein (39, 42, 43), to test whether its localization was affected by LMB. DENV-2-infected cells did not show any significant increase in nuclear or nucleolar accumulation of the capsid protein when treated with LMB at either 24 or 48 h post-infection, suggesting that the capsid protein does not possess a CRM1-mediated NES (Fig. 5, A and C). Because NS5 is the only DENV protein to possess a CRM1-recognized NES and is able to localize in the nucleus to reduce IL-8 induction, it seems highly likely that the effects of LMB on virus and IL-8 production are through inhibition of NS5 nuclear export.
DISCUSSION
This study sheds light for the first time on how despite its strong nuclear localization ability, NS5 can fulfill cytoplasmic functions in replication critical to DENV production. We have shown the active export of NS5 by CRM1 during DENV infection and implicated this pathway in the modulation of host antiviral responses and virus replication. We have mapped the NES responsible to NS5 residues 327–343 within the bNLS and confirmed interaction of NS5 with CRM1. This is the first report of a NES within NS5 for any Flavivirus member. Consistent with the importance of the sequence to the DENV infectious cycle is the fact that viruses possessing mutations within the NES impairing NS5 nuclear export are not viable, although the complexity of the bNLS region with its various binding partners means that we cannot formally conclude that this is due to impairment of NS5 nuclear export alone as opposed to impairment of functions, such as NS3 and/or Imp binding.
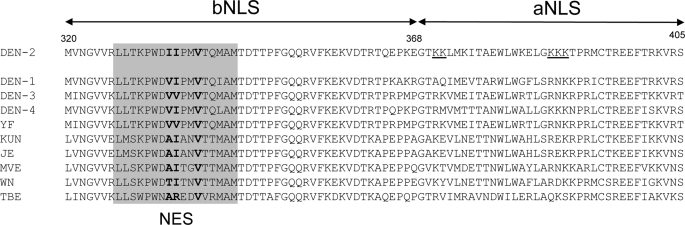
Notably, the NES region within the bNLS of DENV NS5 shows high conservation to the sequences within NS5 of other flaviviruses of interest (Fig. 7), especially with respect to hydrophobic residues (Leu, Val, Ile, and Met); clearly, the interdomain region of NS5 is a conserved “hot spot” for interactions with a range of proteins, including Imp-α/β, Imp-β, NS3, and now CRM1. Intriguingly, it seems likely that Imp-β may compete with CRM1 for binding, thus providing a means by which nuclear import and export may be regulated. Phosphorylation is a common regulator of nuclear targeting pathways (44, 45), including several viral proteins, such as the rabies virus P-protein (46) and chicken anemia virus VP3 (47), both of which regulate their NLS/NES activity via phosphorylation. On the basis of the observation that hyperphosphorylated NS5 is exclusively located in the nucleus (4), we hypothesize that the switch between import and export of NS5 may be mediated by phosphorylation. NS5 dephosphorylation within the nucleus may elicit a conformational change in NS5, allowing CRM1 to bind and hence nuclear export to occur.
FIGURE 7.
Conservation of the NS5 NES within the interdomain linker region (residues 320–405) in Flavivirus members. The NS5 NES (residues 327–343) is highlighted, with the critical residues mutated in this study shown in boldface. The GenBankTM accession numbers of the following sequences are as indicated: DENV-2 TSV01 (DEN-2; AY037116), DENV-1 (DEN-1; U88535), DENV-3 (DEN-3; M93130), DENV-4 (DEN-4; AF32657), yellow fever (YF; X15062), West Nile (WN; M12294), Kunjin (KUN; D00246), Japanese encephalitis virus (JE; M55506), Murray Valley encephalitis virus (MVE; AF161266), and tick-borne encephalitis virus (TBE; U27495).
Significantly, when CRM1 was inhibited in DENV-2-infected cells, we observed increased nuclear accumulation of NS5, confirming that the NS5 NES is active during infection. We also observed an increase in virus production and a decrease in IL-8 induction. IL-8 is a pro-inflammatory CXC chemokine induced by stimuli, such as tumor necrosis factor, viral infection, and lipopolysaccharide (48), and has been implicated in inhibiting virus production (49). We showed previously that nuclear NS5 reduces or dampens IL-8 induction in response to DENV infection, leading to increased virus production (11). The results presented here are consistent with this, whereby increasing nuclear NS5 by inhibiting its nuclear export further dampened the IL-8 induction response and resulted in increased virus production.
Although the contribution of the nucleus and nuclear NS5 to DENV infection is only beginning to be elucidated (11), it seems clear that nuclear NS5 is involved in modulating host cell immune responses and virus production. The majority of NS5 is localized in the nucleus during infection, despite most replication occurring in the cytoplasm (11). This apparent paradox may be explained, at least in part, by the requirement of only small amounts of NS5 for replication of several flaviviruses (50–52). One possibility is that excess NS5 protein within the cytoplasm may be inhibitory to viral processes so that transport to the nucleus may relieve such problems as well as provide opportunities for additional nuclear functions such as those outlined above. This study provides an explanation as to how essential NS5 functions in both the cytoplasm and nucleus may be maintained through regulated NLS/NES activities.
In summary, we have identified a CRM1-mediated NES within NS5 that is active during infection and that, when inhibited, decreases IL-8 production and alters the kinetics of virus production. Current research in our laboratory is aimed at further elucidating the nuclear functions of NS5. The conservation of the identified NS5 NES region (Fig. 7) within related flaviviruses of medical importance such as Japanese encephalitis virus and West Nile virus makes it a promising candidate for the development of urgently needed new therapeutics to combat infection.
Acknowledgments
We thank Belinda Thomas and Phillip Bardin for technical assistance with the IL-8 enzyme-linked immunosorbent assays.
This work was supported by National Health and Medical Research Council Fellowship 384109 (Australia).
- DENV
- dengue virus
- NLS
- nuclear localization sequence
- Imp
- importin
- NES
- nuclear export sequence
- LMB
- leptomycin B
- IL-8
- interleukin-8
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- CLSM
- confocal laser scanning microscopy.
REFERENCES
- 1.Gubler D. J. ( 2002) Arch. Med. Res 33, 330– 342 [DOI] [PubMed] [Google Scholar]
- 2.Lindenbach B. D., Rice C. M. ( 2001) Flaviviridae: The Viruses and Their Replication, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 3.Mackenzie J. M., Westaway E. G. ( 2001) J. Virol 75, 10787– 10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor M., Zhang L., Ramachandra M., Kusukawa J., Ebner K. E., Padmanabhan R. ( 1995) J. Biol. Chem 270, 19100– 19106 [DOI] [PubMed] [Google Scholar]
- 5.Egloff M. P., Benarroch D., Selisko B., Romette J. L., Canard B. ( 2002) EMBO J 21, 2757– 2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koonin E. V. ( 1991) J. Gen. Virol 72, 2197– 2206 [DOI] [PubMed] [Google Scholar]
- 7.Koonin E. V. ( 1993) J. Gen. Virol 74, 733– 740 [DOI] [PubMed] [Google Scholar]
- 8.Ackermann M., Padmanabhan R. ( 2001) J. Biol. Chem 276, 39926– 39937 [DOI] [PubMed] [Google Scholar]
- 9.Bartholomeusz A. I., Wright P. J. ( 1993) Arch. Virol 128, 111– 121 [DOI] [PubMed] [Google Scholar]
- 10.Tan B. H., Fu J., Sugrue R. J., Yap E. H., Chan Y. C., Tan Y. H. ( 1996) Virology 216, 317– 325 [DOI] [PubMed] [Google Scholar]
- 11.Pryor M. J., Rawlinson S. M., Butcher R. E., Barton C. L., Waterhouse T. A., Vasudevan S. G., Bardin P. G., Wright P. J., Jans D. A., Davidson A. D. ( 2007) Traffic 8, 795– 807 [DOI] [PubMed] [Google Scholar]
- 12.Gorlich D., Kutay U. ( 1999) Annu. Rev. Cell Dev. Biol 15, 607– 660 [DOI] [PubMed] [Google Scholar]
- 13.Macara I. G. ( 2001) Microbiol. Mol. Biol. Rev 65, 570– 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried H., Kutay U. ( 2003) CMLS Cell. Mol. Life Sci 60, 1659– 1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutay U., Guttinger S. ( 2005) Trends Cell Biol 15, 121– 124 [DOI] [PubMed] [Google Scholar]
- 16.Mosammaparast N., Pemberton L. F. ( 2004) Trends Cell Biol 14, 547– 556 [DOI] [PubMed] [Google Scholar]
- 17.Fornerod M., Ohno M., Yoshida M., Mattaj I. W. ( 1997) Cell 90, 1051– 1060 [DOI] [PubMed] [Google Scholar]
- 18.la Cour T., Kiemer L., Molgaard A., Gupta R., Skriver K., Brunak S. ( 2004) Protein Eng. Des. Sel 17, 527– 536 [DOI] [PubMed] [Google Scholar]
- 19.Fischer U., Huber J., Boelens W. C., Mattaj I. W., Luhrmann R. ( 1995) Cell 82, 475– 483 [DOI] [PubMed] [Google Scholar]
- 20.Wen W., Meinkoth J. L., Tsien R. Y., Taylor S. S. ( 1995) Cell 82, 463– 473 [DOI] [PubMed] [Google Scholar]
- 21.Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E. P., Wolff B., Yoshida M., Horinouchi S. ( 1999) Proc. Natl. Acad. Sci. U. S. A 96, 9112– 9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo N., Wolff B., Sekimoto T., Schreiner E. P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. ( 1998) Exp. Cell Res 242, 540– 547 [DOI] [PubMed] [Google Scholar]
- 23.Nishi K., Yoshida M., Fujiwara D., Nishikawa M., Horinouchi S., Beppu T. ( 1994) J. Biol. Chem 269, 6320– 6324 [PubMed] [Google Scholar]
- 24.Begitt A., Meyer T., van Rossum M., Vinkemeier U. ( 2000) Proc. Natl. Acad. Sci. U. S. A 97, 10418– 10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murai N., Murakami Y., Matsufuji S. ( 2003) J. Biol. Chem 278, 44791– 44798 [DOI] [PubMed] [Google Scholar]
- 26.Scheifele L. Z., Ryan E. P., Parent L. J. ( 2005) J. Virol 79, 8732– 8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman M. P., de Noronha C. M., Heusch M. I., Greene S., Greene W. C. ( 2001) J. Virol 75, 1522– 1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strunze S., Trotman L. C., Boucke K., Greber U. F. ( 2005) Mol. Biol. Cell 16, 2999– 3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forwood J. K., Brooks A., Briggs L. J., Xiao C. Y., Jans D. A., Vasudevan S. G. ( 1999) Biochem. Biophys. Res. Commun 257, 731– 737 [DOI] [PubMed] [Google Scholar]
- 30.Brooks A. J., Johansson M., John A. V., Xu Y., Jans D. A., Vasudevan S. G. ( 2002) J. Biol. Chem 277, 36399– 36407 [DOI] [PubMed] [Google Scholar]
- 31.Johansson M., Brooks A. J., Jans D. A., Vasudevan S. G. ( 2001) J. Gen. Virol 82, 735– 745 [DOI] [PubMed] [Google Scholar]
- 32.Medin C. L., Fitzgerald K. A., Rothman A. L. ( 2005) J. Virol 79, 11053– 11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gualano R. C., Pryor M. J., Cauchi M. R., Wright P. J., Davidson A. D. ( 1998) J. Gen. Virol 79, 437– 446 [DOI] [PubMed] [Google Scholar]
- 34.Ghildyal R., Ho A., Wagstaff K. M., Dias M. M., Barton C. L., Jans P., Bardin P., Jans D. A. ( 2005) Biochemistry 44, 12887– 12895 [DOI] [PubMed] [Google Scholar]
- 35.la Cour T., Gupta R., Rapacki K., Skriver K., Poulsen F. M., Brunak S. ( 2003) Nucleic Acids Res 31, 393– 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. ( 1989) Gene (Amst.) 77, 51– 59 [DOI] [PubMed] [Google Scholar]
- 37.Pryor M. J., Carr J. M., Hocking H., Davidson A. D., Li P., Wright P. J. ( 2001) Am. J. Trop. Med. Hyg 65, 427– 434 [DOI] [PubMed] [Google Scholar]
- 38.Laemmli U. K. ( 1970) Nature 227, 680– 685 [DOI] [PubMed] [Google Scholar]
- 39.Bulich R., Aaskov J. G. ( 1992) J. Gen. Virol 73, 2999– 3003 [DOI] [PubMed] [Google Scholar]
- 40.Hubner S., Xiao C. Y., Jans D. A. ( 1997) J. Biol. Chem 272, 17191– 17195 [DOI] [PubMed] [Google Scholar]
- 41.Xiao C. Y., Hubner S., Jans D. A. ( 1997) J. Biol. Chem 272, 22191– 22198 [DOI] [PubMed] [Google Scholar]
- 42.Makino Y., Tadano M., Anzai T., Ma S. P., Yasuda S., Fukunaga T. ( 1989) J. Gen. Virol 70, 1417– 1425 [DOI] [PubMed] [Google Scholar]
- 43.Tadano M., Makino Y., Fukunaga T., Okuno Y., Fukai K. ( 1989) J. Gen. Virol 70, 1409– 1415 [DOI] [PubMed] [Google Scholar]
- 44.Alvisi G., Rawlinson S. M., Ghildyal R., Ripalti A., Jans D. A. ( 2008) Biochim. Biophys. Acta 1784, 213– 227 [DOI] [PubMed] [Google Scholar]
- 45.Poon I. K., Jans D. A. ( 2005) Traffic 6, 173– 186 [DOI] [PubMed] [Google Scholar]
- 46.Moseley G. W., Filmer R. P., DeJesus M. A., Jans D. A. ( 2007) Biochemistry 46, 12053– 12061 [DOI] [PubMed] [Google Scholar]
- 47.Poon I. K., Oro C., Dias M. M., Zhang J., Jans D. A. ( 2005) Cancer Res 65, 7059– 7064 [DOI] [PubMed] [Google Scholar]
- 48.Remick D. G. ( 2005) Crit. Care Med 33, S466– S467 [DOI] [PubMed] [Google Scholar]
- 49.Koo B. C., McPoland P., Wagoner J. P., Kane O. J., Lohmann V., Polyak S. J. ( 2006) J. Virol 80, 7885– 7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu P. W., Westaway E. G. ( 1992) Arch. Virol 125, 177– 191 [DOI] [PubMed] [Google Scholar]
- 51.Grun J. B., Brinton M. A. ( 1987) J. Virol 61, 3641– 3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchil P. D., Satchidanandam V. ( 2003) Virology 307, 358– 371 [DOI] [PubMed] [Google Scholar]